Encapsulation of lipase using magnetic
fluorescent calix[4]arene
derivatives; improvement of enzyme activity and stability
Elif Ozyilmaz
a, Sevilay Cetinguney
b, Mustafa Yilmaz
b,⁎
aDepartment of Biochemistry, Selcuk University, 42075 Konya, Turkey
bDepartment of Chemistry, Selcuk University, 42075 Konya, Turkey
a b s t r a c t
a r t i c l e i n f o
Article history: Received 21 January 2019
Received in revised form 26 April 2019 Accepted 27 April 2019
Available online 28 April 2019
In this study, iron magnetic nanoparticles capped withfluorescent calixarene derivatives (Fe3O4@Calix-2 and
Fe3O4@Calix-3) were prepared in one-step using coprecipitation of Fe(II) and Fe(III) in basic solution. Different
techniques were used to characterize the synthesized magnetic nanoparticles, such as Fourier Transform Infrared spectroscopy (FTIR), powder X-ray diffraction (XRD), vibrating sample magnetometer (VSM) and confocal mi-croscopy. Candida rugosa was encapsulated on synthesized nanoparticles following sol–gel method. It has been observed that under the optimum conditions, the activity of encapsulated lipase (Fe3O4@Calix-2E) was
119 U/g of support which is 4.1 times more that of the encapsulated lipase without calix[4]arene derivative (Fe3O4@E). Comparative study show that the encapsulated lipase on nanoparticles has higher thermal and
oper-ational stability than encapsulated lipase without calix[4]arene derivative. Among these encapsulated lipase nanoparticles, Fe3O4@Calix-2 was capable of effectively catalyze hydrolysis of racemic Flurbiprofen methyl
ester with high conversion of 49% and substrate enantiomeric excess (ees) of 85% at optimum pH and tempera-ture. The efficiency of these nanoparticles was assessed by their reusability, for that after five consecutive oper-ational uses these encapsulated lipase nanoparticles retained their conversion ratios up to 38% and 30% respectively, in the hydrolysis of (R,S)-Flurbiprofen methyl ester. The results showed that encapsulated lipases nanoparticle with calixarene moieties lead to increased activity, stability, reusability and enhanced stereoselectivity in kinetic resolution.
© 2019 Elsevier B.V. All rights reserved.
Keywords: Calixarene Fluorescent Fe3O4nanoparticles Lipase encapsulation Flurbiprofen 1. Introduction
Flurbiprofen is a non-steroidal anti-inflammatory drug used to cure osteoarthritis and rheumatoid arthritis, whose activity resides in S-enantiomer and cyclooxygenase (COX) inhibiting activity that is re-sponsible for reducing the production of inflammation-mediating pros-taglandins, thereby providing relief from inflammation [1]. R-Flurbiprofen is used in treatment of Alzheimer's disease and in phase II clinical trial for colon and prostate cancer. [2–4].
Lipases as biocatalysts are very important enzyme that biocatalysts the hydrolysis of long-chain triglycerides and generally exhibit excel-lent chemo-, regio- and enantioselectivity. These enzymes have been widely applied in kinetic resolution [5]. However, lipase as a biocatalyst has some disadvantages, the difficulty of product recovery, poor stabil-ity under operational conditions, and the impossibilstabil-ity of multiple re-uses in industrial processes [6,7].
Calix[n]arenes can act as nanobasket structure when cast with nano-particles, they have been used to carry ions and molecules more ef fi-ciently [8]. In recent years it has been observed that calixarenes are
carrier of amino acid, protein, collagens and enzymes [9,10]. The active site offlap/lid of lipase (CRL) has 31 amino acids, mainly hydrophilic on its external face and hydrophobic on the internal side, directed towards the active site is the hydrophilic surface of the molecule in the open form and the inactive site is the hydrophobic surface of the molecule in the closed form. [11–13]. Calix[n]arenes interact with amino acids on the lid or with strong hydrogen bonds that leads to open up the lid and thus the activity of the enzyme is maintained for a long time [9]. In addition, the calixarenes interact with different functional groups of the enzyme to preserve the conformation [6]. In this regard, different calixarene derivative have been used previously by our group for binding of enzymes and analyzed enzyme activity, stability, and enantioselectivity.
For example, we synthesized calix[n]arenes substituted at the lower rim by carboxylic acid [14], hydrazide [15], pyridyl [16], mercapto [4], and substituted at the upper rim by N-methylglucamine [17], phos-phonic acid or iminodicarboxylic acid [6] immobilized magnetic nano-particles that were used as additives in the encapsulation process. The influence of the synthesized material on the hydrolysis and enantioselectivity of some racemic aromatic methyl esters was per-formed. However, so far, there is no study reported aboutfluorescent calix[4]arene derivatives for immobilization of lipase. Therefore, the
⁎ Corresponding author.
E-mail address:myilmaz42@gmail.com(M. Yilmaz).
https://doi.org/10.1016/j.ijbiomac.2019.04.182
0141-8130/© 2019 Elsevier B.V. All rights reserved.
Contents lists available atScienceDirect
International Journal of Biological Macromolecules
aim of this work was to synthesize two lower rim-functionalized calix [4]arene derivatives carryingfluorescent groups and use these in mon-itoring the enzyme activity.
2. Materials and methods 2.1. Materials
Candida rugosa lipase (CRL) (Type VII), Bovine serum albumin, tetramethoxysilane (TMOS), p-nitrophenyl palmitate (p-NPP), and octyltriethoxysilane (OTES) and the other reagents were supplied from Merck, Sigma-Aldrich or Fluka. All aqueous solutions were pre-pared with deionized water that had been passed through a Millipore Milli-Q Plus water purification system.
2.2. Instrumentation
IR spectra were recorded on a Bruker Vertex FTIR spectrometer. UV/ Vis spectra were measured with a Shimadzu UV-1700. An Agilent 1200 Series High-performance liquid chromatography (HPLC) with a diode array detector was used for enantiomeric excess of Flurbiprofen. The surface morphology of samples was examined by scanning electron mi-croscope (SEM, Jeol, JSM 5310, Japan).1HNMR spectra were recorded on a Varian 400 MHz spectrometer. A Zeiss 510 Meta Confocal Laser Scan-ning Microscope was used to be imaged forfluorescence magnetite nanoparticles. An X-ray diffraction (XRD) analysis was performed using by a Bruker D8 ADVANCE X ray diffractometer. Magnetic property was measured using a vibrating sample magnetometer (VSM, LDJ9600). 2.3. Synthesis
Scheme 1represent the synthesis of different derivative of calix[4] arene in which, calix[4]arene derivatives 1–4 were prepared according to literature methods [18–20] Calix-2 and Calix-3 were synthesized by published method with some modification and their procedure is given below [21,22].
2.3.1. Dansyl derivative of 4 (Calix-2)
Dansyl chloride (0.363 mg, 1.344 mmol) was added dropwise to the stirred solution of diaminopropyl derivative of calix[4]aren (4) (0.544 g, 0.611 mmol) and Et3N (0.136 g, 1.344 mmol) in 50 mL dry
dichloro-methane. The reaction was stirred at room temperature for 6 h. After the completion of reaction, the reaction mixture was washed with 1 M HCl. The organic phase is reacted with NaHCO3and washed with
water. The organic layer was separated, dried over anhydrous Na2SO4
and distilled under reduced pressure to give Calix-2 in 90%. IRνmax (KBr pellet, cm−1) 1614 and 1574 cm−1(C=C).
1HNMR (CDCl
3):δ 1.00 (s, 18H, But), 1.40 (s, 18H, But), 1.90 (bp, 4H,
–CH2-) 2.85 (s, 12H, N-CH3), 3.20 (t, 4H, CH2-N), 3.30 (d, 4H J = 12.6 Hz,
ArCH2Ar)1H NMR (CDCl3): 3.85 (s, 4H, CH2-N), 4.20 (d, 4H J = 12.6 Hz,
ArCH2Ar) 6.80 (s, 4H, ArH), 7.02 (m, 8H, ArH), 7.40–7.55 (m, 4H, ArH),
8.20–8.35 and 8.50 (m, 8H, ArH, NH). 2.3.2. Dansyl derivative of 3 (Calix-3)
Dansyl chloride (2.99 g, 11.09 mmol) was added dropwise to the stirred solution of dihydrazide derivative of p-tert-butylcalix[4]arene (3) (4 g, 5.04 mmol) and Et3N (1.12 g, 11.09 mmol) in 100 mL dry
di-chloromethane. The reaction was stirred at room temperature for 6 h. After the completion of reaction, the reaction mixture was washed with 1 M HCl. The organic phase is reacted with NaHCO3and washed
with water. The organic layer was separated, dried over anhydrous Na2SO4and distilled under reduced pressure to give Calix-3 in 88%
yield.
IRνmax (KBr pellet, cm−1) 1707 and 1675 cm−1(C=O) and 1570
(C=C).
1HNMR (CDCl
3):δ 1.00 (s, 18H, But), 1.25 (s, 18H, But), 3.00 (s, 12H,
N-CH3), 3.25 (bd, 4H, ArCH2Ar), 3.10 (d, 4H J = 12.6 Hz, ArCH2Ar) 3.30
(bs, 4H, CH2-O), 4.15 (bd, 4H J = 12.6 Hz, ArCH2Ar) 6.65–8.90 (m, 20H,
ArH).
2.4. Preparation of Fe3O4@Calix-2 and Fe3O4@Calix-3
Iron oxide nanoparticles were prepared according to the modified literature procedure [23]. Typically, a 0.5 g of dansyl derivative of calix [4]arene (Calix-2 or Calix-3) was dissolved in 20 mL of ethanol by ultra-sonic irradiation for 20 min. The mixture was further stirred vigorously for 30 min at 50 °C. Then 177 mg of FeCl3/FeCl2salts in the mass ratio of
2:1 was added by stirring. Under inert atmosphere, the reaction was fur-ther stirred vigorously for 30 min. At the end of this period, 25 mL of 10% NH4OH solution was added drop by drop into the mixture at 60 °C
dur-ing 1 h and treated for another 2 h. Finally, the black precipitates were obtained and separated by decantation with help of external magnet, washed several times with Milli-Q water, and dried under vacuum at room temperature.
2.5. Sol–gel encapsulation of lipases and their activity assay
The encapsulated lipases were prepared according to the previous method [24]. Briefly, 50 mg the encapsulated lipase was added in a 25 mL reaction vessel containing phosphate buffer (390μL; 0.05 M). The mixture was stirred in the incubator. Fe3O4@Calix-2 or Fe3O4@
Calix-3 (50 mg), 100μL of polyvinyl alcohol (PVA), 0.1 M, 50 μL of NaF, and 500μL isopropyl alcohol were mixed then homogenized on a shaker. Later, octyltriethoxysilane (2.5 mmol) and tetramethoxysilane (0.5 mmol; 120μL) were added until completely homogenous mixture was obtained. The gel-like material was lyophilized. The subsequent en-capsulated lipases were stored at 4 °C before usage. The protein concen-tration of the assay was calculated using Bradford's method [25].
To define the hydrolytic activities of the encapsulated lipase with and without additives, p-NPP was hydrolyzed in 0.05 M of buffer (pH 7.0) and the concentration of p-nitrophenol (p-NP) was measured at 410 nm by means of a spectrophotometer. The quantity of lipase, which releases 1μmol p-NP min−1is defined as one unit enzyme
activ-ity [26].
2.6. Effect of pH and temperature on encapsulated enzyme activity The effect of pH on the activity of encapsulated lipases was analyzed in different pH values ranges from pH 4.0 to pH 9.0. Furthermore, to see the thermal inactivation of the encapsulated lipases, experiment was performed in the temperature range of 30–60 °C by incubating both li-pases in PBS (50 mM) for 20 min.
2.7. Thermal stability
The encapsulated lipases were stored at 60 °C, for different lengths of time (ranging from 0 to 120 min), then the residual activity was assayed as above.
3. Results and discussion 3.1. Synthesis of Calix-2 and Calix-3
Dansylamide (5-dimethylaminonaphthalene-1-sulfonamide) groups asfluorescent probes have been used in many areas of research, including sensors for biological molecules and various metal ions [27–29]. In this work, two newfluorescent magnetite nanoparticles (Fe3O4@Calix-2) and (Fe3O4@Calix-3) containing dansyl derivative of
calix[4]arenes were synthesized. Preliminary, calix[4]arene was func-tionalized with dansyl moiety according to reported method (Scheme 1). In brief, calix[4]arene bearing dansyl groups (Calix-2) was prepared
1043 E. Ozyilmaz et al. / International Journal of Biological Macromolecules 133 (2019) 1042–1050
through the reaction of dansyl chloride with corresponding diaminopropyl derivative of calix[4]arene (4), according to a published procedure [21,22]. Calix-3 was synthesized by the reaction of 1,3-dihydrazide derivative of calix[4]arene (3) and dansyl chloride. The structures of Calix-2 and Calix-3 have been characterized by1HNMR
and FT-IR techniques. From the1HNMR data, two signals for equivalent tert-butyl group are observed (two singlets atδ 1.00 and 1.40 ppm for Calix-2 and atδ 1.00 and 1.25 ppm for Calix-3). Calix-2 and Calix-3 show a single AB spin system for bridging methylene groups [6]. These compounds were confirmed to be present in the cone
conformation by detailed study of the1HNMR spectrum a typical AB
pattern was observed for the splitting pattern of the ArCH2Ar methylene
protons atδ 3.30 ppm and 4.20 ppm for Calix-2; at δ 3.25 ppm and 4.15 for Calix-3 (See, Figs. S1-S5).
3.2. Preparation of Fe3O4@Calix-2 and Fe3O4@Calix-3
Fe3O4@Calix-2 and Fe3O4@Calix-3 were prepared by alkaline
co-precipitation of Fe(II) and Fe(III) precursors in water/ethanol (2:1) solu-tion of Calix-2 or Calix-3 (Fig. 1). To obtain surface modified magnetic
nanoparticles, Molday's procedure [23] was applied. It is a well-known that coordination bonds formed between the calix[4]arene dansyl de-rivative (Calix-2 or Calix-3) and Fe(II)/Fe(III). In this process the calix [4]arene dansyl derivative (Calix-2 or Calix-3) is bound to the nanopar-ticles. The hydrophilic group of calix[4]arene cavity provides an impor-tant role in the preparation of Fe3O4nanoparticles [30,31] (Fig. 1).
Compared with the FTIR spectrum of Fe3O4@Calix-2 and Fe3O4@
Calix-3, exhibited the characteristic absorption of Fe\\O at 789, 569 cm−1for Fe3O4@Calix-2 and at 787, 573 cm−1Fe3O4@Calix-3. In
the FTIR spectrum of magnetic calix-silica composites the band around 1072 and 973 cm−1were attributed to -Si-O stretching vibrations (Fig. S5 and Fig. S6). This indicates that the surface of the encapsulated nanoparticles is covered with silica.
The Fe3O4@Calix-2 and Fe3O4@Calix-3 gave also satisfactory
analyt-ical data, consistent with the proposed formulas integrating residual molecules. According to the elemental analysis, the resulting Fe3O4@
2 contains 2.24% nitrogen, corresponding to 0.38 mmol, of Calix-2/g of support and Fe3O4@Calix-3 contains 3.26% nitrogen,
correspond-ing to 0.40 mmol of Calix-3/g of support [32].
Confocal microscopy is utilized to get information about the surface morphology offluorescent materials [33]. The study demonstrated the fluorescent calixarene labeling for investigation of interactions between the fluorescent calixarene and Fe3O4 nanoparticles and used for
calixarene based lipase immobilization imaging agent. The presence of fluorescence calix[4]arene on iron oxide nanoparticles (Fe3O4@Calix-2
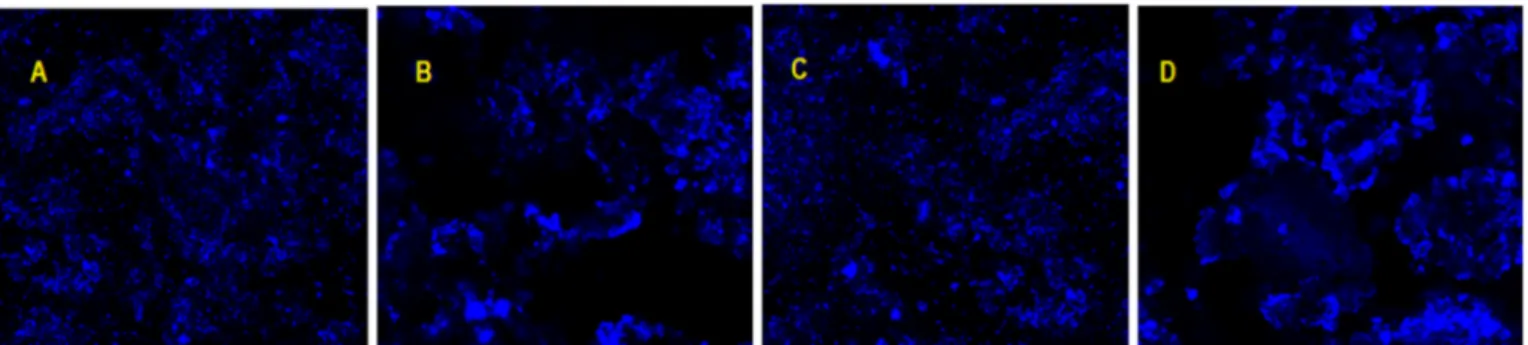
and Fe3O4@Calix-3) was confirmed with confocal microscopy.Fig. 2
shows the confocal microscope image of Fe3O4@Calix-2 and Fe3O4@
Calix-3 nanoparticles with brightfields due to calix[4]arene fluores-cence. However, a homogeneousfluorescence irradiation before lipase encapsulation (Fig. 2A and C), but after lipase encapsulation, it was ob-served that thefluorescence mostly seen in the calixarene interacted with a part of the lipase (Fig. 2B and D).
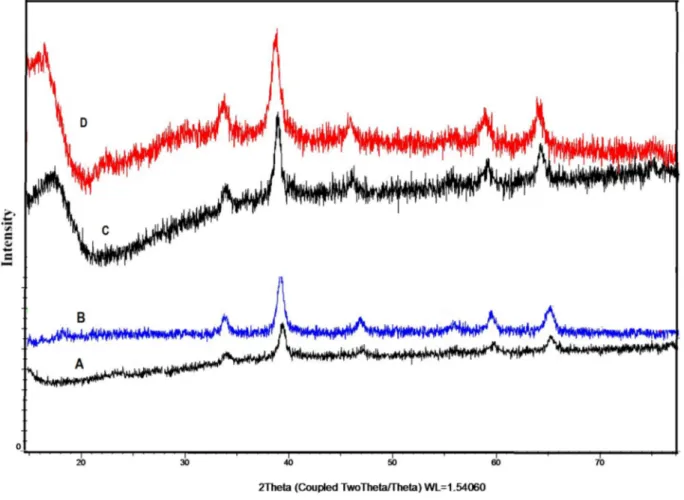
The X-ray diffraction (XRD) patterns of calixarene and Fe3O4grafted
and calixarene, enzyme and-Fe3O4/SiOXnanoparticles are shown in
Fig. 3. It is clear that the diffraction pattern of the calixarene immobilized Fe3O4nanoparticles is close to the standard pattern for
crystalline magnetite (Fig. 3(A and B). XRD data showed the standard Fe3O4crystal with spinel structure has six diffraction peaks. They were
also observed for encapsulated lipases (Fig. 3C and D). The magnetic property of the Fe3O4@Calix-2 and the encapsulated lipase (Fe3O4@
Calix-2E) were investigated by vibrating sample magnetometer (VSM). Maximum saturation supermagnetizations Fe3O4@Calix-2, and
Fe3O4@Calix-2E are obtained at 40 and 30 emu g−1, respectively
(Fig. 4A and B)). Although the addition of the nonmagnetic portion leads to decreased saturation supermagnetizations, the obtained encap-sulated lipase still has a high saturation supermagnetization of 30 emu g−1.
3.3. Determination of hydrolytic activity of encapsulated lipases
It has been well known that disubstituted calixarene derivatives have effective binding sites for ions and molecules [7]. In view of these properties of the calixarene derivatives, two dansyl derivative of calix [4]arenes (Calix-2 or Calix-3) were used as additive for the
OHOH O O HN NH O O S NCH3 H3C O O S N H3C CH3 O O
Calix-3
OH OH O O NH HN S N H3C CH3 O O S NCH3 H3C O OCalix-2
HN NHFe
3
O
4
or
Fig. 1. The proposed structure of Fe3O4@Calix-2 and Fe3O4@Calix-3.
Fig. 2. Confocal microscopy photos a) Fe3O4@Calix-2 b) Fe3O4@Calix-2E c) Fe3O4@Calix-3 d) Fe3O4@Calix-3E.
1045 E. Ozyilmaz et al. / International Journal of Biological Macromolecules 133 (2019) 1042–1050
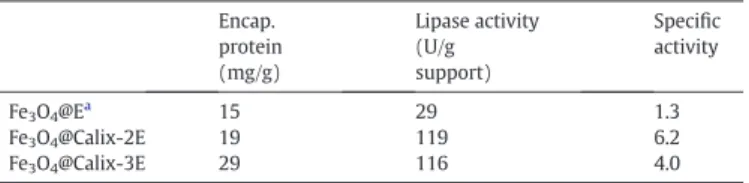
encapsulation of the lipase. The specific activities, the hydrolytic activ-ity, and amounts of the protein of encapsulated lipases are found by the hydrolysis reaction of p-nitrophenyl palmitate (p-NPP) (Table 1).
Table 1shows the data generated from the encapsulated lipases by the sol–gel method for hydrolysis of p-NPP. The specific activities of en-capsulated nanoparticles were observed as 6.2 for Fe3O4@Calix-2E, 4.0
for Fe3O4@Calix-3E, while it was observed as 1.3 when no calixarene
derivatives were used. It was well known that lipase has a certain part of protein and structural activity relationship studies demonstrated that lower-rim arylation, resulting in maintaining cone conformation, is important for calixarene complexation. Calix[4]arenes interact with the amino acid building blocks and with relevant short or longer peptide sequences [34,35]. When we look at these results, it is understood that dansyl groups of Fe3O4@Calix-2E and Fe3O4@Calix-3E nanoparticles
are effective for interaction with proteins and enzymes. In addition, the Fe3O4@Calix-2E showed more efficiency as compared to Fe3O4@
Calix-3E and Fe3O4@E. The efficacy can be attributed due to fact that
Fe3O4@Calix-2E containing hydrazide groups that make more
effective-ness and protecting the active center of the enzyme by electrostatic in-teractions and hydrogen bonds [6,36–38].
3.4. Effect of pH and temperature on encapsulated enzyme activity It has been known that lipase activity is affected with pH of the solu-tion. Residual activities of the encapsulated enzymes were measured in different pH values (4.0–9.0) (Fig. 5A). The optimum pH values for reaching efficient hydrolysis of p-NPP were observed to be 7.0 for both Fe3O4@E and encapsulated lipases (Fe3O4@Calix-2E and Fe3O4@
Calix-3E). The results indicated that pH did not differ by encapsulation because there was no change in the conformation of the lipase and that
the charge density on the lipase surface did not change (at pH 7). Similar results have also been reported in the literature [39–47]. The encapsu-lated lipase with calix[4]arene derivative (Fe3O4@Calix-2E or Fe3O4@
Calix-3E) was more stable than the encapsulated lipase without calix [4]arene derivative (Fe3O4@E) between pH 4 and 5. The increased
toler-ance towards acidic pH for encapsulated lipase may be because the calix [4]arene dansyl derivative complexed with the functional groups of the lipase to provide a suitable microenvironment to reduce the deforma-tion of enzyme structure [42]. However, all encapsulated lipases lost some of their activity at alkaline condition (pH 8–9), remarkably, Fe3O4@Calix-2E lost more activity than Fe3O4@Calix-3E below pH 5.0,
while the pH tolerance of the two encapsulated lipases was comparable in the pH range of 6 to 9, possibly due to the different natures of the calix [4]arene derivatives. The encapsulated lipase without calix[4]arene de-rivative (Fe3O4@E) maintained 80% at pH 8.0 and 70% at pH 9.0 of its
ini-tial activity. Lack of improvement in stability under alkaline pH could be due to the dansyl groups carry amino groups, they do not form com-plexes with enzyme in an alkaline environment.
The optimum temperature was determined by the hydrolysis of p-nitrophenyl palmitate at pH 7.0 from 30 °C to 60 °C (Fig. 5B). The opti-mal reaction temperature of the encapsulated lipase without calixarene derivatives (Fe3O4@E) was nearly 35 °C, whereas it shifted to 45 °C for
the encapsulated lipases (Fe3O4@Calix-2E and Fe3O4@Calix-3E).
This effect can be arise due to conformational mobility of enzyme and consequently multipoint interaction between the calixarene and the enzyme or improved substrate diffusion at a high temperature [48,49]. The results suggested that the sol-gel encapsulation process with calixarene derivative might be able to increase the conformational rigid-ity of enzyme and protect the lipase against denaturation at high temperature.
3.5. Thermal stability of encapsulated enzymes
Thermal stability of encapsulated lipases (Fe3O4@E, Fe3O4@Calix-2E
and Fe3O4@Calix-3E) was incubated at 60 °C for 2 h and analyzed
enzy-matic activity over different periods (Fig. 5C). Encapsulated lipase with-out calixarene derivative (Fe3O4@E) lost its initial activity within about
100 min, after keeping 60 °C for 120 min, while the encapsulated lipases (Fe3O4@Calix-2E and Fe3O4@Calix-3E) retained about 43% and 40% of
their initial activity, respectively.
As a result Fe3O4@Calix-2E and Fe3O4@Calix-3E were more stable
than that of Fe3O4@E. These results have enabled the preservation of
enzyme activity in Fe3O4@Calix-2E, and Fe3O4@Calix-3E nanoparticles
at high temperatures over a long period of time.
3.6. Enantioselective hydrolysis of racemic Flurbiprofen methyl ester Enzymatic resolutions of racemic mixtures are used to obtain enantiopure compounds by chiral chemical synthesis for the pharma-ceutical industry. The reaction was monitored by HPLC, the enantio-meric compositional analysis of the materials obtained and the enantiomeric excesses (% ee) determined by chiral columns in high
pressure liquid chromatography (HPLC). The results were analyzed on HPLC using a mobile phase of hexane/2-propanol/trifluoroacetic acid (100/0.1/0.1) at 254 nm at aflow rate of 1 mL/min (Scheme 2,Table 2). In our earlier study [4], we used two mercapto derivative of calix[4] arenes capped on Fe3O4nanoparticles as additives for the encapsulation
of enzyme to work the possible effects of the calix[4]arene derivatives in the enantioselective hydrolysis reaction of racemic Flurbiprofen methyl ester. It was perceived that enantioselectivity (E = 244) after 72 h was found for most enzyme preparations in this work with an ee % value of S-Flurbiprofen about 98%.
Table 1
The comparison between different encapsulated enzymes. Encap. protein (mg/g) Lipase activity (U/g support) Specific activity Fe3O4@Ea 15 29 1.3 Fe3O4@Calix-2E 19 119 6.2 Fe3O4@Calix-3E 29 116 4.0 a
Encapsulated enzyme without magnetic calixarene derivatives. Fig. 4. A) Vibrating sample magnetometer (VSM) supermagnetization curves of Fe3O4@Calix-2; (B) Encapsulated lipase Fe3O4@Calix-2E;
1047 E. Ozyilmaz et al. / International Journal of Biological Macromolecules 133 (2019) 1042–1050
Enantioselective hydrolysis of (R,S)-Flurbiprofen methyl ester was determined by incubation with encapsulated lipases at different tem-peratures (35 °C, 45 °C) at pH = 7 in the presence of different additives (Fe3O4@Calix-2E and Fe3O4@Calix-3E). Optimum temperature values
for enantioselective hydrolysis studies were best observed at 45 °C and 48 h for both encapsulated enzymes and the best enantioselectivity and conversion. As shown inFig. 6, the encapsulated enzyme with calix [4]arene derivatives (Fe3O4@Calix-2E and Fe3O4@Calix-3E) show high
enantioselectivity and more conversion at pH 7, temperature 45 °C and 48 h than the encapsulated lipase without calix[4]arene derivative (Fe3O4@E).
The reusability of encapsulated enzymes is a key factor for its cost-effective use for industrial application. Therefore, the reusability of the encapsulated lipases (Fe3O4@Calix-2E and Fe3O4@Calix-3E) for
re-peated batch biotransformation of (R,S)-Flurbiprofen methyl esters to (S)-Flurbiprofen was assessed. As shown inFig. 7, the encapsulated en-zymes (Fe3O4@Calix-2E and Fe3O4@Calix-3E) still kept 38% and 30% of
their conversion ratios for the encapsulated enzymes using (R,S)-Flurbiprofen methyl esters as substrate after the 5 cycles, respectively, making the encapsulated enzyme suitable for reuse in industrial applications.
4. Conclusions
In the present work, an encapsulated lipase was prepared using a novel approach involving co-entrapment offluorescent calix[4]arene derivative containing dansyl moiety and enzyme and magnetic nano-particles in a silica matrix using enzyme-assisted direct condensation reactions of silicic acid. It has been examined that the resulting magnetic encapsulated lipases exhibited higher operational and thermal stabili-ties than the encapsulated lipase without calix[4]arene derivative. The encapsulated enzyme could be easily recovered by applying an external
Table 2
Enantioselective hydrolysis of racemic Flurbiprofen methyl ester using encapsulated lipases.-x (%) ees(%) eep(%) E Fe3O4@E (48 h)a 36 55 N98 173 Fe3O4@E (96 h)a 20 22 N98 123 Fe3O4@E (144 h)a 14 16 N98 116 Fe3O4@Calix-2E (48 h) 49 85 N98 270 Fe3O4@Calix-2E (96 h) 34 50 N98 163 Fe3O4@Calix-2E (144 h) 19 20 N98 120 Fe3O4@Calix-3E (48 h) 47 81 N98 249 Fe3O4@Calix-3E (96 h) 32 48 N98 159 Fe3O4@Calix-3E (144 h) 10 10 N98 109 a
Encapsulated enzyme without calixarene derivatives.
Scheme 2. Schematic representation of enantioselective hydrolysis of (R,S)-Flurbiprofen methyl ester with Fe3O4@Calix-2E or Fe3O4@Calix-3E
Fig. 5. The pH activity curves (A), temperature activity curves (B) and thermal stability curves (C) for encapsulated enzymes.
magnet. For this reason, the simple route employed can yield easily recycled biocatalyst with high activity, and this can offer a very promis-ing applications.
Acknowledgments
We would like to thank the Scientific Research Foundation of Selcuk University, Konya, Turkey (BAP Project No. 15201094) forfinancial sup-port of this work.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps://doi. org/10.1016/j.ijbiomac.2019.04.182.
References
[1] J. Miao, Z. Guo, Y. Wang, D. Chen, Y. Li, F. Zhang, Study on the inclusion complex be-tween beta-cyclodextrin derivatives andflurbiprofen by spectrofluorometric, Green Energy and Sustainable Development I, vol. 1864, 2017, pp. 20001–20007.
[2] R. Pignatello, C. Bucolo, G. Spedalieri, A. Maltese, G. Puglisi, Flurbiprofen-loaded ac-rylate polymer nanosuspensions for ophthalmic application, Biomaterials 23 (2002) 3247–3255.
[3] A. Rousseau, P. Chiap, R. Ivanyi, J. Crommen, M. Fillet, A.C. Servais, Validation of a nonaqueous capillary electrophoretic method for the enantiomeric purity determi-nation of R-flurbiprofen using a single-isomer amino cyclodextrin derivative, J. Chromatogr. A 1204 (2008) 219–225.
[4] H. Yildiz, E. Ozyilmaz, A.A. Bhatti, M. Yilmaz, Enantioselective resolution of racemic flurbiprofen methyl ester by lipase encapsulated mercapto calix[4]arenes capped Fe3O4nanoparticles, Bioprocess Biosyst. Eng. 40 (2017) 1189–1196.
[5] F. Secundo, G. Carrea, C. Tarabiono, S. Brocca, M. Lotti, Activity and enantioselectivity of wildtype and lid mutated Candida rugosa lipase isoform 1 in organic solvents, Biotechnol. Bioeng. 86 (2004) 236–240.
[6] E. Ozyilmaz, M. Bayrakci, M. Yilmaz, Improvement of catalytic activity of Candida rugosa lipase in the presence of calix[4]arene bearing iminodicarboxylic/phosphonic acid complexes modified iron oxide nanoparticles, Bioorg. Chem. 65 (2016) 1–8.
[7] H. Tutar, E. Yilmaz, E. Pehlivan, M. Yilmaz, Immobilization of Candida rugosa lipase on sporopollenin from lycopodium Clavatum, Int. J. Biol. Macromol. 45 (2009) 315–320.
[8] C.-P. Li, S. Tan, H. Ye, J. Cao, H. Zhao, A novelfluorescence assay for resveratrol deter-mination in red wine based on competitive host-guest recognition, Food Chem. 283 (2019) 191–198.
[9] M. Yilmaz, S. Sayin, in: P. Neri, J. Sessler, M.X. Wang (Eds.), Calixarenes in Organo and Biomimetic Catalysis, Calixarenes and beyond, Springer, 2016.
[10] Z. Yongxiang, M. Jianzhong, G. Dangge, J. Lu, G. Kaitao, R. Huijun, Modification of col-lagen with three novel tannages, sulfonated calix[4]arenes, Int. J. Biol. Macromol. 116 (2018) 1004–1010.
[11] M. Lotti, A. Tramontano, S. Longhi, F. Fusetti, S. Brocca, E. Pizzi, L. Alberghina, Vari-ability within the Candida rugosa lipase family, Protein Eng. 7 (1994) 531–535.
[12] J.H. Hernández, J.T. del Pozo, P.M. Canelo, A.R. Puebla, S.P. Tejo, J.S. Marcos, Lung can-cer incidence in the province of Avila, Spain in 2002 and decade-long trends, Arch. Bronconeumol. 40 (2004) 304–310.
[13]M. Mathesh, B. Luan, T.O. Akanbi, J.K. Weber, J. Liu, C.J. Barrow, R. Zhou, W. Yang, Opening lids: modulation of lipase immobilization by graphene oxides, ACS Catal. 6 (2016) 4760–4768.
[14] E. Akoz, O.Y. Akbulut, M. Yilmaz, Calix[n]arene carboxylic acid derivatives as regula-tors of enzymatic reactions: enhanced enantioselectivity in lipase-catalyzed hydro-lysis of (R/S)-naproxen methyl ester, App. Biochem. Biotechnol. 172 (2014) 509–523.
[15] E. Akoz, S. Sayin, S. Kaplan, M. Yilmaz, Improvement of catalytic activity of lipase in the presence of calix[4]arene valeric acid or hydrazine derivative, Bioprocess Biosyst. Eng. 38 (2015) 595–604.
[16] E. Ozyilmaz, S. Sayin, A magnetically separable biocatalyst for resolution of racemic naproxen methyl ester, Bioprocess Biosyst. Eng. 36 (2013) 1803–1806.
[17] S. Sayin, E. Yilmaz, M. Yilmaz, Improvement of catalytic properties of Candida rugosa lipase by sol–gel encapsulation in the presence of magnetic calix[4]arene nanopar-ticles, Biomol. Chem. 9 (2011) 4021–4024.
[18] C.D. Gutsche, M. Iqbal, p-tert-Butylcalix[4]arene, Org. Syn. 68 (1990) 234–237.
[19] U. Rodrigues, M. Aguirre, R. Facklam, M. Collins, Specific and intraspecific molecular typing of lactococci based on polymorphism of DNA encoding rRNA, J. Appl. Microbiol. 71 (1991) 509–516.
[20]I.Y. Bakunina, L. Shevchenko, O. Nedashkovskaya, N. Shevchenko, S. Alekseeva, V. Mikhailov, T. Zvyagintseva, Screening of marine bacteria for fucoidanases, Microbi-ology 69 (2000) 303–308.
[21] L.A. Chrisstoffels, F. de Jong, D.N. Reinhoudt, D.S. Sivelli, L. Gazzola, A. Casnati, R. Ungaro, Facilitated transport of hydrophilic salts by mixtures of anion and cation carriers and by ditopic carriers, J. Am. Chem. Soc. 121 (1999) 10142–10151. 0 10 20 30 40 50 1 2 3 4 5 Reuse X ( % ) Fe3O4@Calix-2E Fe3O4@Calix-3E Fe3O4@E
Fig. 7. Reusability on the conversion (x) in the hydrolysis of Flurbiprofen methyl ester. Fig. 6. Effect of temperature on the conversion (x) in the hydrolysis of Flurbiprofen methyl ester.
1049 E. Ozyilmaz et al. / International Journal of Biological Macromolecules 133 (2019) 1042–1050
[22] D. Maity, A. Chakraborty, R. Gunupuru, P. Paul, Calix[4]arene based molecular sen-sors with pyrene asfluoregenic unit: effect of solvent in ion selectivity and colori-metric detection offluoride, Inorg. Chim. Acta 372 (2011) 126–135.
[23]D. Horak, M. Babic, P. Jendelova, V. Herynek, M. Trchova, Z. Pientka, E. Pollert, M. Hajek, E. Sykova, D-mannose-modified iron oxide nanoparticles for stem cell label-ing, Bioconjug. Chem. 18 (2007) 635–644.
[24]M.T. Reetz, P. Tielmann, W. Wiesenhofer, W. Konen, A. Zonta, Second generation sol–gel encapsulated lipases: robust heterogeneous biocatalysts, Adv. Synth. Catal. 345 (2003) 717–728.
[25]M.M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248–254.
[26] S.H. Chiou, W.T. Wu, Immobilization of Candida rugosa lipase on chitosan with acti-vation of the hydroxyl groups, Biomaterials 25 (2004) 197–204.
[27] M.C. Aragoni, M. Arca, A. Bencini, A.J. Blake, C. Caltagirone, A. Decortes, F. Demartin, F.A. Devillanova, E. Faggi, L.S. Dolci, A. Garau, F. Isaia, V. Lippolis, L. Prodi, C. Wilson, B. Valtancoli, N. Zaccheroni, Coordination chemistry of N-aminopropyl pendant arm derivatives of mixed N/S-, and N/S/O-donor macrocycles, and construction of selec-tivefluorimetric chemosensors for heavy metal ions, Dalton Trans. 0 (2005) 2994–3004.
[28] R. Métivier, I. Leray, B. Valeur, Lead and mercury sensing by Calixarene-based fluoroionophores bearing two or four dansyl fluorophores, Chem. Eur. J. 10 (2004) (4880–4490).
[29] B. Valeur, I. Leray, Design principles offluorescent molecular sensors for cation rec-ognition, Coord. Chem. Rev. 205 (2000) 3–40.
[30] M.L. Ben-Ishay, A. Gedanken, Difference in the bonding scheme of calix(6)arene and p-sulfonic calix(6)arene to nanoparticles of Fe2O3and Fe3O4, Langmuir 23 (2007)
5238–5242.
[31] S. Cay, S. Sayin, M.S. Engin, S. Eymur, Preparation and characterization of calix[4] aren-immobilized magnetic microcapsule and its application in heavy metal re-moval, Polycycl. Arom. Comp. (2019)https://doi.org/10.1080/10406638.2017. 1363063(in press).
[32]K.V. Korpany, C. Mottillo, J. Bachelder, S.N. Cross, P. Dong, S. Trudel, T. Friscic, A.S. Blum, One-step ligand exchange and switching from hydrophobic to water-stable hydrophilic superparamagnetic iron oxide nanoparticles by mechanochemical mill-ing, Chem. Commun. 52 (2016) 3050–3057.
[33] M. Jianzhong, H. Xueyan, G. Dangge, Z. Jing, Diffusion and reaction behavior of pro-teases in cattle hide materix via FITC labeled propro-teases, J. Amer. Chem Assoc. 109 (2014) 138–145.
[34] A. W. Coleman, F.Perret1 · Aly Moussa,· M. Dupin,· Y. Guo, · H. Perron, Calix[n] arenes as protein sensors, Top. Curr. Chem. 277 (2007) 31–88.
[35] R. Zadmard, N.S. Alavijeh, Protein surface recognition by calixarenes, RSC Advanced 4 (2014) 41529–41542.
[36]F. Perreta, A.W. Coleman, Biochemistry of anionic calix[n]arenes, Chem. Commun. 47 (2011) 7303–7319.
[37] M. Bayrakci, S. Ertul, M. Yilmaz, Synthesis of new water-soluble phosphonate calixazacrowns and their use as drug solubilizing agents, J. Incl. Phenom. Macrocycl. Chem. 74 (2012) 293–303.
[38]M. Bayrakci, S. Ertul, M. Yilmaz, Transportation of poorly soluble drug molecules from the organic phase to the aqueous phase by using phosphorylated calixarenes, J. Chem. Eng. Data 56 (2011) 4473–4479.
[39] M. Yilmaz, S. Cetinguney, E. Ozyilmaz, Improvement of catalytic activity of lipase in the presence twofluorescent calix[4]arene derivatives-grafted Fe3O4nanoparticles,
2017 IEEE 7th International Conference Nanomaterials: Application & Properties (NAP), 2017.
[40] U. Ozgur, Y.I. Alivov, C. Liu, A. Teke, M. Reshchikov, S. Dogan, V. Avrutin, S.J. Cho, H. Morkoc, A comprehensive review of ZnO materials and devices, J. Appl. Phys. 98 (2005) 11.
[41] C. Berger, Z. Song, X. Li, X. Wu, N. Brown, C. Naud, D. Mayou, T. Li, J. Hass, A.N. Marchenkov, Electronic confinement and coherence in patterned epitaxial graphene, Science 312 (2006) 1191–1196.
[42] A. Uyanik, N. Sen, M. Yilmaz, Improvement of catalytic activity of lipase from Can-dida rugosa via sol–gel encapsulation in the presence of calix(aza)crown, Bioresour. Technol. 102 (2011) 4313–4318.
[43]E. Ozyilmaz, S. Sayin, Utilization of catalytic properties of the encapsulated lipase with calix[4]arene-adorned sporopollenin, Polycycl. Arom. Comp. 38 (2018) 272–281.
[44]E. Poorakbar, A. Shafiee, A.A. Saboury, B.L. Rad, K. Khoshnevisan, L. Ma'mani, H. Derakhshankhah, M.R. Ganjali, M. Hosseini, Synthesis of magnetic gold mesoporous silica nanoparticles core shell for cellulase enzyme immobilization: improvement of enzymatic activity and thermal stability, Process Biochem. 71 (2018) 92–100.
[45] K. Khoshnevisan, F. Vakhshiteh, M. Barkhi, H. Baharifar, E.P. Akbar, N. Zari, H. Stamatish, A.K. Bordbar, Immobilization of cellulase enzyme onto magnetic nano-particles: applications and recent advances, Mol. Catal. 442 (2017) 66–73.
[46] K. Khoshnevisan, A.K. Bordbar, D. Zare, D. Davoodi, M. Noruzi, M. Barkhi, M. Tabatabaei, Immobilization of cellulase enzyme on superparamagnetic nanoparti-cles and determination of its activity and stability, Chem. Eng. J. 171 (2011) 669–673.
[47]K. Khoshnevisan, M. Barkhi, A. Ghasemzadeh, H.M. Tahami, S. Pourmand, Fabrica-tion of coated/uncoated magnetic nanoparticles to determine their surface proper-ties, Mater. Manuf. Process. 31 (2016) 1206–1215.
[48] B.P. Dwivedee, J. Bhaumik, S.K. Rai, J.K. Laha, U.C. Banerjee, Development of nanobiocatalysts through the immobilization of Pseudomonasfluorescens lipase for applications in efficient kinetic resolution of racemic compounds, Bioresour. Technol. 239 (2017) 464–471.
[49] G. Sargazi, D. Afzali, A.K. Ebrahimi, A. Badoe-dalfard, S. Malekabadi, Z. Karami, Ultra-sound assisted reverse micelle efficient synthesis of new Ta-MOF@Fe3O4 core/shell nanostructures as a novel candidate for lipase immobilization, Mat. Sci. Eng. C 93 (2018) 768–775.