Submitted 20 March 2020 Accepted 2 June 2020 Published 17 July 2020 Corresponding author Aydın Kaleli, aydin.kaleli@istanbul.edu.tr Academic editor Jingchun Li
Additional Information and Declarations can be found on page 17
DOI 10.7717/peerj.9406
Copyright 2020 Kaleli et al. Distributed under
Creative Commons CC-BY 4.0
OPEN ACCESS
Biodiversity of carapace epibiont diatoms
in loggerhead sea turtles (Caretta caretta
Linnaeus 1758) in the Aegean Sea
Turkish coast
Aydın Kaleli1,*, Ana Car2,*, Andrzej Witkowski3, Marta Krzywda3,
Catherine Riaux-Gobin4,5, Cüneyt Nadir Solak6, Yakup Kaska7, Izabela
Zgłobicka8, Tomasz Płociński9, Rafał Wróbel10and Krzysztof Kurzydłowski8,9
1Department of Marine and Freshwater Resources Management, Faculty of Aquatic Sciences, Istanbul
University, Istanbul, Turkey
2Institute for Marine and Coastal Research, University of Dubrovnik, Dubrovnik, Croatia 3Institute of Marine and Environmental Sciences, University of Szczecin, Szczecin, Poland 4CNRS-EPHE-UPVD, CRIOBE, PSL Research University, Perpignan, France
5Laboratoire d’Excellence ‘CORAIL’, Université de Perpignan, Perpignan, France
6Department of Biology, Faculty of Science and Arts, Kütahya Dumlupınar University, Kütahya, Turkey 7Department of Biology, Faculty of Science and Arts, Pamukkale University, Denizli, Turkey
8Faculty of Mechanical Engineering, Bialystok University of Technology, Bialystok, Poland 9Faculty of Materials Science and Engineering, Warsaw University of Technology, Warsaw, Poland 10Faculty of Chemical Technology and Engineering, West Pomeranian University of Technology, Szczecin,
Poland
*These authors contributed equally to this work.
ABSTRACT
Background. The Aegean Sea coast of Turkey hosts one of the most important nesting
grounds for loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea. Previous studies have revealed that the sea turtle carapace provides favourable conditions for various epibiontic organisms. Epibionts occurring on the carapace have been examined from different locations in the oceans.
Methods. This is the first time such a high number (39) of samples collected from
nesting turtles during such a long time period (extending from 2011 to 2018) has been used for the study of the diatom component of the microbiome on the turtle carapaces. A total of 33 samples were investigated in terms of light microscopy (LM) and scanning electron microscopy (SEM). Six unprocessed biofilm fragments were subject to SEM observations.
Results. A total of 457 epizoic diatom taxa belonging to 86 genera were identified.
Epizoic forms, e.g., Achnanthes spp., Chelonicola spp. or Tripterion spp. (also identified by SEM observations of the undisturbed pieces of the microbiome) dominated in terms of relative abundance, but the highest numbers of taxa were ubiquitously represented by Navicula (79), Nitzschia (45), Amphora (40), Cocconeis (32), Diploneis (25) and
Mastogloia(23). Navicula perminuta and Delphineis australis were the most frequent taxa, present in 65% of the samples, both with an average relative abundance of 10%. The results of our study revealed that diatoms are an essential component of the loggerhead sea turtles’ microbiome, in terms of high biodiversity and abundance. Although strict epibionts provide a signature of the turtle microbiome, the carapace
How to cite this articleKaleli A, Car A, Witkowski A, Krzywda M, Riaux-Gobin C, Solak C, Kaska Y, Zgłobicka I, Płociński T, Wróbel R, Kurzydłowski K. 2020. Biodiversity of carapace epibiont diatoms in loggerhead sea turtles (Caretta caretta Linnaeus 1758) in the Aegean Sea Turkish coast. PeerJ 8:e9406http://doi.org/10.7717/peerj.9406
as a solid substrate attracts numerous benthic diatom species which are considered opportunistic forms and can be found in the surrounding benthic habitats of the vast ocean littoral space.
SubjectsBiodiversity, Marine Biology, Taxonomy
Keywords Diatoms (Bacillariophyta), Biodiversity, Caretta caretta, Epibionts, The Mediterranean Sea, Turkey
INTRODUCTION
Epibiosis is a relationship between two organisms where an epibiont lives on the surface of a basibiont used as a substrate (Lima et al., 2017). Marine vertebrates (especially whales and sea turtles) are ideal motile substrata for other organisms and are known to host epibiont assemblages (Dodd, 1988;Ernst, Barbour & Lovich, 1994). Although there has been much focus on the epibiont fauna of sea turtles, scientists have begun also to investigate the epibiont flora of sea turtles in recent decades. Kitsos et al. (2005)found seventeen taxa of algae associated with loggerhead sea turtles from Greek coasts. Green and red-algal taxa have been found on sea turtles (Pfaller et al., 2006;Pfaller et al., 2008), including a newly described Rhodophyte species limited in its distribution to turtles inhabiting the Mediterranean Sea (Báez et al., 2001).
Although epizoic diatoms on vertebrates were first described from cetaceans, freshwater and sea turtles can also host very specific diatom floras (Nemoto, 1956;Holmes, Nagasawa & Takano, 1993a;Holmes, Nagasawa & Takano, 1993b;Denys, 1997;Riaux-Gobin et al., 2017a;Riaux-Gobin et al., 2017b). Loggerhead sea turtles (Caretta caretta Linnaeus, 1758) are one of the seven species of sea turtles (Lutz & Musick, 1997), distributed from tropical waters of the Indian and the Pacific Ocean to temperate waters of the Atlantic Ocean and the Mediterranean Sea (Ernst, Barbour & Lovich, 1994). The most recent research on epibionts from extant sea turtle microbiomes showed that diatoms are present on all known species of turtles (Robinson et al., 2016). The same authors found that the sea turtle carapace could be host to several undescribed taxa (Robinson et al., 2016). There have been a number of recent papers with analyses of the epibiont diatom composition on the carapace of the sea turtles (Frankovich, Sullivan & Stacy, 2015;Majewska et al., 2015a;Majewska et al., 2015b;Majewska et al., 2017a;Riaux-Gobin et al., 2017a;Riaux-Gobin et al., 2017b). Several diatom genera and species have been described as new to science from the carapace of sea turtles from different geographic regions.Majewska et al. (2015a) described two genera (Poulinea Majewska, De Stefano & Van de Vijver and Chelonicola Majewska, De Stefano & Van de Vijver) from olive ridley sea turtles (Lepidochelys olivacea Escholtz, 1829) from the Pacific coast of Costa Rica. Chelonicola caribeana Riaux-Gobin, Witkowski, Ector & Chevallier and Tripterion societatis Riaux-Gobin, Witkowski & Ector were identified and described from the Atlantic Ocean from green sea turtle (Chelonia mydas Linnaeus, 1758) population (Riaux-Gobin et al., 2017b). Additionally, Tursiocola yin-yangii Riaux-Gobin & Witkowski and Tursiocola guyanensis Riaux-Gobin & Witkowski were described from green turtles in French Guiana and the eastern Caribbean (Riaux-Gobin et al., 2017a).
Research on Tursiocola and Tripterion species revealed that some epibiont diatoms could live on various animals’ skin or carapaces. In the past Tursiocola species have been observed on Dall’s porpoises (Phocoenoides dalli True, 1885) (Nemoto, 1956,Holmes, Nagasawa & Takano, 1993a;Holmes, Nagasawa & Takano, 1993b;Denys, 1997), on manatee (Trichechus
manatusLinnaeus, 1758) skin (Frankovich, Sullivan & Stacy, 2015) and freshwater turtles (Wetzel et al., 2012). Some Tripterion species were formerly reported from whales and other cetaceans.
In the Mediterranean Sea, the most numerous turtle nesting sites are on the northern Cilician coasts of Turkey. Recently, diatoms associated with the Mediterranean loggerhead sea turtle population have been described. These included an Olifantiella species (Kaleli et al., 2018) and six new species of Proschkinia (Majewska et al., 2019), and a small celled
Catenulataxon from the Adriatic Sea (Robert, Bosak & Van de Vijver, 2019).
The objectives of this study were (i) to describe the species composition and diversity of diatom assemblages on loggerhead sea turtles from a series of survey samples taken between 2011–2014, (ii) to determine functional group of particular diatom taxa e.g., epizoic, epiphytic and (iii) to highlight data on the diatom species associated with the biofilm from the samples collected in 2018 which have been studied in situ with SEM.
MATERIAL & METHODS
Study area
Dalyan beach is located in the province of Muğla (36◦4200200N, 28◦4103100E) (Fig. 1). It has one of the highest numbers of loggerhead sea turtle nests along with the beaches of Belek, Antalya, and Anamur, along the Aegean and the Mediterranean coasts of Turkey (Kaska et al., 2016). As a result, Dalyan beach was assigned as a ‘‘specially protected area’’ in 1988 and has ‘‘flagship beach’’ status for the conservation of loggerhead sea turtles (Türkozan & Yılmaz, 2008). The beach is 4.7 km long and composed of a fine-sand dune and gravel drifted from the Dalyan Delta, which is deposited to the east of the beach. Dalyan Delta is an extensive wetland with a labyrinth of reedy channels opening to Köyceğiz Lake via the Dalyan River where, during the study period (2011–2018), some foraging sea turtles were observed. The wetland complex (Dalyan Delta) opens to the sea through a channel at the northern part of the beach (Türkozan & Yılmaz, 2008).
Sampling
Samples of diatoms were collected from nesting loggerhead sea turtles, at night during the nesting season, between May–August, 2011–2014 and 2018 (Fig. 2). All sampling was carried out in accordance with the regulations of the Ministry of Environment and Urbanization (TR-15/04/2018/39). Sampling was supervised by experts from the Sea Turtle Research Rescue and Rehabilitation Centre (DEKAMER), Ref. B.32.PAU.0.AG.00.00/005. In total, 39 samples were taken. Samples were collected with toothbrushes from 20 cm2of vertebral and coastal carapace scutes of 33 turtles (curved carapace length (CCL) between 67,5–77 cm) between 2011–2014, and pieces of biofilm were scraped with a razor from six different sea turtles (according to the conservation regulations) while the turtles were laying eggs in 2018. A total of 33 samples were processed and used for light microscopy (LM)
Figure 1 Location of the sampling site.
Full-size DOI: 10.7717/peerj.9406/fig-1
and scanning electron microscopy (SEM) (3 samples from 2011; 5 samples from 2012; 20 samples from 2013 and 5 samples from 2014). Six unprocessed fragments of biofilm (from 2014 and 2018) were used for SEM observations (Table 1).
Biofilm pieces were fixed with 70% ethanol for 4 h. Each fixed biofilm was then washed five times with distilled water, followed by washing in increasing alcohol concentration. In each concentration, the biofilm was left for 20 mins, (30 mins in absolute alcohol) at room temperature. After drying, a piece of biofilm was mounted on an aluminium stub with double-adhesive carbon tape. Untreated samples of the dried and dehydrated microbiome were sputter-coated with palladium-gold alloy and observed with a Hitachi SU8020 scanning electron microscope (Hitachi, Tokyo, Japan).
For light (LM) and scanning electron microscopy (SEM) observations, samples were cleaned to remove organic material by washing with 10% HCl, boiling in 30% H2O2
and rinsing with distilled water (Swift, 1967). Permanent slides were air-dried and mounted in Naphrax R
. LM observations were performed with a Zeiss Axio Imager 2 (Carl Zeiss Microscopy Gmbh, Jena, Germany) equipped with a 100× oil immersion Plan apochromatic objective (with numerical aperture = 1.46) at the University of Szczecin (Poland), and a Nikon Eclipse Ci (Nikon Corp. Tokyo, Japan) with a Nikon DS-Fi1 camera at the Kütahya Dumlupınar University. SEM images were taken using a HITACHI S-5500 at Warsaw University of Technology (Poland). Slides and processed material are deposited at the Department of Marine and Freshwater Resources Management, Istanbul University, Istanbul (Turkey) and the diatom collection (SZCZ) of the Institute of Marine and Environmental Sciences, University of Szczecin, Szczecin (Poland).
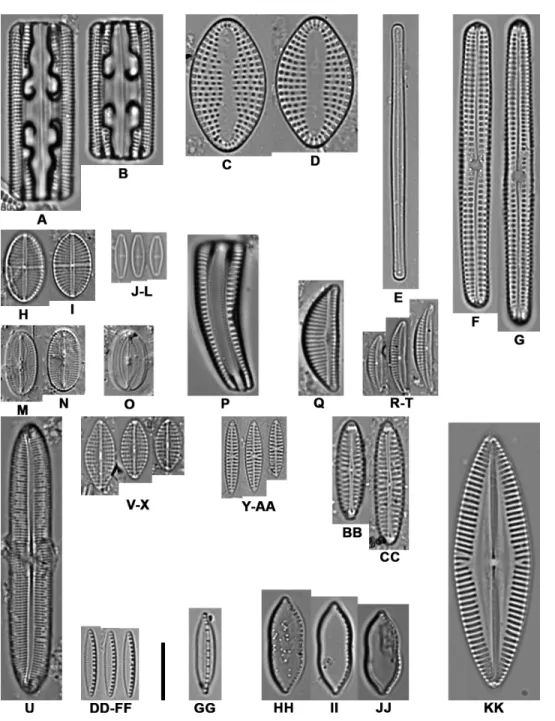
Figure 2 Light microscope images of the most abundant epibiont diatoms associated with Caretta
caretta. (A, B) Grammatophora angulosa; (C, D) Delphineis australis; (E) Neosynedra provincialis; (F, G)
Achnanthes elongata; (H, I) Mastogloia crucicula var. alternans; (J–L) Olifantiella seblae; (M, N) Fallacia
cassubiae; (O) Fallacia florinae; (P) Rhoicosphenia abbreviata; (Q) Encyonema minutum; (R–T)
Halam-phora tenerrima; (U) Caloneis liber; (V–X) Navicula vimineoides; (Y–AA) Navicula perminuta; (BB, CC)
N.cf. borowkae; (DD–FF) Nitzschia frustulum; (GG) N. volvendirostrata; (HH–JJ) Psammodictyon rudum; (KK) N. palpebralis var. angulosa. Scale bar: 10µm.
Full-size DOI: 10.7717/peerj.9406/fig-2
Table 1 Sampling codes of the carapaces. Note that CAR_2018_5 was taken from a dead sea turtle cara-pace, CAR_2013_10 is the cleaned material, and CAR_2018_4 is the biofilm fragment from the same turtle that was sampled in 2013 and 2018.
Fieldwork—culture collection code names
Codes names adjusted for this study
Sampling date 18866 / TRYB-404 CAR_2011_1 2011 18867 / TRY-0074 CAR_2011_2 2011 18868 / TRY-0075 CAR_2011_3 2011 19772 CAR_2012_1 2012 19776 CAR_2012_2 2012 19780 CAR_2012_3 2012 19781 CAR_2012_4 2012 19782 CAR_2012_5 2012 20679 / TRY-0200 CAR_2013_1 2013 20690 / TRY-0008 CAR_2013_2 2013 20694 / TRY-0141 CAR_2013_3 2013 20698 / TRY-0412 CAR_2013_4 2013 20705 / TRY-0027 CAR_2013_5 2013 20707 / TRY-0175 CAR_2013_6 2013 20714 / TRY-0130 CAR_2013_7 2013 20715 / TRY-0138 CAR_2013_8 2013 20735 / TRY-0174 CAR_2013_9 2013 TRC-2300 CAR_2013_10 2013 TRY-0154 CAR_2013_11 2013 TRY-0165 CAR_2013_12 2013 TRY-0184 CAR_2013_13 2013 TRY-0438 CAR_2013_14 2013 TRY-0439 CAR_2013_15 2013 TRY-0442 CAR_2013_16 2013 TRY-0451 CAR_2013_17 2013 TRY-0452 CAR_2013_18 2013 TRY-0457 CAR_2013_19 2013 TRY-0467 CAR_2013_20 2013 Caretta 2014-1 CAR_2014_1 2014 Caretta 2014-2 CAR_2014_2 2014 Caretta 2014-3 CAR_2014_3 2014 Caretta 2014-4 CAR_2014_4 2014 Caretta 2014-6 CAR_2014_5 2014 Biofilm fragments TRY-0520 CAR_2014_6 2014 TRY-0627 CAR_2018_1 2018 TRY-1180 CAR_2018_2 2018 TRY-2012 CAR_2018_3 2018 TRC-2300 CAR_2018_4 2018 TRY-Carapace-1801 CAR_2018_5 2018
Data analysis
The abundance of diatom species was expressed as a percentage of the total number of valves counted (relative abundances in %). The relative abundance (RA) of particular taxa and the taxa richness of the assemblages were estimated on the basis of at least 300 diatom valves counted per sample. Frequency of the most abundant taxa and their maximum RA during the four-year period (2011–2014) and for each of the years were determined.
Raw diatom counts were expressed as a relative abundance and were square-root transformed to normalize data. A resemblance matrix of the data was generated using Bray–Curtis analysis. The Bray–Curtis similarity matrix (Legendre & Legendre, 1983;Clarke & Gorley, 2006) of the relative abundance data of 457 taxa over 33 samples was constructed. Similarity percentage analysis (SIMPER, (Clarke & Warwick, 1994)) was used to identify the taxa making the most significant contribution to the similarities between epibiontic diatom assemblages. All statistical analyses were performed using the Primer v6 software (Clarke & Gorley, 2006) and Statistica 7.0 (StatSoft, Inc. 2004).
Identifications were made following Witkowski, Lange-Bertalot & Metzeltin (2000). Terminology followsRound, Crawford & Mann (1990), and nomenclature of recorded taxa follows AlgaeBase (Guiry & Guiry, 2019) and Diatombase (Kociolek et al., 2019).
RESULTS
Diatom composition & distribution
A total of 457 diatom taxa belonging to 86 diatom genera were identified from 33 samples (Table S1). Among them, 62, 95, 253 and 275 taxa were identified in 2011, 2012, 2013 and 2014, respectively. Among the 457 diatom taxa, 27 taxa were observed exclusively in 2011, 26 taxa in 2012, 111 taxa in 2013, and 129 taxa in 2014, while 174 taxa were found only once (sporadic).
The genera with the highest number of taxa represented were Navicula (79), Nitzschia (45), Amphora (40), Cocconeis (32), Diploneis (25), Mastogloia (23), Fallacia (14) and
Achnanthes(12), followed by Halamphora (10) and Psammodictyon (10). Although Navicula and Nitzschia had the highest numbers of taxa, they occurred with an average RA of 3%. Amongst the genera which were recorded in all four sampling years, the most abundant was Achnanthes (Avg RA = 7%) (Tables 2and3).
The results revealed that there were 16 taxa common to all four sampling years. These taxa were Achnanthes elongata Majewska & Van de Vijver, Cocconeis sp. 8, Dimmeregramma
minusvar. nanum (Gregory) Van Heurck, Diplomenora cocconeiformis (Schmidt) Blazé,
Diploneis bombus(Ehrenberg) Ehrenberg, Halamphora acutiuscula (Kützing) Levkov, H.
tenerrima (Aleem & Hustedt) Levkov, Karayevia submarina (Hustedt) Bukhtiyarova,
Meloneis mimallis Louvrou, Danielidis & Economou-Amilli, Navicula normaloides Cholnoky, N. perminuta Grunow, Nitzschia elegantula Grunow, N. liebetruthii Rabenhorst,
Pinnunavis yarrensis(Grunow) Okuno, Tryblionella pararostrata (Bertalot) Lange-Bertalot, T. granulata (Grunow) Mann. Navicula perminuta and Delphineis australis (Petit) Watanabe, Tanaka, Reid, Kumada & Nagumo were recorded in 65% of samples, both with an average RA of 10% (Figs. 2and3,Tables S2–S5).
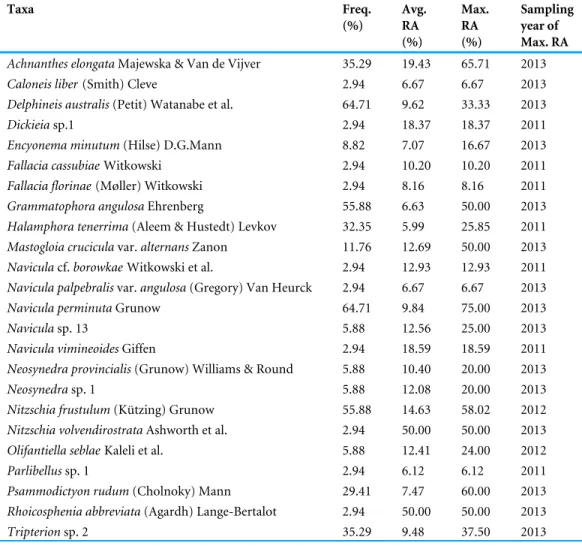
Table 2 Dominant diatom taxa collected during a four-year period (2011–2014) from turtles with the frequency of appearance (Freq.) >3%, average relative abundance (Avg. RA) >6% and maximum rela-tive abundance (Max. RA) >6%. N = 33.
Taxa Freq. (%) Avg. RA (%) Max. RA (%) Sampling year of Max. RA
Achnanthes elongataMajewska & Van de Vijver 35.29 19.43 65.71 2013
Caloneis liber(Smith) Cleve 2.94 6.67 6.67 2013
Delphineis australis(Petit) Watanabe et al. 64.71 9.62 33.33 2013
Dickieiasp.1 2.94 18.37 18.37 2011
Encyonema minutum(Hilse) D.G.Mann 8.82 7.07 16.67 2013
Fallacia cassubiaeWitkowski 2.94 10.20 10.20 2011
Fallacia florinae(Møller) Witkowski 2.94 8.16 8.16 2011
Grammatophora angulosaEhrenberg 55.88 6.63 50.00 2013
Halamphora tenerrima(Aleem & Hustedt) Levkov 32.35 5.99 25.85 2011
Mastogloia cruciculavar. alternans Zanon 11.76 12.69 50.00 2013
Naviculacf. borowkae Witkowski et al. 2.94 12.93 12.93 2011
Navicula palpebralisvar. angulosa (Gregory) Van Heurck 2.94 6.67 6.67 2013
Navicula perminutaGrunow 64.71 9.84 75.00 2013
Naviculasp. 13 5.88 12.56 25.00 2013
Navicula vimineoidesGiffen 2.94 18.59 18.59 2011
Neosynedra provincialis(Grunow) Williams & Round 5.88 10.40 20.00 2013
Neosynedrasp. 1 5.88 12.08 20.00 2013
Nitzschia frustulum(Kützing) Grunow 55.88 14.63 58.02 2012
Nitzschia volvendirostrataAshworth et al. 2.94 50.00 50.00 2013
Olifantiella seblaeKaleli et al. 5.88 12.41 24.00 2012
Parlibellussp. 1 2.94 6.12 6.12 2011
Psammodictyon rudum(Cholnoky) Mann 29.41 7.47 60.00 2013
Rhoicosphenia abbreviata(Agardh) Lange-Bertalot 2.94 50.00 50.00 2013
Tripterionsp. 2 35.29 9.48 37.50 2013
According to the SIMPER analysis (Tables S2–S5), samples collected from turtles in 2014 had the highest observed within-group average similarities (37.96%). As revealed by SIMPER analyses, the group of taxa contributing the most (cumulatively 50.63%) to similarity between diatom assemblages from the five samples collected in 2014 included
Navicula perminuta, Nitzschia frustulum, Cocconeis placentula Ehrenberg, Navicula sp. 54,
Naviculasp. 55, Nitzschia liebetruthii, Melosira moniliformis (Müller) Agardh, Tryblionella
granulataand Seminavis strigosa (Hustedt) Danielidis & Economou-Amilli (Table S5). Biofilm observations
During SEM analysis of the unprocessed biofilm samples (Figs. 4,5) diatoms were found mixed with other microorganisms, e.g., cyanobacteria, organic detritus, broken pieces of the carapace, mineral detritus and diatomaceous detritus. However, in the carapace fragments (CAR_2018_1 and CAR_2018_5), which had sparse biofilm components, diatoms were observed as pioneer epibionts attached directly to the carapace. In the well-developed biofilm (CAR-2018_3) diatoms were abundant, well preserved and represented by epizoic
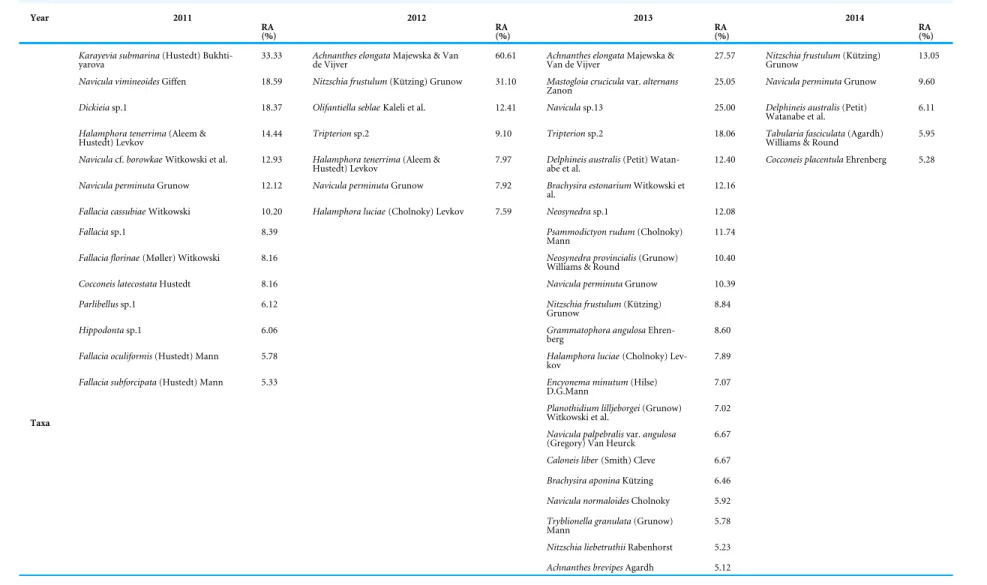
Table 3 List of diatom taxa and their percentage contribution to total diatom community composition (taxa with relative abundances, RA ≥ 5% are only shown) from 2011 till 2014.
Year 2011 2012 2013 2014
RA
(%) RA(%) RA(%) RA(%)
Karayevia submarina(Hustedt)
Bukhti-yarova 33.33 Achnanthes elongatade Vijver Majewska & Van 60.61 Achnanthes elongataVan de Vijver Majewska & 27.57 Nitzschia frustulumGrunow (Kützing) 13.05
Navicula vimineoidesGiffen 18.59 Nitzschia frustulum(Kützing) Grunow 31.10 Mastogloia cruciculavar. alternans
Zanon 25.05 Navicula perminutaGrunow 9.60
Dickieiasp.1 18.37 Olifantiella seblaeKaleli et al. 12.41 Naviculasp.13 25.00 Delphineis australis(Petit)
Watanabe et al. 6.11
Halamphora tenerrima(Aleem &
Hustedt) Levkov 14.44 Tripterionsp.2 9.10 Tripterionsp.2 18.06 Tabularia fasciculataWilliams & Round (Agardh) 5.95
Naviculacf. borowkae Witkowski et al. 12.93 Halamphora tenerrima(Aleem &
Hustedt) Levkov 7.97 Delphineis australisabe et al. (Petit) Watan- 12.40 Cocconeis placentulaEhrenberg 5.28
Navicula perminutaGrunow 12.12 Navicula perminutaGrunow 7.92 Brachysira estonariumWitkowski et
al. 12.16
Fallacia cassubiaeWitkowski 10.20 Halamphora luciae(Cholnoky) Levkov 7.59 Neosynedrasp.1 12.08
Fallaciasp.1 8.39 Psammodictyon rudum(Cholnoky)
Mann 11.74
Fallacia florinae(Møller) Witkowski 8.16 Neosynedra provincialis(Grunow)
Williams & Round 10.40
Cocconeis latecostataHustedt 8.16 Navicula perminutaGrunow 10.39
Parlibellussp.1 6.12 Nitzschia frustulum(Kützing)
Grunow 8.84
Hippodontasp.1 6.06 Grammatophora angulosa
Ehren-berg 8.60
Fallacia oculiformis(Hustedt) Mann 5.78 Halamphora luciae(Cholnoky)
Lev-kov 7.89
Fallacia subforcipata(Hustedt) Mann 5.33 Encyonema minutum(Hilse)
D.G.Mann 7.07
Planothidium lilljeborgei(Grunow)
Witkowski et al. 7.02
Navicula palpebralisvar. angulosa
(Gregory) Van Heurck 6.67
Caloneis liber(Smith) Cleve 6.67
Brachysira aponinaKützing 6.46
Navicula normaloidesCholnoky 5.92
Tryblionella granulata(Grunow)
Mann 5.78
Nitzschia liebetruthiiRabenhorst 5.23 Taxa
Achnanthes brevipesAgardh 5.12
K aleli e t al. (2020), P eerJ , DOI 10.7717/peerj.9406 9/22
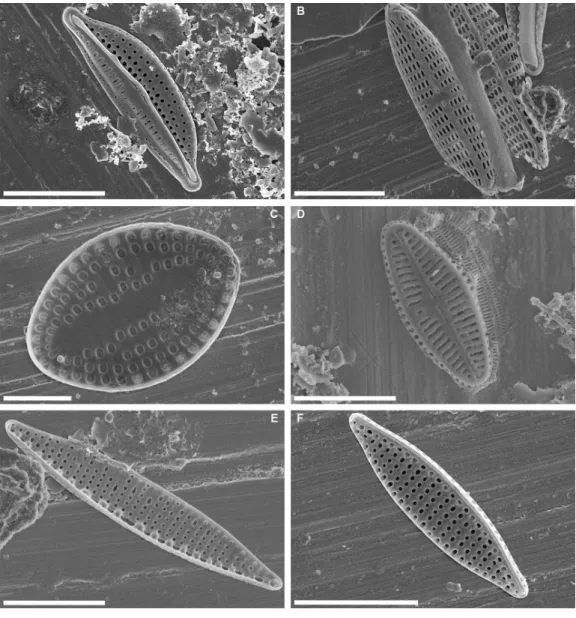
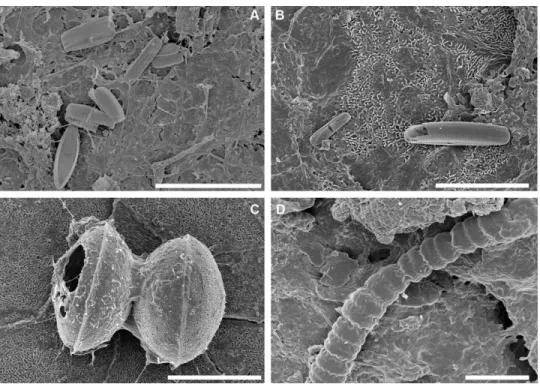
Figure 3 Scanning electron micrographs of some abundant taxa in epibiont diatom assemblages asso-ciated with Caretta caretta. (A) Halamphora tenerrima; (B) Navicula perminuta; (C) Delphineis australis; (D) Olifantiella seblae; (E, F) Nitzschia frustulum. Scale bars: (A, B, C, E, F): 5µm; (D): 3µm.
Full-size DOI: 10.7717/peerj.9406/fig-3
forms: Achnanthes elongata, A. squaliformis (Majewska et al., 2017a), Chelonicola sp. and
Tripterionspp. Another biofilm was dominated by cosmopolitan species such as Navicula
perminuta and small Nitzschiae sect. Lanceolatae (N. frustulum, N. liebethrutii), with lesser participation of the above-mentioned epizoic forms. It appeared as if the layers of diatoms were bound between microlayers of a mucilage composed of unidentifiable organic matter, possibly containing microfungi. In the well-developed biofilm fragments, low occurrence of diatoms was observed. Biofilm sample CAR_2018_2 (Fig. 4D) was mostly composed of mineral and fine organic detritus along with relatively rare, usually broken, diatom frustules. Interestingly, in CAR_2018_4 (Fig. 5D), we observed organic compounds, filamentous cyanobacteria (Anabaena sp.) and fine-mineral detritus, whereas
Figure 4 SEM observations of intact biofilm fragments from Caretta caretta. (A) aggregate of Navicula sp. with Achnanthes sp. and solitary valves of epizoic diatoms between mucilage and pieces of the carapace. (B, C) same biofilm rich in epizoic diatoms mainly Tripterion sp. with solitary specimens of Navicula and
Nitzschiaspp. intercalated with mucilage. (D) Diatom poor example of biofilm with rare fragmented di-atoms; note the presence of mineral detritus. ((A–C) CAR_2018_3; D. CAR_2018_2). Scale bars: (A): 50
µm; (B): 20µm; (C): 30µm; (D): 40µm.
Full-size DOI: 10.7717/peerj.9406/fig-4
diatoms were absent. The differences on the biofilm of several loggerhead sea turtles may give ideas of the development of biofilms, also diatom composition should be taken into consideration. However there are not any data on sea turtles’ health regarding diatoms, diatom composition especially freshwater and brackish taxa may be monitored in foraging areas.Komoroske et al. (2011)observed concentrations of pollutants of carapace like metals, in further studies diatom composition and pollutants could be monitored to reveal any possible relation.
DISCUSSION
Diatom composition
In this study, we present the first detailed floristic list of epibiont diatoms observed on the carapace of loggerhead sea turtles in the Mediterranean Sea. The number of taxa (457) was higher than any floristic surveys conducted on turtles or similar biotic habitats (e. g., whales and cetaceans) in the Mediterranean and over a wider geographic area (Nemoto, 1956;Nemoto, 1958;Majewska et al., 2015b;Robinson et al., 2016). The number of diatom taxa recorded in this study was considerably larger than those recorded on the carapace of olive ridley sea turtles (21 diatom taxa) inMajewska et al. (2015b)and green sea turtles carapace (57 diatom taxa) inRivera et al. (2018). Number of diatom taxa difference might
Figure 5 SEM observations of intact biofilm fragments from Caretta caretta. (A) Biofilm rich in epizoic diatoms mainly Tripterion sp. with solitary specimens of Navicula and Nitzschia spp. intercalated with mucilage. (B) Exposed surface of carapace with rare broken diatom frustules and mucilage. (C) Chain-forming Melosira sp. attached with mucilage to the carapace surface. (D) Close up of filamentous Cyanobacteria - Anabaena sp. Note the presence of mucilage and absence of diatoms. ((A) CAR_2018_2; (B) CAR_2018_5; (C) CAR_2018_1; (D) CAR_2018_4 samples). Scale bars: (A, B, C): 20µm; (D): 10
µm.
Full-size DOI: 10.7717/peerj.9406/fig-5
be related with some factors such as sample numbers, sampling techniques (razoring or brushing the carapce), sea turtles’ foraging areas (Robinson et al., 2016) or the possible difficulties in marine diatom identification. However common diatom taxa of the three sea turtle species might suggest that not only the epizoic diatoms addressed to sea turtles but also other marine diatoms could occur on different sea turtle species, whereas further studies on sea turtle species may reveal the similarity or difference on diatom dispersal.
Of the 457 diatom taxa, the genus Navicula was the most diversified, with several unidentified Navicula spp. abundant in the samples analysed. However, most of the unidentified Navicula spp. were similar in morphology with only very minor differences in some characteristics. This might be a result of adaptation in the biofilm (e.g., some valves were heteropolar with a narrower valve end on one side of the valve). The second-largest group in the diatom community was Nitzschia spp., with N. frustulum as the dominant taxon overall. We observed some small-celled taxa, e.g., Nitzschia inconspicua, Halamphora
tenerrimaand Navicula spp. on the turtle carapaces, in accordance with previous studies from various regions (Majewska et al., 2015b;Robinson et al., 2016;Rivera et al., 2018). Occurrence on the turtle carapace might be related to the small cell size of the frustules, which may lead to rapid reproduction, as has been observed in Navicula perminuta
(an opportunistic species). Mastogloia species were found in low abundance, but were represented by numerous species (e.g., M. adriatica Voigt, M. corsicana Grunow, and M.
decussataGrunow). Therefore, Mastogloia species demonstrated the ability to survive under conditions in the biofilm, but were unable to reach high abundance.
Comparison with the local diatom flora
Some of the taxa observed in the biofilm on the loggerhead sea turtle carapace have been found in diatom flora on different substrata in the same region and along the Aegean Sea coast, and do not seem to have a preference either for a geographic region or for the substrate type (Kaleli, 2019;Kaleli, Kociolek & Solak, 2020). In a shallow coastal lake (Iztuzu Lake), in the same area as the beach occupied by sea turtles during the nesting season, diatoms were abundant (Kaleli, 2019), and some of the species were the same as those found associated with the C. caretta carapace (e.g., Diplomenora cocconeiformis, Diploneis bombus,
Fallacia schaeferae (Hustedt) D.G. Mann, Mastogloia lanceolata, Meloneis mimallis). It is possible that diatoms were transferred by the sea turtles during the nesting season. These taxa have also been observed from different locations in the adjacent coasts and also in the Western Indian Ocean (Kaleli et al., 2018, unpublished observations).
Despite the fact that marine taxa strongly dominated the assemblages (Table S3), a few freshwater taxa were observed. The freshwater forms were usually observed as solitary valves (e.g., Encyonema minutum (Hilse) D.G. Mann and Lindavia balatonis (Pantocsek) Nakov, Guillory, Julius, Theriot & Alverson). The presence of taxa associated with fresh to brackish waters (Table S6) was not particularly surprising as Köyceğiz Lake, which is a typical freshwater lake, is located nearby, and connected through the delta, to Dalyan beach. Both male and female turtles have been observed in the shallow waters close to the banks of the channels connecting the beach to Köyceğiz Lake. Some of the turtles were also observed feeding in the lake and this could be why freshwater taxa were incorporated into the biofilm. Some taxa may be able to tolorate change in salinity (freshwater-brackish, brackish-marine) despite their apparent freshwater preference, and results also support the idea that some species could have different responses to environmental conditions, resulting in a better or worse adaptation (Underwood, Phillips & Saunders, 1998;Ribeiro et al., 2003;
Miho & Witkowski, 2005;Hafner, Jasprica & Car, 2018) to variable conditions, which could be explained by the number of the freshwater forms observed on the carapace. It was also suggested byMajewska et al. (2017b)that lakes and rivers could make exclusive epibiosis where specific species could attach and grow in the biofilm and environment affects the dispersal on sea turtle carapace. The abundance of freshwater and brackish water species, presumably reveal that important amount of sea turtles access to shallow freshwaters of Dalyan and spend long periods in the surrounding areas. Nutrient enrichment in these waters provide favourable conditions for the ubiquitous taxa. Navicula perminuta and Nitzschia frustulum, which are found in marine and brackish waters, dominated the assemblages and this may indicate that species with similar ecological tolerances can settle on the carapace, but species with better adaptation (small cell size, attaching to the carapace, broad tolerance of changes in salinity and light intensity) can thrive.
Epizoic diatoms
Among the dominant taxa, Achnanthes elongata and Olifantiella seblae (Kaleli et al., 2018), recently described from the same biofilm samples, were observed, as obligately epizoic diatoms together with unidentified Chelonicola sp. and Tripterion spp. which we consider potentially new to science (Kaleli et al. in preparation). Representatives of Chelonicola and Tripterion have been described and observed as obligately epizoic forms on sea-turtle carapaces from oceanic waters (Majewska et al., 2015a;Riaux-Gobin et al., 2017a;
Riaux-Gobin et al., 2017b) and have not yet been found on other substrates. Achnanthes
elongatawas described from samples from the Pacific coasts and with this study observed for the first time in the Mediterranean Sea, O. seblae has only been observed in the Mediterranean Sea (Kaleli et al., 2018). The epizoic taxa observed in this study (O. seblae,
A. elongata) have a broad range of valve morphology in terms of outline. Morphological plasticity is common in diatom species observed on other sea turtle carapaces or whale skin (Nemoto, 1956;Nemoto, 1958;Riaux-Gobin et al., 2019). For example, Olifantiella showed high plasticity in the Mediterranean samples, and Olifantiella seblae was observed with a length range of 4.5–14.5µm with elliptic–lanceolate valves. A recent study on Olifantiella species from the South Pacific found similar results on valve plasticity (Riaux-Gobin et al., 2019), valve outline had a wide range of polymorphisms and changes were observed also in valve structure, such as stria formation and counts and the buciniportula, though it was indicated that Olifantiella seblae and Labellicula lecohuianaMajewska, Stefano & Van
de Vijver (2017) could be conspecific in Olifantiella gorandiana complex (Riaux-Gobin
et al., 2019). Likewise, Achnanthes elongata and A. squaliformis valves were 20.3–70µm and 12.3–63.1µm long respectively and showed high plasticity in this study. These two
Achnanthesspecies were described with quite similar lengths to our samples inMajewska et al. (2017a); 15–75µm for A. elongata and 11.5–45µm for A. squaliformis).
Biofilm composition
Our SEM observations of intact biofilms highlight that the biofilm is composed of microorganisms and mineral detritus along with micro-detritus from the carapace (Figs. 4and5). The formation of the biofilm seems to be a stochastic process, with the early colonisers (we observed diatoms) serving as a foundation for the subsequent deposition of organic and mineral detritus. A similar ‘‘messy’’ microstructure of the biofilm was also observed on the carapace of several species of sea turtles in Robinson et al. (2016). The biofilm observed on carapaces of olive ridley turtles from Costa Rica had a quite different spatial organisation (Majewska et al., 2015b). A relatively low diatom species number was reported (Majewska et al., 2015b), there was stable species composition with low inter-sample dissimilarities, and the epizoic microalgae were either partly immersed or entirely encapsulated within an exopolymeric coat. Here, the biofilm was formed by a massive occurrence of several diatom species with a dozen more taxa being sporadically observed. In addition, our observations on a clean carapace fragment revealed that among the micro-epibionts, diatoms might play a pioneering role as they attach with mucilage (Fuller et al., 2010) to the relatively smooth surface of the carapace.
The colonization of an existing surface by epibionts, organisms living attached to the body surface of a basibiont (host or substratum organism), constitutes one of the most substantial modifications of the basibiont’s body surface (Molino & Wetherbee, 2008;Wahl, 2008). Small epibionts, although generally ignored in the description of marine organisms, may have profound effects on the basibiont by causing a variety of either beneficial or detrimental effects. These effects should be taken into account when the ecology of the host is studied (Gillan & Cadee, 2000). Among the early settlers, microalgae play a crucial role in biofilm development and are able to settle on even the most fouling-resistant surfaces (Molino & Wetherbee, 2008).
Biogeography
Our findings of biofilms composed of epizoic diatoms (e.g., Achnanthes elongata,
A. squaliformis, Olifantiella seblae, Tursiocola sp. and Tripterion sp.) showed that the carapace of loggerhead sea turtles in the Mediterranean Sea was a suitable environment for diatom growth and further distribution. It was not possible to determine from which turtle population these epizoic diatoms originated, or from which substrata diatoms were introduced to the carapace (e.g., by grazing). There have not yet been sufficient comparable observations of sea turtle epizoic diatoms and the diatom flora from coasts or coral reefs of nesting grounds in general. However, the presence of epizoic diatom taxa of sea turtles from locations such as the Pacific Coast of Costa Rica, or the Caribbean and South American coasts (Majewska et al., 2015a;Majewska et al., 2015b;Riaux-Gobin et al., 2017a;Riaux-Gobin et al., 2017b;Riaux-Gobin et al., 2019), and the Mediterranean Sea might show that populations of basibionts meet somewhere in the oceans during their foraging migrations, as in the example of Achnanthes squaliformis or the similar species,
O. seblae, L. lecohuiana both observed from the Atlantic and the Mediterranean sea turtles. In the Mediterranean C. caretta were tracked and it was found that turtles spent time foraging in the Eastern Mediterranean basin (Casale et al., 2012;Casale et al., 2013). It is possible that the Mediterranean loggerhead population and the Atlantic population could exchange diatom flora (Revelles et al., 2007). Genetic data have shown that the C. caretta populations from the west Atlantic coast spend time foraging with the population from the Eastern Mediterranean (Laurent et al., 1998;Carreras et al., 2006). Species distribution comparison of green sea turtles in Costa Rica and Iran showed a remarkable difference (Majewska et al., 2017b). Different characteristics of water column in Iranian coast of the Persian Gulf and Atlantic Ocean was found as a possible effect of diatom dispersal. Water chemistry and nutrients play a role in diatom community and their growth forms, where in the Persian Gulf species numbers were lower in the challenging environment. On the contrary, the Mediterranean loggerhead sea turtles comprised high biodiversity. However, tracking of these C. caretta is challenging and more detail is needed from the coasts of the Mediterranean for a comparison. But in the oligotrophic waters of the Eastern Mediterranean Sea diatom assemblage composition was significantly richer in species with epizoic diatoms present and characterized by high frequencies. Nonetheless, our study indicated that diatoms could adapt to the sea turtle’s microbiome and form a highly diversified facultative epibiont community. The dominant taxa in RA observed here
were mostly raphid diatoms (in particular Navicula spp.). Raphid diatoms are generally among the earliest and most abundant primary colonizers of natural and artificial surfaces (Hoagland, Zlotsky & Peterson, 1986).
In general, the intensity of fouling pressure varies between season, latitude, depth, and local ecological factors; however, any permanently exposed, non-defended surface will eventually become fouled (Wahl, 1989). To determine whether seasonal and spatial variability is a relevant structuring factor, observations of the epibiont diatom community structure should be conducted involving more locations (e.g., different nesting grounds) in the Mediterranean, and over a more extended time period. Due to the fact that the nesting season only happens over a few months each year, there is little opportunity to study the seasonality of the diatom assemblages on the carapaces. However, as more and more turtles are tagged and followed with remote sensing (although it is still difficult to locate the tagged sea turtle as GPS trackers could be damaged or stop sending signals, and the turtle may not come back to ashore or to the same coasts for nesting in 3–4 years’ time), it could be possible and fruitful to repeat the analysis of the microbiome composition in terms of changes in the diatom assemblages over the time on the same sea turtle.
CONCLUSIONS
Our study provides the first detailed information of the diatom assemblage from the C.
carettacarapace. The results contain the diatom composition of sea turtle biofilm of the carapace and the specific taxa attached to carapace fragments. The most significant result from this study lies in the information about epibiont diatoms from turtles in this part of the Mediterranean Sea: there was great diatom diversity on the carapace, even if only some of them are considered epizoic forms. It seems that some of the epibionts are occurring on various host sea turtles e.g., Achnanthes elongata and have broad biogeography. This suggests that epibiont diatoms of the sea turtles have a broad range of ecological adaptation, even though some species were low in individuals, most species can survive in the biofilm, while the rare, typically epizoic, are well adapted.
The results of our study enhance the existing knowledge on the diatom-species composition and community structure of the microbiome of C. caretta carapaces in the Mediterranean, and will be a comparable dataset for C. caretta distributed in other geographic regions of ocean. Nesting sea turtles spend winters in the southeast Mediterranean (Isreal, Egypt, Tunisia), and as the diatom flora of these coasts has not been fully described, in general it is not possible to evaluate each individual sea turtle’s foraging area. Therefore, this study brings data for future comparison of nesting coasts from various locations, and contributes to sea turtle carapace flora of the Mediterranean C. caretta for further diatom investigations. Such knowledge would be useful for future investigations of sea turtles from different waters (East Atlantic coasts of Africa for the Mediterranean Sea turtle population).
ACKNOWLEDGEMENTS
The authors express their gratitude to Prof. Alistair W.R. Seddon and Caroline Maggil for their critical reading, useful comments and English edits. Doğan Sözbilen, Erhan Kabuk and Ahmet Yavuz Candan are acknowledged for field sampling assistance. Genowefa Daniszewska-Kowalczyk, Agnieszka Kierzek, Dr Małgorzata Bąk (Institute of Marine and Environmental Sciences, University of Szczecin, Szczecin, Poland) are acknowledged for literature help and LM, SEM assistance. Finally we thank Jingchun Li and anonymous reviewers for their valuable comments which improved this manuscript.
ADDITIONAL INFORMATION AND DECLARATIONS
FundingThis research was supported by the Turkish Council of Higher Education Foundation (Project No: MEV-2016-04); Croatian Ministry of Science, Education and Sports (Project No: 275-0000000-3186) and by the Croatian science foundation (HRZZ AdriaMedPLan Project No: IP-2014-09-2945); The National Science Centre, Cracow, Poland (Project No: 2017/25/N/NZ8/02740, 2012 Maestro) and ERASMUS+ Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Grant Disclosures
The following grant information was disclosed by the authors: Turkish Council of Higher Education Foundation: MEV-2016-04. Croatian Ministry of Science, Education and Sports: 275-0000000-3186. Croatian Science Foundation: IP-2014-09-2945.
The National Science Centre, Cracow, Poland: 2017/25/N/NZ8/02740, 2012 Maestro. ERASMUS+ Program.
Competing Interests
The authors declare there are no competing interests. Author Contributions
• Aydın Kaleli and Ana Car conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
• Andrzej Witkowski conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
• Marta Krzywda performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
• Catherine Riaux-Gobin, Cüneyt Nadir Solak, Yakup Kaska, Izabela Zgłobicka, Tomasz Płociński, Rafał Wróbel and Krzysztof Kurzydłowski analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Conservation and Monitoring Project of Sea Turtles was approved by the Ministry of Environment and Urbanization (TR-15/04/2018/39).
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Field experiments were approved by DEKAMER- Sea Turtle Research, Rescue and Rehabilitation Centre (Ref. B.32.PAU.0.AG.00.00/005).
Data Availability
The following information was supplied regarding data availability: The raw data are available in theSupplementary Files.
Supplemental Information
Supplemental information for this article can be found online athttp://dx.doi.org/10.7717/ peerj.9406#supplemental-information.
REFERENCES
Báez JC, Camisias J, Valeiras J, Conde F, Flores-Moya A. 2001. First record of the
Epizoic Red Seaweed Polysiphonia carettia Hollenberg in the Mediterranean Sea. Acta
Botanica Malacitana26:197–201DOI 10.24310/abm.v26i0.7417.
Carreras C, Pont S, Maffucci F, Pascual M, Barceló A, Bentivegna F, Cardona L, Alegre F, San Félix M, Fernández G, Aguilar A. 2006. Genetic structuring of immature
loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea reflects water cir-culation patterns. Marine Biology 149:1269–1279DOI 10.1007/s00227-006-0282-8.
Casale P, Broderick AC, Freggi D, Mencacci R, Fuller WJ, Godley BJ, Luschi P. 2012.
Long-term residence of juvenile loggerhead turtles to foraging grounds: a potential conservation hotspot in the Mediterranean. Aquatic Conservation Marine and
Freshwater Ecosystems22:144–154DOI 10.1002/aqc.2222.
Casale P, Freggi D, Cina A, Rocco M. 2013. Spatio-temporal distribution and migration
of adult male loggerhead sea turtles (Caretta caretta) in the Mediterranean Sea: further evidence of the importance of neritic habitats off North Africa. Marine
Biololgy160:703–718DOI 10.1007/s00227-012-2125-0.
Clarke KR, Gorley RN. 2006. PRIMER v6: use manual/tutorial. Plymouth: PRIMER-E. Clarke KR, Warwick RM. 1994. Change in marine communities: an approach to statistical
analysis and interpretation. Plymouth: Natural Environmental Research Council, Plymouth Marine Laboratory.
Denys L. 1997. Morphology and taxonomy of epizoic diatoms (Epiphalaina and
Tursio-cola) on a sperm whale (Physeter macrocephalus) stranded on the coast of Belgium.
Dodd CK. 1988. Synopsis of the biological data on the loggerhead sea turtle. U.S.
Department of the Interior, Fish and Wildlife Service Biological Report 88:110. Ernst CH, Barbour RW, Lovich JE. 1994. Turtles of the United States and Canada.
Washington and London: Smithsonian Institute Press.
Frankovich TA, Sullivan MJ, Stacy NI. 2015. Three new species of Tursiocola
(Bacillar-iophyta) from the skin of the West Indian manatee (Trichechus manatus). Phytotaxa
204:33–48DOI 10.11646/phytotaxa.204.1.3.
Fuller WJ, Broderick AC, Enever R, Thorne P, Godley BJ. 2010. Motile homes: a
com-parison of the spatial distribution of epibiont communities on Mediterranean sea turtles. Journal of Natural History 44:1743–1753DOI 10.1080/00222931003624820.
Gillan DC, Cadee GC. 2000. Iron-encrusted diatoms and bacteria epibiotic on
Hy-drobia ulvae(Gastropoda: Prosobranchia). Journal of Sea Research 43:83–91 DOI 10.1016/S1385-1101(99)00041-6.
Guiry MD, Guiry GM. 2019. AlgaeBase. Galway: World-wide electronic publication.
National University of Ireland. Available athttp:// www.algaebase.org(accessed on 16 December 2019).
Hafner D, Jasprica N, Car A. 2018. Taxonomic survey of benthic diatoms in Neum Bay,
southeastern Adriatic. Natura Croatica 27:1–26DOI 10.20302/NC.2018.27.1.
Hoagland KD, Zlotsky A, Peterson CG. 1986. The source of algal colonizers on rock
substrates in a freshwater impoundment. In: Evans LV, Hoagland KD, eds. Algal
biofouling. Amsterdam: Elsevier, 21–39.
Holmes RW, Nagasawa S, Takano H. 1993a. The morphology and geographic
distri-bution of epidermal diatoms of the Dall’s Porpoise (Phocoenoicies dalli True) in the Northern Pacific Ocean. Bulletin of the National Science Museum Series B 19:1–18.
Holmes RW, Nagasawa S, Takano H. 1993b. A re-examination diatom samples obtained
from cetaceans of collected off South Africa. Bulletin of the National Science Museum
Series B19:127–135.
Kaleli A. 2019. Benthic diatom composition of Iztuzu coastal lake, Dalyan (Aegean Sea,
Turkey). Aquatic Sciences and Engineering 34(4):122–130 DOI 10.26650/ASE2019575987.
Kaleli A, Kociolek J, Solak C. 2020. Taxonomy and distribution of diatoms on the
Turkish Mediterranean Coast, Dalyan (Muğla). Mediterranean Marine Science
21(1):201–215DOI 10.12681/mms.17293.
Kaleli A, Krzywda M, Witkowski A, Riaux-Gobin C, Nadir Solak C, Zgłobicka I, Płociński T, Grzonka J, Kurzydłowski KJ, Car A, Desrosiers C, Kaska Y, Mc-Cartney K. 2018. A new sediment-dwelling and epizoic species of Olifantiella
(Bacillariophyceae), with an account on the genus ultrastructure based on Focused Ion Beam nanocuts. Fottea 18:212–226DOI 10.5507/fot.2018.007.
Kaska Y, Baskale E, Katılmıs Y, Sözbilen D, Azmaz M. 2016. Monitoring and
conserva-tion studies of sea turtles (caretta caretta) during the 2016 nesting season on Muğla Sea Turtle nesting beaches. Denizli: MacArt.
Kitsos M-S, Christodoulou M, Arvanitidis C, Mavidis M, Kirmitzoglou I, Kouk-ouras A. 2005. Composition of the organismic assemblage associated with
Caretta caretta. Journal of the Marine Biological Association of the UK 85:257–261 DOI 10.1017/S0025315405011136h.
Kociolek JP, Balasubramanian K, Blanco S, Coste M, Ector L, Liu Y, Kulikovskiy M, Lundholm N, Ludwig T, Potapova M, Rimet F, Sabbe K, Sala S, Sar E, Taylor J, Van de Vijver B, Wetzel CE, Williams DM, Witkowski A, Witkowski J. 2019.
DiatomBase. Available athttp:// www.diatombase.org (accessed on 24 December 2019).
Komoroske LM, Lewison RL, Seminoff JA, Deheyn DD, Dutton PH. 2011. Pollutants
and health of green sea turtles resident to urbanized estuary in San Diego, CA.
Chemosphere84:544–552DOI 10.1016/j.chemosphere.2011.04.023.
Laurent L, Casale P, Bradai MN, Godley BJ, Gerosa G, Broderick AC, Schroth W, Schierwater B, Levy AM, Freggi D, Abd el-Mawla EM, Hadoud DA, Gomati HE, Domingo M, Hadjichristophorou M, Kornaraki L, Demirayak F, Gautier C. 1998. Molecular resolution of marine turtle stock composition in fishery
bycatch: a case study in the Mediterranean. Molecular Ecology 7:1529–1542 DOI 10.1046/j.1365-294x.1998.00471.x.
Legendre L, Legendre P. 1983. Numerical ecology. In: Developments in environmental
modelling. Amsterdam: Elsevier.
Lima SFB, Lucena RA, Queiroz V, Guimarães CRP, Breves A. 2017. The first finding
of Ostrea cf. puelchana (Bivalvia) living as epibiont on Callinectes exasperates (Decapoda). Acta Scientiarum. Biological Sciences 39:79–85
DOI 10.4025/actascibiolsci.v39i1.33629.
Lutz PL, Musick JA. 1997. The biology of sea turtles Vol I. Boca Raton: CRC Press. Majewska R, Bosak S, Frankovich TA, Ashworth MP, Sullivan MJ, Robinson NJ,
Lazo-Wasem EA, Pinou T, Nel R, Manning SC, Van de Vijver B. 2019. Six new epibiotic
Proschkinia(Bacillariophyta) species and new insights into the genus phylogeny.
European Journal of Phycology 54(4):609–631DOI 10.1080/09670262.2019.1628307.
Majewska R, De Vijver BV, Nasrolahi A, Ehsanpour M, Afkhami M, Bolaños F,
Iamunno F, Santoro M, De Stefano M. 2017b. Shared epizoic taxa and differences in
diatom community structure between Green Turtles (Chelonia mydas) from distant habitats. Microbial Ecology 74:969–978DOI 10.1007/s00248-017-0987-x.
Majewska R, Kociolek JP, Thomas EW, De Stefano M, Santoro M, Bolaños F, Van de Vijver B. 2015a. Chelonicola and Poulinea, two new gomphonemoid diatom genera
(Bacillariophyta) living on marine turtles from Costa Rica. Phytotaxa 233:236–250 DOI 10.11646/phytotaxa.233.3.2.
Majewska R, Santoro M, Bolaños F, Chaves G, De Stefano M. 2015b. Diatoms and other
epibionts associated with Olive Ridley (Lepidochelys olivacea) sea turtles from the Pa-cific Coast of Costa Rica. PLOS ONE 10:e0130351DOI 10.1371/journal.pone.0130351.
Majewska R, De Stefano M, Ector L, Bolaños F, Frankovich TA, Sullivan MJ, Ashworth MP, Van de Vijver B. 2017a. Two new epizoic Achnanthes species (Bacillariophyta)
living on marine turtles from Costa Rica. Botanica Marina 60:303–318.
Majewska R, De Stefano M, Van de Vijver B. 2017. Labellicula lecohuiana, a new epizoic
Miho A, Witkowski A. 2005. Diatom (Bacillariophyta) flora of Albanian coastal wetlands
taxonomy and ecology: a review. Proceedings of the California Academy of Sciences
56:129–145.
Molino PJ, Wetherbee R. 2008. The biology of biofouling diatoms and their role in the
development of microbial slimes. Biofouling 24:365–379 DOI 10.1080/08927010802254583.
Nemoto T. 1956. On the diatoms of the skin film of whales in the northern Pacific. The
Scientific Reports of the Whales Research Institute11:99–132.
Nemoto T. 1958. Cocconeis diatoms infected on whales in the Antarctic. The Scientific
Reports of the Whales Research Institute Tokyo13:185–192.
Pfaller JB, Bjorndal KA, Reich KJ, Williams KL, Frick MG. 2006. Distribution patterns
of epibionts on the carapace of loggerhead turtles, Caretta caretta. Marine Biodiversity
Records1:E36.
Pfaller JB, Frick MG, Reich KJ, Williams KL, Bjorndal KA. 2008. Carapace epibionts of
loggerhead turtles (Caretta caretta) nesting at Canaveral National Seashore, Florida.
Journal of the Natural History 42:1095–1102DOI 10.1080/00222930701877565.
Revelles M, Carreras C, Cardona L, Marco A, Bentivegna F, Castillo JJ, De Martino G, Mons JL, Smith MB, Rico C, Pascual M, Aguilar A. 2007. Evidence for an
asymmetrical size exchange of loggerhead sea turtles between the Mediterranean and the Atlantic through the Straits of Gibraltar. Journal of Experimental Marine Biology
and Ecology349:261–271DOI 10.1016/j.jembe.2007.05.018.
Riaux-Gobin C, Witkowski A, Chevallier D, Daniszewska-Kowalczyk G. 2017a. Two
new Tursiocola species (Bacillariophyta) epizoic on green turtles (Chelonia mydas) in French Guiana and Eastern Caribbean. Fottea 17:150–163DOI 10.5507/fot.2017.007.
Riaux-Gobin C, Witkowski A, Kociolek JP, Ector L, Chevallier D, Compère P. 2017b.
New epizoic diatom (Bacillariophyta) species from sea turtles in the Eastern Caribbean and South Pacific. Diatom Research 32:109–125
DOI 10.1080/0269249X.2017.1299042.
Riaux-Gobin C, Witkowski A, Kociolek JP, Saenz-Agudelo P, Daniszewska-Kowalczyk G, Grzonka J. 2019. Diatom phenotypic plasticity: Olifantiella gorandiana epizoic
on ‘G5-Manahere’ (Society Archipelago, South Pacific), a case study. Phytotaxa
415(2):89–104DOI 10.11646/phytotaxa.415.2.1.
Ribeiro L, Brotas V, Mascarell G, Couté A. 2003. Taxonomic survey of the
microphy-tobenthic communities of two Tagus estuary mudflats. Acta Oecologica 24:117–123 DOI 10.1016/S1146-609X(03)00012-2.
Rivera SF, Vasselon V, Ballorain K, Carpentier A, Wetzel CE, Ector L, Bouchez A, Rimet F. 2018. DNA metabarcoding and microscopic analyses of sea turtles biofilms:
complementary to understand turtle behaviour. PLOS ONE 13(4):e0195770 DOI 10.1371/journal.pone.0195770.
Robert K, Bosak S, Van de Vijver B. 2019. Catenula exigua sp. nov., a new marine
diatom (Bacillariophyta) species from the Adriatic sea. Phytotaxa 414:113–118 DOI 10.11646/phytotaxa.414.2.3.
Robinson NJ, Majewska R, Lazo-Wasem EA, Nel R, Paladino FV, Rojas L, Zardus JD, Pinou T. 2016. Epibiotic diatoms are universally present on all sea turtle species.
PLOS ONE11:1–8.
Round FE, Crawford RM, Mann DG. 1990. The Diatoms; biology & morphology of the
genera. Cambridge: University Press.
Swift E. 1967. Cleaning diatom frustules with ultraviolet radiation and peroxide 1.
Phycologia6:161–163DOI 10.2216/i0031-8884-6-2-161.1.
Türkozan O, Yılmaz C. 2008. Loggerhead turtles, Caretta caretta, at Dalyan Beach,
Turkey: nesting activity (2004–2005) and 19-year abundance trend (1987–2005).
Chelonian Conservation Biology7:178–187DOI 10.2744/CCB-0719.1.
Underwood GJC, Phillips J, Saunders K. 1998. Distribution of estuarine benthic
diatom species along salinity and nutrient gradients. European Journal of Phycology
33:173–183DOI 10.1080/09670269810001736673.
Wahl M. 1989. Marine epibiosis, I. Fouling and antifouling: some basic aspects. Marine
Ecology Progress Series58:175–189DOI 10.3354/meps058175.
Wahl M. 2008. Ecological lever and interface ecology: epibiosis modulates the
interac-tions between host and environment. Biofouling 24:427–438 DOI 10.1080/08927010802339772.
Wetzel CE, Van de Vijver B, Cox EJ, Bicudo DDC, Ector L. 2012. Tursiocola
podocne-micolasp. nov. a new epizoic freshwater diatom species from the Rio Negro in the Brazilian Amazon Basin. Diatom Research 27:1–8DOI 10.1080/0269249X.2011.642498.
Witkowski A, Lange-Bertalot H, Metzeltin D. 2000. Diatom Flora of Marine Coasts I.
In: Lange-Bertalot H, ed. Iconographia Diatomologica 7. Konigstein: Koeltz Scientific Books.