Contents lists available atScienceDirect
Industrial Crops & Products
journal homepage:www.elsevier.com/locate/indcropMetabolomics pro
filing, bio-pharmaceutical properties of Hypericum
lanuginosum extracts by in vitro and in silico approaches
Mohamad Fawzi Mahomoodally
a,1, Gokhan Zengin
b,1,⁎, Dimitrina Zheleva-Dimitrova
c,
Adriano Mollica
d, Azzurra Stefanucci
d, Kouadio Ibrahime Sinan
b,
Muhammad Zakariyyah Aumeeruddy
aaDepartment of Health Sciences, Faculty of Science, University of Mauritius, 230 Réduit, Mauritius bDepartment of Biology, Faculty of Science, Selcuk University, Turkey
cDepartment of Pharmacognosy, Faculty of Pharmacy, Medical University of Sofia, Bulgaria dDepartment of Pharmacy, University“G. d’Annunzio” of Chieti-Pescara, 66100 Chieti, Italy
A R T I C L E I N F O Keywords: Docking Antioxidant Enzyme inhibition Phenolic Tyrosinase LC-HRMS A B S T R A C T
Hypericum species are important as a source of natural-bioactive compounds in the Turkish folk medicine. Among them, Hypericum lanuginosum has not been explored so far for its biological properties. The current study aimed to determine the antioxidant activity, enzyme inhibitory potential, and phenolic content (spectro-photometric and liquid chromatography/high resolution mass spectrometry (LC-HRMS) analysis) of different solvent extracts (ethyl acetate, methanol, and aqueous) of H. lanuginosum aerial parts. Twenty one phenolic compounds including phenolic acids, acylquinic acids,flavonoids and bioflavonoids were identified by LC-HRMS profiles. Quinic acid was the dominant compound in all H. lanuginosum extracts. The highest total phenolic (168.56 mg gallic acid equivalent (GAE)) andflavonoid (53.22 mg rutin equivalent (RE)) contents were observed in the aqueous extract. Also, the aqueous extract was the best antioxidant, showing the highest 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS) scavenging, ferric re-ducing antioxidant power (FRAP), cupric rere-ducing antioxidant capacity (CUPRAC), and activity in the phos-phomolybdenum assay. The methanol extract exhibited the strongest metal chelating and inhibitory effect on α-amylase and tyrosinase. In contrast, the most efficient inhibitor of cholinesterases and α-glucosidase was the ethyl acetate extract. Docking showed that the selected compounds are all possible inhibitor candidates of tyrosinase. To conclude, each solvent extract of H. lanuginosum varied in its chemical and biological profile but overall, possess a good source of many natural agents which can be used to manage ailments inked with oxi-dative stress.
1. Introduction
Recently, there has been emerging number of studies confirming a strong correlation between oxidative stress, free radicals over-produc-tion, cellular redox imbalance, decreased activity of endogenous anti-oxidant enzymes, and the onset of several pathologies including dia-betes (Marginean et al., 2018; Li et al., 2014). Hyperglycemia and the resulting glucose autoxidation are regarded as major hallmarks in the production of reactive oxygen species and its associated-damage in Type-2 diabetes mellitus. Oxidative stress can impact negatively on a couple of transcription factors and pathways, ultimately resulting in insulin resistance. In Alzheimer's disease, Aβ plaques and
hyper-phosphorylated tau neurofibrillary tangles (T-NFTs) are linked in the production and promotion of oxidative damage thereby enhancing mitochondrial dysfunction and membrane damage (Ahmad et al., 2017).
Exploring the pharmacological propensities of natural products have attracted much interest in the past decades. Such bio-resources have been found to possess broad spectrum of biological effects in-cluding antioxidant, antimicrobial, anticancer or antiviral. In addition, public concerns have emerged against synthetics (Alvarez-Rivera et al., 2019; Awortwe et al., 2019). For instance, synthetic antioxidants have been reported as carcinogenic and hepatotoxic (da Cruz et al., 2019). From this perspectives, the search of novel bioactive compounds from
https://doi.org/10.1016/j.indcrop.2019.03.033
Received 13 November 2018; Received in revised form 12 March 2019; Accepted 12 March 2019
⁎Corresponding author.
E-mail address:gokhanzengin@selcuk.edu.tr(G. Zengin).
1These authors contributed equally.
Available online 24 March 2019
0926-6690/ © 2019 Elsevier B.V. All rights reserved.
natural products to replace synthetic ones is one of the most attractive research avenues in an endeavor to develop new drug regimen.
The quest for enzyme inhibitors to manage these chronic disorders is high on the research agenda. For instance, cholinesterase inhibitors are the major class of drugs currently employed for the symptomatic management of Alzheimer's disease, in which there is an impairment in signal transmission due to acetylcholine deficit in the brain (Korabecny et al., 2015).α-Glucosidase and α-amylase inhibitors are now being used to control postprandial hyperglycemia (Thilagam et al., 2013). In addition, inhibitors of tyrosinase, involved in melanin biosynthesis, is a therapeutic target for human hyperpigmentation disorders including post-inflammatory states, melisma, lentigo, ephelides, and nevus (Kao et al., 2013).
Hypericum is the main genus of the Hypericaceae family, comprising 458 accepted species names (The Plant List, 2013). These species gen-erally possess pale to dark yellowflowers together with transparently dotted leaves having red or black colored gland (Tekin, 2017). A broad spectrum of bioactive compounds are known to be produced by Hy-pericum species such as naphthodianthrones (hypericin and pseudohy-pericin), phloroglucinols (hyperforin and adhyperforin), flavonoids (hyperoside, rutin or quercitrin), xanthones, and essential oils (Zorzetto et al., 2015). The naphthodianthrone, hypericin, is one of the most important compounds isolated from H. perforatum and later identified in other Hypericum species (Napoli et al., 2018).
Theflora of Turkey is an important gene center for Hypericum spe-cies. Bingol et al. (2011)reported that the genus of Hypericum is re-presented by 82 species or 98 taxa, 45 of which are endemic; the en-demism ratio being 45.92%. However, according to latest report by Tekin (2017), the genus is represented by 96 species and 104 taxa, of which 45 are endemic with an endemism ratio of 43%. The most popular member of this genus is H. perforatum (also known as St. John's wort), which is fully authorized for marketing in many European countries as traditional medicine to manage depression primarily (González et al., 2010; Jarić et al., 2015; Rao et al., 2015; Sõukand and Pieroni, 2016; Zorzetto et al., 2015). In Turkish folk medicine, a number of Hypericum species are used including H. perforatum, H. tri-quetrifolium, H. tetrapterum, H. tritri-quetrifolium, H. thymifolium, H. lydium, H. montbretii, H. scabrum, H. venustum, H. microcalycinum, and H. poly-phyllum (Altundag and Ozturk, 2011; Dalar et al., 2018; Güzel et al., 2015; Sargin, 2015).
Nonetheless, not all species are fully exploited for their biological potentials, such as in the case of Hypericum lanuginosum. Forty one compounds were detected in the essential oils of H. lanuginosum var. lanuginosum Lam. by GC and GC–MS techniques, the main constituents identified as spathulenol (17.3%), caryophyllene oxide (13.1%), α-pinene (11.7%) and undecane (6.2%) (Yüce, 2016). High performance liquid chromatography (HPLC) analysis of the methanolic extract also revealed a number of secondary metabolites in its different parts. The leaf was abundant in neochlorogenic acid and quercitrin while the flower was abundant in hyperoside, isoquercetin, quercitrin, quercetin, (+)-catechin, and (−)-epicatechin. The flower also contained low amount of hypericin, pseudohypericin, and avicularin, which were not detected in neither the leaf nor stem (Odabas et al., 2016).
So far, only two phytochemical studies were conducted on this species while its biological properties have not been investigated thoroughly as far as the recent literature is concerned. Therefore, this aims to document the antioxidant activity, enzyme inhibitory potential, and phenolic content (using spectrophotometric and LC-HRMS techni-ques) of several solvent extracts of H. lanuginosum.
2. Materials and methods 2.1. Plant material
Hypericum lanuginosum was collected in Antalya-Gundogmus in June 2018. The taxonomical identification was performed by the
botanist Dr. Murad Aydin Sanda (Muş Alpaslan University, Department of Molecular Biology, Muş, Turkey) and one representative specimen was kept in the herbarium of Selcuk University. The aerial parts (as mixed) were divided and dried for 10 days at the room temperature. Then, these samples were powdered with a laboratory mill.
2.2. Extraction
To prepare ethyl acetate and methanol extracts, the plants samples were stirred overnight (24 h) at room temperature (5 g in 100 mL sol-vent). Afterfiltration, the extracts were concentrated using a rotary evaporator under vacuum at 40 °C. For water extract, infusion was prepared (5 g of plants were kept in 100 mL of boiled water for 20 min). After that, the infusion wasfiltered and dried by using a lyophilizator. The restudies were stored at +4 °C until further analysis.
2.3. Profile of bioactive compounds
With reference to our previous studies (Uysal et al., 2017), the total amount of phenolics (TPC) (by standard Folin–Ciocalteu method) and flavonoids (TFC) (by AlCl3method) were determined. Thefinal results
were expressed as equivalents of standard compounds (gallic acid (mg GAE/g) for TPC and rutin (mg RE/g) for TFC), respectively).
2.4. LC-HRMS method
The LC-HRMS analyses were achieved on a Q Exactive Plus heated electrospray ionization (HESI-II)– high resolution mass spectrometer (HRMS) (ThermoFisher Scientific, Inc., Bremen, Germany) equipped with an ultra-high-performance liquid chromatography (UHPLC) system Dionex Ultimate 3000RSLC (ThermoFisher Scientific, Inc.).
Operating conditions for the HESI source used in a negative ioni-zation mode were:−2.5 kV spray voltage, 320 °C capillary tempera-ture, 300 °C probe heater temperatempera-ture, sheath gasflow rate 38 units, auxiliary gasflow 12 units (units refer to arbitrary values set by the Exactive Tune software) and S-Lens RF level 50.00. Nitrogen was used for sample nebulization and collision gas in HCD cell. The LC-HRMS method was operating in Full scan-dd MS2/Top 5 with the following
settings: 70,000 FWHM resolution (at m/z 200), AGC target 1e6, max. IT 50 ms and mass range 100–1000 m/z, while ddMS2
conditions were set to resolution 17,500 FWHM (at m/z 200), AGC target 1e3, max. IT 50 ms, isolation window 2.0 m/z and normalized collision energy (NCE) 30. The UHPLC separations were performed on a Kromasil EternityXT C18, 1.8μm, 2.1 × 100 mm (AkzoNobel, Sweeden) with a binary mo-bile phase consisting of solution A: 0.1% HCOOH and solution B: MeCN (0.1% HCOOH). The following step gradient profile was used: 5% B for 1.0 min, increasing up to 25% B in 14 min, held isocratic at 25% B for 2.0 min, increasing up to 50% B in 1.0 min, held isocratic at 50% B for 2.0 min, increasing up to 95% B in 2.0 min, held isocratic for 2.0 min finally brought back down to 5% B over 0.5 min (Zengin et al., 2017a). Equilibration time was 4.5 min, the flow rate was 0.3 mL/min. The column compartment temperature was set at 40 °C. Data were pro-cessed with Xcalibur software ver. 3.0. The calculation of the exact masses and mass measurement errors, prediction of molecular formulas and simulation of monoisotopic profiles were carried out with Xcalibur 3.0 software (ThermoScientific).
2.5. Experiments for enzyme inhibitory and antioxidant abilities
Hypericum lanuginosum extracts were tested as sources of enzyme inhibitors on some enzymes, includingα-amylase, α-glucosidase, cho-linesterases and tyrosinase. The procedures of these assays were given reported in our earlier work (Uysal et al., 2017). The enzyme inhibitor effects were evaluated as equivalents of acarbose (for amylase and α-glucosidase), galantamine (for AChE and BChE), and kojic acid (for tyrosinase).
Antioxidant capacity of H. lanuginosum extracts were spectro-photometrically screened by different experiments as phosphomo-lybdenum, quenching of radicals (DPPH and ABTS), reduction poten-tials (FRAP and CUPRAC), and ferrous ion chelating. Thefindings were expressed as standard compounds equivalents (mg trolox equivalent (TE)/g and mg ethylenediamine tetra acetate equivalent (EDTAE)/g). The procedures of assays were given reported in our earlier work (Uysal et al., 2017).
2.6. In silico evaluation 2.6.1. Enzymes preparation
The enzyme tyrosinase had been crystallized by several authors, among these, we have selected a suitable structure being cosrystallized with tits standard inhibitor tropolole. Thus the protein structure was downloaded the raw file from the Protein Data Bank RCSB PDB (Berman et al., 2000): Tyrosinase pdb:2Y9X.
The protein was prepared by isong the tools ProtPrepWizard (Sastry et al., 2013) implemented in Maestro suite 10.2 (Schrödinger, 2015). This software was useful tofix the several problems present in the raw enzyme structure, such as missing side chains and loops (Prime module). The pH wasfixed at 7.4 using Prime (Jacobson et al., 2002). The crystallization water was deleted, the protons were assigned and a minimization was conducted by using PROPKA at pH 7.4 with OPLS-3 force filed. After preparation the protein was used for molecular docking without further modifications.
2.6.2. Ligand preparation
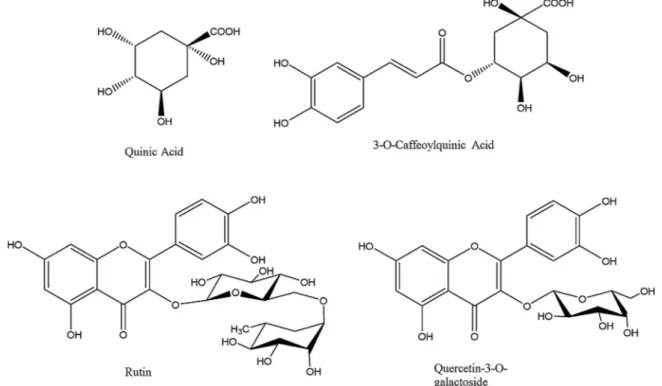
Chlorogenic acid, Quinic acid, Quercetin-3-O-rutinoside (Rutin) and Quercetin-3-O-galactoside (Hyperoside) were used for the computa-tional study, being the most abundant bioactive substances in the herb extracts (Fig. 1). Their 3D structures were obtained online from Zinc database and used for the docking experiments after preparation. The ligands were prepared by the LigPrep software. This software is able to neutralize the structures at pH 7.4 by the module Ionizer and to mini-mize the structures by OPLS3 forcefield.
2.6.3. Molecular docking
The selected polyphenols, have been docked to the enzymatic cavity of tyrosinase. The docking software Gold 6.0 was selected for the docking experiments, being already validated for docking on this en-zyme by our previous work. The“Gold Score” scoring method was used to rank the best poses, being the most accurate among all the scoring methods embedded in GOLD, for all the ligands (Llorent-Martínez et al., 2017; Mocan et al., 2016; Picot et al., 2017; Zengin et al., 2017b). The binding pocket was determined by positioning the center of the grid on the crystallographic inhibitor and by extending the grit in a radius of 10 Angstroms from the center.
2.7. Statistical analysis
One-way ANOVA was done to determine any differences between the tested samples following by Tukey's test. p < 0.05 were assigned to be statistically significant. The heat map and Pearson linear correlation
Fig. 1. Chemical structure of the selected compounds for molecular modeling experiments.
Fig. 2. Total bioactive compounds of H. lanuginosum extracts. (Values expressed are means ± S.D. of three parallel measurements. GAE: gallic acid equivalent; RE: rutin equivalent. Different letters indicate differences in the tested extracts (p < 0.05)).
(r) was employed to recognize any relationship between phytochemical contents and the observed biological activities. The statistical proce-dures were performed by R software v. 3.5.1.
3. Results and discussion
3.1. Total amounts of phenolics,flavonoids and LC-HRMS analysis In recent years, phytochemistry has developed as a distinct dis-cipline, closely related to natural product organic chemistry and plant biochemistry, dealing with a variety of organic compounds accumu-lated in plants, their chemical structures, biosynthesis, turnover and metabolism, distribution, and most importantly their biological prop-erties. A number of techniques are currently employed for the separa-tion, purification, and identification of these compounds and these phytochemical analyses have played a major part in the identification of lead compounds for the development of novel potent drugs
(Harborne, 1984).
In the present study, the total bioactive compounds of the solvent extracts of H. lanuginosum were determined based on TPC and TFC (see Fig. 2). TPC was found in the order: aqueous extract (168.56 mg GAE) > methanol extract (72.03 mg GAE) > ethyl acetate extract (52.19 mg GAE). Similarly, the aqueous extract was richest in TFC (53.22 mg RE) followed by the methanolic and ethyl acetate extract (30.96 and 11.80 mg RE, respectively).
LC-HRMS profile of H. lanuginosum extracts demonstrated the pre-sence of 21 phenolic compounds including phenolic acids, acylquinic acids, flavonoids and bioflavonoids (Table 1, Fig. 3). Protocatechuic (1), 3-CQA (2), caffeic acid (5), 5-CQA (6), catechin (7), rutin (9), hyperoside (10), isoquercitrine (11), miquelianin (12), myricetin-3-O-glucoside (15), kaepferol-3-O-rhamnoside (16), apigenin-7-O-myricetin-3-O-glucoside (19), as well as the aglycons kaemferol (17) and quercetin (18) were identified by comparison of their retention time (tR), and
LC-HRMS-MS-fragmentation with standard compounds. All others derivatives were Table 1
Peak assessment of phenolic compounds in Hypericum lanuginosum extracts.
Peak no. [M–H]−m/z
Molecular formula
MS/MS data m/z tRmin Theoret. mass Delta ppm Proposed compound
1 153.0181 C7H5O4 109.0281 (100), 153.0181 (15.31) 2.06 153.0182 −1.232 Protocatechuic acid1,2,3 2 353.0876 C16H17O9 191.0552 (100), 179.0340 (64.10), 135.0438 (54.87), 353.0876 (35.82), 161.0231 (4.25), 173.0443 (3.06) 2.48 353.0867 −0.155 3-Caffeoylquinic acid1,2,3 3 191.0553 C7H11O6 191.0553 (100), 93.0330 (6.39), 173.0448 (1.90) 3.21 191.0550 −0.781 Quinic acid1,2,3 4 353.0879 C16H17O9 173.0446 (100), 191.0554 (87.45), 179.0341 (68.04), 135.0439 (55.38), 353.0879 (31.86), 161.0232 (3.09) 3.42 353.0867 0.085 4-Caffeoylquinic acid1,2,3 5 179.0342 C9H7O4 135.0439 (100), 179.0342 (18.20) 3.59 179.0338 −0.822 Caffeic acid1,2,3 6 353.0881 C16H17O9 191.0552 (100), 353.0881 (4.82), 161.0233 (2.38), 179.0338 (0.65) 3.92 353.0867 0.295 5-Caffeoylquinic acid1,2,3 7 289.0719 C15H13O6 289.0719 (100), 245.0818 (44.26), 203.0706 (16.98), 179.0403 (11.63), 165.0185 (7.26), 139.0388 (5.90), 137.0231 (16.47), 125.0231 (26.70) 3.96 289.0706 0.376 Catechin2,3 8 337.0931 C16H17O8 337.0931 (7.62), 191.0553 (100), 173.0445 (6.83), 163.0389 (5.38), 4.05 337.0917 0.651 5-p-coumaroylquinic acid1,2,3 9 609.1466 C27H29O16 609.1467 (100), 300.0273 (82.14), 301.0351 (44.79), 271.0254 (24.55), 255.0287 (17.65) 5.12 609.1450 0.923 Quercetin-3-O-rutinoside (rutin) 1,2,3 10 463.0883 C21H19O12 463.0883 (100), 316.0222 (35.51), 301.0349 (22.31), 300.0276 (56.14), 271.0248 (33.91), 255.0302 (9.85), 243.0298 (7.50), 151.0024 (4.54), 121.0282 (1.38), 107.0124 (1.48) 5.28 463.0871 0.091 Quercetin-3-O-galactoside (hyperoside)1,2,3 11 463.0882 C21H19O12 463.0882 (100), 316.0226 (7.50), 301.0353 (86.51), 300.0275 (30.19), 272.0324 (9.33) 271.0241 (3.67), 255.0304 (1.50), 243.0297 (1.41), 151.0024 (6.19), 149.0232 (10.75), 107.0124 (2.06) 5.63 463.0871 0.031 Quercetin-3-O-glucoside (isoquercitrine)1,2,3 12 477.1025 C22H21O12 477.1026 (100), 315.0518 (52.12), 301.5468 (12.74), 300.0276 (22.90), 133.0287 (16.67) 5.71 477.1027 −2.681 Quercetin-3-O-glucuronide (miquelianin)1,2 13 515.1194 C25H23O12 191.0553 (100), 353.0880 (97.82), 135.0438 (53.97), 179.0340 (52.13), 515.1194 (13.03), 173.0441 (4.99), 161.0230 (4.91) 5.91 515.1184 −0.119 1,3-DiCaffeoylquinic acid1,2,3 14 515.1190 C25H23O12 173.0441 (100), 179.0341 (66.23), 353.0881 (65.01), 515.1190 (62.71), 191.0553 (34.46), 135.0438 (60.79), 161.0236 (4.53) 6.26 515.1184 −0.479 4,5-DiCaffeoylquinic acid1,2,3 15 479.0622 C24H15O11 479.0622 (100), 317.0296 (93.60), 311.5435 (7.31), 303.8556 (7.42) 6.60 479.0608 0.513 Myricetin-3-O-glucoside1,2,3 16 431.0984 C21H19O10 431.0984 (100), 285.0403 (80.59), 284.0327 (62.93), 269.1396 (14.74), 255.0299 (52.00), 227.0347 (45.17), 151.0023 (1.67) 6.65 431.0972 0.116 Kaempferol-3-O-rhamnoside1,2,3 17 285.0406 C15H9O6 285.0406 (100), 276.9118 (0.67), 183.6113 (0.75), 175.0388 (2.83), 169.6354 (0.60), 151.0025 (5.22), 133.0282 (23.97), 107.0125 (3.50) 7.64 285.0393 0.346 Kaempferol1,2,3 18 301.0355 C15H9O7 301.0355 (100), 179.9977 (27.64), 151.0025 (52.58), 149.0232 (2.01), 139.0388 (1.93), 121.0281 (18.23), 107.0123 (13.75) 7.67 301.0342 0.279 Quercetin1,2,3 19 431.0982 C21H19O10 431.0982 (100), 341.0660 (3.47), 311.0556 (18.48), 295.1524 (3.36), 283.0621 (19.57), 269.0459 (72.07), 255.0645 (4.19), 240.0435 (2.75), 225.0554 (20.55), 8.10 431.0972 −0.232 Apigenin-7-O-glucoside1,2,3 20 537.0882 C30H17O10 537.0832 (100), 443.0407 (21.29), 385.0718 (42.16), 151.0025 (77.35), 117. 0333 (10.67) 9.41 537.0816 0.875 Biapigenin1,2,3 21 537.0832 C30H17O10 537.0883 (100), 443.0394 (12.88), 417.0617 (18.83), 399.0508 (4.34), 375.0510 (61.70), 331.0623 (11.73), 307.0612 (5.47), 161.8439 (3.12), 117. 0330 (5.16) 10.02 537.0816 1.099 Amentoflavone1,2,3 tRRetention time. 1Ethyl acetate extract. 2Methanol extract. 3Aqueous extract.
readily characterized by their MS2patterns as well as chromatographic behavior. The hierarchical key for identification of phenolic acids was used to identify acylquinic acids (Clifford et al., 2003; Clifford et al., 2005) supporting by UVλmaxand retention time relative to
commer-cially available 3-caffeoylquinic acid (neochlorogenic acid) and 5-caf-feoylquinic acid (chlorogenic acid), while quinic acid (3), biflavones biapigenin (20) and amentoflavone (21) were identified by comparison of their LC-HRMS-MS-fragmentation with literature data (Michler et al., 2011; Saldanha et al., 2013). All Hypericum extracts demonstrated si-milar phenolic LC-HRMS profile. However, catechin was found only in the methanol and aqueous extracts, while miquelianin was absent in the water extract.
Neochlorogenic acid (2), quinic acid (3), rutin (9), hyperoside (10), 1,3-dicaffeoylquinic acid (13), kaempferol (17) and quercetin (18) were the major compounds in the studied extracts. These major com-ponents have been reported as significant bioactive agents, including antioxidants and enzyme inhibitors (Tadera et al., 2006; Yang et al., 2008; Calderon-Montano et al., 2011; Zhang et al., 2014; Raza et al., 2017; Enogieru et al., 2018). Based on thesefindings, these compounds could be considered as main contributors to observed biological abil-ities.
3.2. Antioxidant effects
The antioxidant effects of H. lanuginosum extracts were also assessed based on different mechanisms including scavenging, reducing, and chelating effect (Table 2). DPPH•and ABTS+•scavenging techniques are among the most widely used antioxidant assays. Moreover, transi-tion metals namely copper and iron are known to trigger oxidative stress by reacting with hydrogen peroxide to produce the hydroxyl ra-dical•OH, which is the most dangerous radical. This radical can directly react with proteins and other macromolecules to produce carbonyls (aldehydes and ketones), cross-linking, and lipid peroxidation. There-fore, chelation of these metal ions can inhibit their activity thereby reducing the production of free radicals (Lobo et al., 2010). Also, the reductive potential of the H. lanuginosum extracts was determined by their ability to reduce Fe(III) and Cu(II) (FRAP and CUPRAC assay, respectively), while the total antioxidant activity was assessed using the phosphomolybdenum method.
Among the tested extracts, the aqueous one exhibited the strongest antioxidant effect in five out of the six assays conducted (DPPH: 354.60 mg TE; ABTS: 638.86 mg TE; CUPRAC: 964.91 mg TE; FRAP: 760.27 mg TE; phosphomolybdenum: 3.59 mmol TE) while the most effective metal chelator was the methanol extract (46.91 mg EDTAE/g extract). In contrast, the aqueous extract showed the lowest metal Fig. 3. Total ion chromatograms (TIC) of Hypericum lanuginosum extracts. (A) Ethyl acetate extract, (B) Methanol extract, (C) aqueous extract. (1) Protocatechuic acid, (2) 3-Caffeoylquinic acid, (3) Quinic acid, (9) Rutin, (10) Hyperoside, (13) 1,3-Dicaffeoylquinic acid, (17) Kaempferol, (18) Quercetin, (19) Apigenin-7-O-glucoside, (20) Biapigenin, (21) Amentoflavone. For other peak numbering seeTable 2.
Table 2
Antioxidant properties of H. lanuginosum extracts*.
Extracts Phosphomolybdenum (mmol TE/g extract) DPPH (mg TE/g extract) ABTS (mg TE/g extract) CUPRAC (mg TE/g extract) FRAP (mg TE/g extract)
Metal chelating activity (mg EDTAE/g extract)
Ethyl acetate 2.26 ± 0.02c 73.49 ± 1.20c 57.66 ± 1.16c 135.05 ± 4.53c 45.81 ± 4.49c 33.58 ± 1.15b
Methanol 3.01 ± 0.29b 105.93 ± 2.07b 142.11 ± 6.48b 212.65 ± 5.99b 127.96 ± 7.93b 46.91 ± 2.65a
Aqueous 3.59 ± 0.03a 354.60 ± 7.29a 638.86 ± 9.37a 964.91 ± 10.72a 760.27 ± 4.03a 14.47 ± 0.77c
* Values expressed are means ± S.D. of three parallel measurements. DPPH: 1,1-diphenyl-2-picrylhydrazyl; ABTS: 2,2 ′-azino-bis(3-ethylbenzothiazoline)-6-sul-fonic acid; FRAP: Ferric reducing antioxidant power; CUPRAC: Cupric reducing antioxidant capacity; TE: Trolox equivalent; EDTAE: Ethylenediamine tetra acetat equivalent.*Superscripts indicate differences in the tested extracts (p < 0.05).
chelating effect while the ethyl acetate extract was the weakest anti-oxidant in all the rest of the assays conducted. The high antianti-oxidant activity of the aqueous extract in most assays (except metal chelating assay) may be linked by its highest TPC and TFC compared with the other solvent extracts. On the other hand, the ethyl acetate one, which contained the lowest level of TPC and TFC, exhibited the weakest an-tioxidant properties. Indeed, data obtained from the present work re-vealed a strong positive correlation between TPC andfive antioxidant mechanisms including scavenging (DPPH and ABTS), reducing (CUPRAC and FRAP), and phosphomolybdenum assay (R values in the
range 0.906–0.999). TFC also was found to be positively correlated with these antioxidant assays (R values in the range 0.923–0.993). On the contrary, metal chelating effect was negatively correlated with TPC (−0.836) and TFC (−0.620) (Fig. 4).
Previous studies tend to also support the claim that plants posses-sing high amount of polyphenols are more effective antioxidants (Jiao et al., 2018; Karamać et al., 2018; Pang et al., 2018; Um et al., 2017). High intake of foods rich in polyphenols has been linked to a lower risk of common degenerative and chronic diseases caused by oxidative stress (Zhang and Tsao, 2016). Interestingly, the extracts were also Fig. 4. Statistical evaluations (A) Correlation coefficients between total bioactive compounds and biological activities (Pearson Correlation Coefficient (R), p < 0.05). (B) Heatmap of extracts in according to bioactive compounds and biological activities; TPC: total phenolic content; TFC: totalflavonoid content.
Table 3
Enzyme inhibitory effects of H. lanuginosum extracts*.
Extracts AChE inhibition (mg GALAE/g extract)
BChE inhibition (mg GALAE/g extract)
Tyrosinase inhibition (mg KAE/ g extract)
Amylase inhibition (mmol ACAE/g extract)
Glucosidase inhibition (mmol ACAE/g extract)
Ethyl acetate 5.03 ± 0.04a 6.02 ± 0.01 260.40 ± 2.10b 0.47 ± 0.06a 1.71 ± 0.01a
Methanol 2.72 ± 0.13b 2.57 ± 0.20 276.34 ± 2.42a 0.51 ± 0.03a 1.41 ± 0.04b
Aqueous 0.98 ± 0.03c na 118.85 ± 3.44c 0.08 ± 0.01b 1.06 ± 0.07c
* Values expressed are means ± S.D. of three parallel measurements. GALAE: Galatamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; na: not active.*Superscripts indicate differences in the tested extracts (p < 0.05).
found to contain twenty one phenolic compounds including phenolic acids, acylquinic acids,flavonoids and bioflavonoids which might jus-tify the observed antioxidant properties. For instance, protocatechuic acid and caffeic acid were previously found to display scavenging effect against some radicals such as DPPH, ABTS, reducing power and che-lating ability (Gülçin, 2006; Li et al., 2011). Chlorogenic acid isomers including caffeoylquinic acid and dicaffeoylquinic acid isomers ex-hibited antioxidant and DNA-protective activities in an earlies study (Xu et al., 2012). Catechin is also an effective antioxidant present in many dietary products, plants, fruits, green tea, chocolate, cocoa, among others (Zanwar et al., 2014). Quercetin, a member of the fla-vonoids family, is known as one of the most effective antioxidant compounds as evidenced by a plethora of studies reviewed by Boots et al. (2008). Apigenin was also found to protect against oxidative stress in Wistar albino rats (Singh et al., 2004). Amentoflavone, a biflavonoid, exhibited potent antioxidant effect on scavenging hydroxyl, superoxide, DPPH and ABTS radicals and also protected against•OH-induced DNA damage (Bajpai et al., 2019; Li and Huang, 2010). All these compounds could act individually and in combination to contribute to the overall antioxidant power of H. lanigosum.
3.3. Enzyme inhibitory activity
Enzymes remain prime targets in designing novel drugs since al-tering enzyme activity has an immediate and defined effects. Although there is an increasing use of drugs acting on receptors to regulate sig-nals from outside the cell, 47% of all present drugs are reported to inhibit enzyme targets (Ramsay and Tipton, 2017).
From the present investigation (seeTable 3), the ethyl acetate ex-tract of H. lanuginosum was the most effective inhibitor of acet-ylcholinesterase (AChE) (5.03 mg galatamine equivalent (GALAE)/g extract), butyrylcholinesterase (BChE) (6.02 mg GALAE), and α-gluco-sidase (1.71 mmol acarbose equivalent (ACAE)). In addition, the most efficient tyrosinase and α-amylase inhibitor was the methanolic extract (276.34 mg kojic acid equivalent (KAE) and 0.51 mmol ACAE,
respectively), followed by the ethyl acetate extract which showed a less effective but quite similar inhibitory effect (260.40 mg KAE/and 0.47 mmol ACAE, respectively). On the contrary, the aqueous extract displayed the weakest inhibitory properties against all enzymes, showing no activity against BChE.
It should be highlighted that although the ethyl acetate extract displayed high enzyme inhibitory activity, it possessed the lowest TPC and TFC compared with the methanolic and aqueous extracts, which indicate that the total bioactive constituents of a plant does not always represent the strength of its biological activity. In fact, a negative cor-relation was observed between the TPC of the extracts and its enzyme inhibitory effects (r values in the range −0.159 to 0.970). Similarly, TFC was negatively correlated, with r values ranging from−0.462 to −0.999 (Fig. 4). Therefore, the presence of specific compounds could be responsible for the high enzyme inhibitory activity of the ethyl acetate extract. These compounds, though might be present in small amount, may interact together in proper ratios to exhibit a synergistic effect as highlighted above.
The variations observed in the phytochemical composition, and biopharmaceutical effects of the H. lanuginosum extracts clearly indicate that the extraction solvent used is one major factor which affect the extraction efficiency of bioactive compounds from plant materials and their subsequent biological properties. The differences could be ex-plained by different polarities of phenolic components (Dailey and Vuong, 2015; Złotek et al., 2016). Thus, the selection of the extraction solvent for H. lanuginosum compounds is one important step to de-signing novel pharmaceutics.
Besides, a number of compounds detected in H. lanuginosum were previously found to possess enzyme inhibitory properties. For instance, Khan et al. (2009)observed that quercetin dose-dependently inhibited AChE and BChE in a competitive manner. Quercetin also inhibited both the monophenolase and diphenolase activities of tyrosinase. Its diphe-nolase inhibitory effect occurred in a reversible and competitive manner. Molecular docking revealed that quercetin bound to the tyr-osinase active site and chelated a copper with the 3′,4′-dihydroxy Fig. 5. Best poses of 3-O-caffeoylquinic acid (A), quinic acid (B), quercetin-3-O-rutinoside (Rutin) (C) and quercetin-3-O-galactoside (Hyperoside) (D).
groups (Fan et al., 2017). In addition, amentoflavone showed a high binding affinity (−11.6 kcal/mol) compared with acarbose (−9.3 kcal/ mol) on humanα-amylase (Ogunwa and Ayenitaju, 2017). Moreover, apigenin, isolated from theflowers of Hemisteptia lyrata (Bunge) Fisch. & C.A.Mey., showed significant inhibitory effect against tyrosinase with an IC50value of 4.56μg/ml (Ha et al., 2003). 4,5-O-Dicaffeoylquinic
acid also dose-dependently inhibited melanin synthesis and tyrosinase activity in melanocytes and affected as negatively the expression of tyrosinase-related protein-1 (Tabassum et al., 2016).
3.4. Molecular docking
The enzyme inhibitory tests conducted on the plant extracts may not provide a detailed explanation of binding modes of active compounds
in the enzymatic pocket of tyrosinase. Docking experiments are useful tools to predict and analyze the most energetically favorite interactions between the bioactives and enzymes. In this present work, the best docking pose obtained was for 3-O-caffeoylquinic acid docked to tyr-osinase.
It was found that this pose is stabilized by several interactions namely, two hydrogen bonds to His244 and His296, twoπ–π stacks formed to the aromatic side chains of His85 and His259 and two co-ordinative bonds with the copper atoms Cu400 and Cu401 as shown in Fig. 5(A). These latter interactions are particularly important being tyrosinase a metallo-enzyme. 3-O-Caffeoylquinic acid presented a docking Fitness value of 55.82. FromFig. 5(B) it can be observed that quinic acid is docked to tyrosinase enzymatic cavity by forming one hydrogen bond to Met280 and three coordinative bonds involving Fig. 6. Best interactions of 3-O-caffeoylquinic acid (A), quinic acid (B), quercetin-3-O-rutinoside (Rutin) (C) and (D) quercetin-3-O-galactoside (Hyperoside).
Cu400 and Cu401. In this case the docking Fitness value was 56.57. Fig. 5(C) presents the best pose found for rutin, with a docking Fitness value of 63.24 to the studied enzyme. This in silico study found that rutin is able to form one hydrogen bond with Asn81, oneπ–π stack to the aromatic side chain of His85 and bond to the copper Cu401 of tyrosinase. Moreover, rutin, which have shown the best Fitness values within the selected compounds, was previously reported as competitive inhibitor of tyrosinase with an IC50 of 6.8 mM (Si et al., 2012), this
finding further validate our in silico experiments.
The best docking pose for quercetin-3-O-galactoside (Hyperoside) Fig. 6(D) docked to tyrosinase is stabilized by three hydrogen bonds respectively to His244, Gly281 and His296 and three π–π stacks to His61, His85 and His263 and one coordinative bond to copper Cu401. Hyperoside showed a dockingfitness value of 45.86, the lowest of this series. These docking experiments demonstrated that the selected compounds are all possible inhibitor candidates of tyrosinase, being able to efficaciously interact with the amino acid side chains sur-rounding the enzymatic cavity of tyrosinase. They were also able to directly bind the catalytic copper atoms presents at the bottom of the enzymatic pocket of tyrosinase. These data, together with the observed biological activity, are also in agreement with previous literature data (Uysal et al., 2016, 2017; Zengin et al., 2018).
4. Conclusion
This study presented newfindings on the biological properties of H. lanuginosum. In summary, our results indicate that H. lanuginosum ex-tracts are rich in a great diversity of phenolic compounds, including phenolic acids, acylquinic acids, flavonoids and bioflavonoids. They also exhibited prominent antioxidant and enzyme inhibitory properties. 3-Caffeoylquinic acid, quinic acid, rutin, hyperoside, 1,3-di-caffeoylquinic acid, kaempferol and quercetin seem to be major com-pounds in the extracts. Our findings suggested that H. lanuginosum could be considered as a great source of biologically-active compounds for pharmaceutical purposes which warrants to be further investigated using in vivo model. In addition, preclinical and clinical studies should be conducted along with further phytochemical characterization for the development of new pharmaceutical products.
References
Ahmad, W., Ijaz, B., Shabbiri, K., Ahmed, F., Rehman, S., 2017. Oxidative toxicity in diabetes and Alzheimer's disease: mechanisms behind ROS/RNS generation. J. Biomed. Sci. 24, 76–85.https://doi.org/10.1186/s12929-017-0379-z. Altundag, E., Ozturk, M., 2011. Ethnomedicinal studies on the plant resources of east
Anatolia, Turkey. Proc. Soc. Behav. Sci. 19, 756–777.https://doi.org/10.1016/j. sbspro.2011.05.195.
Alvarez-Rivera, G., Ballesteros-Vivas, D., Parada-Alfonso, F., Ibañez, E., Cifuentes, A., 2019. Recent applications of high resolution mass spectrometry for the character-ization of plant natural products. Trends Anal. Chem. 112, 87–101.https://doi.org/ 10.1016/j.trac.2019.01.002.
Awortwe, C., Bruckmueller, H., Cascorbi, I., 2019. Interaction of herbal products with prescribed medications: a systematic review and meta-analysis. Pharmacol. Res. 14, 397–408.https://doi.org/10.1016/j.phrs.2019.01.028.
Bajpai, V.K., Park, I., Lee, J., Shukla, S., Nile, S.H., Chun, H.S., Khan, I., Oh, S.Y., Lee, H., Huh, Y.S., Na, M., Han, Y.-K., 2019. Antioxidant and antimicrobial efficacy of a bi-flavonoid, amentoflavone from Nandina domestica in vitro and in minced chicken meat and apple juice food models. Food Chem. 271, 239–247.https://doi.org/10. 1016/j.foodchem.2018.07.159.
Berman, H., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T., Weissig, H., Shindyalov, I., Bourne, P., 2000. The protein data bank. Nucleic Acids Res. 28, 235–242.
Bingol, U., Cosge, B., Gurbuz, B., 2011. Hypericum species inflora of Turkey. Hypericum. Med. Aromat. Plant Sci. Biotechnol. 5, 86–90.
Boots, A.W., Haenen, G.R., Bast, A., 2008. Health effects of quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 585, 325–337.https://doi.org/10.1016/j.ejphar. 2008.03.008.
Calderon-Montano, M., Burgos-Morón, J., Pérez-Guerrero, E., López-Lázaro, M., 2011. A review on the dietaryflavonoid kaempferol. Mini Rev. Med. Chem. 11 (4), 298–344.
https://doi.org/10.2174/138955711795305335.
Clifford, M.N., Johnston, K.L., Knight, S., Kuhnert, N., 2003. Hierarchical scheme for LC–MS n identification of chlorogenic acids. J. Agric. Food Chem. 51, 2900–2911.
https://doi.org/10.1021/jf026187q.
Clifford, M.N., Knight, S., Kuhnert, N., 2005. Discriminating between the six isomers of
dicaffeoylquinic acid by LC–MS n. J. Agric. Food Chem. 53, 3821–3832.https://doi. org/10.1021/jf050046h.
da Cruz, R.G., Beney, L., Gervais, P., de Lira, S.P., de Souza Vieira, T.M.F., Dupont, S., 2019. Comparison of the antioxidant property of Acerola extracts with synthetic antioxidants using an in vivo method with yeasts. Food Chem. 277, 698–705.https:// doi.org/10.1016/j.foodchem.2018.10.099.
Dailey, A., Vuong, Q.V., 2015. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric. 1, 1115646.https://doi.org/10.1080/23311932.2015. 1115646.
Dalar, A., Mukemre, M., Unal, M., Ozgokce, F., 2018. Traditional medicinal plants of Ağrı Province, Turkey. J. Ethnopharmacol. 226, 56–72.https://doi.org/10.1016/j.jep. 2018.08.004.
Enogieru, A.B., Haylett, W., Hiss, D.C., Bardien, S., Ekpo, O.E., 2018. Rutin as a potent antioxidant: implications for neurodegenerative disorders. Oxid. Med. Cell Longev. 1–17.https://doi.org/10.1155/2018/6241017.
Fan, M., Zhang, G., Hu, X., Xu, X., Gong, D., 2017. Quercetin as a tyrosinase inhibitor: inhibitory activity, conformational change and mechanism. Food Res. Int. 100, 226–233.https://doi.org/10.1016/j.foodres.2017.07.010.
González, J.A., García-Barriuso, M., Amich, F., 2010. Ethnobotanical study of medicinal plants traditionally used in the Arribes del Duero, western Spain. J. Ethnopharmacol. 131, 343–355.https://doi.org/10.1016/j.jep.2010.07.022.
Gülçin,İ., 2006. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 217, 213–220.https://doi.org/10.1016/j.tox.2005.09.011. Güzel, Y., Güzelşemme, M., Miski, M., 2015. Ethnobotany of medicinal plants used in
Antakya: a multicultural district in Hatay province of Turkey. J. Ethnopharmacol. 174, 118–152.https://doi.org/10.1016/j.jep.2015.07.042.
Ha, T.J., Jang, D.S., Lee, J.R., Lee, K.D., Lee, J., Hwang, S.W., Jung, H.J., Nam, S.H., Park, K.H., Yang, M.S., 2003. Cytotoxic effects of sesquiterpene lactones from the flowers of Hemisteptia lyrata B. Arch. Pharm. Res. 26, 925.https://doi.org/10.1007/ BF02980201.
Harborne, J., 1984. Methods of Plant Analysis, Phytochemical Methods. Springer pp. 1–36.
Jacobson, M.P., Kaminski, G.A., Friesner, R.A., Rapp, C.S., 2002. Forcefield validation using protein side chain prediction. J. Phys. Chem. B. 106, 11673–11680.https://doi. org/10.1021/jp021564n.
Jarić, S., Mačukanović-Jocić, M., Djurdjević, L., Mitrović, M., Kostić, O., Karadžić, B., Pavlović, P., 2015. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 175, 93–108.https:// doi.org/10.1016/j.jep.2015.09.002.
Jiao, Y., Kilmartin, P.A., Fan, M., Quek, S.Y., 2018. Assessment of phenolic contributors to antioxidant activity of new kiwifruit cultivars using cyclic voltammetry combined with HPLC. Food Chem. 268, 77–85.https://doi.org/10.1016/j.foodchem.2018.06. 046.
Kao, Y.-Y., Chuang, T.-F., Chao, S.-H., Yang, J.-H., Lin, Y.-C., Huang, H.-Y., 2013. Evaluation of the antioxidant and melanogenesis inhibitory properties of Pracparatum mungo (Lu-Do Huang). J. Tradit. Complement. Med. 3, 163–170.https://doi.org/10. 4103/2225-4110.113443.
Karamać, M., Orak, H.H., Amarowicz, R., Orak, A., Piekoszewski, W., 2018. Phenolic contents and antioxidant capacities of wild and cultivated white lupin (Lupinus albus L.) seeds. Food Chem. 258, 1–7.https://doi.org/10.1016/j.foodchem.2018.03.041. Khan, M.T.H., Orhan, I.,Şenol, F., Kartal, M., Şener, B., Dvorská, M., Šmejkal, K.,
Šlapetová, T., 2009. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chem. Biol. Interact. 181, 383–389.https://doi.org/10.1016/j.cbi.2009.06.024.
Korabecny, J., Zemek, F., Soukup, O., Spilovska, K., Musilek, K., Jun, D., Nepovimova, E., Kuca, K., 2015. Pharmacotherapy of Alzheimer's disease: current state and future perspectives. Drug Design and Discovery in Alzheimer's Disease. Elsevier pp. 3–39.
Li, P., Huo, L., Su, W., Lu, R., Deng, C., Liu, L., Deng, Y., Guo, N., Lu, C., He, C., 2011. Free radical-scavenging capacity, antioxidant activity and phenolic content of Pouzolzia zeylanica. J. Serbian Chem. Soc. 76, 709–717.
Li, S., Chen, G., Zhang, C., Wu, M., Wu, S., Liu, Q., 2014. Research progress of natural antioxidants in foods for the treatment of diseases. Food Sci. Human Well. 3, 110–116.https://doi.org/10.1016/j.fshw.2014.11.002.
Li, S., Huang, K., 2010. Study advances on Selaginella doederleinii. Lishizhen Med. Mat. Med. Res. 21, 2637–2639.
Llorent-Martínez, E.J., Zengin, G., Fernández-de Córdova, M.L., Bender, O., Atalay, A., Ceylan, R., Mollica, A., Mocan, A., Uysal, S., Guler, G.O., 2017. Traditionally used Lathyrus species: phytochemical composition, antioxidant activity, enzyme inhibitory properties, cytotoxic effects, and in silico studies of L. czeczottianus and L. nissolia. Frontiers Pharmacol. 8, 83.https://doi.org/10.3389/fphar.2017.00083. Lobo, V., Patil, A., Phatak, A., Chandra, N., 2010. Free radicals, antioxidants and
func-tional foods: impact on human health. Pharmacogn Rev. 4, 118.https://doi.org/10. 4103/0973-7847.70902.
Marginean, C., Popescu, M.S., Vladaia, M., Tudaroscu, D., Pirvu, D.C., Petrescu, F., 2018. Involvement of oxidative stress in COPD. Curr. Health Sci. J. 44, 48–54.https://doi. org/10.12865/CHSJ.44.01.08.
Michler, H., Laakmann, G., Wagner, H., 2011. Development of an LC–MS method for simultaneous quantitation of amentoflavone and biapigenin, the minor and major biflavones from Hypericum perforatum L., in human plasma and its application to real blood. Phytochem. Anal. 22, 42–50.https://doi.org/10.1002/pca.
Mocan, A., Zengin, G., Uysal, A., Gunes, E., Mollica, A., Degirmenci, N.S., Alpsoy, L., Aktumsek, A., 2016. Biological and chemical insights of Morina persica L.: a source of bioactive compounds with multifunctional properties. J. Funct. Foods 25, 94–109.
https://doi.org/10.1016/j.jff.2016.05.010.
Saija, A., Cristani, M., 2018. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species– A comparative study. Phytochemistry 152, 162–173.https://doi.org/10.1016/j.phytochem.2018.05.003.
Odabas, M.S., Radusiene, J., Ivanauskas, L., Jakstas, V., Camas, N., Kayikci, S., 2016. Secondary metabolites in Hypericum species and their distribution in different plant parts. Agriculture 103, 193–198.https://doi.org/10.13080/z-a.2016.103.025. Ogunwa, T.H., Ayenitaju, F.C., 2017. An insight into the precise molecular interaction
and inhibitory potential of amentoflavone and its substituted derivatives on human α-amylase. Arch. Curr. Res. Int. 10, 1–14.https://doi.org/10.9734/ACRI/2017/ 35759.
Pang, Y., Ahmed, S., Xu, Y., Beta, T., Zhu, Z., Shao, Y., Bao, J., 2018. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 240, 212–221.https://doi.org/10.1016/j.foodchem.2017.07. 095.
Picot, M.C., Zengin, G., Mollica, A., Stefanucci, A., Carradori, S., Mahomoodally, M., 2017. In vitro and in silico studies of mangiferin from Aphloia theiformis on key en-zymes linked to diabetes type 2 and associated complications. Med. Chem. 13, 633–640.https://doi.org/10.2174/1573406413666170307163929.
Ramsay, R.R., Tipton, K.F., 2017. Assessment of enzyme inhibition: a review with ex-amples from the development of monoamine oxidase and cholinesterase inhibitory drugs. Molecules 22, 1192.
Rao, P., Hasan, S., Bhellum, B., Manhas, R., 2015. Ethnomedicinal plants of Kathua dis-trict, J&K, India. J. Ethnopharmacol. 171, 12–27.https://doi.org/10.1016/j.jep. 2015.05.028.
Raza, A., Xu, X., Sun, H., Tang, J., Ouyang, Z., 2017. Pharmacological activities and pharmacokinetic study of hyperoside: a short review. Trop. J. Pharm. Res. 16 (2), 483–489.https://doi.org/10.4314/tjpr.v16i2.30.
Saldanha, L.L., Vilegas, W., Dokkedal, A.L., 2013. Characterization offlavonoids and phenolic acids in Myrcia bella cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 18, 8402–8416.https://doi.org/10.3390/ molecules18078402.
Sargin, S.A., 2015. Ethnobotanical survey of medicinal plants in Bozyazı district of Mersin, Turkey. J. Ethnopharmacol. 173, 105–126.https://doi.org/10.1016/j.jep. 2015.07.009.
Sastry, G.M., Adzhigirey, M., Day, T., Annabhimoju, R., Sherman, W., 2013. Protein and ligand preparation: parameters, protocols, and influence on virtual screening en-richments. J. Comput. Aided Mol. Des. 27, 221–234.https://doi.org/10.1007/ s10822-013-9644-8.
Schrödinger, 2015. 2017-3: Maestro. Schrödinger, LLC, New York.
Si, Y.-X., Yin, S.-J., Oh, S., Wang, Z.-J., Ye, S., Yan, L., Yang, J.-M., Park, Y.-D., Lee, J., Qian, G.-Y., 2012. An integrated study of tyrosinase inhibition by rutin: progress using a computational simulation. J. Biomol. Struct. Dyn. 29, 999–1012.https://doi. org/10.1080/073911012010525028.
Singh, J.P., Selvendiran, K., Banu, S.M., Padmavathi, R., Sakthisekaran, D., 2004. Protective role of apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine 11, 309–314.
https://doi.org/10.1078/0944711041495254.
Sõukand, R., Pieroni, A., 2016. The importance of a border: medical, veterinary, and wild food ethnobotany of the Hutsuls living on the Romanian and Ukrainian sides of Bukovina. J. Ethnopharmacol. 185, 17–40.https://doi.org/10.1016/j.jep.2016.03. 009.
Tabassum, N., Lee, J.-H., Yim, S.-H., Batkhuu, G.J., Jung, D.-W., Williams, D.R., 2016. Isolation of 4,5-O-dicaffeoylquinic acid as a pigmentation inhibitor occurring in Artemisia capillaris Thunberg and its validation in vivo. J. Evid. Based Complementary Altern. Med.https://doi.org/10.1155/2016/7823541.
Tadera, K., Minami, Y., Takamatsu, K., Matsuoka, T., 2006. Inhibition ofα-glucosidase andα-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 52 (2), 149–153.https://doi. org/10.3177/jnsv.52.149.
Tekin, M., 2017. Pharmacobotanical study of Hypericum thymopsis. Rev. Bras. Farmacogn. 27, 143–152.https://doi.org/10.1016/j.bjp.2016.09.004.
The Plant List, 2013. Version 1.1. Published on the Internet.http://www.theplantlist.org/
(accessed 1st January).
Thilagam, E., Parimaladevi, B., Kumarappan, C., Mandal, S.C., 2013.α-Glucosidase and α-amylase inhibitory activity of Senna surattensis. J. Acupunct. Meridian Stud. 6, 24–30.https://doi.org/10.1016/j.jams.2012.10.005.
Um, M., Shin, G.-J., Lee, J.-W., 2017. Extraction of total phenolic compounds from yellow poplar hydrolysate and evaluation of their antioxidant activities. Ind. Crops Prod. 97, 574–581.https://doi.org/10.1016/j.indcrop.2016.12.062.
Uysal, A., Zengin, G., Mollica, A., Gunes, E., Locatelli, M., Yilmaz, T., Aktumsek, A., 2016. Chemical and biological insights on Cotoneaster integerrimus: a new (–)-epicatechin source for food and medicinal applications. Phytomedicine 23, 979–988.https://doi. org/10.1016/j.phymed.2016.06.011.
Uysal, S., Zengin, G., Locatelli, M., Bahadori, M.B., Mocan, A., Bellagamba, G., De Luca, E., Mollica, A., Aktumsek, A., 2017. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Frontiers Pharmacol. 8, 290.https://doi.org/10.3389/fphar.2017.00290. Xu, J.-G., Hu, Q.-P., Liu, Y., 2012. Antioxidant and DNA-protective activities of
chloro-genic acid isomers. J. Agric. Food Chem. 60, 11625–11630.https://doi.org/10.1021/ jf303771s.
Yang, J., Guo, J., Yuan, J., 2008. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 41 (6), 1060–1066.https://doi.org/10.1016/j.lwt.2007.06.010. Yüce, E., 2016. Analysis of the essential oils of two Hypericum species (H. lanuginosum var.
lanuginosum Lam. and H. perforatum L.) from Turkey. Hacettepe J. Biol. Chem. 44, 29–34.https://doi.org/10.15671/HJBC.20164417564.
Zanwar, A.A., Badole, S.L., Shende, P.S., Hegde, M.V., Bodhankar, S.L., 2014. Antioxidant Role of Catechin in Health and Disease, Polyphenols in Human Health and Disease. Elsevier pp. 267–271.
Zengin, G., Aktumsek, A., Ceylan, R., Uysal, S., Mocan, A., Guler, G.O., Mahomoodally, M.F., Glamoclija, J., Ciric, A., Sokovic, M., 2017a. Shedding light on the biological and chemicalfingerprints of three Achillea species (A. biebersteinii, A. millefolium and A. teretifolia). Food Funct. 8, 1152–1165.https://doi.org/10.1039/c6fo01847e. Zengin, G., Mollica, A., Aktumsek, A., Picot, C.M.N., Mahomoodally, M.F., 2017b. In vitro
and in silico insights of Cupressus sempervirens, Artemisia absinthium and Lippia tri-phylla: Bridging traditional knowledge and scientific validation. Eur. J. Integr. Med. 12, 135–141.https://doi.org/10.1016/j.eujim.2017.05.010.
Zengin, G., Senkardes, I., Mollica, A., Picot-Allain, C.M.N., Bulut, G., Dogan, A., Mahomoodally, M.F., 2018. New insights into the in vitro biological effects, in silico docking and chemical profile of clary sage – Salvia sclarea L. Comp. Biol. Chem. 75, 111–119.https://doi.org/10.1016/j.compbiolchem.2018.05.005.
Zhang, X.D., Liu, X.Q., Kim, Y.H., Whang, W.K., 2014. Chemical constituents and their acetyl cholinesterase inhibitory and antioxidant activities from leaves of Acanthopanax henryi: potential complementary source against Alzheimer's disease. Arch. Pharm. Res. 37 (5), 606–616.https://doi.org/10.1007/s12272-013-0252-x. Zhang, H., Tsao, R., 2016. Dietary polyphenols, oxidative stress and antioxidant and
anti-inflammatory effects. Curr. Opin. Food Sci. 8, 33–42.https://doi.org/10.1016/j.cofs. 2016.02.002.
Złotek, U., Mikulska, S., Nagajek, M., Świeca, M., 2016. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 23, 628–633.https:// doi.org/10.1016/j.sjbs.2015.08.002.
Zorzetto, C., Sánchez-Mateo, C.C., Rabanal, R.M., Lupidi, G., Petrelli, D., Vitali, L.A., Bramucci, M., Quassinti, L., Caprioli, G., Papa, F., 2015. Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum, Hypericum canariense and Hypericum grandifolium). Fitoterapia 100, 95–109.https://doi.org/10.1016/j.fitote.2014.11.013.