ORIGINAL ARTICLE
Comparing Partial and Total
Tibial-Nerve Axotomy:
Long-Term Effects on Prevalence
and Location of Evoked
Pain Behaviors
Isin Unal-Cevik, MD, PhD; Anne Louise Oaklander, MD, PhD
Departments of Neurology and Pathology, Nerve Injury Unit, Massachusetts General
Hospital, Harvard Medical School, Boston, Massachusetts, U.S.A.
䊏 Abstract: Monophasic (one-time) nerve injuries heal without clinically significant residua in most cases, but rare individuals are left with neuropathic pain, even after seem-ingly minor lesions. The effects of lesion size on risk for chronic pain persistence are not well understood, particularly as concerns the complex regional pain syndrome, which is defined in part by pain “disproportionate” to the severity of the causative lesion, and extending outside the autonomous territory of a single nerve. To better clarify the expected prevalence of pain behaviors after nerve injury, we compared the effects in rats of different-sized axotomies on the preva-lence and location of evoked pain behaviors. To highlight clinical relevance, we also describe a patient with iatrogenic tibial-nerve injury causing similar chronic neuralgia. Adult male Sprague-Dawley rats were anesthetized and had either 1/3, 2/3 or their entire left tibial nerves tightly ligated at two sites just below the sciatic trifurcation and the interposed nerve was cut. Unoperated rats provided controls. Sensory function in the tibial and sural-innervated territories of both
plantar hindpaws was assessed for as long as 6 months post-operatively. Soon after surgery, evoked pain behavior devel-oped in the ipsilesional sural-innervated site in a subset of axotomized rats and recovery was variable. The relationship between lesion size and prevalence and severity of hyperal-gesia varied for different pain behaviors, with pinprick hype-ralgesia clearly more likely after larger axotomies. In summary, partial tibial-nerve injury in rats models human disease and suggests that expectations of proportionality between lesion size and development of neuropathic pain may need revision. 䊏
Key Words: rat, preclinical pain model, human, complex regional pain syndrome, neuropathic pain, diagnostic criteria, hyperalgesia, tibial neuropathy, partial tibial axotomy
INTRODUCTION
Neuropathic pain is pathological pain that develops as a direct consequence of a lesion or disease of the soma-tosensory nervous system.1 Peripheral nerve or root
injuries are far more common causes than central lesions. Epidemiologic studies show that few among the many people with nerve injuries experience chronic pain.2Even then, pain severity usually decreases rapidly
over time, leaving only rare individuals with pain for Address correspondence and reprint requests to: Isin Unal-Cevik, MD,
PhD, Department of Neurology, Faculty of Medicine, Ufuk University, Balgat, Ankara, 06520, Turkey. E-mail: isin.unalcevik@gmail.com.
Submitted: April 23, 2010; Revision accepted: September 28, 2010 DOI. 10.1111/j.1533-2500.2010.00429.x
© 2010 The Authors
Pain Practice © 2010 World Institute of Pain, 1530-7085/11/$15.00 Pain Practice, Volume 11, Issue 2, 2011 109–119
longer than a year.2Multiple determinants influence the
risk of this disabling complication. Intrinsic risk factors (eg, age, sex, and genetics) cannot be changed, but lesion characteristics deserve consideration, as they are poten-tially modifiable, particularly for the iatrogenic neural-gias. There is no one pattern of lesion size effects for neuropathic pain. With shingles (herpes zoster), mul-tiple lines of evidence show that the severity of the neurological damage is the major biological risk factor for the development of postherpetic neuralgia.3,4In
con-trast, a very different story is emerging for complex regional pain syndrome (CRPS), a form of post-traumatic neuralgia where the pain is by definition dis-proportionately severe, prolonged, or widespread compared with the initiating injury.5The vast majority
of CRPS is caused by minor injuries to small nerve branches or to a minority of axons within an injured nerve (CRPS-I), rather than major injuries to large nerves (CRPS-II).
Several groups have developed rat models of post-injury neuralgia after experimental post-injury to the spinal nerves or common sciatic nerve. The stimulus-evoked pain behaviors that develop in such rats’ hindpaws may model many CRPS features.6–8But these lesions produce
stimulus-evoked pain behaviors in almost all of the nerve-injured rats. The few that do not develop pain behaviors are usually excluded from the analysis, or their data may be obscured in the standard summary statistics that aggregate data from all lesioned rats. The spared nerve injury (SNI) model where the common peroneal and tibial nerves are ligated and cut has experi-mental advantages.9Locating the lesion on a distal nerve
rather than the common sciatic permits evaluation of the peripheral and central termination zones of nearly largely uninjured or “spared” nerve branches (ie, the sural) or roots.10 But SNI diverges from the clinical
situation because the nerves are completely severed, all rats are severely affected, and none recover.9
Further-more, its total axotomy leaves the skin exclusively innervated by the injured nerves desensate rather than hyperalgesic as is characteristic of patients.9 We
attempted to build on the strengths of the SNI model to create smaller SNI-type lesions that permit us to compare the effects of partial and total axotomies on pain behaviors within the innervated and “spared” ter-ritories of the rat plantar hindpaw. Furthermore, in addition to the customary data analysis depicting mean sensory thresholds from groups of similarly lesioned rats (Figure 2), we include a novel analysis; the proportion of rats left with mechanical hyperalgesia after the
experimental injuries (Figure 3). This enables us to better analyze the different possible outcomes (hyperal-gesia, no change, hypoalgesia) from the same experi-mental injury.
CASE REPORT
A 50-year-old woman with osteoporosis and several years of bilateral foot pain attributed to plantar fasciitis had been anesthetized for extracorporeal shock wave treatment at an outside hospital. Posterior tibial nerve block with 1% xylocaine plus epinephrine provided anesthesia, but the injection caused immediate pain, bruising, and blister formation at the injection site, fol-lowed by chronic ulceration and itching of her medial hind-foot, dysesthesia, and heel hyperalgesia. Oxyc-odone, nortriptyline, and gabapentin were administered for pain and her eschar ultimately required surgical excision and split-thickness skin grafting at the Massa-chusetts General Hospital (MGH). She was hospitalized twice and remembers her pain as 10/10 severity. She was examined at the MGH Pain Center 12 months postin-jury. Her pain intensity had lessened to 4/10 with time and medication. Weight bearing worsened her pain. Her left toes were dorsiflexed and the strength of toe plantar-flexion was 5-/5, consistent with relative weakness of tibial-innervated muscles. Mechanical allodynia and pinprick hyperalgesia affected only her left lateral hind-foot (innervated predominantly by the sural nerve), which was not known to be injured. Magnetic reso-nance imaging of her ankle disclosed no injury or inflammation of her sural or tibial nerve or visualized plantar branches. Neuropathic pain and hyperalgesia centered in her predominantly sural-innervated lateral foot caused by iatrogenic tibial-nerve injury was medi-cally treated as she continued to improve. In 2010, seven years postinjury, she reported only mild residua. She no longer used pain medications and usually had no foot pain. Her foot often felt “odd” in the mornings until she began to use it; an exceptionally long day on her feet often caused foot “achiness” rated 3/10, and once or twice a week she felt brief “electric shock” sensations in her foot.
MATERIALS AND METHODS Study Design and Animal Use
Male Sprague-Dawley rats (400 to 450 g; Charles River Laboratories, Wilmington, MA) were studied using pro-cedures approved by the Animal Care and Use Commit-tees and the International Association for the Study of
Pain.11 Rats were housed in smooth-bottom cages at
211 0.5°C with a 12-hour light/dark cycle and free access to food and water. After the collection of baseline sensory data (see next), rats were anesthetized (50 mg/ kg/intraperitoneal Nembutal, Abbott, Northville, MI) and the left common sciatic nerve and its trifurcation into tibial, sural, and peroneal branches at the knee were surgically exposed. A variable amount of the tibial nerve (identified as the largest branch) was tightly ligated with 8.0 silk at two sites 2 mm apart; interposed nerve was transected and the sutures were left in place. Twenty rats had ~1/3rd of the tibial nerve axotomized (tibial nerve injury [TNI]); 16 rats had ~2/3 of the tibial nerve cut; and 17 rats underwent total tibial axotomy. Five same-age rats were housed, tested, and euthanized among experimental rats to provide unoperated controls (naïve group). Overlying muscles were sutured with 5.0 silk and the skin wound was closed with surgical clip. No postoperative medications were administered and rats were sacrificed by Nembutal overdose at 2 weeks, 6 weeks, or 12 weeks postoperatively. All rats were ran-domly grouped for time of sacrifice prior to surgery in order to investigate the evolution of pathological changes of the epidermal sensory nerve endings, nerves, dorsal root ganglia, and the spinal cord. Four 1/3rd axotomized rats were randomly selected preoperatively for a prolonged study that lasted 184 days (6 months).
Sensory Testing and Criteria for Pain-Related Behaviors
Rats were habituated to the sensory testing environment and protocol for 1 week, then underwent two baseline measurements (separated by 4 days), the mean of which provided the baseline value. Sensory testing was per-formed in a quiet room in random order by a single examiner who was blinded to experimental groups. Established protocols were used to gather data from four separate sites; the tibial- and sural-innervated zones of rats’ left and right proximal plantar hindpaw.9 The
paw center between tori represented tibial-innervated skin and proximo-lateral glabrous skin represented pre-dominantly sural-innervated skin.9 Sensation in tibial
and sural-innervated hindpaw was assessed at postop-erative days 3, 7, 14, 21, 28, 42 and 63 days. In 4 rats with 1/3rd tibial axotomy, sensory testing was assessed until 6 months postoperatively.
Evaluation of Mechanosensation. Punctate static
allo-dynia was assessed using calibrated Semmes-Weinstein von Frey (vF) monofilaments that applied between 0.08
and 1,680 milliNewtons (mN) force (Stoelting, Wood Dale, IL). The threshold for paw withdrawal was defined as the thinnest monofilament that produced hindpaw withdrawal at least twice in 10 (2/10) consecu-tive applications.12Monofilaments apply forces that are
on a logarithmic scale so to calculate the decrease in force necessary to elicit hindpaw withdrawal, we calcu-lated the logarithmic force difference between the base-line force (A) and the force applied by the thinnest vF filament (B = 0.0818 mN). Log A–log B provides the total distance to reach 100% reduction from the base-line threshold force. Postoperative force was represented by C. The logarithmic force difference from baseline to postoperative value was (log A–log C). The percent decrease in threshold force is then calculated as (log A–log C)/(log A–log B) ¥ 100. Mechanical allo-dynia was defined as3 50% postoperative decrease from each individual rat’s preoperative baseline threshold for hindpaw withdrawal. Punctate mechanical hyperalgesia was evaluated by a safety pin and stopwatch measure-ment of the time that rats kept their hindpaw elevated after a single pinprick.9Normal withdrawal duration is
less than 0.5 second, which was used as an arbitrary minimum value. An arbitrary maximum cutoff of 10 seconds was used for longer withdrawals.9Mechanical
hyperalgesia was defined as withdrawal duration3 2 s.
Evaluation of Thermosensation. Cold sensitivity was
tested by applying a drop of acetone to the mid-plantar hindpaw surface with a blunt needle held away from the skin. Because acetone spreads, it is not possible to gather data separately from tibial and sural-innervated sites. Paw withdrawal duration was timed by stopwatch. Normal rats withdraw very briefly and 0.5 second was used as an arbitrary minimum.13An arbitrary cutoff of
20 seconds was used for longer withdrawals.9Cold
allo-dynia was defined as hindpaw withdrawal duration of 3 2 s. Responses to heat were tested using radiant heating through a Perspex floor, but preliminary experi-ments established that lesioned rats often held their ipsilateral hindpaws without full contact with the testing floor, so these data were deemed unreliable and discarded.14
Data Analysis
Analyses were conducted using SPSS version 16.0 (SPSS Inc., Chicago, IL). Data from groups are summarized as means1 standard errors. Statistical significance was defined by P< 0.05. Kruskal–Wallis analysis of variance test followed by Mann–Whitney U-test were used for
comparisons of the group means at defined time points. Fisher’s exact test was used to compare the proportion of individual rats with hypersensitivity between groups at all time points.
RESULTS
Nerve-injured rats maintained apparently normal activ-ity, grooming, sleep–wake cycles, and social interaction. No autotomy or weight loss developed. After recovery from anesthesia, some operated rats developed abnor-mal paw postures and pale, dry skin on their ipsilesional hindpaw. These were never observed in unoperated rats or contralateral to nerve injury. Among 1/3rd axoto-mized rats, the prevalent posture was tonic flexion of digits with ambulation on the volar surface of the digits (Figure 1A). Among the long-term 1/3rd axotomy rats, one of four rats displayed this posture tonically until sacrifice 6 months postoperatively. Elevation of the lateral hindpaw margin and hindpaw eversion was more common among total-axotomized rats, consistent with weakness of tibial-innervated intrinsic hindpaw muscles (Figure 1B).
Effects of Different-Sized Lesions on Average Magnitude of Evoked Pain Responses
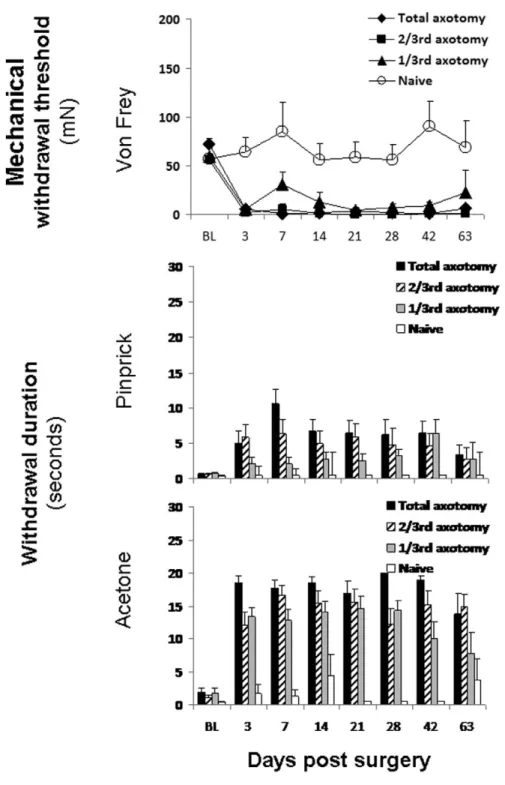
Figure 2, which depicts the mean scores for the three types of sensory testing at the ipsilateral sural-innervated territory over time, demonstrates different relationships between lesion size and severity of sensory abnormality for different stimuli. The top panel demon-strates that all 3 lesions caused overall significant changes in withdrawal thresholds from vF compared with unoperated rats at all time points when tested at the ipsilateral sural test site. Post hoc analysis revealed no differences between total and 2/3rd axotomy but both groups were different when compared with 1/3rd axotomized rats (P< 0.05). The 1/3rd axotomized group had reduced withdrawal thresholds between
post-operative day 3 (from 59.61 8.3 mN at baseline to 5.11 2.4 mN, P = 0.000) and day 42. The middle panel depicts the analysis of data on response to pinprick at the ipsi-sural test-site. The 2/3rd and total-axotomized rats had significantly elevated withdrawal durations at the ipsi-sural test site as compared with unoperated rats between days 3 and 42, whereas 1/3rd axotomy only caused significant differences at the later time points, days 28 and 42. Pinprick caused different effects at the ipsilateral tibial test site. Among the total-axotomized rats, none responded to safety pin until day 21 postop-eratively when 2/12 responded with mean withdrawal duration of 15.51 4.5 seconds. By postoperative day 63, 6/12 rats responded with a mean duration of 8.21 3.8 seconds. Findings from the total axotomy group were overall statistically different than from unoperated rats and 1/3rd axotomized rats (all P< 0.05). The response to acetone was significantly dif-ferent in all 3 groups of axotomized rats as compared with unoperated rats. A “dose-response” relationship was statistically evident in comparing the groups of axotomized rats, with mean duration of hindpaw with-drawal usually proportional to lesion size.
Comparison of Lesion Sizes on Prevalence of Evoked-Pain Responses in Different
Hindpaw Locations
We assessed the effects of our distal nerve injuries on different locations on both hindpaws. Figure 3 depicts the prevalence of mechanical allodynia to vF filaments at all 4 hindpaw sites studied. No rats developed pro-longed contralesional or ipsi-tibial withdrawal. The proportion of rats with hypersensitivity to vF monofila-ments at the ipsi-sural test site was overall different after axotomy when compared with the group of unoperated rats at all study time points (all P< 0.05). The preva-lence of mechanical allodynia in total and 2/3rd axoto-mized rats at postoperative week 1 was 83% and 88%,
Figure 1. Panel A demonstrates a rat
with abnormal ipsilateral tonic hindpaw toe flexion and hyperalgesia, 6 months after 1/3rd tibial-nerve axotomy. Panel B was photographed from below the mesh floor used for sensory testing. It depicts a rat 4 weeks after left total tibial axotomy with weak left hindpaw adduction and hyperalgesia.
respectively, both significantly elevated as compared with naïve rats (P = 0.002; P = 0.001). Sixty percent of the 1/3rd axotomized rats had sural mechanical allo-dynia at this time point, a prevalence different from that in unoperated rats (P = 0.024). Two of the 4 long-term rats (1/3rd axotomized) had mechanical allodynia at ipsilateral sural innervated area in the first postoperative week, but by 6 months, only 1 remained allodynic.
At the ipsilateral tibial study site, analysis of grouped data from the different lesion groups identified none with overall significant mechanical allodynia, but data from the total tibial-transection group had been ren-dered desensate, without any response to either safety pin or monofilaments at early time points. Interestingly, mechanosensation returned, and by postoperative day 14, 2/17 of the total axotomized rats had mean
with-Figure 2. Summary of baseline and
postoperative ipsi-sural hindpaw responses to different sensory stimuli for each of the 4 different study groups of rats. Data shown represent means1 standard errors. Acetone data represent the entire ipsilesional hindpaw. BL refers to the mean of the 2 baseline sensory measures tested pre-operatively 4 days apart. All lesion sizes induced abnormalities in mechanosen-sation (von Frey and pinprick) in ipsi-sural and thermosensation (acetone) in ipsilesional hindpaw.
drawal thresholds of 61.41 0.0 mN and by postopera-tive day 63, 6/6 rats had a mean response of 103.61 21.2 mN, not meeting the criteria for mechani-cal allodynia. In concordance with the reappearance of ipsi-tibial responses of the total axotomized rats, pre-liminary studies of a few axotomized rats’ tissues showed re-innervation by small nerve endings immuno-labeled with PGP9.5 at innervated skin and tibial-nerve regeneration (Figure 4).
Figure 5 shows the same analysis for pinprick. There was greater variability in these measures with even unoperated control rats occasionally exhibiting pro-longed withdrawal. Contralesional hypersensitivity was not different among all 4 groups. The likelihood of developing pinprick hyperalgesia appeared to be influ-enced by lesion size. The prevalence of hyperalgesia at the ipsi-sural innervated area at postoperative day 7 was 20% in the 1/3rd, 50% in the 2/3rd, and 76% in the
Figure 3. Prevalence of mechanical allodynia in all experimental groups at all study sites. Baseline (preoperative) and postoperative time points are resented on the x axis. The y axis rep-resents the percent of rats in the group who meet the defined criteria for mechanical allodynia (3 50% reduction from each individual rat’s baseline threshold). Significant mechanical allodynia developed only in the ipsilesional sural study site.
total axotomy group. Both total and 2/3rd axotomized rats displayed mechanical hyperalgesia compared with unoperated rats almost throughout the study (all P< 0.05). Total axotomized rats were also statistically different from 1/3rd axotomized rats at days 7, 14 and 21 (all P< 0.05). Among the 4 long-term rats (1/3rd axotomized), 1 had pinprick hyperalgesia at the ipsi-sural innervated area within the first postoperative week and two during the second week. At 6 months, only one of these rats still had fixed abnormal hindpaw posture and hyperalgesia (see Figure 1A).15,16 While
sural-territory hyperalgesia was present at the earliest post-operative time points, tibial-territory pinprick hyperalgesia developed in a delayed pattern. Among totally axotomized rats, the maximum prevalence (67%) was at day 63 postoperative, at a time when prevalence at the sural-innervated site was declining.
Figure 6 depicts the analysis for acetone. Hindpaw withdrawal to acetone can only be assessed for the entire hindpaw, as the acetone spreads to cover both the sural- and tibial-innervated study sites. The prevalence of rats with prolonged ipsilesional hindpaw withdrawal from acetone at postoperative day 7 was 100% in total, 100% in 2/3rd, and 90% in 1/3rd axotomized rats (all
P< 0.05 when compared with unoperated controls). Although there was a suggestion that rats with smaller lesions may have had prolonged withdrawal of their contralateral hindpaws as well, the results were not significantly different than those seen in unoperated control rats. Among the four 1/3rd axotomized rats observed for 6 months, all had ipsilesional cold allo-dynia during the first postoperative week that started to resolve by postoperative week 8, when allodynia had resolved in one rat, and by 6 months, all 4 had recov-ered. In contrast, 90% of 2/3rd axotomized rats and all of the total axotomized rats still had cold allodynia on postoperative day 63.
DISCUSSION
This long-term study demonstrates prospectively that smaller, partial axotomies can cause similar evoked-pain behaviors as larger axotomies in outbred male rats. All forms of axotomy were able to trigger sural-territory abnormalities in response to vF monofilaments, pin-prick, and acetone. Symptom prevalence was propor-tional to lesion size only for pinprick (Figure 5). Even after the smallest lesion, a few rats had sensory abnor-malities that persisted throughout 6 months of study.
Figure 4. The upper and middle panels are photomicrographs of PGP9.5 immunolabeled, 50-mm thick, vertical sections of tibial-nerve injured hindpaw skin biopsies. Panels A, B, and C were all taken at week 2 post-operatively to show proportional levels of loss of cutaneous innervation after 1/3 axotomy (A), 2/3 axotomy (B), and total axotomy (C). Panel D depicts partial reinnervation of the affected area 6 weeks after total axotomy. The bottom panels depict epoxy-embedded, 1mm sections of injured distal tibial nerve (1% toluidine blue stained). Panel E depicts Wallerian degeneration 2 weeks after 1/3 axotomy. Panel F depicts widespread regeneration (axons with dispropor-tionately thin myelin sheaths) 12 weeks after 2/3rd axotomy.
A B
C D
Partial as well as total tibial-nerve axotomies produced rapid and prolonged mechanical and thermal hypersen-sitivities in a subset of rats, mainly confined to the ipsilateral sural nerve territory. The presence of ipsilat-eral sural hypipsilat-eralgesia confirms and extends those from a prior study from our group that compared smaller tibial-nerve lesions made by needlestick.17 Sensory
abnormalities were more widespread there, often
affect-ing the ipsi-tibial and contralesional hindpaw as well. It is hard to know whether the discrepancies relate to the different types of lesioning or the proportion of axons cut. The lesions used here included ligating the cut nerve, which prevents or slows axon regeneration and introduces suture material into the lesion. With regard to “mirror” spread to the contralesional hindpaw, uni-lateral nerve ligation/axotomies can cause profound
Figure 5. Prevalence of mechanical hyperalgesia to safety pin at all experi-mental groups and all study sites. Baseline (preoperative) and postop-erative time points are represented on the x axis. The y axis represents the percent of rats in the group who meet the defined criteria for mechanical hyperalgesia (withdrawal duration3 2 seconds). Pinprick hyperalgesia was not restricted to the ipsilesional sural-innervated study site, although still more prevalent there. The prevalence of this specific abnormality was pro-portional to lesion size.
contralesional axonal loss without behavioral changes, but needlestick appears more likely to cause mirror hyperalgesia.17,18
The patient reported here with iatrogenic partial TNI from a nerve block had a similar distribution of neuro-pathic pain symptoms characterized by hyperalgesia pri-marily outside the autonomous territory of an injured tibial nerve. Her pain also persisted with gradual improvement throughout 7 years of follow-up. Her nerve injury was attributed to ischemia from epinephrine-induced vasospasm, a known complication of distal nerve blocks.19–22
Partial TNI is a rodent model that resembles human post-traumatic neuralgia in several ways. Partial nerve injury is far more common than total nerve transection in most non-military settings, as demonstrated in our case. Importantly, as in humans, not all nerve-injured rats develop pain behaviors, and not all individuals are affected, in contrast to the widely used rat models dis-cussed in the Introduction. By characterizing each rat’s response individually and reporting the data as preva-lence of specific abnormalities (a standard measure in human research) rather than as aggregate means from groups, one can tease out subtle changes within groups
Figure 6. Prevalence of cold allodynia
in all experimental groups at all study sites. Baseline (preoperative) and post-operative time points are represented on the x axis. The y axis represents the percent of rats in the group who meet the defined criteria for cold allodynia (withdrawal duration 3 2 seconds). The prevalence of cold allodynia was largely independent of lesion size.
of individual rats with variable outcomes. Use of partial axotomies also avoids a major complication of total axotomy, namely loss of all sensation in the autono-mous nerve territory with gradual recovery of sensory function. At postoperative week 1, no total-axotomy rats responded to safety pin or monofilaments there. By week 3, ipsi-tibial pinprick hyperalgesia was present in 17% of these rats, with results congruent with those of Hofmann et al.23 Two to three weeks after total
tibial-nerve transection, their rats developed mechanical allo-dynia lasting at least for 2 months.23 This late tibial
hyperalgesia must reflect sprouting from adjacent nerves (see Figure 4) as the proximal tibial stump was ligated in our model.18,23–26Neuroplasticity in the dorsal horn or
higher centers may also contribute.24,25,27–29
The spread of multimodal hypersensitivity that we and others detect ipsilaterally in the primarily sural-innervated territory9 likely has several mechanisms.
Although the sural nerve was not axotomized, the sural territory of rats is not autonomous, being partially innervated by the tibial nerve as well, so this area was partly denervated.30There is an additional lateral
diffu-sion of pain-associated changes throughout the periph-eral and central nervous systems, particularly at synapses such as in the dorsal horn.10,31,32In conclusion,
partial tibial axotomy causes extraterritorial hyperalge-sia and allodynia whose features correlate with lesion size and inversely correlates with the probability of recovery, and even 1/3rd axotomy to tibial nerve can be associated with long-lasting pain behaviors.
ACKNOWLEDGEMENTS
This study is supported in part by the Reflex Sympa-thetic Dystrophy Association, the National Organiza-tion for Rare Disorders, the Public Health Service (R01NS42866, K24NS059892). It was presented in abstract form to the Society for Neuroscience. The tech-nical assistance of Ralph Gott, MA, and the statistical assistance of E. Burcu Mamak-Ekinci, MS, are greatly appreciated.
REFERENCES
1. Treede RD, Jensen TS, Campbell JN, et al. Neuro-pathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635.
2. Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study.
Pain. 2003;103:199–207.
3. Jung BF, Johnson RW, Griffin DR, Dworkin RH. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545–1551.
4. Oaklander AL. The density of remaining nerve endings in human skin with and without postherpetic neural-gia after shingles. Pain. 2001;92:139–145.
5. Merskey H, Bogduk N. Classification of Chronic
Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle: IASP Press; 1994.
6. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107.
7. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363.
8. Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218.
9. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158.
10. Lee JW, Siegel SM, Oaklander AL. Effects of distal nerve injuries on dorsal-horn neurons and glia: relationships between lesion size and mechanical hyperalgesia.
Neuro-science. 2009;158:904–914.
11. Zimmermann M. Ethical guidelines for investiga-tions of experimental pain in conscious animals. Pain. 1983;16:109–110.
12. Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve.
Pain. 1994;57:375–382.
13. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376.
14. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nocicep-tion in cutaneous hyperalgesia. Pain. 1988;32:77–88.
15. Oaklander AL. Progression of dystonia in complex regional pain syndrome. Neurology. 2004;63:751.
16. Reich SG, Weiner WJ. Progression of dystonia in com-plex regional pain syndrome. Neurology. 2005;64:2162–2163. 17. Siegel SM, Lee JW, Oaklander AL. Needlestick distal nerve injury in rats models symptoms of complex regional pain syndrome. Anesth Analg. 2007;105:1820–1829.
18. Oaklander AL, Brown JM. Unilateral nerve injury produces bilateral loss of distal innervation. Ann Neurol. 2004;55:639–644.
19. Aycock BG, Hawtof DB, Moody SB. Treatment of peripheral ischemia secondary to lidocaine containing epi-nephrine. Ann Plast Surg. 1989;23:27–30.
20. Emsen IM. Catastrophic complication of the circum-cision that carried out with local anesthesia contained adrena-line. J Trauma. 2006;60:1150.
21. Ribald MS, Whitman PL, Lemont H. Traumatic pos-terior tibial nerve injury. A case report. J Am Podiatry Assoc. 1975;65:50–54.
22. Torrente-Castells E, Gargallo-Albiol J, Rodriguez-Baeza A, Berini-Aytes L, Gay-Escoda C. Necrosis of the skin of the chin: a possible complication of inferior alveolar nerve block injection. J Am Dent Assoc. 2008;139:1625– 1630.
23. Hofmann HA, De VJ, Siegling A, Spreyer P, Denzer D. Pharmacological sensitivity and gene expression analysis of the tibial nerve injury model of neuropathic pain. Eur J
Phar-macol. 2003;470:17–25.
24. Devor M, Schonfeld D, Seltzer Z, Wall PD. Two modes of cutaneous reinnervation following peripheral nerve injury. J Comp Neurol. 1979;185:211–220.
25. Inbal R, Rousso J, Ashur H, Wall PD, Devor M. Collateral sprouting in skin and sensory recovery after nerve injury in man. Pain. 1987;28:141–154.
26. Kinnman E, Aldskogius H. Collateral sprouting of sensory axons in the glabrous skin of the hindpaw after chronic sciatic nerve lesion in adult and neonatal rats: a mor-phological study. Brain Res. 1986;377:73–82.
27. LaMotte CC, Kapadia SE, Kocol CM.
Deafferentation-induced expansion of saphenous terminal field labelling in the adult rat dorsal horn following pronase injec-tion of the sciatic nerve. J Comp Neurol. 1989;288:311–325.
28. Markus H, Pomeranz B, Krushelnycky D. Spread of saphenous somatotopic projection map in spinal cord and hypersensitivity of the foot after chronic sciatic denervation in adult rat. Brain Res. 1984;296:27–39.
29. McMahon SB, Kett-White R. Sprouting of peripher-ally regenerating primary sensory neurones in the adult central nervous system. J Comp Neurol. 1991;304:307–315.
30. Bajrovic F, Sketelj J. Extent of nociceptive der-matomes in adult rats is not primarily maintained by axonal competition. Exp Neurol. 1998;150:115–121.
31. Costigan M, Moss A, Latremoliere A, et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J
Neurosci. 2009;29:14415–14422.
32. Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol. 1985;231:66–77.