A R T I C L E
Design of phenanthrimidazole
‐pendant cyclam and cyclen
macrocyclic complex ligands as inorganic hosts:
Photophysics, electrochemistry, and bimetallic complex
formation
Aslihan Yilmaz Obali
Department of Chemistry, Science Faculty, Selcuk University, Selcuk, TurkeyCorrespondence
Aslıhan Yilmaz Obali, Department of Chemistry, Science Faculty, Selcuk University, Selcuk, Turkey. Email: aslihanyilmaz84@gmail.com
Funding information
Selcuk University (Konya/TURKEY), Grant/Award Number: 18401062
Abstract
Novel ruthenium(II)bpy complexes of the phenanthrimidazole‐pendant cyclen and cyclam macrocyclic ligands were synthesized as inorganic host molecules. Inorganic host design was planned as a“complex ligand” form because of its tetraaza macrocyclic unit, which has the ability to coordinate to the metals. Photophysical properties and metal selectivity of inorganic hosts RuL1A and RuL2 complex ligands were investigated by UV‐vis and fluorescence spectros-copy in aqueous medium. Among Ag(I), Li(I), Na(I), K(I), Cd(II), Cr(II), Fe(II), Hg(II), Ni(II), Pb(II), Zn(II), Cu(II), Mn(III), and Co(III) metal ions, Fe(II) addition causes spectral changes for both RuL1A and RuL2 complex ligands. Electrochemical studies were performed for RuL1B and also for cis‐ [Ru(bpy)2Cl2]. Bimetallic complexes [[Ru(bpy)2([Ni(2 ‐(4‐((1,4,8,11‐tetraaza-cyclotetradecan‐1‐yl)methyl)phenyl)‐1H‐imidazo[4,5‐ f ][1,10]phenanthroline])] (ClO4)4, RuL1‐NiA and [Ru(bpy)2([Ni(2‐(4‐((1,4,8,11‐tetraazacyclotetradecan‐1‐ yl)methyl)phenyl)‐1H‐imidazo[4,5‐ f ][1,10]phenanthroline])]Cl4, RuL1‐NiB solid complexes were also obtained by the reaction of the RuL1B and Nickel (II).
1 | I N T R O D U C T I O N
The coordination chemistry of macrocyclic ligands has been an important area for inorganic chemists for over years.[1–5] The photophysical properties of macrocyclic ligands with metal ions have led to applications of metal ion sensing, inorganic excited states in solar energy con-version, modelling for protein‐metal binding sites in a range of metalloproteins, host‐guest interactions, cataly-sis, and environmental studies.[6,7] Tetraazamacrocycles such as cyclen (1,4,7,10‐tetraazacyclododecane) and
cyclam (1,4,8,11‐tetraazacyclotetradecane) attract a great deal of research interest with their wide range of metal
ion complexations and high thermodynamic and kinetic stability.[8–10]
Recently, supramolecular host‐guest interactions and molecular recognitions of tetraazamacrocycles have an increasing attention paid on the design of“inorganic host” molecules of macrocyclic units with their valuable photophysical properties.[11,12]Inorganic host molecules have macrocyclic binding site/s for sensing of alkaline and alkaline‐earth metal ions and transition metal con-taining unit/s in design.[13,14]According to this knowledge, the light‐harvesting ruthenium(II)bpy polypyridines cova-lently linked to a macrocyclic unit is a perfect example for the areas of metal recognition. Herein, design of two novel
DOI: 10.1002/jhet.3793
J Heterocyclic Chem. 2020;57:400–412. © 2019 Wiley Periodicals, Inc. wileyonlinelibrary.com/journal/jhet
inorganic host molecules; ruthenium(II)bpy polypyridine coordinated phenanthrimidazole‐pendant cyclam and cyclen macrocycles are reported. Ruthenium(II)bpy con-tributes additional and interesting photophysical proper-ties involved in metal‐to‐ligand charge transfer MLCT excited states to the host molecules.[15–17] The synthesis, characterization, photophysics, and electrochemistry are described.
2 | E X P E R I M E N T A L S E C T I O N
2.1 | Reagents and instrumentation
All reagents used were of standard analytical grade from Sigma Aldrich, Merck, and used without further purifica-tion. All aqueous solutions were prepared with deionized water that passed through a Millipore Milli‐Q Plus water purification system.1H‐NMR spectra were recorded on a Varian 400‐MHz spectrometer. FT‐IR spectra were recorded using a Perkin Elmer Spectrum 100 FT‐IR spec-trometer. Elemental analyses were carried out using a LECO‐CHNS‐932 elemental analyzer. Magnetic suscepti-bilities of metal complexes were determined using a Sheerwood Scientific MX Gouy magnetic susceptibility apparatus using the Gouy method with Hg[Co(SCN)4] as calibrant. UV‐vis spectra were recorded on Perkin Elmer Lambda 25 UV‐vis spectrometer. Fluorescence measurements were performed using a Perkin Elmer LS 55 Luminescence spectrometer. All the electrochemical experiments were performed using a Gamry Reference 600 workstations (Gamry, Pennsylvania) electrochemical analyser (Model 600C series) equipped with BAS C3 cell stand. The working electrode was a bare, glassy carbon disk (BAS Model MF‐2012) with a geometric area of 0.027 cm2. The reference electrode was Ag/AgCl (0.01 M) in nonaqueous media, and the counter electrode was a Pt wire.
2.2 | Methods
1,10‐Phenanthroline‐5,6‐dione,[18] 2‐(4‐(bromomethyl) phenyl)‐1H‐imidazo[4,5‐ f ][1,10] phenanthroline, Phen‐ Br,[19]and cis‐[Ru(bpy)2Cl2].H2O[20]were synthesized by the literature methods. Cyclen (1,4,7,10 ‐tetraaza-cyclododecane), cyclam (1,4,8,11 ‐tetraazacyclo-tetradecane), and other materials were commercially available and purchased. Sodium perchlorate salt (NaClO4) was used in some complex precipitations. All of reagents were used without purification.
2.2.1 | Caution
Perchlorate salts of metal complexes with organic ligands are potentially explosive, and only small amounts of the material should be prepared and handled with great care.
2.3 | Synthesis
2.3.1 | 2‐(4‐((1,4,8,11‐
Tetraazacyclotetradecan
‐1‐yl)methyl)phe-nyl)‐1H‐imidazo[4,5‐f][1,10]phenanthroline,
L1
2‐(4‐(Bromomethyl)phenyl)‐1H‐imidazo[4,5‐ f ][1,10] phenanthroline, Phen‐Br (0.14 g, 0.375 mmol) was dis-solved in 50 mL chloroform and then added dropwise to the chloroform solution of cyclam (0.3 g, 1.5 mmol) and triethylamine (0.062 mL, 0.45 mmol). The mixture was refluxed at 62°C for 48 hours under N2. After cooling to room temperature, solution was washed with 1M NaOH (3 × 5 mL) to remove the excess of cyclam and then washed with water (3 × 5 mL), dried with MgSO4, and evaporated to yield a light yellow solid. Yield 0.34 g, % 45, FT‐IR ν (cm−1): 3064.17‐3059.02 (NHstretch), 2919.84‐ 2850.57 (CHstretch), 1735.70 (C═Nstretch), 1606.07‐1562.94 (NHbend), 1338.43 (C–Nstretch), 1445.15 (Aromatic C –C-stretch).1H‐NMR (400 MHz, DMSO‐d6) δ (ppm): 1.25 (m, 4H, CH2CH2CH2), 2.52 (s, 3H, cyclam‐NH), 2.80 (t, 8H, NHCH2CH2CH2), 3.05 (s, 8H, NHCH2CH2NH), 3.56 (s, 2H, CH2N), 5.01 (s, 1H, (imidazole‐NH), 7.31‐8.98 (m, 10H, aromatic‐CH). Elemental analysis (C30H36N8, 508.31 g/mol), Calculated (Found) %: C: 70.00 (71.94), H: 7.13 (7.03), N: 22.03 (21.78).2.3.2 | [Ru(bpy)
2(2
‐(4‐((1,4,8,11‐
tetraazacyclotetradecan‐1‐yl)methyl)phe-nyl)
‐1H‐imidazo[4,5‐f] [1,10]
phenanthroline)](ClO
4)
2, RuL1A
cis‐[Ru(bpy)2Cl2] (0.1 g, 0.02 mmol) and L1 (0.1 g, 0.02 mmol) were dissolved in 20 mL ethanol. The mixture was refluxed at 79°C for 12 hours under N2. The cooled reaction mixture was diluted with water (20 mL) and fil-tered to remove solid impurities. The complex was then precipitated by concentrated solution of NaClO4. Light yellow complex was filtered and washed with water and dried. Yield 0.013 g, % 59, FT‐IR ν (cm−1): 3064.10‐ 3058.20 (NHstretch), 2918.58‐2850.50 (CHstretch), 1735.70 (C═Nstretch), 1599.20‐1570.79 (NHbend), 1376.45 (C– Nstretch), 1459.41 (Aromatic C–C stretch), 1015.36 (ClO4− -stretch), 652.53 (RuNstretch).1H‐NMR (400 MHz, CDCl3) δ (ppm): 1.26 (m, 4H, CH2CH2CH2), 2.02 (s, 3H,
cyclam‐NH), 2.98 (t, 8H, NHCH2CH2CH2), 2.71 (s, 8H, NHCH2CH2NH), 3.05 (s, 2H,CH2N), 3.78 (s, 1H, (imid-azole‐NH), 6.76‐8.34 (m, 26H, aromatic‐CH). Elemental analysis (C50H52Cl2N12O8Ru, 1120.25 g/mol), Calculated (Found) %: C: 53.57 (52.94), H: 4.68 (4.87), N: 14.99 (14.84).
2.3.3 | [Ru(bpy)
2(2
‐(4‐((1,4,8,11‐
tetraazacyclotetradecan
‐1‐yl)methyl)phe-nyl)
‐1H‐imidazo[4,5‐f] [1,10]
phenanthroline)]Cl
2, RuL1B
cis‐[Ru(bpy)2Cl2] (0.2 g, 0.04 mmol) was dissolved in 20 mL methanol. And then poured into 10 mL methanol solution of L1 (0.2 g, 0.04 mmol). The mixture was refluxed at 65°C for 24 hours under N2. After the reaction was completed, methanol were evaporated. And resulted dark red solid was recrystallized by methanol‐ether (1:1) solvent mixture. The crude product was purified by
col-umn chromatography on a silica by using
chloroform/methanol/NH3 (15: 4: 1) as the eluent to afford RuL1B as clear red solid. Yield 0.015 g, % 38, FT‐ IR ν (cm−1): 3064.17‐3058.20 (NHstretch), 2918.58‐2850.50 (CHstretch), 1735.70 (C═Nstretch), 1599.20‐1570.79 (NHbend), 1376.45 (C–Nstretch), 1459.41 (Aromatic C –C-stretch), 652.53 (RuNstretch), 329.13 (RuCl). 1H‐NMR (400 MHz, CDCl3)δ (ppm): 1.25 (m, 4H, CH2CH2CH2), 2.01 (s, 3H, cyclam‐NH), 2.98 (t, 8H, NHCH2CH2CH2), 2.74 (s, 8H, NHCH2CH2NH), 3.05 (s, 2H, CH2N), 3.78 (s, 1H, (imidazole‐NH), 6.75‐8.33 (m, 26H, aromatic‐CH). Elemental analysis (C50H52Cl2N12Ru, 992.29 g/mol), Calculated (Found) %: C: 60.48 (61.01), H: 5.28 (5.12), N: 16.93 (16.64).
2.3.4 | [Ru(bpy)
2([Ni(2
‐(4‐((1,4,8,11‐
tetraazacyclotetradecan‐1‐yl)methyl)phe-nyl)
‐1H‐imidazo[4,5‐f][1,10]
phenanthroline])](ClO
4)
4, RuL1‐NiA
NiCl2.6H2O (0.028 g, 0.11 mmol) was added to RuL1B (0.116 g, 0.11 mmol), which was dissolved in 10 mL meth-anol. The reaction mixture was refluxed at 65°C for 15 hours. After filtration of impurities, the solvent were evaporated; 20 mL of water was added, and the complex was then precipitated by concentrated solution of NaClO4. The mixture was kept for 2 days at room temper-ature and then light yellow precipitates were formed and filtrated. Yield 0.023 g, % 17, FT‐IR ν (cm−1): 3064.17‐ 3058.20 (NHstretch), 2918.73‐2850.27 (CHstretch), 1726.96 (C═Nstretch), 1658.34‐1604.68 (NHbend), 1376.45 (C– Nstretch), 1461.52 (Aromatic C–Cstretch), 1098.47 (ClO4− -stretch), 622.14 (RuNstretch).1H‐NMR (400 MHz, CDCl3)δ
(ppm): 1.26 (m, 4H, CH2CH2CH2), 2.01 (s, 3H, cyclam‐NH), 2.98 (t, 8H, NHCH2CH2CH2), 2.74 (s, 8H, NHCH2CH2NH), 3.05 (s, 2H,CH2N), 3.78 (s, 1H, (imid-azole‐NH), 6.75‐8.33 (m, 26H, aromatic‐CH). Elemental analysis (C51H52Cl4N12NiO4Ru, 1196.14 g/mol), Calcu-lated (Found) %: C: 51.11 (51.04), H: 4.37 (4.32), N: 14.02 (14.11).
2.3.5 | [Ru(bpy)
2([Ni(2
‐(4‐((1,4,8,11‐
tetraazacyclotetradecan
‐1‐yl)methyl)phe-nyl)
‐1H‐imidazo[4,5‐f] [1,10]
phenanthroline])]Cl
4, RuL1
‐NiB
NiCl2.6H2O (0.028 g, 0.11 mmol) was added to RuL1B (0.116 g, 0.11 mmol) was dissolved in 10 mL methanol. The reaction mixture was refluxed at 65°C for 15 hours. After filtration of impurities, the solvent were evaporated. The dark yellow solid was recrystallized from methanol. The crude product was purified by column chromatogra-phy on a silica by using chloroform/methanol/NH3 (15: 10: 1) as the eluent. Yield 0.035 g, % 28, FT‐IR ν (cm−1): 3064.17‐3058.20 (NHstretch), 2918.73‐2850.27 (CHstretch), 1726.96 (C═Nstretch), 1658.34‐1604.68 (NHbend), 1376.45 (C–Nstretch), 1461.52 (Aromatic C–Cstretch), 622.14 (RuNstretch), 325.14 (RuCl). 1H‐NMR (400 MHz, CDCl3) δ (ppm): 1.25 (m, 4H, CH2CH2CH2), 2.01 (s, 3H, cyclam‐NH), 2.99 (t, 8H, NHCH2CH2CH2), 2.72 (s, 8H, NHCH2CH2NH), 3.05 (s, 2H,CH2N), 3.78 (s, 1H, (imid-azole‐NH), 6.75‐8.33 (m, 26H, aromatic‐CH). Elemental analysis (C51H52Cl4N12NiRu, 1132.16 g/mol), Calculated (Found) %: C: 53.99 (54.08), H: 4.62 (4.44), N: 14.81 (14.63).
2.3.6 | 2‐(4‐((1,4,7,10‐
Tetraazacyclododecan
‐1‐yl)methyl)phe-nyl)‐1H‐imidazo[4,5‐f][1,10]phenanthroline,
L2
2‐(4‐(Bromomethyl)phenyl)‐1H‐imidazo[4,5‐ f ][1,10] phenanthroline (0.056 g, 0.145 mmol) was dissolved in 20 mL chloroform and then added dropwise to the chlo-roform solution of cyclen (0.1 g, 0.580 mmol) and triethylamine (0.024 mL, 0.174 mmol). The mixture was refluxed at 62°C for 36 hours under N2. After cooling to room temperature, the solution was washed with 1M NaOH (3 × 5 mL) to remove the excess of cyclen and then washed with water (3 × 5 mL), dried with MgSO4, and evaporated to yield a dark yellow solid. Yield 0.043 g, % 53, FT‐IR ν (cm−1): 3086.15‐3065.49 (NHstretch), 2918.27‐ 2850.58 (CHstretch), 1737.04 (C═Nstretch), 1602.73‐1556.53 (NHbend), 1262.73 (C–Nstretch), 1458.18 (Aromatic C –C-stretch). 1H‐NMR (400 MHz, DMSO‐d6) δ (ppm): 2.42 (s,3H, cyclen‐NH), 3.12 (m, 16H, NHCH2CH2NH), 4.15 (s, 2H, CH2N), 5.23 (s, 1H, (imidazole‐NH), 7.22‐8.82 (m, 10H, aromatic‐CH). Elemental analysis (C28H32N8, 480.27 g/mol), Calculated (Found) %: C: 69.97 (69.44), H: 6.71 (6.32), N: 23.31 (22.98).
2.3.7 | [Ru(bpy)
2(2
‐(4‐((1,4,7,10‐
tetraazacyclododecan‐1‐yl)methyl)phenyl)‐
1
H‐imidazo[4,5‐f] [1,10]phenanthroline)]
(ClO
4)
2, RuL2
cis‐[Ru(bpy)2Cl2] (0.1 g, 0.02 mmol) and L2 (0.1 g, 0.02 mmol) were dissolved in 20 mL ethanol. The mixture was refluxed at 79°C for 12 hours under N2. The cooled reaction mixture was diluted with water (20 mL) and fil-tered to remove solid impurities. The complex was then precipitated by concentrated solution of NaClO4. Light yellow complex was filtered and washed with water and dried. Yield 0.012 g, % 55, FT‐IR ν (cm−1): 3086.15‐ 3065.49 (NHstretch), 2918.15‐2850.48 (CHstretch), 1721.20 (C═Nstretch), 1602.73‐1599.38 (NHbend), 1266.55 (C– Nstretch), 1461.44 (Aromatic C–Cstretch), 1114.48 (ClO4− -stretch), 617.14 (RuNstretch).
1
H‐NMR (400 MHz, CDCl3)δ (ppm): 2.21 (s, 3H, cyclen‐NH), 2.98 (m, 16H, NHCH2CH2NH), 3.52 (s, 2H, CH2N), 4.00 (s, 1H, (imid-azole‐NH), 6.72‐8.23 (m, 26H, aromatic‐CH). Elemental analysis (C48H48Cl2N12O8Ru, 1092.21 g/mol), Calculated (Found) %: C: 52.75 (52.93), H: 4.43 (3.99), N: 15.38 (15.11).
3 | R E S U L T A N D D I S C U S S I O N
3.1 | Synthesis and characterizations
Main initial reagent“phenanthrimidazole unit” of the syn-thesis path, 2‐(4‐(bromomethyl)‐phenyl)‐1H‐imidazo[4,5‐
f][1,10]phenanthroline,Phen‐Br, was obtained by the condensation reaction of 1,10‐phenanthroline‐5,6‐dione and 4‐(bromomethyl)benzaldehyde with the addition of ammonium acetate in glacial acetic acid medium.
Cyclam and cyclen macrocyclic units then mono‐ alkylated with that phenanthrimidazole unit to obtain L1, 2‐(4‐((1,4,8,11‐tetraazacyclotetradecan‐1‐yl)methyl) phenyl)‐1H‐imidazo[4,5‐ f ][1,10]phenanthroline, and L2, 2‐(4‐((1,4,7,10‐tetraazacyclododecan‐1‐yl)methyl)phenyl)‐ 1H‐imidazo[4,5‐ f ][1,10]phenanthroline, ligands in one step. By the reaction of cis‐[Ru(bpy)2Cl2].H2O and L1 and L2 ligands, RuL1A and RuL2 were isolated as per-chlorate salts, and RuL1B was isolated with chlorine anionic part (Figures 1 and2).
Selective mono‐alkylation was an important step in the preparation of ligand L1 and L2 designs. Several
routes for mono‐alkylation were reported as addition of protecting groups such as tert‐butyloxy‐carbonyl, tosyl, and formyl before alkylation to protect other three amines in the cyclic tetraamine or blocking three of nitrogen atoms from the inside of macrocycle by the addition of sterically hindered reagents such as glyoxal, phosphoryl
species, and metal carbonyls in the previous studies.[21– 23]
Direct alkylation method was preferred in this study. Herein, using excess amount of cyclam or cyclen gave opportunity to have mono‐alkylation with one‐side bond-ing and at one step. For the excess usage of high cost cyclam or cyclen, recycling is needed. In washing step with alkaline solution and water, the unreacted cyclen or cyclam could be recycled by concentrating the alkaline solution under reduced pressure adjusting the pH: 7 with 1M HCl. This gave white precipitates as cyclam or cyclen reagent.[24]
Thence, there are free aza groups in the complex ligands, metal coordination can be expected, and RuL1‐ NiA and RuL1‐NiB complexes were designed and syn-thesized as solid compounds by the reaction of RuL1B and NiCl2.6H2O. RuL1‐NiA was isolated as perchlorate salt, and RuL1‐NiB was isolated with chlorine anionic part.
All the compounds were characterized by 1H‐NMR spectroscopy, FT‐IR spectroscopy, and elemental analysis. The chemical shifts ofCH2‐N protons at 3.56 ppm for L1 and at 4.15 ppm for L2 proves the alkylation of the cyclam/cyclen macrocyclic compounds. Additionally, the chemical shifts of imidazole‐NH protons at 5.01 ppm for L1 and at 5.2 ppm for L2 were characteristic data for the ligands. The ruthenium(II) complex ligands RuL1A, RuL1B, and RuL2 and bimetallic complexes RuL1‐NiA and RuL1‐NiB have also 1H‐NMR data because of their diamagnetic nature. The data were given in experimental part with the synthesis procedures. Dia-magnetic properties were proved by the Dia-magnetic suscep-tibility studies of the bimetallic complexes RuL1‐NiA and RuL1‐NiB. They show diamagnetic properties with electronic structures of t2g6eg2.
The summary of the FT‐IR data were given in Table 1. In FT‐IR spectrum of the ligands, the frequency peaks of cyclam‐NH stretch/cyclen‐NH stretch were seen at 3064.17‐3059.02 cm−1/3085.15‐3065.49 cm−1, and cyclam‐NH bend/cyclen‐NH bend were seen at 1606.07‐ 1562.94 cm−1/1602.73‐1556.53 cm−1. After complexation with cis‐[Ru(bpy)2Cl2].H2O, the peaks for RuN stretch were seen at 652.53 cm−1 for RuL1A and RuL1B and at 617.14 cm−1 for RuL2. The perchlorate frequency peaks were observed at 1015.36 cm−1 for RuL1A and 1114.48 cm−1for RuL2.
After coordination of Nickel(II) to the complex ligand RuL1B, some changes were observed in all
FIGURE 2 Synthesis route of 2‐(4‐((1,4,7,10‐tetraazacyclododecan‐1‐yl)methyl)phenyl)‐1H‐imidazo[4,5‐f][1,10]phenanthroline, L2 macrocyclic ligand and [Ru(bpy)2(2‐(4‐((1,4,7,10‐tetraazacyclododecan‐1‐yl)methyl)phenyl)‐1H‐imidazo[4,5‐f][1,10]phenanthroline)](ClO4)2,
RuL2 ligand‐complex. Reaction conditions were (i) chloroform, 62°C, 36 hours, N2; (ii) ethanol, 79°C, 12 hours, N2
FIGURE 1 Synthesis route of 2‐(4‐((1,4,8,11‐tetraazacyclotetradecan‐1‐yl)methyl)phenyl)‐1H‐imidazo[4,5‐f][1,10]phenanthroline, L1 macrocyclic ligand, and [Ru(bpy)2(2‐(4‐((1,4,8,11‐tetraazacyclotetradecan‐1‐yl)methyl) phenyl)‐1H‐imidazo[4,5‐f][1,10]phenanthroline)]
(ClO4)2, RuL1A, [Ru(bpy)2(2‐(4‐((1,4,8,11‐tetraazacyclo tetradecan‐1‐yl)methyl)phenyl)‐1H‐imidazo[4,5‐f][1,10]phenanthroline)]Cl2, RuL1B
ligand‐complexes. Reaction conditions were (i) chloroform, 62°C, 48 hours, N2; (ii) ethanol, 79°C, 12 hours, N2; (iii) methanol, 65°C,
spectra.[25–27] 1H‐NMR and FT‐IR results shows nickel(II) coordination to all the NH fragments and C═N fragment in RuL1B. The peaks of cyclam‐NH bend were shifted to 1658.34‐1604.68 cm−1 for both bimetallic complexes RuL1‐NiA and RuL1‐NiB. Additionally, the frequency peak of C═N stretch of RuL1B was shifted from 1735.70 cm−1to 1726.96 cm−1for bimetallic complexes. The presence of NH bands in all cases shows that depro-tonation of the NH‐nitrogen has not occurred. FT‐IR spectra superpositions of the perchlorate salts of complex ligand RuL1A/RuL1‐NiA and also RuL1‐NiA/RuL1‐ NiB were also displayed in Figures 3 and 4. Elemental analysis data were given in experimental part with the synthesis procedures.
3.2 | Photophysical properties
Absorption studies of the macrocylic ligands (L1, L2) (1 × 10−4M in chloroform) and inorganic host complex ligands (RuL1A and RuL1B) and metal binding abilities were studied by UV‐vis spectroscopy (1 × 10−4M in water). Metal‐binding studies were performed with RuL1A/RuL2 hosts (1 × 10−4M in water, 1equiv.) in the presence of different metal ions: Ag(I), Li(I), Na(I), K(I), Cd(II), Cr(II), Fe(II), Hg(II), Ni(II), Pb(II), Zn(II), Cu(II), Mn(III), and Co(III) (1 × 10−4M in water, 2equiv.). Superpositions of the L1/RuL1A and L2/RuL2 absorption spectra are displayed in Figures 5 and 6. UV‐ vis spectra of RuL1A and RuL1B and the appearance
TABLE 1 Summary of the FT‐IR frequencies (ν) of the functional groups of ligands L1 and L2, ligand‐complexes RuL1A, RuL1B, and
RuL2, and bimetallic complexes RuL1‐NiA, RuL1‐NiB
Compounds
ν (cm−1)
NHstretch CHstretch C═Nstretch NHbend
C– Nstretch
C–Cstretch
(Ar) ClO4−stretch RuNstretch RuClstretch L1 3064.17‐3059.02 2919.84‐2850.57 1735.70 1606.07‐1562.94 1338.43 1445.15 ‐ ‐ ‐ RuL1A 3064.10‐3058.20 2918.58‐2850.50 1735.70 1599.20‐1570.79 1376.45 1459.41 1015.36 652.53 ‐ RuL1B 3064.17‐3058.20 2918.58‐2850.50 1735.70 1599.20‐1570.79 1376.45 1459.41 ‐ 652.53 329.13 RuL1‐NiA 3064.17‐3058.20 2918.73‐2850.27 1726.96 1658.34‐1604.68 1376.45 1461.52 1098.47 622.14 RuL1‐NiB 3064.17‐3058.20 2918.73‐2850.27 1726.96 1658.34‐1604.68 1376.45 1461.52 ‐ 622.14 325.14 L2 3085.15‐3065.49 2918.27‐2850.58 1737.04 1602.73‐1556.53 1262.73 1458.18 ‐ ‐ ‐ RuL2 3086.15‐3065.49 2918.15‐2850.48 1721.20 1602.73‐1599.38 1266.55 1461.44 1114.48 617.14 ‐
FIGURE 3 FT‐IR spectrum of [Ru(bpy)2([Ni(2‐(4‐((1,4,8,11‐tetraazacyclotetradecan‐1‐yl)methyl)phenyl)‐1H‐imidazo [4,5‐f][1,10]
phenanthroline])](ClO4)4, RuL1‐NiA (top, green color) and [Ru(bpy)2(2‐(4‐((1,4,8,11‐tetraazacyclo tetradecan‐1‐yl)methyl)phenyl)‐1H‐
FIGURE 4 FT‐IR spectrum of [Ru(bpy)2([Ni(2‐(4‐((1,4,8,11‐tetraazacyclotetradecan‐1‐yl)methyl)phenyl)‐1H‐imidazo[4,5‐f][1,10]
phenanthroline])](ClO4)4, RuL1‐NiA (top, blue color) and [Ru(bpy)2([Ni(2‐(4‐((1,4,8,11‐tetraazacyclotetradecan‐1‐yl)methyl)phenyl)‐1H‐
imidazo[4,5‐f][1,10]phenanthroline])]Cl4, RuL1‐NiB (bottom, red color) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 5 The absorption spectra of macrocyclic ligand, L1 (1 × 10−4M in chloroform) and complex ligand RuL1A (1 × 10−4M in water) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 6 The absorption spectra of macrocyclic ligand, L2 (1 × 10−4M in chloroform) and complex ligand RuL2 (1 × 10−4M in water) [Color figure can be viewed at wileyonlinelibrary.com]
of their water solutions were also displayed in Figures 7 and 8. Because the appearance of the two complex ligands, RuL1A and RuL1B, is very different from each under daylight, absorbances were also expected as differ-ent. According to the data, RuL1B is darker in color and absorps UV light more than RuL1A under the same
conditions and concentrations (1 × 10−4M, in water, at room temperature).
As seen from the absorption spectra of L1, the bands appearing at 275, 329, and 393 nm could be assigned to cyclam‐phenanthrimidazole centered π → π* transitions, and for the ligand L2, the bands appearing at 281, 331, and 393 nm were assigned to cyclen‐phenanthrimidazole centered π → π* transitions. The complex RuL1A has three bands at 289, 340, and 461 nm and the complex RuL2 has also three bands at 290, 345, and 461 nm. These bands were assigned to the intraligand bpy/ligand based π → π*, π → π* transitions and metal‐ligand charge transfer MLCT. For the complex ligand, two distinct MLCT bands [dπRu → π*bpy and dπRu → π*L1/L2 transitions] might be expected. Because of the broad band nature, one broad shoulder was observed as MLCT band.[28–30] Additionally, the MLCT bands of the ruthenium(II) complex ligands are red‐ shifted in comparison with that of [Ru(bpy)3]2+ com-plex, which has maximum absorbance atλ: 450 nm[31,32]
FIGURE 7 The absorption spectra of the complex ligands RuL1A and RuL1B (1 × 10−4M in water) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 8 The appearance of the macrocyclic ligands L1 and L2 (1 × 10−4M in chloroform) and complex ligands RuL1A, RuL1B, and RuL2 under daylight (1 × 10−4M in water) [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2 Absorption data of the L1 and L2 ligands, RuL1A and RuL1B complex ligands, and Ru(bpy)32+complex at their maximum
wavelength in nanometer
Compounds
λmax(nm)
π → π* transitions π → π* transitions π → π* transitions dπ→ π* (MLCT) transitions
L1 275 329 393 RuL1A 289 340 461 RuL1B 291 342 485 L2 281 331 393 RuL2 290 345 461 Ru(bpy)32+ 285 344 450
(Table 2). This 11 nm shift can be attributed to the increased π‐delocalization and π‐acceptor capacity of the ligands (L1, L2) resulting in decrease of electron den-sity on ruthenium(II) center.
Metal‐binding abilities of RuL1A and RuL2 inorganic hosts (1 × 10−4M in water, 1equiv.) were performed with the titration of various metal ions such as Ag(I), Li(I), Na (I), K(I), Cd(II), Cr(II), Fe(II), Hg(II), Ni(II), Pb(II), Zn (II), Cu(II), Mn(III), and Co(III) (1 × 10−4M in water, 2equiv.). Maximum absorbance of Fe(II) titration was observed atλ: 280 nm and RuL1A and other metal ions atλ: 285 nm. Fe(II) addition increases the absorbance of RuL1A, and it was observed that other metal ion addi-tions decrease the absorbances of RuL1A at their maxi-mum wavelengths and Co(II) addition also decreases the absorbance, most (Figure 9).
It is nearly the same Fe(II) effect for RuL2 complex ligand. Maximum absorbance of Fe(II) titration was observed atλ: 285 nm, and RuL2 and other metal ions were observed at λ: 290 nm. Fe(II) was increasing the absorbance of RuL2, and Cu(II) was decreasing the absorbance most (Figure 10).
Fluorescence properties of the macrocylic ligands (L1, L2) with the concentration of 1 × 10−6M in chloroform and inorganic hosts (RuL1A and RuL1B complex ligands)
FIGURE 9 The absorption spectra of the complex ligand RuL1A (1 × 10−4M in water, 1equiv.) in the presence of different metal ions: Ag(I), Li(I), Na(I), K(I), Cd(II), Cr(II), Fe(II), Hg(II), Ni(II), Pb(II), Zn(II), Cu(II), Mn(III), and Co(III) (1 × 10−4M in water, 2equiv.) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 10 The absorption spectra of the complex ligand RuL2 (1 × 10−4M in water, 1equiv.) in the presence of different metal ions: Ag(I), Li(I), Na(I), K(I), Cd(II), Cr(II), Fe(II), Hg(II), Ni(II), Pb(II), Zn(II), Cu(II), Mn(III), and Co(III) (1 × 10−4M in water, 2equiv.) [Color figure can be viewed at wileyonlinelibrary.com]
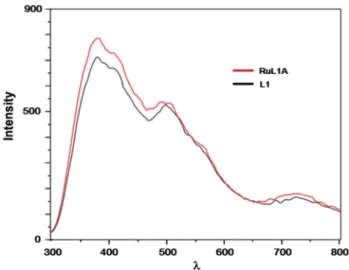
FIGURE 11 The fluorescence spectra of macrocyclic ligand L1 (1 × 10−6M in chloroform) and complex ligand RuL1A
(1 × 10−6M in water) [Color figure can be viewed at wileyonlinelibrary.com]
and metal binding abilities were also studied with the con-centration of 1 × 10−6M in water atλexcitation: 270 nm.
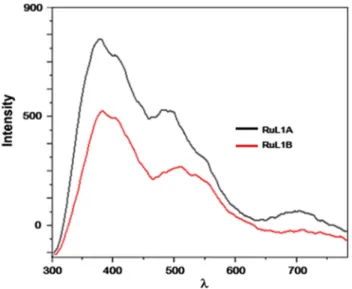
Fluorescence intensity superpositions of the ligands, L1 and L2, and their complex ligands, RuL1A and RuL2, are displayed in Figures 11 and 12. The spectra of RuL1A and RuL1B comparison are also investigated. RuL1B has lower fluorescence than RuL1A under the same conditions and concentrations (1 × 10−4M, in water, at room temperature) (Figure 13). When excitated at λexcitation: 270 nm, the maximum emission intensities of L1 was observed at λ: 383, 497, 710 nm; RuL1A was observed atλ: 383, 487, 710 nm; and L2 was observed at
λ: 392, 411, 723 nm; RuL2 was observed at λ: 378, 496, 700 nm.
Metal‐binding titrations were performed with the RuL1A and RuL2 inorganic hosts (1 × 10−4M in water, 1equiv.) in the presence of different metal ions: Ag(I), Li (I), Na(I), K(I), Cd(II), Cr(II), Fe(II), Hg(II), Ni(II), Pb (II), Zn(II), Cu(II), Mn(III), and Co(III) (1 × 10−4M in water, 2equiv.) (λexcitation: 270 nm). Upon addition of Fe
FIGURE 12 The fluorescence spectra of macrocyclic ligand L2 (1 × 10−6M in chloroform) and complex ligand RuL2 (1 × 10−6M in water) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 13 The fluorescence spectra of the complex ligands
RuL1A and RuL1B (1 × 10−6M in water) [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 14 The fluorescence intensities of the complex ligand
RuL1A (1 × 10−5M in water, 1equiv.) in the presence of different metal ions: Ag(I), Li(I), Na(I), K(I), Cd(II), Cr(II), Fe(II), Hg(II), Ni(II), Pb(II), Zn(II), Cu(II), Mn(III), and Co(III) (1 × 10−5M in water, 2equiv.) [Color figure can be viewed at wileyonlinelibrary. com]
FIGURE 15 The fluorescence intensities of the complex ligand
RuL2 (1 × 10−5M in water, 1equiv.) in the presence of different metal ions: Ag(I), Li(I), Na(I), K(I), Cd(II), Cr(II), Fe(II), Hg(II), Ni(II), Pb(II), Zn(II), Cu(II), Mn(III), and Co(III) (1 × 10−5M in water, 2equiv.) [Color figure can be viewed at wileyonlinelibrary. com]
(II) ion to RuL1A, the fluorescence maximum were observed atλ:412, 476, 710 nm. RuL1A and other metal ions were observed at λ: 383, 487, 710 nm (Figure 14). And upon addition of Fe(II) ion to RuL2, the fluores-cence maximum were observed at λ: 413, 482, 710 nm. RuL2 and other metal ions were observed at λ: 378, 496, 700 nm (Figure 15). For both complex ligands RuL1A and RuL2, upon addition of Fe(II) metal ions, the emission maxima underwent red‐shifts, and the intensities were a little increased.
3.3 | Binding stoichiometry
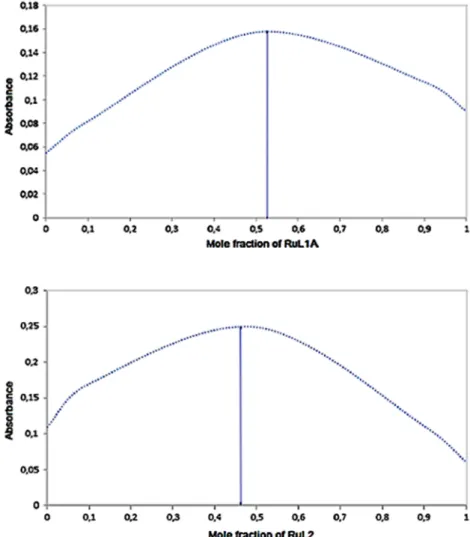
The stoichiometry of the bimetallic complexations of RuL1A + Fe(II) and RuL2 + Fe(II) in water were deter-mined by using Job's plot method. Maximum plots of the absorbance were recorded onλ: 285 nm for RuL1A + Fe (II) andλ: 296 nm for RuL2 + Fe(II). The Job graphs are shown in Figures 16 and 17. The result of Job's plot identified as 1:1 complexation between RuL1A and Fe (II), because the RuL1A‐Fe(II) bimetallic complex concentration approaches a maximum when the mole
fraction of RuL1A {[RuL1A]/([RuL1A] + [Fe(II)])} is about 0.57.
Nearly the same approach that the binding stoichiom-etry is calculated for the RuL2 and Fe(II) as a bimetallic complex at a 1:1 ratio on 0.46 mole fraction.
3.4 | Electrochemistry
The electrochemical experiments were performed by cyclic voltammetry. The working electrode was a glassy carbon disk. The reference electrode was Ag/AgCl (0.01M) in nonaqueous media, and the counter electrode was a Pt wire. The glassy carbon electrodes were prepared by first polishing them with fine, wet emery papers (grain size 4000) and then 0.1 and 0.05 lm alumina slurry on polishing pads in order to give them a mirror‐like appear-ance. The electrodes were sonicated for 5 minutes in water and in a 50:50 (v/v) isopropyl alcohol and acetonitrile (IPA + MeCN) solution purified over activated carbon. Prior to the electrochemical experiments, the electrodes were dried with an argon gas stream, and the solutions were purged with pure argon gas (ie, 99.999 %) for at least
FIGURE 16 Job plot of the RuL1A‐Fe (II) bimetallic complex in water with the ratio 1:1. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 17 Job plot of the RuL2‐Fe(II) bimetallic complex in water with the ratio 1:1. [Color figure can be viewed at wileyonlinelibrary.com]
10 minutes; additionally, an argon atmosphere was main-tained over the solution during the experiments.
Cyclic voltammetric measurements of cis‐[Ru(bpy) 2Cl2] (which is one of the initial reagents of the complex ligand, RuL1B, in synthesis path) and [Ru(bpy)2(2‐(4‐ ((1,4,8,11‐tetraaza cyclotetradecan‐1‐yl)methyl)phenyl)‐ 1H‐imidazo[4,5‐ f ][1,10]phenanthroline)]Cl2, RuL1B were conducted in CH3CN:H2O solution mixture (50:50) containing 0.1M KH2PO4as the supporting electrolyte at room temperature. Oxidation peaks were observed in Figure 18. RuL1B exhibits one oxidation at E ° = +1.68 V vs Ag/AgCl but initial reagent cis‐[Ru (bpy)2Cl2] has no oxidation on this potential but one oxi-dation at E° = +0.85 V. As a reference, [Ru(bpy)3]2+ complex is known to exhibit one oxidation at E ° = +1.26 V vs Ag/AgCl.[33] According to the data, RuL1B is more positive than [Ru(bpy)3]2+and cis‐[Ru (bpy)2Cl2] in CH3CN:H2O solution mixture.
4 | C O N C L U S I O N
Phenanthrimidazole‐pendant cyclam and cyclen macro-cyclic ligands (L1, L2) were synthesized in good yields. Inorganic hosts, ruthenium(II)bpy coordinated phenanthrimidazole complex ligands with macrocyclic units (RuL1A, RuL1B, and RuL2), were designed and obtained. Macrocyclic units have four aza groups, and phenanthrimidazole groups have also two aza groups to catch metals targets. According to the FT‐IR and 1H‐ NMR data, it was estimated that bimetallic ruthenium (II)‐nickel(II) complexes RuL1‐NiA and RuL1‐NiB were obtained, which is compatible with previous literature data. Metal binding properties of inorganic hosts were investigated by absorption and fluorescence studies. For both complex ligands RuL1A and RuL2, upon addition
of Fe(II) metal ions, the emission maxima underwent red‐shifts, and the intensities were a little increased.
A C K N O W L E D G M E N T
We thank the Scientific Research Projects Foundation (BAP) of Selcuk University (Konya/TURKEY) with grand number 18401062 for financial support of this work.
O R C I D
Aslihan Yilmaz Obali https://orcid.org/0000-0001-6753-2958
R E F E R E N C E S
[1] A. Prasanna de Silva, H. Q. Nimal Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher, T. E. Rice, Chem. Rev. 1997, 97, 1515.
[2] B. Yibing Shen, P. Sullivan, Inorg. Chem. 1995, 34, 6235. [3] H. Bang, E. J. Lee, E. Y. Lee, J. Suh, M. P. Suh, Inorg. Chim.
Acta2000, 308, 150.
[4] P. Deveci, J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 319. [5] Özpınar K, Deveci P, Kılınc M¸ Özcan E, Karaman BD,
Karaarslan H, Taner B, Kılıc Z. , J. Incl. Phenom. Macrocycl.
Chem., 2015, 82, 407–415.
[6] A. Gans, R. Belda, J. Pitarch, R. Goddard, E. Garcia‐Espana, S. Kubik, Org. Lett. 2015, 17, 5850.
[7] D. Dischino, E. J. Delaney, J. E. Emswiler, G. T. Gaughan, J. S. Prasad, S. K. Srivastava, M. F. Tweedle, Inorg. Chem. 1991, 30, 1265.
[8] C. Li, W.‐T. Wong, Tetrahedron Lett. 2002, 43, 3217.
[9] W. J. Kruper Jr., P. R. Rudolf, C. A. Langhoff, J. Of Org. Chem.
1993, 58, 3869.
[10] I. Meunier, A. K. Mishra, B. Hanquet, P. Cocolios, R. Guilard,
Can.J. Chem.1995, 73, 685.
FIGURE 18 Cyclic voltammogram of (A) cis‐[Ru(bpy)2Cl2] initial reagent and
(B) [Ru(bpy)2(2‐(4‐((1,4,8,11‐
tetraazacyclotetradecan‐1‐yl)methyl) phenyl)‐1H‐imidazo[4,5‐f][1,10] phenanthroline)]Cl2, RuL1B complex
ligand in acetonitrile‐water (50:50) (and 0.1M KH2PO4) at room temperature
[Color figure can be viewed at wileyonlinelibrary.com]
[11] C. Li, W.‐T. Wong, J. Org. Chem. 2003, 68, 2956.
[12] A. El Majzoub, C. Cadiou, I. Déchamps‐Olivier, F. Chuburu, M. Aplincour, Eur. J. Inorg. Chem. 2007, 5087.
[13] M.‐J. Li, B. W.‐K. Chu, N. Zhu, V. W.‐W. Yam, Inorg. Chem.
2007, 46, 720.
[14] V. W.‐W. Yam, V. W.‐M. Lee, F. Ke, M. S. Kam‐Wing, Inorg.
Chem.1997, 36, 2124.
[15] B. Yibing Shen, P. Sullivan, J. Chem. Educ. 1997, 74, 685. [16] B. W.‐K. Chu, V. W.‐W. Yam, Langmuir 2006, 22, 7437. [17] E. Tfouni, K. Q. Ferreira, F. G. Doro, R. S. da Silva, Z. N. da
Rocha, Coord. Chem. Rev. 2005, 249, 405.
[18] R. H. Zheng, H. C. Guo, H. J. Jiang, K. H. Xu, B. B. Liu, W. L. Sun, Z. Q. Shen, Chin. Chem. Lett. 2010, 21, 1270.
[19] A. Y. Obali, H. I. Ucan, J. Fluoresc. 2016, 26, 1685.
[20] B. P. Sullivan, D. J. Salmon, T. J. Meyer, Inorg. Chem. 1978, 17 (12), 3334.
[21] E. Kimura, S. Aoki, T. Koike, A. Shiro, J. Am. Chem. Soc. 1997,
119, 3068.
[22] J. A. Halfen, V. G. Young Jr., Chem. Commun. 2003, 2894. [23] V. Boldrini, G. B. Giovenzana, R. Pagliarin, G. Palmisano, M.
Sisti, Tetrahedron Lett. 2000, 41, 6527.
[24] J. Massue, S. E. Plush, C.'l. S. Bonnet, D. A. Moore, T. Gunnlaugsson, Tetrahedron Lett.2007, 48, 8052.
[25] E. Kimura, S. Wada, M. Shionaya, Y. Okazaki, Inorg. Chem.
1994, 33, 770.
[26] K. W. Wellington, P. T. Kaye, G. M. Watkins, ARKIVOC 2008 (xvii), 248.
[27] Z. Cai, Z. Mao, J. Xu, J. Mol. Struct. 2011, 1006, 282.
[28] D. Amilan Jose, P. Kar, D. Koley, B. Ganguly, W. Thiel, H. N. Ghosh, A. Das, Inorg. Chem. 2007, 46, 5576.
[29] A. Ghosh, B. Ganguly, A. Das, Inorg. Chem. 2007, 46, 9912. [30] D. Atindra, Inorg. Chim. Acta 1999, 285, 89.
[31] A. Juris, V. Balzani, F. Barigelletti, S. Campagna, P. Belser, A. Von Zelewsky, Coord. Chem. Rev. 1988, 84, 85.
[32] J. V. Caspar, T. J. Meyer, J. Am. Chem. Soc. 1983, 105, 5583. [33] B. D. Muegge, M. M. Richter, Anal. Chem. 2002, 74, 547.
S U P P O R T I N G I N F O R M A T I O N
Additional supporting information may be found online in the Supporting Information section at the end of the article.
How to cite this article: Obali AY. Design of phenanthrimidazole‐pendant cyclam and cyclen macrocyclic complex ligands as inorganic hosts: Photophysics, electrochemistry, and bimetallic complex formation. J Heterocyclic Chem. 2020;57: 400–412. https://doi.org/10.1002/jhet.3793
![FIGURE 2 Synthesis route of 2‐(4‐((1,4,7,10‐tetraazacyclododecan‐1‐yl)methyl)phenyl)‐1H‐imidazo[4,5‐f][1,10]phenanthroline, L2 macrocyclic ligand and [Ru(bpy) 2 (2‐(4‐((1,4,7,10‐tetraazacyclododecan‐1‐yl)methyl)phenyl)‐1H‐imidazo[4,5‐f][1,10]phenanthroline](https://thumb-eu.123doks.com/thumbv2/9libnet/4973155.100697/5.892.191.708.72.605/figure-synthesis-tetraazacyclododecan-phenanthroline-macrocyclic-tetraazacyclododecan-imidazo-phenanthroline.webp)
![FIGURE 3 FT‐IR spectrum of [Ru(bpy) 2 ([Ni(2‐(4‐((1,4,8,11‐tetraazacyclotetradecan‐1‐yl)methyl)phenyl)‐1H‐imidazo [4,5‐f][1,10]](https://thumb-eu.123doks.com/thumbv2/9libnet/4973155.100697/6.892.68.832.111.344/figure-ft-ir-spectrum-tetraazacyclotetradecan-methyl-phenyl-imidazo.webp)
![FIGURE 4 FT ‐IR spectrum of [Ru(bpy) 2 ([Ni(2 ‐(4‐((1,4,8,11‐tetraazacyclotetradecan‐1‐yl)methyl)phenyl)‐1H‐imidazo[4,5‐f][1,10]](https://thumb-eu.123doks.com/thumbv2/9libnet/4973155.100697/7.892.191.701.70.382/figure-ft-ir-spectrum-tetraazacyclotetradecan-methyl-phenyl-imidazo.webp)
![FIGURE 7 The absorption spectra of the complex ligands RuL1A and RuL1B (1 × 10 −4 M in water) [Color figure can be viewed at wileyonlinelibrary.com]](https://thumb-eu.123doks.com/thumbv2/9libnet/4973155.100697/8.892.82.546.67.582/figure-absorption-spectra-complex-ligands-color-viewed-wileyonlinelibrary.webp)
![FIGURE 18 Cyclic voltammogram of (A) cis ‐[Ru(bpy) 2 Cl 2 ] initial reagent and (B) [Ru(bpy) 2 (2 ‐(4‐((1,4,8,11‐](https://thumb-eu.123doks.com/thumbv2/9libnet/4973155.100697/12.892.84.565.64.363/figure-cyclic-voltammogram-cis-ru-bpy-initial-reagent.webp)