http://journals.tubitak.gov.tr/biology/ © TÜBİTAK
doi:10.3906/biy-1404-55
Evaluation of the genotoxic or mutagenic effects of thermal stress on
cultured human lymphocytes
Hasan Basri İLA1,*, Mehmet TOPAKTAŞ1, Mehmet ARSLAN2, Mehmet BÜYÜKLEYLA3, Erman Salih İSTİFLİ1
1Department of Biology, Faculty of Science and Letters, Çukurova University, Adana, Turkey 2Department of Nursing, School of Health Sciences, Ardahan University, Ardahan, Turkey 3Natural and Applied Science Institute, Department of Biology, Çukurova University, Adana, Turkey
1. Introduction
All organisms in the ecosystem are in engaged in the effort of maintaining the optimal balance in their internal environment as long as they survive. Acid–base balance and body temperature regulation are 2 of these balances. This concept, defined for the first time by C. Bernard in 1865 (Cross and Albury, 1987), was named homeostasis by W.B. Cannon (1926). In particular, balance in the body temperature of an organism is important for life activities. The normal internal body temperature of a human is in the range of 36.3 to 37.3°C (Mackowiak et al., 1992), but it is possible for these ranges to have small deviations because of physiological reasons.
Temperature changes in an organism may be caused by exogenous factors, but they may also depend on endogenous sources like pathological and hormonal phenomena. Whatever the reason is, development of thermal stress (heat shock) as a result of sudden temperature changes is unavoidable. As a result of thermal stress, transcriptional discrepancies and extraordinary RNA processing merge, synthesis of mRNAs is stimulated, and various molecular responses like heat shock protein accumulation occur (Wu, 1995). In one of the limited studies investigating the cytotoxic and genotoxic effects of thermal stress in Chinese hamster ovary (CHO) cells treated with 7 mM procaine HCl under alkaline conditions, temperature-dependent
lethality was observed to increase. In other words, it was stated that there was an increased sensitivity to heat at pH 7.4 and the temperature sensitivity of the cells was low at pH 6.9 (Li et al., 1990). While temperature applications in Japanese fish exposed to heat shock at various levels (34 °C, 36 °C, and 38°C) produced DNA single-strand break and micronuclei, 38 °C heat inhibited cell proliferation. In addition, chromosome abnormalities were detected in metaphase at temperatures of 34°C and 36 °C (Anitha et al., 2000). Similarly, in Saccharomyces cerevisiae cells exposed to lethal heat stress (50 °C), oxidative stress was observed and anaerobic cells were observed to be more resistant to this stress than aerobic cells. It has been reported that aerobic heat stress causes degradation in the mitochondrial membrane (Davidson and Schiestl, 2001). In addition, it was stated that for triploid induction in shrimp, which have economic importance, heat shock (29–32 °C) was very effective (Li et al., 2003). In another study performed on the same organism in fertilized shrimp (Fenneropenaeus chinensis), some abnormal chromosome behaviors (3 pronuclei and triploid embryo development) were observed in eggs exposed to 10 min of heat shock (30 ± 0.5 °C) (Zhang et al., 2003). The small number of publications on this subject and the inadequate number of studies investigating the effects of heat shock on known mutagens acted as the inspiration for our study.
Abstract: This study was performed to determine the cytogenetic effects of short-term thermal stress in human cultured lymphocytes. Experimental heat shock (39 °C) was performed alone or with the addition of mitomycin C (MMC) to determine the synergistic or antagonistic effects of heat shock on genotoxicity induced by MMC. In this study, during culture periods of 72 h, human peripheral blood lymphocytes were exposed to heat shock for specified durations (30, 60, 120, and 240 min) 24 or 48 h before harvest. According to our results, the selected temperatures did not show genotoxic or mutagenic effects. In summary, the heat shock tested did not show any cytogenetic effect on the cultured blood cells and did not cause significant alterations in genotoxicity induced by MMC.
Key words: Heat shock, mitomycin C, in vitro, sister chromatid exchange, chromosome aberration, micronucleus
Received: 15.04.2014 Accepted: 14.07.2014 Published Online: 02.01.2015 Printed: 30.01.2015
Therefore, in this study, in order to determine the genotoxic or mutagenic effect of heat shock alone or heat shock + mitomycin C (MMC) treatment on cultured human peripheral blood cells, in vitro sister chromatid exchange (SCE), chromosomal aberration (CA), and micronuclei (MN) tests were performed.
2. Materials and methods
In this study, human peripheral blood was used in vitro as the test material. Whole blood was taken from healthy volunteer blood donors (1 female and 1 male) who did not smoke or use drugs, and the ages (25–30 years) of the donors were close to each other. Blood samples were taken from the donors in sterile conditions and were added to a chromosome medium (GIBCO cat. no. 12552-013) in a volume of 2.5 mL. For incubation of the cultures and heat shock treatment, an adjustable incubator (Incucell) was used. In order to determine the alteration caused by heat shock on the genotoxic effects of mutagens, MMC (Sigma, CAS number 50-07-7; 0.25 µg/mL), a known mutagen, was used (Erboğa and İla, 2013).
2.1. In vitro sister chromatid exchange and chromosome aberration assay
Determination of the genotoxic effects of mutagens and carcinogens that may have genotoxic effects on humans is possible primarily through the use of SCE and CA tests. When this type of study is planned and executed, there is a requirement to follow international guidelines. Therefore, this study was performed according to the International Programme on Chemical Safety instructions published by Albertini et al. (2000).
In this study, to determine SCE and CA, the preparation and cell cultures were conducted according to the methods modified by Evans (1984) and Perry and Thomson (1984). In order to determine the genotoxic and mutagenic effects of heat shock producing stress, peripheral blood samples taken from the volunteer donors were transplanted as 6 drops (0.2 mL) into chromosome media that were heparinized at a 1/10 ratio in sterile cabins (Labormed). To determine SCE, the cells were incubated for 72 h at 37 ± 0.5 °C by adding fresh 10 µg/mL 5′-bromo-2′-deoxyuridine solution (Sigma, CAS number: 59-14-3) into culture tubes at the beginning of the incubation.
For the heat shock treatment (24 or 48 h after blood transplantation), tubes were exposed to 39 °C for 30, 60, 120, and 240 min in a water bath (BM 302). In a parallel series, in addition to the heat shock, an effective dose of MMC (0.25 µg/mL) was added. In order to block mitosis in the metaphase stage, colchicine (Sigma, CAS number: 64-86-8) was added (0.06 µg/mL) 2 h before harvesting (i.e. at the 70th hour of the culture).
At the end of 72 h, the length of culture time employed, cells were precipitated by centrifugation for 5 min at 2000
rpm and then the supernatant was removed. The precipitate was homogenized, a warmed (37 °C) hypotonic solution (0.4% KCl) was added, and the cells were treated at 37 °C for 5 min. At the end of the period, the suspension was precipitated by centrifuging the tubes for 15 min at 1200 rpm and the supernatant was removed. After the addition of a cold fixative (1:3 glacial acetic acid and methanol), the cells were held at room temperature for 15 min and centrifugation was performed again for 10 min at 1200 rpm. This process was repeated 3 times in total. The cell pellet in the tube was homogenized and dropped onto a cold slide from a height of 50 cm. After the slides dried under room temperature, they were stained with a 5% Giemsa stain prepared in a Sorensen buffer and covered with Entellan. In order to determine chromosome aberrations, 100 well-spread metaphase chromosomes from each tube were evaluated. Chromatid and chromosome gaps were not evaluated as chromosome abnormalities (Mace et al., 1978). Mitotic index (MI) was calculated by counting 3000 cells in total from the slides of each donor.
To investigate SCE, the fluorescence plus Giemsa method, developed by Speit and Haupter (1985) and Speit (1984), was modified and used. From each tube, 25 well-spread metaphase chromosomes were evaluated for SCE average. Proliferation index (PI) was determined according to the following formula by evaluating 100 total cells undergoing first, second, and third mitosis from each application.
1 × (M1) + 2 × (M2) + 3 × (M3) PI =
100 ,
where M1 is the number of cells undergoing the first mitosis, M2 is the number of cells undergoing the second mitosis, and M3 is the number of cells undergoing the third mitosis.
The significances between percentages of the mean for SCE, CA, PI, and MI in the treated cultures and their controls were evaluated using the t-test. At P < 0.05, the results were interpreted as statistically significant.
2.2. In vitro micronucleus assay
The potential of heat shock to exert a genotoxic effect was also investigated using a micronucleus test in human peripheral lymphocytes. For the in vitro micronucleus test, the method developed by Rothfuss et al. (2000) was modified and used. In this test, blood taken from the same donors was added to the chromosome medium and incubated for 68 h at 37 °C. Cytochalasin B (Sigma, CAS Number: 14930-96) was added at the 44th hour of incubation to a final concentration of 6 µg/mL in order to block cytokinesis.
After the culture period elapsed, the tubes were centrifuged (2000 rpm for 5 min) and the precipitated cells were incubated for 5 min in a warm (37°C) hypotonic solution. The cell culture was then centrifuged (1200 rpm for 10 min) and the precipitated cells were treated with a cold first fixative (1:5:6 glacial acetic acid, methanol, and 0.9% NaCl) for 20 min. With the second and third cold fixatives, treatment was repeated with the same principle (1:5 glacial acetic acid and methanol). Finally, slides were prepared by dropping cells onto the cold slides at close range. These slides were stained with a 5% Giemsa stain prepared in a Sorensen buffer and covered with Entellan to be made permanent.
In order to determine MN binuclear cells in the slides prepared from the blood cultures of each group, 1000 binuclear cells were examined. A total of 1000 cells were scored to determine the frequency of the cells with 1, 2, 3, or 4 nuclei and to calculate the nuclear division index (NDI) for the nucleus proliferation: NDI = (MI + 2MII + 3MIII + 4MIV) / total, where MI, MII, MIII, and MIV represent the number of cells with 1–4 nuclei, respectively (Eastmond and Tucker, 1989).
Micronucleated binuclear cell percentages and NDI values were determined in the controls and treated cultures and the significance of the values between the treated and control cultures was evaluated using the t-test. At P < 0.05, the results were considered statistically significant. 3. Results
3.1. Effects of heat shock upon sister chromatid exchange In this study, the findings of the group treated with heat shock alone were compared to the findings of the control group. Furthermore, findings of the heat shock + MMC treated group were compared with the findings of the MMC control.
No significant differences were determined between SCE frequencies in the control group and in the cultures exposed to heat shock alone for various time durations (30, 60, 120, and 240 min) after 24 or 48 h (P > 0.05). Although there were increases in SCE frequencies in the cultures where MMC was applied in addition to heat shock, no significant difference was observed when compared to the MMC controls (Table 1).
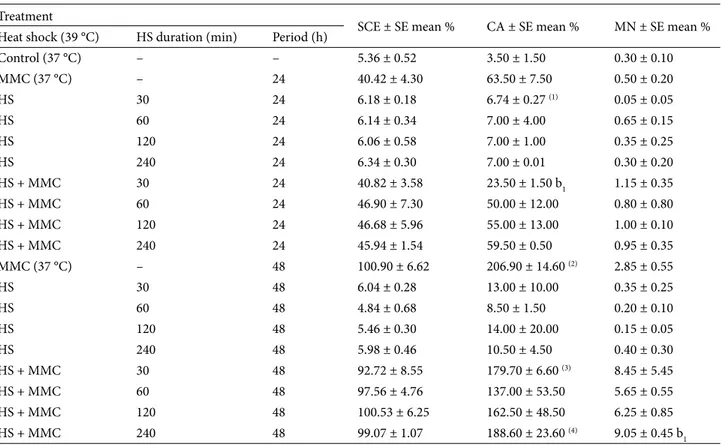
Table 1. Frequency of chromosomal alterations (SCE, CA, and MN)* in human cultured blood cells treated with heat shock (HS) alone or heat shock + MMC 24 or 48 h after blood transplantation.
Treatment
SCE ± SE mean % CA ± SE mean % MN ± SE mean % Heat shock (39 °C) HS duration (min) Period (h)
Control (37 °C) – – 5.36 ± 0.52 3.50 ± 1.50 0.30 ± 0.10 MMC (37 °C) – 24 40.42 ± 4.30 63.50 ± 7.50 0.50 ± 0.20 HS 30 24 6.18 ± 0.18 6.74 ± 0.27 (1) 0.05 ± 0.05 HS 60 24 6.14 ± 0.34 7.00 ± 4.00 0.65 ± 0.15 HS 120 24 6.06 ± 0.58 7.00 ± 1.00 0.35 ± 0.25 HS 240 24 6.34 ± 0.30 7.00 ± 0.01 0.30 ± 0.20 HS + MMC 30 24 40.82 ± 3.58 23.50 ± 1.50 b1 1.15 ± 0.35 HS + MMC 60 24 46.90 ± 7.30 50.00 ± 12.00 0.80 ± 0.80 HS + MMC 120 24 46.68 ± 5.96 55.00 ± 13.00 1.00 ± 0.10 HS + MMC 240 24 45.94 ± 1.54 59.50 ± 0.50 0.95 ± 0.35 MMC (37 °C) – 48 100.90 ± 6.62 206.90 ± 14.60 (2) 2.85 ± 0.55 HS 30 48 6.04 ± 0.28 13.00 ± 10.00 0.35 ± 0.25 HS 60 48 4.84 ± 0.68 8.50 ± 1.50 0.20 ± 0.10 HS 120 48 5.46 ± 0.30 14.00 ± 20.00 0.15 ± 0.05 HS 240 48 5.98 ± 0.46 10.50 ± 4.50 0.40 ± 0.30 HS + MMC 30 48 92.72 ± 8.55 179.70 ± 6.60 (3) 8.45 ± 5.45 HS + MMC 60 48 97.56 ± 4.76 137.00 ± 53.50 5.65 ± 0.55 HS + MMC 120 48 100.53 ± 6.25 162.50 ± 48.50 6.25 ± 0.85 HS + MMC 240 48 99.07 ± 1.07 188.60 ± 23.60 (4) 9.05 ± 0.45 b 1
*A total of 50 metaphase chromosomes were examined for the detection of SCE, a total of 200 metaphase chromosomes were examined for CA, and a total of 2000 binuclear cells were examined for MN. Due to toxicity, a total of (1)193, (2)120, (3)195, and (4)198 cells were
3.2. Heat shock effects on chromosome aberration and micronucleus
When CA and MN percentages obtained from the cultures exposed to heat shock alone 24 or 48 h after initiating incubation were compared with the control, no significant differences were found (Table 1). No significant difference was observed between the positive control and the group where heat shock + MMC were applied, with 2 exceptions. CA frequencies obtained over 30 min in the heat shock + MMC applied cultures 24 h after treatment were found to be significantly lower when compared to the MMC control. Furthermore, in the group in which 240 min of heat shock + MMC was applied 48 h after initiating of the culture, MN percentage was found to be significantly higher (P < 0.05) when compared to the positive control. Relatively high CA as well as abnormal cells and MN values observed
in the other treatments were found not to be statistically significant (Table 1).
3.3. Effects of thermal stress on cell proliferation parameters
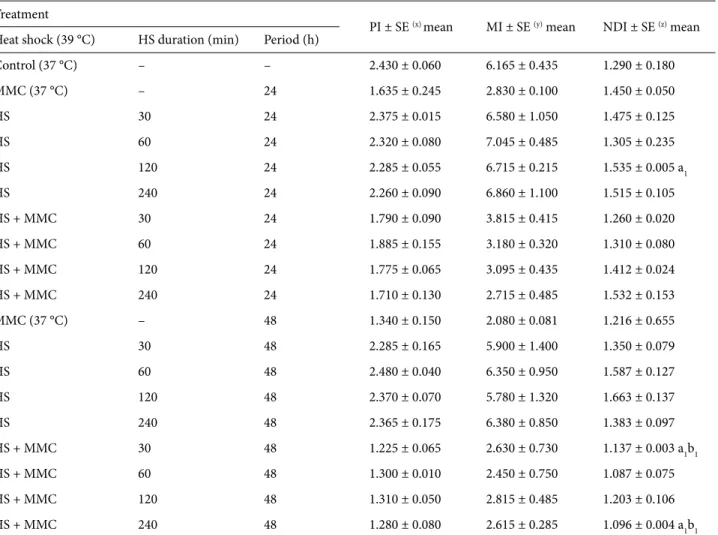
There were slight but not significant differences in PI and MI in the groups treated with heat shock + MMC or heat shock alone (Table 2).
The values calculated in the 3 applications in terms of NDI showed significant alterations compared to the controls. From these, in the treatment where 120 min of heat shock was applied alone after 24 h of initiation of the culture, the NDI value was found to be higher compared to the control; however, in the cultures that were exposed to 30 and 240 min of heat shock + MMC 48 h after initiation of the culture, NDI was found to be significantly reduced compared to its own control (Table 2).
Table 2. Evaluation of cell proliferation parameters (PI, MI, and NDI) in human cultured blood cells treated with heat shock (HS) alone or heat shock + MMC 24 or 48 h after blood transplantation.
Treatment
PI ± SE (x) mean MI ± SE (y) mean NDI ± SE (z) mean
Heat shock (39 °C) HS duration (min) Period (h)
Control (37 °C) – – 2.430 ± 0.060 6.165 ± 0.435 1.290 ± 0.180 MMC (37 °C) – 24 1.635 ± 0.245 2.830 ± 0.100 1.450 ± 0.050 HS 30 24 2.375 ± 0.015 6.580 ± 1.050 1.475 ± 0.125 HS 60 24 2.320 ± 0.080 7.045 ± 0.485 1.305 ± 0.235 HS 120 24 2.285 ± 0.055 6.715 ± 0.215 1.535 ± 0.005 a1 HS 240 24 2.260 ± 0.090 6.860 ± 1.100 1.515 ± 0.105 HS + MMC 30 24 1.790 ± 0.090 3.815 ± 0.415 1.260 ± 0.020 HS + MMC 60 24 1.885 ± 0.155 3.180 ± 0.320 1.310 ± 0.080 HS + MMC 120 24 1.775 ± 0.065 3.095 ± 0.435 1.412 ± 0.024 HS + MMC 240 24 1.710 ± 0.130 2.715 ± 0.485 1.532 ± 0.153 MMC (37 °C) – 48 1.340 ± 0.150 2.080 ± 0.081 1.216 ± 0.655 HS 30 48 2.285 ± 0.165 5.900 ± 1.400 1.350 ± 0.079 HS 60 48 2.480 ± 0.040 6.350 ± 0.950 1.587 ± 0.127 HS 120 48 2.370 ± 0.070 5.780 ± 1.320 1.663 ± 0.137 HS 240 48 2.365 ± 0.175 6.380 ± 0.850 1.383 ± 0.097 HS + MMC 30 48 1.225 ± 0.065 2.630 ± 0.730 1.137 ± 0.003 a1b1 HS + MMC 60 48 1.300 ± 0.010 2.450 ± 0.750 1.087 ± 0.075 HS + MMC 120 48 1.310 ± 0.050 2.815 ± 0.485 1.203 ± 0.106 HS + MMC 240 48 1.280 ± 0.080 2.615 ± 0.285 1.096 ± 0.004 a1b1 A total of (x) 200, (y) 6000, and (z) 2000 cells were counted for PI, MI, and NDI, respectively. a
1 = significant differences compared to control
4. Discussion
The body heats of organisms are prone to instantaneous rises owing to internal and external reasons. It is inevitable that these sudden and transient rises, occurring within the thermal tolerance limits of the organism, will trigger some molecular and cellular response mechanisms.
The temperature tested in this study (39 °C) was lower than the denaturation temperature of biological molecules. However, in some studies, it was stated that endonucleases (S1 nuclease) that were activated in the presence of denatured DNA (a single-stranded molecule) that arose by increased temperature caused DNA breakages (Hunter et al., 1976), and also that the DNA of a metaphase cell is denatured at approximately 8–10°C lower temperatures than are interphase cell DNAs (Darzynkiewicz et al., 1977). Expression increases in the genes of heat shock proteins come at the beginning of the molecular mechanism alterations as a response to the temperature increases (Kelley and Schlesinger, 1978). It was reported that heat shock applied to Drosophila melanogaster caused expression of a small number of RNA transcripts; some of them coded proteins (Bonner and Kerby, 1982). In CHO cells, molecular changes such as aggregation or protein denaturation as a result of exposure to temperature and also radiation for various times at 43 °C caused cell cycle delay, chromosome aberrations, and cell death events. In addition, the S phase was found to be more sensitive to heat shock than the G1 phase (Dewey et al., 1990). Temperature-dependent increases in the expression levels of some genes like estrogen in some salamander larvae and the early stages of reptile embryos were determined to play an important role in sex differentiation (Dournon et al., 1990). It was stated that, in CHO cells, the effect of lethality and chromosomal damage after cisplatin treatment at 37 °C or 41.5 °C were similar and the S phase was detected to be more sensitive in terms of damage affinities than the G1 phase (Krishnaswamy and Dewey, 1993). Proliferation activity in the root meristem cells of sunflower exposed
to 40°C heat shock in the first hour of seed germination was reduced, and a preheating process was determined not to change nitrosomethylurea sensitivity (Mashkina and Gus’kov, 2002). Various stresses such as heat shock induced germ cell apoptosis of Caenorhabditis elegans but did not involve genotoxicity (Salinas et al., 2006). However, it was reported that 30 min of preheat shock can inhibit γH2AX foci formation induced by an alkylating agent, N-methyl-N′-nitro-N-nitrosoguanidine. These data suggest that although heat shock might influence the γH2AX foci formation process, it does not stimulate DNA damage in the different cells lines (including HeLa, CHL, HepG2, and 293 other cells, as well as human spermatozoa) (Dong et al., 2007). Likewise, heat can induce γH2AX foci formation in many mammalian cell lines (Takahashi et al., 2008).
The type of alterations in the parameters related to chromosome morphology in cells exposed to heat shock (39°C) for certain time periods and the alterations in the response of cells to heat stress + a genotoxic compound (MMC) constitute the backbone of this study. The temperature level tested in this study, 39°C, is a level that can induce stress for humans. However, according to our study, it would be plausible to say that the tested temperature generally does not have any clastogenic effect. The insignificant effects shown by heat shock on various indexes (PI, MI, and NDI) lead us to the impression that thermal stress does not affect the cell proliferation course. In summary, it was concluded that the heat shock (39°C) did not have any genotoxic or mutagenic effect on cultured human peripheral lymphocytes, and it also failed to alter the sensitivity of lymphocytes to MMC. The clear effect of heat shock in this study was not apparent.
Acknowledgment
This work was funded by the Çukurova University Research Fund: FEF2011BAP4.
References
Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DE, Tice R et al. (2000). IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. International Programme on Chemical Safety. Mutat Res 463: 111–172.
Anitha B, Chandra N, Gopinath PM, Durairaj G (2000). Genotoxicity evaluation of heat shock in gold fish (Carassius auratus). Mutat Res 469: 1–8.
Bonner JJ, Kerby RL (1982). RNA polymerase II transcribes all of the heat shock induced genes of Drosophila melanogaster. Chromosoma 85: 93–108.
Cannon WB (1926). Physiological regulation of normal states: some tentative postulates concerning biological homeostasis. In: Pettit A, editor. À Charles Richet: Ses Amis, Ses Collègues, Ses Élèves. Paris, France: Éditions Médicales, pp. 91–93.
Cross SJ, Albury WR (1987). Walter B. Cannon, L.J. Henderson, and the organic analogy. Osiris 3: 165–192.
Darzynkiewicz Z, Traganos F, Sharpless T, Melamed MR (1977). Different sensitivity of DNA in situ in interphase and metaphase chromatin to heat denaturation. J Cell Biol 73: 128–138.
Davidson JF, Schiestl RH (2001). Cytotoxic and genotoxic consequences of heat stress are dependent on the presence of oxygen in Saccharomyces cerevisiae. J Bacteriol 183: 4580–4587. Dewey WC, Li XL, Wong RS (1990). Cell killing, chromosomal
aberrations, and division delay as thermal sensitivity is modified during the cell cycle. Radiat Res 122: 268–274. Dong Z, Hu H, Chen W, Li Z, Liu G, Yang J (2007). Heat shock does
not induce γH2AX foci formation but protects cells from N-methyl-N′-nitro-N-nitrosoguanidine-induced genotoxicity. Mutat Res 629: 40–48.
Dournon C, Houillon C, Pieau C (1990). Temperature sex-reversal in amphibians and reptiles. Int J Dev Biol 34: 81–92.
Eastmond DA, Tucker JD (1989). Identification of aneuploidy-inducing agents using cytokinesis blocked human lymphocytes and an antikinetochore antibody. Environ Mol Mutagen 13: 34–43.
Erboğa H, İla HB (2013). Cytogenetic effects of endogenous sex hormones depending on smoking habits. Turk J Biol 37: 709– 715.
Evans HJ (1984). Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. In: Kilbey BJ, Legator M, Nicholson W, Ramel C, editors. Handbook of Mutagenicity Test Procedures. 2nd ed. Amsterdam, the Netherlands: Elsevier Science Publishers, pp. 405–427.
Hunter JD, Bodner AJ, Hatch FT, Balhorn RL, Mazrimas JA, McQueen AP, Gledhill BL (1976). Single-strand nuclease action on heat-denatured spermiogenic chromatin. J Histochem Cytochem 24: 901–907.
Kelley PM, Schlesinger MJ (1978). The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell 15: 1277–1286.
Krishnaswamy G, Dewey WC (1993). Cell killing and chromosomal aberrations induced in Chinese hamster ovary cells by treating with cisplatin at 41.5°C during the G1 or late S phase. Cancer Res 53: 1239–1243.
Li FH, Xiang JH, Zhou LH, Wu CG, Zhang XJ (2003). Optimization of triploid induction by heat shock in Chinese shrimp Fenneropenaeus chinensis. Aquaculture 219: 221–231. Li XL, Wang ZH, Chu GL, Dewey WC (1990). Effects of pH on heat
sensitization of mammalian cells with procaine hydrochloride. Int J Radiat Oncol Biol Phys 18: 933–935.
Mace ML Jr, Daskal Y, Wray W (1978). Scanning-electron microscopy of chromosome aberrations. Mutat Res 52: 199–206.
Mackowiak PA, Wasserman SS, Levine MM (1992). A critical appraisal of 98.6°F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA 268: 1578–1580.
Mashkina EV, Gus’kov EP (2002). Cytogenetic effect of temperature on the sunflower varieties. Tsitologiia 44: 1220–1226.
Perry P, Thomson EJ (1984). The methodology of sister chromatid exchanges. In: Kilbey BJ, Legator M, Nicholson W, Ramel C, editors. Handbook of Mutagenicity Test Procedures. 2nd ed. Amsterdam, the Netherlands: Elsevier Science Publishers, pp. 495–529.
Rothfuss A, Schutz P, Bochum S, Volm T, Eberhardt E, Kreienberg R, Vogel W, Speit G (2000). Induced micronucleus frequencies in peripheral lymphocytes as a screening test for carriers of a BRCA1 mutation in breast cancer families. Cancer Res 60: 390–394.
Salinas LS, Maldonado E, Navarro RE (2006). Stress-induced germ cell apoptosis by a p53 independent pathway in Caenorhabditis elegans. Cell Death Differ 13: 2129–2139.
Speit G (1984). Considerations on the mechanism of differential Giemsa staining of BrdU-substituted chromosomes. Hum Genet 67: 264–269.
Speit G, Haupter S (1985). On the mechanism of differential Giemsa staining of bromodeoxyuridine-substituted chromosomes. II. Differences between the demonstration of sister chromatid differentiation and replication patterns. Hum Genet 70: 126– 129.
Takahashi A, Mori E, Somakos GI, Ohnishi K, Ohnishi T (2008). Heat induces γH2AX foci formation in mammalian cells. Mutat Res 656: 88–92.
Wu C (1995). Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol 11: 441–469.
Zhang X, Lu F, Xiang J (2003). Chromosome behavior of heat shock induced triploid in Fenneropenaeus chinensis. Chinese J Oceanol Limn 21: 222–228.