original article
Peginterferon plus Adefovir versus Either
Drug Alone for Hepatitis Delta
Heiner Wedemeyer, M.D., Cihan Yurdaydìn, M.D., George N. Dalekos, M.D., Andreas Erhardt, M.D., Yilmaz Çakaloğlu, M.D., Halil Değertekin, M.D.,
Selim Gürel, M.D., Stefan Zeuzem, M.D., Kalliopi Zachou, M.D., Hakan Bozkaya, M.D., Armin Koch, M.D., Thomas Bock, M.D., Hans Peter Dienes, M.D., and Michael P. Manns, M.D., for the HIDIT Study Group*
From Hannover Medical School, Han nover (H.W., A.K., M.P.M.); Heinrich Heine University, Düsseldorf (A.E.); Johann Wolfgang Goethe University, Frankfurt (S.Z.); Robert Koch Institute, Berlin (T.B.); and University Köln, Cologne (H.P.D.) — all in Germany; the University of Ankara Medical School (C.Y., H.B.) and Ufuk Uni versity Medical School (H.D.), Ankara; Memorial Hospital Istanbul, Istanbul (Y.C.); and University of Uludağ Medical School, Bursa (S.G.) — all in Turkey; and University Larissa, Larissa, Greece (G.N.D., K.Z.). Address reprint requests to Dr. Manns at the Department of Gastroenterology, Hepatology, and Endocrinology, Medizi nische Hochschule Hannover, CarlNeu berg Str. 1., D30625 Hannover, Germany, or at manns.michael@mhhannover.de. Drs. Wedemeyer and Yurdaydìn contrib uted equally to this work.
*The HepNet–International Delta Hepati tis Intervention Trial (HIDIT) Study Group collaborators are listed in the Supplemen tary Appendix, available at NEJM.org. N Engl J Med 2011;364:32231. Copyright © 2011 Massachusetts Medical Society.
Abs tr act
Background
Chronic infection with hepatitis B virus and hepatitis delta virus (HDV) results in the most severe form of viral hepatitis. There is no currently approved treatment. We investigated the safety and efficacy of 48 weeks of treatment with peginterferon alfa-2a plus adefovir dipivoxil, peginterferon alfa-alfa-2a alone, and adefovir dipivoxil alone.
Methods
We conducted a randomized trial in which 31 patients with HDV infection received treatment with 180 μg of peginterferon alfa-2a weekly plus 10 mg of adefovir daily, 29 received 180 μg of peginterferon alfa-2a weekly plus placebo, and 30 received 10 mg of adefovir alone weekly for 48 weeks. Follow-up was conducted for an additional 24 weeks. Efficacy end points included clearance of HDV RNA, normalization of alanine aminotransferase levels, and a decline in levels of hepatitis B surface antigen (HBsAg).
Results
The primary end point — normalization of alanine aminotransferase levels and clear-ance of HDV RNA at week 48 — was achieved in two patients in the group receiving peginterferon alfa-2a plus adefovir and two patients in the group receiving pegin-terferon alfa-2a plus placebo but in none of the patients in the group receiving ad-efovir alone. At week 48, the test for HDV RNA was negative in 23% of patients in the first group, 24% of patients in the second, and none of those in the third (P = 0.006 for the comparison of the first and third groups; P = 0.004 for the com-parison of the second and third). The efficacy of peginterferon alfa-2a was sus-tained for 24 weeks after treatment, with 28% of the patients receiving peginter-feron alfa-2a plus adefovir or peginterpeginter-feron alfa-2a alone having negative results on HDV-RNA tests; none of the patients receiving adefovir alone had negative results. A decline in HBsAg levels of more than 1 log10 IU per milliliter from baseline to week 48 was observed in 10 patients in the first group, 2 in the second, and none in the third (P<0.001 for the comparison of the first and third groups and P = 0.01 for the comparison of the first and second).
Conclusions
Treatment with peginterferon alfa-2a for 48 weeks, with or without adefovir, resulted in sustained HDV RNA clearance in about one quarter of patients with HDV infec-tion. (Funded by Hep-Net [the German Network of Excellence on Viral Hepatitis] and others; Current Controlled Trials number, ISRCTN83587695.)
H
epatitis delta virus (HDV) is a defec-tive RNA virus that requires coinfection with hepatitis B virus (HBV) to replicate. The HDV genome is encapsidated by hepatitis B surface antigen (HBsAg), which forms the viral envelope. The virus was first identified in 1977 in the serum of long-term carriers of HBsAg and was noted to have an increased prevalence among patients with liver damage.1In long-term carriers of HBV, HDV infection can result in fulminant acute hepatitis or severe chronic hepatitis, often progressing to cirrhosis and hepatocellular carcinoma.2-6 Eight HDV ge-no types have been suggested,7,8 and both HDV and HBV genotypes affect the clinical outcome.9,10 Infection with HDV genotype 1, the most preva-lent genotype in Europe, the Middle East, North America, and North Africa, is associated with more severe disease than infection with geno-type 2.9,11 Although the prevalence of HDV in-fection in southern Europe has declined since the introduction of vaccination against HBV,3,12 HDV infection — and HDV-related disease — con-tinues to be a major health problem in several regions of the world.13 Immigration to central Europe from areas where HDV is highly endemic has put a substantial burden on that part of the continent, where 8 to 14% of patients infected with HBV are reported to be positive for anti-HDV antibodies.13-15
There is currently no approved therapy for infection with HDV. Oral antiviral agents, in-cluding ribavirin, lamivudine, and famciclovir, are ineffective against it.16-19 Clinical data for the more potent oral antiviral agents widely used for the treatment of chronic HBV infection, such as adefovir dipivoxil, entecavir, telbivudine, and tenofovir, are currently lacking. The effective-ness of treatment of HDV infection with inter-feron alfa is limited by the need for prolonged administration and high doses that are poorly tolerated. However, high-dose interferon alfa-2a therapy has been shown to reduce levels of ala-nine aminotransferase and HDV RNA20 and to be associated with improved long-term clinical outcomes and survival.21 The addition of lamivu-dine or ribavirin to interferon-based therapy has not improved efficacy, as compared with the use of interferon alone.19,22-24
Pegylated interferon (peginterferon) has been shown to be more beneficial than conventional interferon in the treatment of both chronic
hepa-titis C25 and hepatitis B.26 Three pilot studies using peginterferon alfa-2b (1.5 μg per kilogram of body weight per week) showed a sustained response to treatment (defined as un detectable levels of HDV RNA 6 months after the end of treatment) in 17 to 43% of patients treated for periods of 48 to 72 weeks.27-29 Adefovir dipivoxil has been reported to have an effect on levels of covalently closed circular DNA (cccDNA) within infected hepatocytes in HBV infection; cccDNA acts as a template for HBsAg production.30 A de-crease in the HBsAg level may deprive HDV of the helper function of the HBV envelope protein and may therefore provide a benefit. The aim of the current study was to investigate the safety and efficacy of peginterferon alfa-2a plus adefovir dipivoxil, as compared with either drug alone, in patients with chronic HDV infection.
Methods Study Protocol and Oversight
The Hep-Net–International Delta Hepatitis Inter-vention Trial (HIDIT) was an investigator-initiat-ed, randomizinvestigator-initiat-ed, controlled trial that was ap-proved by the German Network of Excellence on Viral Hepatitis (Hep-Net) and operated under its auspices.31 F. Hoffmann–La Roche and Gilead Sciences provided financial support for the study and approved the study design proposed by the investigators. The authors vouch for the veracity and completeness of the data and data analyses. The manuscript was written by the first, second, and last authors with the assistance of a repre-sentative of Elements Communication. Writing assistance was funded by F. Hoffmann–La Roche. Patients were recruited between March 2004 and September 2006, and data collection and analysis were completed in August 2009. The study was approved by the ethics committee at each participating institution. Written informed consent was obtained from each patient. Study Population
Patients between 18 and 70 years of age who had HDV infection were eligible for inclusion if they had compensated liver disease, had been positive for HBsAg for at least 6 months and positive for anti-HDV antibodies for at least 3 months, and were positive for HDV RNA on polymerase-chain-reaction assay. A description of the eligibility cri-teria can be found in the Supplementary
Appen-dix and in the study protocol, both available with the full text of this article at NEJM.org. The study was conducted in accordance with the protocol. Study Design
Patients were stratified according to country and presence or absence of a history of interferon treatment before undergoing randomization to one of three treatment groups, and they received the assigned medication for 48 weeks. The first group included a total of 31 patients who re-ceived peginterferon alfa-2a (180 μg once week-ly) plus adefovir (10 mg daiweek-ly), the second group included a total of 29 patients who received peg-interferon alfa-2a (180 μg once weekly) plus pla-cebo, and the third had a total of 30 patients who received adefovir (10 mg daily) alone. Assess-ments for virologic end points were made at a central laboratory at weeks 24 and 48 (end of treatment), with a final clinic visit at week 72 (24 weeks after the end of treatment). At each clinic visit, all patients underwent a complete physical examination and safety assessment, and blood was taken for biochemical and virologic analysis. Safety and Efficacy
Safety was assessed by the study investigators on the basis of adverse events reported spontane-ously by study participants. In addition, plasma samples were routinely assessed for hematologic variables (e.g., leukocyte and platelet counts and levels of creatinine, alanine aminotransferase, as-partate aminotransferase, γ-glutamyl transferase, and bilirubin).
The primary end point was the achievement of undetectable levels of HDV RNA and normal levels of alanine aminotransferase at week 48. Secondary end points were based on HDV RNA levels and alanine aminotransferase levels at week 48 and week 72; levels of HBV DNA, ala-nine aminotransferase, and HBsAg through week 72; and histologic analysis (evaluation of biopsy specimens for fibrosis and histologic activity). Outcome measures are described in detail in the Supplementary Appendix.
Statistical Analysis
Detailed information on the procedures used for the statistical analysis is available in the Supple-mentary Appendix. In brief, an intention-to-treat efficacy analysis was performed for all patients receiving at least one dose of the study medica-tion (i.e., 90 patients). Patients with missing
val-ues were categorized as not having had a re-sponse. Comparisons of specific variables were made with the use of analysis of variance or the Kruskal–Wallis test when appropriate, with P val-ues of less than 0.05 considered to indicate sta-tistical significance. Continuous variables were evaluated with the use of a t-test or a nonpara-metric test.
R esults Characteristics of the Patients
Demographic and baseline clinical characteris-tics of the patients in the three treatment groups are shown in Table 1 and are described in further detail in the Supplementary Appendix.
Adverse Events
Of the 90 patients included in the study, 10 with-drew (5 in the group receiving peginterferon alfa-2a plus adefovir dipivoxil, 3 in the group receiv-ing peginterferon alfa-2a plus placebo, and 2 in the group receiving adefovir alone). Six of the withdrawals were due to disease progression (in-cluding 1 death from intraperitoneal bleeding as a result of hepatocellular carcinoma, 1 case of hepatic decompensation [with the patient placed on the waiting list for liver transplantation], and 1 hospitalization because of suspected hepato-cellular carcinoma); 2 patients left the country, 1 patient did not comply with the treatment regi-men, and 1 patient decided to withdraw because of severe malaise from interferon alfa–related ad-verse events.
The remaining 80 patients (89%) completed the study. Overall, 66 patients (73%) reported a total of 318 adverse events, which were more common in patients treated with peginterferon alfa-2a than in patients receiving adefovir (Table 2). The ad-verse events were those typically associated with interferon alfa or adefovir treatment, and no un-expected adverse events occurred. The 17 serious adverse events that occurred in the study are de-scribed in detail in the Supplementary Appendix. Outcomes
The primary outcome of the intention-to-treat analysis (normal serum levels of alanine amino-transferase and undetectable levels of HDV RNA at week 48) occurred in two patients (7%) receiv-ing peginterferon alfa-2a plus adefovir, two (7%) receiving peginterferon alfa-2a plus placebo, and none receiving adefovir alone (P = 0.16 for
pegin-terferon alfa-2a plus adefovir vs. adefovir dipivox-il, P = 0.14 for peginterferon alfa-2a plus placebo vs. adefovir dipivoxil, and P = 0.95 for peginter-feron 2a plus adefovir vs. peginterpeginter-feron alfa-2a plus placebo).
HDV RNA
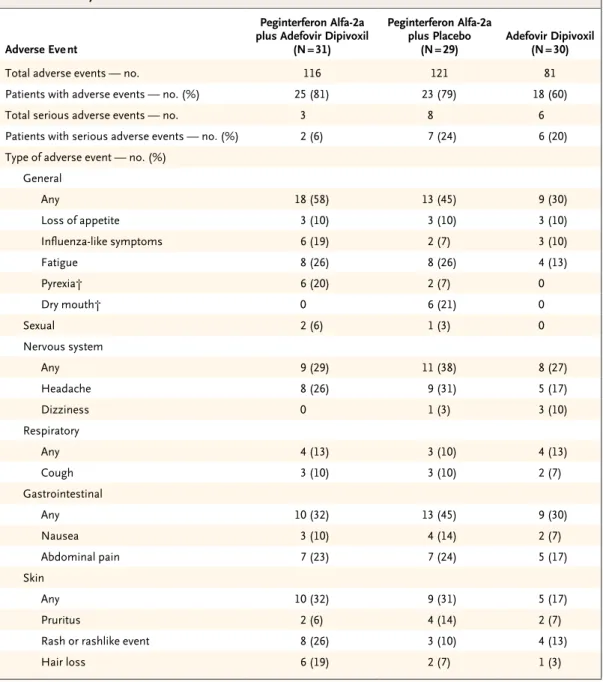
At week 48, test results for HDV RNA were nega-tive in 7 of the 31 patients (23%) treated with peginterferon alfa-2a plus adefovir dipivoxil, 7 of the 29 patients (24%) treated with peginterferon alfa-2a plus placebo, and none of the 30 patients treated with adefovir alone (P = 0.006 for pegin-terferon alfa-2a plus adefovir vs. adefovir dipiv ox-il, and P = 0.004 for peginterferon alfa-2a plus pla-cebo vs. adefovir dipivoxil) (Fig. 1A). At week 72, tests for HDV RNA were negative in 8 (26%) of the patients treated with peginterferon alfa-2a plus adefovir dipivoxil, 9 (31%) of those treated with peginterferon alfa-2a plus placebo, and none of those treated with adefovir alone (P = 0.006 for peginterferon alfa-2a plus adefovir vs. adefovir dipivoxil, and P = 0.004 for peginterferon alfa-2a
plus placebo vs. adefovir dipivoxil) (Fig. 1A). Over-all, the proportion of patients treated with pegin-terferon alfa-2a (with or without adefovir dipiv-oxil) who had HDV-RNA clearance was 23% (14 of 60) at week 48 and 28% (17 of 60) at week 72.
There was a reduction from baseline in me-dian HDV RNA levels in both of the groups re-ceiving peginterferon alfa-2a (P<0.001 for weeks 24 and 48, and P = 0.02 and 0.03, respectively, for week 72) (Fig. 1B). A reduction in median HDV RNA levels of more than 2 log10 copies per mil-liliter from baseline to week 72 was observed in 8 (26%) of the patients treated with peginter-feron alfa-2a plus adefovir dipivoxil, 9 (31%) of those treated with peginterferon alfa-2a plus placebo, and none of those treated with adefovir alone (P = 0.003 for peginterferon alfa-2a plus adefovir vs. adefovir dipivoxil, and P = 0.001 for peginterferon alfa-2a plus placebo vs. adefovir dipivoxil). Only 2 patients had a virologic relapse, with the reappearance of HDV RNA, after the end of treatment (1 patient in each peginterferon alfa-2a group), whereas 9 patients (4 treated with
Table 1. Baseline Characteristics of the Study Participants.*
Characteristic
Peginterferon Alfa-2a plus Adefovir Dipivoxil
(N = 31) Peginterferon Alfa-2a plus Placebo (N = 29) Adefovir Dipivoxil(N = 30) Age — yr Median 42 38 33 Range 23–59 17–62 21–55
Male sex — no. (%) 20 (65) 17 (59) 19 (63)
HBeAgpositive — no. (%) 5 (16) 5 (17) 4 (13)
HBV DNA
Median — log10 IU/ml 1.4‡ 2.6 2.1
≥100 IU/ml — no./total no. (%) 13/31 (42) 17/27 (63) 15/28 (54)
HDV RNA
Median — log10 copies/ml 6.3 5.9 5.7
Above median — no./total no. (%) 16/28 (57) 12/23 (52) 11/26 (42)
Alanine aminotransferase
Median — IU/liter 88 73 111
Above median — no./total no. (%) 12/31 (39) 11/27 (41) 19/29 (66)
Cirrhosis — no./total no. (%) 4/29 (14) 5/25 (20) 7/29 (24)
HDV genotype 1 — % 100 100 100
Previous interferon treatment — no. (%) 12 (38) 15 (52) 12 (40)
* HBeAg denotes hepatitis B e antigen, HBV hepatitis B virus, and HDV hepatitis delta virus.
† P = 0.01 for the comparison of adefovir dipivoxil with peginterferon alfa2a plus adefovir, and P = 0.04 for the compari son of adefovir with peginterferon alfa2a plus placebo.
peginterferon alfa-2a plus adefovir and 5 treated with peginterferon alfa-2a plus placebo) became negative for HDV RNA during follow-up. No follow-up data on HDV RNA were available for 4 patients who were negative for HDV RNA at the end of treatment.
Alanine Aminotransferase
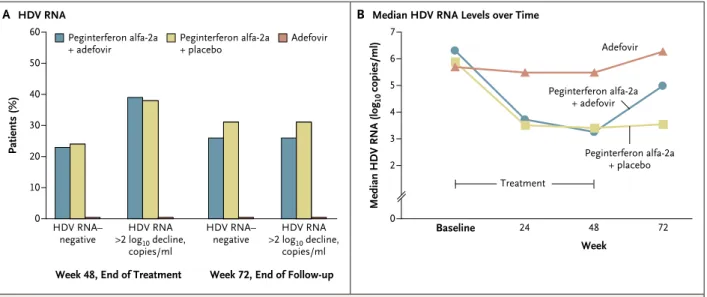
At week 48, the level of alanine aminotransferase was normal in 10 of 31 patients (32%) receiving peginterferon alfa-2a plus adefovir dipivoxil, 8 of 29 (28%) receiving peginterferon alfa-2a plus
pla-cebo, and 2 of 30 (7%) receiving adefovir alone (P = 0.01 for peginterferon alfa-2a plus adefovir vs. adefovir dipivoxil, and P = 0.03 for peginter-feron alfa-2a plus placebo vs. adefovir dipivoxil) (Fig. 2A). At week 72, a total of 11 patients (35%) receiving peginterferon alfa-2a plus adefovir and 13 patients (45%) receiving peginterferon alfa-2a plus placebo had normal alanine aminotransfer-ase levels, as compared with 3 patients (10%) re-ceiving adefovir alone (P = 0.02 for peginterferon alfa-2a plus adefovir vs. adefovir dipivoxil, and P = 0.003 for peginterferon alfa-2a plus placebo
Table 2. Summary of Adverse Events and Incidence of Most Common Adverse Events.*
Adverse Eve nt
Peginterferon Alfa-2a plus Adefovir Dipivoxil
(N = 31)
Peginterferon Alfa-2a plus Placebo
(N = 29) Adefovir Dipivoxil(N = 30)
Total adverse events — no. 116 121 81
Patients with adverse events — no. (%) 25 (81) 23 (79) 18 (60)
Total serious adverse events — no. 3 8 6
Patients with serious adverse events — no. (%) 2 (6) 7 (24) 6 (20)
Type of adverse event — no. (%) General Any 18 (58) 13 (45) 9 (30) Loss of appetite 3 (10) 3 (10) 3 (10) Influenzalike symptoms 6 (19) 2 (7) 3 (10) Fatigue 8 (26) 8 (26) 4 (13) Pyrexia† 6 (20) 2 (7) 0 Dry mouth† 0 6 (21) 0 Sexual 2 (6) 1 (3) 0 Nervous system Any 9 (29) 11 (38) 8 (27) Headache 8 (26) 9 (31) 5 (17) Dizziness 0 1 (3) 3 (10) Respiratory Any 4 (13) 3 (10) 4 (13) Cough 3 (10) 3 (10) 2 (7) Gastrointestinal Any 10 (32) 13 (45) 9 (30) Nausea 3 (10) 4 (14) 2 (7) Abdominal pain 7 (23) 7 (24) 5 (17) Skin Any 10 (32) 9 (31) 5 (17) Pruritus 2 (6) 4 (14) 2 (7)
Rash or rashlike event 8 (26) 3 (10) 4 (13)
vs. adefovir dipivoxil) (Fig. 2A). After the end of treatment, mean alanine aminotransferase levels increased slightly in the two groups receiving ad-efovir (Fig. 2B).
HBV DNA
Median HBV DNA levels decreased until week 48 and rebounded at week 72 in all three treat-ment groups (for details, see the Suppletreat-mentary Appendix).
HBsAg
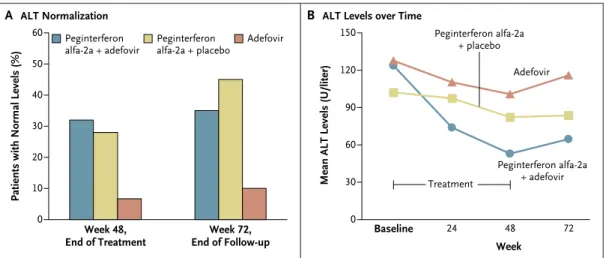
HBsAg levels showed a marked decline only in patients receiving combination therapy, with a median decline of 0.89 log10 IU per milliliter from baseline to week 48 (P = 0.002) and 1.12 log10 IU per milliliter from baseline to week 72 (P<0.001). In contrast, there was no substantial effect on median HBsAg levels in patients treated with peginterferon alfa-2a plus placebo or adefo-vir alone, and levels remained largely stable in these two groups throughout therapy and during follow-up. A decline of more than 1 log10 IU per milliliter in HBsAG levels from baseline to week 48 was observed in 10 patients treated with peg-in ter fer on alfa-2a plus adefovir dipivoxil, 2 pa-tients treated with peginterferon alfa-2a plus pla-cebo, and none treated with adefovir (Fig. 3) (P<0.001 for peginterferon alfa-2a plus adefovir
vs. adefovir, and P = 0.02 for peginterferon alfa-2a plus adefovir vs. peginterferon alfa-2a plus pla-cebo). In total, 2 patients treated with peginter-feron alfa-2a plus adefovir had clearance of HBsAg by week 72; both had seroconversion to anti-HBsAg antibodies. Patients older than 38 years (median age) were more likely to have a decline in HBsAg of more than 1 log10 IU per milliliter at week 72 if they had received treatment with peg-in ter fer on alfa-2a (29% vs. 8%, P = 0.052), but no association was found between a decline in HBsAg and sex, status with respect to alanine aminotransferase, HBV DNA, HDV RNA, HBeAg, histologic grade, or stage.
Histologic Analysis
Specimens from biopsies performed before and after treatment were available for a total of 59 patients (65% of all treated patients). Analysis of paired specimens revealed approximately equal distributions of patients with deterioration, those with improvement, and those with no change in each treatment group (for details, see the Supple-mentary Appendix).
Discussion
Our study showed that a 48-week course of peg-in ter fer on alfa-2a, alone or peg-in combpeg-ination with
Table 2. (Continued.)
Adverse Eve nt
Peginterferon Alfa-2a plus Adefovir Dipivoxil
(N = 31) Peginterferon Alfa-2a plus Placebo (N = 29) Adefovir Dipivoxil(N = 30) Psychiatric 2 (6) 5 (17) 1 (3) Insomnia 2 (6) 4 (14) 1 (3) Musculoskeletal Any‡ 11 (35) 16 (55) 1 (3) Myalgia 7 (23) 8 (26) 1 (3) Arthralgia 4 (13) 6 (21) 0 Circulatory Any 8 (26) 3 (10) 3 (10) Neutropenia 3 (10) 2 (7) 0 Thrombocytopenia 5 (16) 2 (7) 0
* The adverse events listed are those that were reported by at least three patients in at least one of the three study groups. The term “serious adverse event” is defined in the protocol, available at NEJM.org.
† For these events there was a significant difference in the number per study group (P = 0.03 for pyrexia and 0.01 for dry mouth, calculated with the use of chisquare analysis).
‡ P = 0.004 for the comparison of the groups receiving peginterferon alfa2a, with or without adefovir dipivoxil, with the group receiving adefovir dipivoxil alone.
adefovir dipivoxil, significantly reduced HDV RNA levels, with 28% of patients receiving the drug having clearance of HDV RNA 24 weeks af-ter the end of treatment. In contrast, treatment with adefovir dipivoxil alone had no appreciable effect on HDV replication; none of the patients who received this treatment had clearance of HDV RNA, and none had a decline from baseline HDV RNA levels of more than 2 log10 copies per milliliter. The predefined primary end point of the study, normal serum levels of alanine amino-transferase and undetectable HDV RNA at week 48, was achieved in few patients — 7% of those treated with peginterferon alfa-2a, with or with-out adefovir dipivoxil, and none of those treated with adefovir dipivoxil alone. Since alanine ami-notransferase levels frequently remain elevated during treatment with peginterferon alfa,25 this finding was not completely unexpected. On the other hand, levels of alanine aminotransferase normalized in several patients in our study, who nonetheless remained positive for HDV RNA, a finding also reported in the pilot studies of pe-ginterferon alfa-2b.29
Clearance of HDV RNA in patients treated with interferon has been reported previously.
Considerable decreases in HDV RNA levels have been reported after 1 year of treatment with high-dose interferon alfa (9 million units three times a week),20 results that were associated with long-term improvements in hepatic func-tion and histologic findings.21 A number of case reports and reports on small studies have noted reductions in or clearance of HDV RNA as a re-sult of treatment with peginterferon.27-29,32 With regard to efficacy, the results of our larger study are in general accordance with these reports.
In contrast with the findings for peginter-feron in the treatment of HDV and HBV infec-tion, we observed that in several patients, HDV RNA cleared between the end of treatment and follow-up 24 weeks later. Earlier studies also showed that a decrease in HDV RNA levels may occur late in the course of therapy, and in some cases even after the end of treatment, with both conventional interferon and peginterferon.27-29 Further studies are needed to determine whether patients with a slow response to treatment who have clinically significant declines in HDV RNA levels during treatment without becoming HDV RNA–negative would benefit from a longer pe-riod of treatment.33 Patients (%) 60 40 30 10 0 50 20 HDV RNA–
negative >2 logHDV RNA10 decline,
copies/ml
HDV RNA–
negative >2 logHDV RNA10 decline,
copies/ml
B Median HDV RNA Levels over Time A HDV RNA
Peginterferon alfa-2a
+ adefovir Peginterferon alfa-2a+ placebo Adefovir
Week 48, End of Treatment Week 72, End of Follow-up
Median HDV RNA (log
10 copies/ml) 7 5 4 2 0 6 3 24 48 72 Adefovir Baseline Week Peginterferon alfa-2a + adefovir Peginterferon alfa-2a + placebo Treatment
Figure 1. Virologic Response to Treatment as Determined by Serum Level of HDV RNA, According to Treatment Group.
Panel A shows the percentage of patients with a decline in hepatitis delta virus (HDV) RNA of at least 2 log10 copies per milliliter or
clearance of HDV RNA at the end of treatment (week 48) or followup (week 72). Panel B shows the change in median HDV RNA levels over time. In both groups receiving peginterferon alfa2a, HDV RNA levels were significantly lower at week 48 than at baseline (P<0.001 for both groups). At week 72, HDV RNA levels were slightly reduced as compared with baseline in both these groups (P = 0.02 for the group receiving peginterferon alfa2a plus adefovir dipivoxil, and P = 0.03 for the group receiving peginterferon alfa2a plus placebo), but levels were increased in the group receiving adefovir dipivoxil alone (P = 0.02).
In line with earlier studies of the use of oral anti-HBV agents, monotherapy with adefovir dipivoxil had no appreciable effect on HDV RNA levels in our study, and none of the patients treated with adefovir alone had clearance of HDV RNA. Currently, we would not recommend the use of nucleoside or nucleotide monotherapy for HDV infection in patients with suppressed or very low HBV replication.13
The clearance of HBsAg is considered to be the closest outcome to a cure in chronic HBV infection and is therefore recognized as the ideal end point of therapy.34 Since the virus needs HBsAg to produce infectious viral parti-cles, reducing HBsAg levels, or even achieving clearance of HBsAg, is of considerable relevance in the treatment of infection with HBV and HDV. Patients with chronic HBV infection in whom a sustained virologic response to interferon-based therapy is achieved have an increased likelihood of HBsAg clearance during long-term follow up.35,36 HBsAg clearance is not generally seen in HBeAg-negative patients treated with nucleoside or nucleotide analogues, including lamivudine, entecavir, and telbivudine.32,37,38 In our study, the combination of peginterferon alfa-2a and adefovir resulted in a more pronounced decrease in serum HBsAg levels than did either agent
alone; approximately one third of patients re-ceiving combination therapy had a decline in the HBsAg level of at least 1 log10 IU per milliliter. The apparently higher rate of HBsAg decline in patients receiving combination therapy may be related to differences in patient characteristics or to the mechanisms of action or the potency of adefovir as compared with lamivudine.
In the current study, the absence of any appar-ent differences in histologic scores in analyses of paired biopsy samples may reflect the shorter follow-up period in our study, as compared with that in previous studies, or sample bias due to the small numbers of patients with paired biopsy samples. More patients in the combination-therapy group had a worsening of fibrosis scores and a higher number of patients receiving peginter-feron alfa-2a alone had a worsening of scores for histologic activity. It is noteworthy that biopsies were performed at the end of treatment, rather than 24 weeks after the end of treatment, as has been the case in most trials investigating the use of peginterferon alfa-2a for hepatitis B and C. Thus, more data on the results of biopsies per-formed at follow-up after the completion of treat-ment would be needed to answer the question of whether peginterferon alfa-2a worsens certain histologic features of infection with HDV.
Patients with Normal Levels (%)
60 40 30 10 0 50 20
B ALT Levels over Time A ALT Normalization
Week 48,
End of Treatment End of Follow-upWeek 72,
Mean ALT Levels (U/liter)
150 90 60 0 120 30 24 48 72 Adefovir Baseline Week Peginterferon alfa-2a + adefovir Peginterferon alfa-2a + placebo Treatment Peginterferon
alfa-2a + adefovir Peginterferonalfa-2a + placebo Adefovir
Figure 2. Biochemical Response to Treatment as Determined by Serum Level of Alanine Aminotransferase, According to
Treatment Group.
Panel A shows the percentage of patients who had normal levels of alanine aminotransferase (ALT) at week 48 or week 72. Panel B shows the change in mean levels of ALT over time. ALT levels were significantly lower than base line values at week 48 in patients treated with peginterferon alfa2a plus adefovir dipivoxil (P = 0.008) and in those treated with adefovir dipivoxil alone (P = 0.002). At week 72, ALT levels were not significantly different from baseline values in all three treatment groups.
The withdrawal rate for the patients in our study (all of whom had HDV infection) who were treated with peginterferon alfa-2a was 11% — a rate that is not dissimilar to that in large-scale studies of peginterferon alfa-2a in the treatment of chronic HBV monoinfection.39 The one patient in our study who had hepatic decompensation during treatment with peginterferon alfa-2a had a baseline platelet count of 74,000 per cubic mil-limeter after an initial screening count of 75,000 per cubic millimeter, which was in line with the study inclusion criteria. Thus, we would recom-mend caution in the use of peginterferon alfa-2a therapy for patients whose platelet counts are below 90,000 per cubic millimeter.
In conclusion, treatment with peginterferon alfa-2a for 48 weeks resulted in sustained HDV RNA clearance in more than 25% of patients and a sustained biochemical response in 40%.
Supported by Hep-Net (a national network sponsored by the German Ministry for Education and Research, BMBF-Förderkenn-zeichen), F. Hoffmann–La Roche, and Gilead Sciences.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank Hep-Net members U. Drebber (pathology), B. Bremer, P. Magerstedt, and R. Raupach (virology), T. Müller and K. Peter (study management team), U. Alshuth of F. Hoffmann– La Roche, and C. Fischer of Gilead Sciences for their contribu-tions to the study; and K. Searle for support in writing and edit-ing an earlier version of the manuscript.
Patients (%) 60 40 30 10 0 50 20
HBsAg-negative >1 logHBsAg10 IU/µl
decline
HBsAg-negative >1 logHBsAg10 IU/µl
decline
B HBsAg Levels over Time A HBsAg
Week 48, End of Treatment Week 72, End of Follow-up
Median HBsAg (log
10 IU/ml) 5 4 2 0 3 24 48 72 Baseline Week Treatment Peginterferon alfa-2a
+ adefovir Peginterferon alfa-2a+ placebo Adefovir
Adefovir
Peginterferon alfa-2a + adefovir Peginterferon alfa-2a
+ placebo
Figure 3. Change in Levels of Hepatitis B Surface Antigen According to Treatment Group.
Panel A shows the percentage of patients in each treatment group who had levels of hepatitis B surface antigen (HBsAg) that declined by more than 1 log10 IU per milliliter or in whom HBsAg clearance was achieved at week 48 or week 72. Panel B shows the change from
baseline in median levels of HBsAg over time. The decline in patients treated with peginterferon alfa2a plus adefovir dipivoxil was sig nificant at week 48 and week 72 (P = 0.002 for week 48 and P<0.001 for week 72).
References
1. Rizzetto M, Canese MG, Aricò S, et al. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977; 18:997-1003.
2. Rizzetto M. Hepatitis D: virology, clin-ical and epidemiologclin-ical aspects. Acta Gastroenterol Belg 2000;63:221-4.
3. Değertekin H, Yalçın K, Yakut M. The prevalence of hepatitis delta virus infec-tion in acute and chronic liver diseases in Turkey: an analysis of clinical studies. Turk J Gastroenterol 2006;17:25-34.
4. Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis
delta infection, a risk factor for cirrhosis and hepatocellular carcinoma. Gastroen-terology 2009;136:1629-38.
5. Sheng WH, Hung CC, Kao JH, et al. Impact of hepatitis D virus infection on the long-term outcomes of patients with hepatitis B virus and HIV coinfection in the era of highly active antiretroviral ther-apy: a matched cohort study. Clin Infect Dis 2007;44:988-95.
6. Castellares C, Barreiro P, Martín-Carbonero L, et al. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat 2008; 15:165-72.
7. Radjef N, Gordien E, Ivaniushina V,
et al. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J Virol 2004;78:2537-44.
8. Makuwa M, Caron M, Souquière S, Malonga-Mouelet G, Mahé A, Kazanji M. Prevalence and genetic diversity of hepati-tis B and delta viruses in pregnant women in Gabon: molecular evidence that hepati-tis delta virus clade 8 originates from and is endemic in central Africa. J Clin Micro-biol 2008;46:754-6.
9. Su CW, Huang YH, Huo TI, et al. Geno-types and viremia of hepatitis B and D viruses are associated with outcomes of
mynejminthejournalonline
Individual subscribers can store articles and searches using a feature on the Journal’s Web site (NEJM.org) called “My NEJM.” Each article and search result links to this feature. Users can create personal folders and move articles into them for convenient retrieval later. chronic hepatitis D patients.
Gastroenter-ology 2006;130:1625-35.
10. Kiesslich D, Crispim MA, Santos C, et al. Influence of hepatitis B virus (HBV) genotype on the clinical course of disease in patients coinfected with HBV and hep-atitis delta virus. J Infect Dis 2009;199: 1608-11.
11. Shakil AO, Hadziyannis S, Hoofnagle JH, Di Bisceglie AM, Gerin JL, Casey JL. Geographic distribution and genetic vari-ability of hepatitis delta virus genotype I. Virology 1997;234:160-7.
12. Gaeta GB, Stroffolini T, Chiaramonte M, et al. Chronic hepatitis D: a vanishing disease? An Italian multicenter study. Hepatology 2000;32:824-7.
13. Wedemeyer H, Manns MP. Epidemiol-ogy, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol 2010;7:31-40.
14. Wedemeyer H, Heidrich B, Manns MP. Hepatitis D virus infection — not a van-ishing disease in Europe! Hepatology 2007;45:1331-2.
15. Heidrich B, Deterding K, Tillmann HL, Raupach R, Manns MP, Wedemeyer H. Virological and clinical characteristics of delta hepatitis in Central Europe. J Viral Hepat 2009;16:883-94.
16. Garripoli A, Di Marco V, Cozzolongo R, et al. Ribavirin treatment for chronic hepatitis D: a pilot study. Liver 1994;14: 154-7.
17. Yurdaydin C, Bozkaya H, Gürel S, et al. Famciclovir treatment of chronic delta hepatitis. J Hepatol 2002;37:266-71.
18. Niro GA, Ciancio A, Tillman HL, et al. Lamivudine therapy in chronic delta hepa-titis: a multicentre randomized-controlled pilot study. Aliment Pharmacol Ther 2005; 22:227-32.
19. Yurdaydin C, Bozkaya H, Onder FO, et al. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + inter-feron vs interinter-feron. J Viral Hepat 2008; 15:314-21.
20. Farci P, Mandas A, Coiana A, et al. Treatment of chronic hepatitis D with in-terferon alfa-2a. N Engl J Med 1994;330: 88-94.
21. Farci P, Roskams T, Chessa L, et al. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenter-ology 2004;126:1740-9.
22. Gunsar F, Akarca US, Ersoz G, et al. Two-year interferon therapy with or with-out ribavirin in chronic delta hepatitis. Antivir Ther 2005;10:721-6.
23. Wolters LM, van Nunen AB, Honkoop P, et al. Lamivudine–high dose interferon combination therapy for chronic hepatitis B patients co-infected with the hepatitis D virus. J Viral Hepat 2000;7:428-34.
24. Kaymakoglu S, Karaca C, Demir K, et al. Alpha interferon and ribavirin com-bination therapy of chronic hepatitis D. Antimicrob Agents Chemother 2005;49: 1135-8.
25. Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006;55: 1350-9.
26. Cooksley WG, Piratvisuth T, Lee SD, et al. Peginterferon alpha-2a (40 kDa): an advance in the treatment of hepatitis B e antigen-positive chronic hepatitis B. J Viral Hepat 2003;10:298-305.
27. Erhardt A, Gerlich W, Starke C, et al. Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver Int 2006;26:805-10.
28. Castelnau C, Le Gal F, Ripault MP, et al. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepa-tology 2006;44:728-35.
29. Niro GA, Ciancio A, Gaeta GB, et al. Pegylated interferon alpha-2b as mono-therapy or in combination with ribavirin in chronic hepatitis delta. Hepatology 2006; 44:713-20.
30. Werle-Lapostolle B, Bowden S, Locar-nini S, et al. Persistence of cccDNA during
the natural history of chronic hepatitis B and decline during adefovir dipivoxil ther-apy. Gastroenterology 2004;126:1750-8.
31. Manns MP, Meyer S, Wedemeyer H. The German network of excellence for vi-ral hepatitis (Hep-Net). Hepatology 2003; 38:543-4.
32. Ferenci P, Formann E, Romeo R. Suc-cessful treatment of chronic hepatitis D with a short course of peginterferon alfa-2a. Am J Gastroenterol 2005;100:1626-7.
33. Farci P, Chessa L, Balestrieri C, Serra G, Lai ME. Treatment of chronic hepatitis D. J Viral Hepat 2007;14:Suppl 1:58-63.
34. European Association for the Study of the Liver. EASL Clinical Practice Guide-lines: management of chronic hepatitis B. J Hepatol 2009;50:227-42.
35. Buster EH, Flink HJ, Cakaloglu Y, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg positive patients treated with peginterferon alpha 2b. Gastroenterology 2008;135:459-67.
36. Moucari R, Korevaar A, Lada O, et al. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B pa-tients responding to interferon: a long-term follow-up study. J Hepatol 2009;50: 1084-92.
37. Lai C-L, Shouval D, Lok AS, et al. Ente-cavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006;354:1011-20. [Erratum, N Engl J Med 2006;354:1863.]
38. Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus ade-fovir dipivoxil for chronic hepatitis B. N Engl J Med 2008;359:2442-55.
39. Marcellin P, Lau GKK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in pa-tients with HBeAg-negative chronic hepa-titis B. N Engl J Med 2004;351:1206-17. Copyright © 2011 Massachusetts Medical Society.