ContentslistsavailableatScienceDirect
Sensors
and
Actuators
B:
Chemical
j ou rn a l h o m ep a g e :w w w . e l s e v i e r . c o m / l o c a t e / s n b
Microfluidic
droplet
content
detection
using
integrated
capacitive
sensors
Pelin
Kubra
Isgor,
Merve
Marcali,
Mert
Keser,
Caglar
Elbuken
∗UNAM-NationalNanotechnologyResearchCenter,InstituteofMaterialsScienceandNanotechnology,BilkentUniversity,06800Ankara,Turkey
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received12August2014
Receivedinrevisedform
18December2014
Accepted7January2015
Availableonline14January2015
Keywords: Microdroplets
Capacitivesensor
Coplanarelectrodes
Dropletcontentsensing
a
b
s
t
r
a
c
t
Microfluidiccapacitivesensorshavebeenusedfordetectionofdroplets,howevertheyhavebeenlacking thesensitivityrequiredfordetectingthecontentofdroplets.Inthisstudy,wedevelopedascalable, portable,robustandhighsensitivitycapacitivemicrodropletcontentdetectionsystemusingcoplanar electrodeswithnanometerthicksilicondioxide(SiO2)passivationlayerandoff-the-shelfcapacitive
sensors.Themicrofluidicchipwehavedesignedprovideseasyandrapidmodificationofdropletcontent bymixingtwoaqueousliquidsatanygivenratio.Thechangeindielectricconstantofthedropletcontent leadstothechangeincapacitivesignal.Thedielectriccontentofdropletswasmodifiedcontinuously whilecorrespondingcapacitancesignalwasmeasured.Theresolutionofthesystemwasmeasuredas 3dielectricpermittivityunits.Theresultswereverifiedusingasemiconductorparameteranalyzer.The applicationspecificintegratedcircuitusedinthisworkenablesaportable,low-costdetectionsystem andmatchestheperformanceofbench-topanalyzers.Automatedandprecisemeasurementofdielectric contentindropletsforbiochemicalassaymonitoringisamajorapplicationofthepresentedsystem.
©2015ElsevierB.V.Allrightsreserved.
1. Introduction
Microdroplet based microfluidic systems are very handy platformsforapplicationsthatrequireexperimentationonlarge libraries of samples. The fundamental requirements of most microdroplet systems are formation of monodisperse droplets, measuringtherateofdropletformationanddetectingthe analyt-icalcontentofdroplets.Opticalcountingofdropletsandoptical detectionofdropletcontentarethemostprevalenttechniquesin theliterature[1,2].Opticaldetectionoftenrequiresbulky compo-nentsandfluorescentlabelingthatincreasesthecomplexityand costofthesystem[3].Quenchingoflabelsandsterical interfer-encewithmolecularbindingareotherdrawbacksoffluorescent labeling techniques [4]. Although, optical systems can detect dropletsatveryhighrates(ontheorderofkHz),thesesystemsare notscalable[5].Ontheotherhand,electricalsensingtechniques provideascalableand labelfree alternativefordropletcontent detection,whichallowsmultiplesensorsina smallfootprintat very low cost. Using off-the-shelf and low cost electrical com-ponents for highsensitivity droplet contentdetection can take
∗ Correspondingauthor.Tel.:+903122903550;fax:+903122664365.
E-mailaddress:elbuken@unam.bilkent.edu.tr(C.Elbuken).
microdroplet-based microfluidic systems one step further, and turnthemintoprogrammableandeasy-to-useplatforms.
Electricalsensingfordropletbasedmicrofluidicswasinitially focused ondropletcountingusingeither resistiveor capacitive measurements.Somegroupshavemeasuredthechangein resis-tanceduetothepresenceofdropletsbetweentheelectrodes[6,7]. Luoetal.utilizedanelectrochemicaldetectiontechniqueto mea-suredropletsizeanditsioniccontent[8].Duetothesignificant changeinconductivityofthedispersedandcontinuousphase,very highsensitivitycanbeobtainedinresistivedropletmonitoring. However,direct contact betweenthedropletand theelectrode surfaceshouldbeavoidedtominimizetheriskofelectrolysisand pinningofdropletsonelectrodesurfacewhicheventuallyleadsto cross-contaminationbetweendroplets[9].
Capacitivedetectionofdropletsisalabelfreeandnon-contact detectionmethod [10].Chen etal. andRen etal. have demon-strated capacitivesensingof droplets usingcoplanarelectrodes
[11,12].Usingelectro-wettingondielectric(EWOD)systems,they have showed droplet detection and droplet volume metering. Othergroupshavedemonstrateddetectionofdropletsandtheir contentbyusingthecapacitivecomponentofimpedancesignal
[13,14].However,thesestudiesutilizecustom-madeelectronics thatrequirehighlyspecializedexpertise[10].Inaddition,these sys-temscanonlydetectthepresenceofthedropletandtheyarenot sensitiveenoughtodetectthecontentofthedroplets.
http://dx.doi.org/10.1016/j.snb.2015.01.018
change.
Inthisstudy,wehavedesignedaY-junctionmicrofluidicdevice that allows rapid and easy modification of droplet content by mixing two liquids,ethanol and distilled (DI)water. High sen-sitivitylabel-freedroplet contentdetectionwas achievedusing coplanarelectrodescoatedwithananometerthickSiO2
passiv-ation layer,commercially available,low-cost capacitivesensors andamicroprocessor.Thesystemwascharacterizedby modify-ingthedielectriccontentofthedropletsontherunandmeasuring thecorrespondingcapacitance signal. Theresults wereverified by comparative measurements of our detection system and a semiconductor parameter analyzer. The capacitive signal was enhanced by minimizingthe thicknessof the passivationlayer that separates the coplanar electrodes from the microchannel. Thissystemprovideslabelfree capacitivedropletcontent mea-surementswhichenablesdielectricspectroscopyindropletbased systems.
2. Experimental
Inthisstudy,weconductedexperimentsusingamicrofluidic deviceschematicallyshowninFig.1.Dropletswereformedusing aT-junctiongeometry[17,18].Inordertochangethedroplet con-tentontherun,weutilizedaY-junctionthatfeedstwodifferent aqueousphases.Atthedownstreamofthedropletgeneration sec-tion,amixingregionandadetectionregionwereplaced.Detection regionconsistsofcoplanarelectrodesthatwereplacedafterthe mixingregion.Theseelectrodeswerepassivatedby360nmSiO2
dielectriclayer.Aphotographofthefabricateddeviceisshownin
Fig.2.
Duringexperiments,dropletswereformedatvaryingethanol andDIwaterconcentrations.Mixingregionensuredthatethanol andDIwaterwereproperlymixedtoobtainhomogeneousdroplets. Themeasuredcapacitancesignalchangesduetothestarkcontrast betweendielectricpermittivity ofthecontinuousand dispersed phasewhenadropletentersthesensingregion.
Itisimportanttopassivatetheelectrodesinordertoprevent cross-contamination between droplets and pinning of droplets ontotheelectrodes.Forpassivationofelectrodes,thereare fun-damentallytwo fabrication options: spin-coating and thin film deposition.We havestudiedbothmethodsindetail inorderto obtainathin,pin-holefreeuniformpassivationlayercoatingto enhancethecapacitancesignal.Wehavepassivatedelectrodesby spincoatingofpolydimethylsiloxane(PDMS),andalsobyplasma enhancedchemicalvapordeposition(PECVD)ofSiO2.Weshowed
thatthethicknessof thepassivationlayercanbedecreased by addingtoluenetothePDMSmixture.Westudiedtheeffectofspin speedonthethicknessofthePDMSand toluene-thinnedPDMS coatedlayer[18].
modeinairunderambienttemperature.ATap190Al-Gprobewith aforceconstantofapproximately48N/mandresonancefrequency of190kHzwasused.Byusingtheanalyticalmodelinourprevious study,electrodewidthandgapweredeterminedas200mand 50m,respectively[19].
Microfabricated electrodes werepassivated witheither spin coatingofPDMS,toluenethinnedPDMSorthinfilmdepositionof SiO2usingPECVD.Forstudyingtheeffectofspinspeedonthe
thick-nessofthecoatedlayer,thePDMSmixturewasspunonmicroscope slidesfor5minatspinratesvaryingfrom1000rpmto7000rpm.In ordertogetthetoluenethinnedPDMS,PDMSmixturewasmixed withtoluenein1:3(w/w)ratio.Then,thepreparedmixturewas spuncoatedonmicroscopeslidesfor2minatsamespinrates.After spincoating,glassslideswerebakedat110◦Cfor2h.Variationof passivationlayerthicknesswithrespecttospinrateisdiscussed inSection3.2.SiO2passivationlayerwasdepositedusingPECVD
(Vaksis,CVD-Handy).Targetpressure,RFpowerandheaterwere setas1Torr,10W,and200◦C,respectively.Thethicknessofthe passivationlayerwasadjustedbythedepositionduration.
ThicknessoftheSiO2,PDMSandtoluene-thinnedPDMS
passiv-ationlayersweremeasuredusingaVariableAngleSpectroscopic Ellipsometer(J.A.Woollam,V-VASE).Ellipsometerwasusedatan incidenceangleof 65◦.Cauchydispersionfunctionwasusedto determinepassivationlayerthicknesses.Inordertofit experimen-taldata,refractiveindicesofPDMSandtoluene-thinnedPDMSwere takenas1.42,andrefractiveindexofSiO2wastakenas1.55.
Themicrochannelswerefabricatedusingstandardsoft lithog-raphy methods[20].Molds werefabricatedby patterningSU-8 photoresistona4inchsiliconwaferusingphotolithography.The channelheightwasmeasuredas100musingStylusProfilometer (KLATencor,P6SurfaceProfiler).
Formoldingprocess,PDMSmixturewaspreparedbymixing siliconeelastomerandcuringagent(DowCorning,Sylgard184)in 10:1(w/w)ratio.Followingdegassingofthemixture,PDMSwas cross-linkedat100◦Cfor4h.Aftercuring,inlet/outletholeswere punchedusingabiopsypunch.Finally,microchannelswerebonded totheglassslideswithpassivatedcoplanarelectrodesusing oxy-genplasma.ThebondingbetweenPDMSmicrochannelsandSiO2
passivationlayerwascompletedusingaplasmacleaner(Nanoplas, DSB6000)at50W,30◦Cfor1min.Inordertoenhancethebonding, thedeviceswerebakedat100◦Cfor12himmediatelyafterplasma activatedbonding.
2.2. Measurementsetup
Microdropletswereformed usingtheT-junction microchan-nelgeometryanda pressurepump(Elveflow).Siliconeoilwith 50mPasviscosity(Ultrakim) wasusedasthecontinuousphase. ThedispersedphasewascomposedofethanolandDIwater.
Fig.1.Schematicofthemicrofluidicdevice.Theinsetsshowthe(a)dropletgenerationregionandmixingregion,(b)detectionregion.
Fig.2.Photographofthefabricatedmicrofluidicdevice.Channelswerefilledwithdyesolutionsforclarity.
Thedielectric permittivity contrast betweencontinuous and dispersed phase is the basis of capacitive content detection of microdroplets.Across-sectionofthedetectionregionisshownin
Fig.3.Thedielectricpermittivitydifferencebetweensiliconeoil (εr=2.5)andmicrodroplet(εrethanol=24<εr<εrwater=80)causes
anincreasein thecapacitancesignalamplitude.Thesignalwas measuredusingacapacitive-to-digitalconverterintegratedcircuit (AnalogDevices,AD7746)whichprovidesalow-cost,portableand scalablesolutionasopposedtobench-topmeasurementsystems suchasLCRanalyzers.Full-scalelinearcapacitancemeasurement rangeof AD7746is±4pFwitha precisionof4fF. AD7746 per-formsthemeasurement usinganexcitationsignalataconstant frequencyof32kHz.Theexcitationvoltagecanbedigitallytuned byusingtheexcitationset-upregister.Inordertoprevent impre-cisionofthesignalduetoelectricalwiringandkeepthesignalin
Fig.3.Schematicofthecross-sectionofthedetectionregion.
thehigh-accuracylinearmeasurementrange,internaloffset capac-itorwastunedforself-calibrationduringtheteststart-upcycle. AD7746hastwomeasurementmodesbutduringthisstudy,only single-endedmodewasused.Theread-outdataratewaskept con-stantat50Hztohaveenoughsamplingrateandlowmeasurement noise.Thediscussiononselectingtheread-outdataratecanbe seeninSupplementaryMaterial.AD7746wascommunicatedwith LabViewusinga USB-poweredmicrocontroller(Arduino Duemi-lanoveATmega328).Thismicrocontrollerwasusedfordisplaying real-timecapacitancesignalthroughaLabViewinterface.
During theelectrical measurements,droplets wereobserved usinganinvertedcompoundmicroscopeinordertomaintainthe dropletsizeassimilaraspossiblebetweendifferentruns.
2.3. Experimentalprocedure
ThedevicethatisshowninFig.2wasusedforthedielectric contentmeasurementofdroplets.EthanolandDIwaterwere sepa-ratelydrivenfromaqueoussolutioninletsusingapressurepumpin ordertochangethedielectriccontentofthedroplets.Bluedyewas addedtoDIwaterinordertodistinguishtheseparatingboundary betweenDIwaterandethanol(Fig.4).Duringthemeasurements, thestreamlineseparatingtheDIwater(withbluedye)andethanol wasobserved.ThepositionoftheseparatingboundarybetweenDI waterandethanolisusedtodeterminethemixingratio.In addi-tion,thebluecolorcontentofthedropletsinthedetectionregion wasobservedtoformtheaqueousdropletsofpredetermined con-centrations.
For droplet contentdetection,coplanar electrodesthat have awidthof200mandagapof50mwereused.Experiments
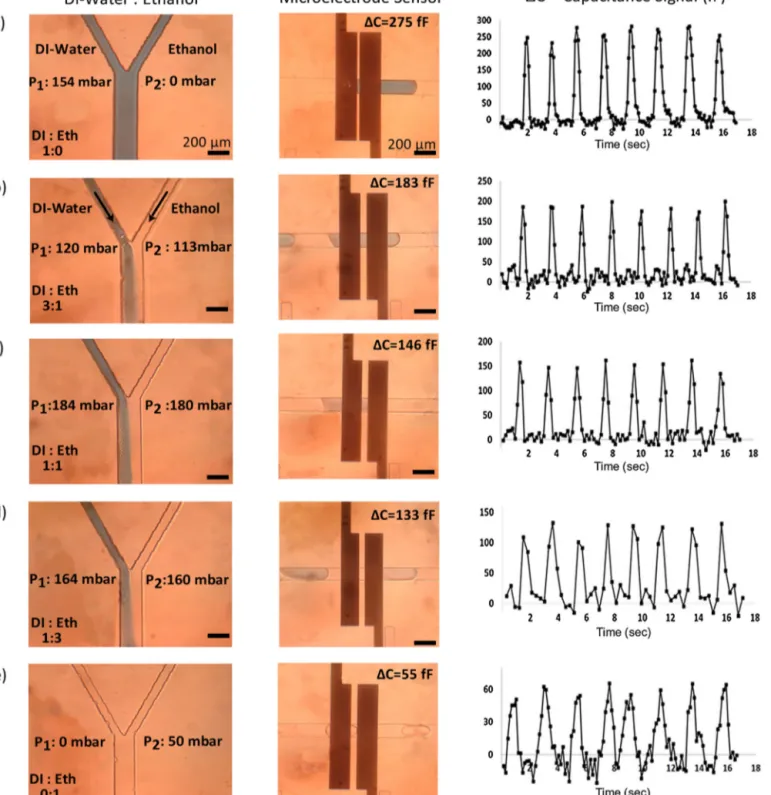
Fig.4. CapacitancesignalforvariedDIethanolmixtures(a)onlyDI,(b)25%ethanol,(c)50%ethanol,(d)75%ethanol,(e)onlyethanol.Eachpeakcorrespondstoasingle
dropletinthesensingregion.Theplotsshowthedatafor17s.
wereperformedatfivedifferentconcentrationsofethanolDIwater mixtures(0%, 25%,50%,75%,100% (v/v)ethanol concentration). Thereal-timecapacitancesignalwasdisplayedthroughthe Lab-Viewinterfaceandrecordedwhendropletenterstothedetection region.Then,signalamplitudeforeachdropletismeasuredfrom therecorded data. For each experiment snapshot imageswere captured at the Y-junction region and the detection region as showninFig.4.Theflowrateswerefinetunedinordertohave dropletsofsimilarsizeandspeed.Inthisstudy,plug-likedroplets whoselengthislargerthanthespacingbetweenelectrodeswere
formed.Thisensuredthatdropletscompletelyoccupythesensing domain.
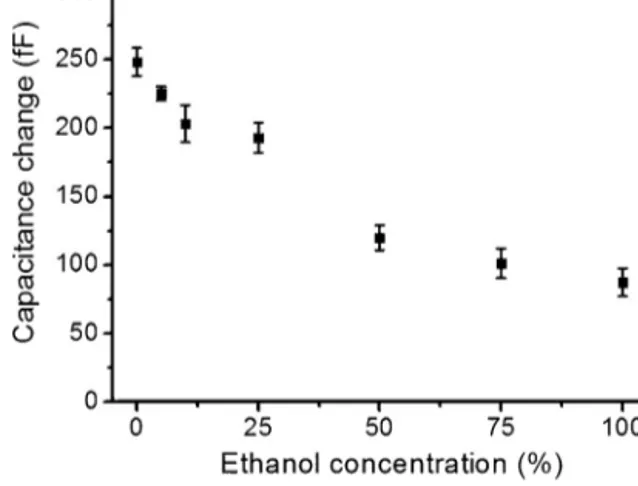
Forverificationoftheresultsandtodeterminetheresolution ofthesystem,sevendifferentsolutionsofethanolandDIwater mixtures werepreparedoff thedevicewithethanol concentra-tionsof0%,5%,10%,25%,50%,75%and100%(v/v).Thesesolutions werefedintooneofthedispersedphaseinletswhilekeepingthe otherinletplugged. Dropletsofthesesolutions weregenerated separatelyandcapacitivedropletcontentmeasurementswere per-formedasexplainedabove.Theseresultsformthecalibrationplot
Fig.5. SolutiondependentcapacitancesignalamplitudesobtainedfromFig.4.Error
barsdenoteonestandarddeviationacross50dropletpeaks.
thatshowsdependenceofcapacitancesignalamplitudeonethanol concentrationasshowninFig.6.
This calibration plot was also verified by recording capaci-tancesignalamplitudeusingahighendbench-topsemiconductor parameteranalyzer(Keithley4200).Thesamepre-mixedsolutions weredispensedontocoplanarelectrodesaslargedroplets(50l) usingamicropipette.Thedispensedvolumewashighenoughto completelyfillthesensingdomainof200mwidthand 50m spacingelectrodes.TheresultscanbeseeninSupplementary Mate-rial.
3. Resultsanddiscussion
3.1. Dropletdielectriccontentmeasurement
Fig. 4 summarizes the capacitive droplet content detection experiments.AsseeninFig.4,DIdroplets (0%ethanol)(εr=80)
causedthehighestcapacitancechangewithanaverageof275fF, whereasethanoldroplets(100%ethanol)(εr=24)causedthelowest
capacitancechangewithanaverageof55fF.DecreaseinDIwater pressurecausedashifttowardsleftatseparatingboundaryatthe Y-junction.Therefore,dropletswithhigherethanolcontentledto smallercapacitancesignal.
ForthefivecasesdemonstratedinFig.4,thecapacitance sig-nalswereanalyzed.Fig.5demonstratestherelationshipbetween ethanolconcentrationofdropletsandaveragecapacitancesignal amplitudestakenover50droplets.Increasingethanol concentra-tioncausesasteadydecreaseinthedetectedsignal.
Fig.6showsthecalibrationplotobtainedbyusingpre-mixed solutions.Thisplotconfirmsthatincreaseinethanolconcentration causeslineardecreaseincapacitivesignalamplitude.DIethanol mixtureswerepreparedinpredeterminedratioswithethanol con-centrationsof0%,5%,10%,25%,50%,75%and100%(v/v).Foreach ethanolconcentration,capacitivesignalamplitudewasaveraged over50droplets.
AsseenfromFig.5 andFig.6,thepeakamplitudedecreases withincreasingethanolconcentration.Thestandarddeviationis higherwhenmixingisperformedontherun(Fig.5)asopposed topre-mixedsolutions(Fig.6).Thisismainlyduetothefactthat thepressure fluctuationsduringdropletbreak-upprocessaffect theupstreampressuresandcausefluctuationinrelativeflowrates ofethanolandDIwater.WhentheresultsinFig.5areanalyzed,it canbeseenthatthelargeststandarddeviationisobtainedfor75% ethanolconcentration.Itwasobservedthatgenerating monodis-persedropletsatthisconcentrationisverychallenging.Asethanol concentrationofdropletswasincreased,thedropletformationat
Fig.6. Calibrationplotobtainedbypre-mixedsolutions.Errorbarsdenoteone
standarddeviationacross50dropletpeaks.
theT-junctionswitchedfromsqueezingregimetodrippingregime (showninSupplementaryMaterial).Indrippingregime,thedroplet break-uppointisafunctionoftheethanolandDIwaterratio. Dur-ingtheformationof75%ethanolconcentrationdroplets,wehave observedhighervariationinthebreak-uppoint.Thisleadstohigher variationindropletsize,causingthehigheststandarddeviationin
Fig.5.It isalsoimportanttonotethatethanolchangesthe sur-facepropertiesofthePDMSmicrofluidicdevicesovertime,which causespartialdropletwetting.Afterafewhoursofcontinuous run-ning,wehaveobservedsomedropletpinningonthesidewallsof channelsasseeninFig.4d.
Inarecentstudy,thissensorwasusedtomeasuredropletsize andspeed[19].Wehaveshownthatanychangeindropletsizeand speedaffectsthecapacitancesignalamplitude.Increasingdroplet sizeordecreasingdropletspeedleadstohighercapacitancesignal amplitude.Therefore,thevariationinFig.5andFig.6ispartially duetothevariationindropletsizeandspeed.Duringallofthe mea-surements,thepressuresystemwasadjustedtoformdropletsof approximately450minlength.Themonodispersityofdroplets wasmeasuredas5–10%fromtherecordedimagesofdroplets.It wasalsoobservedthatmonodispersitywashigherforthedroplets thatweregeneratedusingthepre-mixedsolutions.Thisexplains thesmallerstandarddeviations observedin Fig.6compared to
Fig.5.Otherthanthemeasurementsat75%ethanolconcentration, allthemeasurementsfollowalineartrendlineinboth measure-ments.Itisalsoimportanttonotethatthe5%changeinthedroplet ethanolcontentcanberesolved,whichcorrespondstoadielectric constantunitresolutionoflessthan3.
Thelineardecreaseof capacitancesignaldue tothe increas-ing ethanol concentration was verified using a semiconductor parameteranalyzer.Duringtheseverificationmeasurements50l dropletswerepreciselypipettedontothe375nmSiO2passivated
electrodesusing thepre-mixedDIwater ethanol solutions.The resultantplotcanbefoundasSupplementaryMaterialtogether withthephotographofthefabricateddevice.Sincethesensing domainis completely covered withthesolutions, thevariation duetochangingdropletsizewaseliminated.Weobservedalinear responsewithR2=0.99.Thesemeasurementsareinperfect
agree-mentwiththetheoreticalpredictionsthatthecapacitancesignal amplitudelinearlydecreaseswithincreasingethanol concentra-tions. The signalamplitudefor DIsample (εr=80)was1054fF,
whereasitwas276fF forethanolsample(εr=24).Theseresults
ensuredthatthevariationandnonlinearityinthemicrofluidic sys-temisduetothevariationindropletgenerationdynamics.The changingcontentofdispersedphaseaffectstheviscosityaswellas
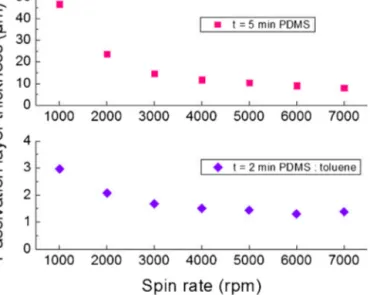
Fig.7. PDMSandtoluenethinnedPDMSthickness.PDMSthickness(pink,square)
for5min.spinningtimeandPDMS:toluene1:3(w/w)thickness(blue,diamond)
for2min.spinningtime(Forinterpretationofthecolorinformationinthisfigure
legend,thereaderisreferredtothewebversionofthearticle.).
thewettingconditions,whichinturnaltersdropletformationat theT-junction.
Inordertoperformhighsensitivitydropletcontent measure-ments,itiscriticaltominimizethebaselinenoiselevels.Accuracy ofAD7746dependsontheshieldingofthesystemandelectrodes aswellasthemeasurement read-outrate.Theread-outrateof AD7746canbevariedbetween10Hzand90Hz. Wehave char-acterizedthebaselinenoiselevelforDIwaterdropletsmeasured atdifferentdatarates.TheplotsaregivenasSupplementary Mate-rial.Wehaveobservedthatlowdataratesdecreasebaselinenoise levels,howeverthesamplingrateatthesevalues(10Hz)isnot suf-ficienttosamplethedroplets.At10Hzsamplingrate,dropletspass overthesensingregioninapproximately500ms,whichyieldsonly fourorfivedatapointsforeachdroplet.Thisleadstovariationin thecapacitancesignalamplitude.Therefore,wehaveincreasedthe dataread-outrateofAD7746,untilwehaveobserveduniform sig-nalforidenticalDIdroplets.Wehavesetourdatarateas50Hz.The baselinenoiselevelatthisspeedwas15fF.Itisworthmentioning thatthedatarateforthesensorshouldbetailoreddependingon theflowrateofthedroplets.
3.2. Signalenhancementthroughthinnerpassivationlayer
Formicrofluidicsystemsthatcontaincapacitivedetectionusing coplanarelectrodes,havingapassivationlayerisimportantto pre-ventcross-contaminationbetweensamples.Sincethethicknessof thepassivationlayerdeterminesthedistanceofthe microchan-nelfromtheelectrodes(Fig.3),itiscriticaltokeepthethickness aslowas possibletoimprove thesignalamplitude.In orderto comparedifferentpassivationlayertypesinterms ofthickness, uniformityand quality, we used PDMS, toluene-thinned PDMS andSiO2.WehavestudiedtheeffectofspinspeedforPDMSand
toluene-thinnedPDMStoobtainthinnerpassivationlayers. Thick-nessof PDMSdepends onspin speed, duration,mixing ratioof siliconeelastomerandcuringagent,ambientconditions (tempera-ture,humidity),timebetweenPDMSpreparationandspincoating. AsitcanbeseenfromFig.7,fora5minspinningtime,increasing spinspeedexponentiallydecreasesPDMSpassivationlayer thick-ness.
Itshouldbenotedthatinordertogetconsistentcoating thick-nessesformultipledevices,careshouldbetakenfortheduration
Therefore, weuseda thin filmdepositiontechnique, PECVD,in ordertoachieveuniformityandnanometerscalethickness.
WecoatedthecoplanarelectrodeswithtwodifferentSiO2
pas-sivation layerthicknesses and measuredthe capacitancesignal amplitudesforDIdropletsusingthesameY-junctionchannel.For 180nm thickSiO2 passivationlayer,weachieved514fF
capaci-tancesignalamplitude,whereaswegot275fFcapacitancesignal amplitudefor 360nmthick SiO2 passivationlayeras shown in
Fig.4a. These resultsprovea 10-foldcapacitance signal ampli-tudeenhancementfor DIdropletsascompared toourprevious studywhere toluene-thinnedPDMS passivationlayerwasused andthecapacitancesignalwasmeasuredas26fF[19].Wehave also confirmedthe effect of passivation layerthickness onthe signal level by measuring the capacitance change signal using PDMScoatedelectrodes.WecancontrolthethicknessofPDMS in a wide range as shown in Fig. 7. We have increased the thickness of PDMS passivation layer by successive spin coat-ing steps in between each measurement. We have observed thatthesignalamplitudedecreasessignificantlywithincreasing passivationlayerthickness(Fig. S3inSupplementaryMaterial). Therefore,forapplicationsrequiringhighsensitivity,itis impor-tanttominimizethethicknessofthepassivationlayer,preferably using thin film deposition techniques to maximize the signal amplitude.
3.3. Outlook
AsrecentlydiscussedindetailbyHolgerBecker, commercial-izationoflab-on-a-chipdevicesarefacingseveralhurdles[21].As themicrofluidiccommunity,itisanadditionalassignmentforusto considerthemanufacturabilityandscalabilityofthetechnologies wedeveloptoovercomethesehurdles.Thisstudytakesastepin thatdirectionbyutilizingverylowcostelectronicsforhigh sensi-tivitymeasurementofdropletcontent(ArduinoNano$20,AD7746 $4).Dropletcontentdetectionisrequiredformostofthedroplet basedbiochemicalassays.Inadditiontothedropletdielectric con-tentmeasurement,thissystemcanbeusedforveryprecisedroplet sizeandspeeddetectionaswellasdropletcounting.Monitoring metabolicactivityof cellsencapsulatedindropletsusing copla-narelectrodeswillbeaninterestingapplicationofthissystem[5]. Anotherpotentialapplicationofthissystemisdielectricindexingof dropletsasanalternativetochemicalindexingthatisaccomplished byencodedparticlesorlabeledbeads[22].
4. Conclusions
Inthis study,wehave demonstratedhighsensitivity capaci-tivedropletcontentdetectionusingascalableandcost-effective method.We showedthatthereisa linearrelationshipbetween
thedecreaseindielectricpermittivityofdropletcontentandthe capacitivesignal.Weverifiedtheseresultsbyusinga semiconduc-torparameteranalyzer.Inordertogobeyondtheroutineusesof capacitivesensorsasdropletdetectionsensors,itwasrequiredto increasethesystemsensitivitysothatdropletcontent measure-mentswerealsopossible.Wehavedemonstratedthatsensitivity canbeimprovedbyminimizingthethicknessofthepassivation layer. We showed that using commercially available, low-cost electricalcomponents,dropletcontentcanbedetectedinrobust, preciseandscalablemannerathighsensitivity.Thepresented sys-temisanalternativeforbulkyandcostlybench-topanalyzers.
Acknowledgment
TheauthorsacknowledgesupportfromEuropeanUnionFP7 MarieCurieCareerIntegrationGrant(no.322019)andState Plan-ningOrganizationofTurkeyfor thesupportofUNAM–National NanotechnologyResearchCenter.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,athttp://dx.doi.org/10.1016/j.snb.2015.01.018.
References
[1]M.R.deSaintVincent,S.Cassagnere,J.Plantard,J.P.Delville,Real-timedroplet caliperfordigitalmicrofluidics,Microfluid.Nanofluid.13(2012)261–271.
[2]Y.Zhao,Z.G.Xu,M.Parhizkar,J.Fang,X.G.Wang,T.Lin,Magneticliquidmarbles, theirmanipulationandapplicationinopticalprobing,Microfluid.Nanofluid.13 (2012)555–564.
[3]J.Schemberg,A.Grodrian,R.Romer,G.Gastrock,K.Lemke,Onlineoptical detec-tionoffoodcontaminantsinmicrodroplets,Eng.LifeSci.9(2009)391–397.
[4]U.Resch-Genger,M.Grabolle,S.Cavaliere-Jaricot,R.Nitschke,T.Nann, Quan-tumdotsversusorganicdyesasfluorescentlabels,NatureMethods5(2008) 763–775.
[5]H.N.Joensson,M.Uhlén,H.A.Svahn,Dropletsizebasedseparationby deter-ministiclateraldisplacement—separatingdropletsbycell-inducedshrinking, LabChip11(2011)1305–1310.
[6]N.Srivastava,M.A.Burns,Electronicdropsensinginmicrofluidicdevices: auto-matedoperationofananoliterviscometer,LabChip6(2006)744–751.
[7]M.C.Cole,P.J.A.Kenis,Multiplexedelectricalsensorarraysinmicrofluidic networks,Sens.ActuatorsB-Chem.136(2009)350–358.
[8]C.X.Luo,X.J.Yang,O.Fu,M.H.Sun,Q.Ouyang,Y.Chen,etal.,Picoliter-volume aqueousdropletsinoil:Electrochemicaldetectionandyeastcell electropora-tion,Electrophoresis27(2006)1977–1983.
[9]B.P.Cahill,R.Land,T.Nacke,M.Min,D.Beckmann,Contactlesssensingofthe conductivityofaqueousdropletsinsegmentedflow,Sens.ActuatorsB-Chem. 159(2011)286–293.
[10]M.Demori,V.Ferrari,P.Poesio,D.Strazza,Amicrofluidiccapacitancesensorfor fluiddiscriminationandcharacterization,Sens.ActuatorsA-Phys.172(2011) 212–219.
[11]H.Ren,R.B.Fair,M.G.Pollack,Automatedon-chipdropletdispensingwith volumecontrolbyelectro-wettingactuationandcapacitancemetering,Sens. ActuatorsB-Chem.98(2004)319–327.
[12]J.Z.Chen,A.A.Darhuber,S.M.Troian,S.Wagner,Capacitivesensingofdroplets formicrofluidicdevicesbasedonthermocapillaryactuation,LabChip4(2004) 473–480.
[13]X.Z.Niu,M.Y.Zhang,S.L.Peng,W.J.Wen,P.Sheng,Real-timedetection,control, andsortingofmicrofluidicdroplets,Biomicrofluidics1(2007).
[14]E.Ghafar-Zadeh,M.Sawan,D.Therriault,A0.18-mumCMOScapacitivesensor lab-on-chip,Sens.ActuatorsA-Phys.141(2008)454–462.
[15]M.F.Abdullah,P.Leow,M.A.Razak,F.C.Harun,On–chipdropletssensingusing capacitivetechnique,J.Teknologi61(2013).
[16]J.Li,Y.X.Wang,E.K.Dong,H.S.Chen,USB-drivenmicrofluidicchipsonprinted circuitboards,LabChip14(2014)860–864.
[17]T.Thorsen,R.W.Roberts,F.H.Arnold,S.R.Quake,Dynamicpatternformation inavesicle-generatingmicrofluidicdevice,Phys.Rev.Lett.86(2001)4163.
[18]P.Garstecki,M.J.Fuerstman,H.A.Stone,G.M.Whitesides,Formationofdroplets andbubblesinamicrofluidicT-junction-scalingandmechanismofbreak-up, LabChip6(2006)437–446.
[19]C.Elbuken, T.Glawdel,D. Chan,C.L.Ren,Detectionofmicrodroplet size and speed usingcapacitive sensors, Sens. Actuators A-Phys. 171 (2011) 55–62.
[20]D.C.Duffy,J.C.McDonald,O.J.A.Schueller,G.M.Whitesides,Rapid prototyp-ingofmicrofluidicsystemsinpoly(dimethylsiloxane),Anal.Chem.70(1998) 4974–4984.
[21]H.Becker,Chips,money,industry,educationandthekillerapplication,LabChip 9(2009)1659–1660.
[22]K.Braeckmans,S.C.DeSmedt,M.Leblans,R.Pauwels,J.Demeester,Encoding microcarriers:presentandfuturetechnologies,Nat.Rev.DrugDiscov.1(2002) 447–456.
Biographies
PelinKubraIsgorreceivedherB.Sc.degreeinElectricalandElectronicsEngineering
fromKocUniversity,Turkeyin2012.ShepursuesherM.Sc.degreeinDepartment
ofMaterialsScienceandNanotechnologyatNationalNanotechnologyResearch
Center–UNAMatBilkentUniversity,Ankara,Turkey.Herresearchinterestsinclude
microdroplet-basedmicrofluidicsystemsandlab-on-a-chipdevices.
MerveMarcalireceivedherB.Sc.degreeinBiomedicalEngineeringfromBaskent
University,Turkeyin2013.In2012,sheworkedasasummerresearcheratJade
UniversityofAppliedScienceinGermanyandatKocUniversity.Shepursuesher
M.ScdegreeinDepartmentofMaterialsScienceandNanotechnologyatNational
NanotechnologyResearchCenter–UNAMatBilkentUniversity,Ankara,Turkey.
MertKeserreceivedhisB.Sc.degreeinBiomedicalEngineeringfromBaskent
Uni-versity,Turkeyin2013.In2012,heworkedasasummerresearcheratKocUniversity
aboutmicrodropletdetection.Duringsummerof2013,heworkedatNational
Nano-technologyResearchCenter–UNAMasavisitingresearcher.
CaglarElbukenreceivedhisB.Sc.degreeinElectricalandElectronicsEngineering
fromBilkentUniversity,Turkeyin2004andhisPh.D.degreeinMechanicaland
MechatronicsEngineeringfromUniversityofWaterloo,Canadain2008.Heworked
asapostdoctoralassociateattheWaterlooMicrofluidicsLaboratoryfortwoyears
beforejoiningtoAbbottPoint-of-CareasaseniorR&Dscientist.Later,hejoinedKoc
Universityasaresearchassistantprofessor.Since2012,heisworkingatBilkent
University,NationalNanotechnologyResearchCenterasanassistantprofessor.His
researchinterestsincludelab-on-a-chipdevices,microdroplet-basedmicrofluidic