Contents lists available atScienceDirect
Interdisciplinary Neurosurgery
journal homepage:www.elsevier.com/locate/inatCase Reports & Case Series
Proximalmigration of a lumboperitoneal shunt into the cerebello medullary
cisterns
Burak Gezer
a,⁎, H. Karabagli
b, E. Koktekir
b, M. Sahinoglu
b aTatvan State Hospital, Department of Neurosurgery, Bitlis, TurkeybSelcuk University, Faculty of Medicine, Department of Neurosurgery, Konya, Turkey
A R T I C L E I N F O Keywords: Hyrocephalus Lumboperitoneal Mişlgration Shunt A B S T R A C T
Background: Lumboperitoneal (LP) shunt is a type of treatment commonly used in the surgical treatment of pseudotumor cerebri, comorbid hydrocephalus, cerebrospinal fluid (CSF) fistula. Despite the promising results of the LP shunt blockage of %7 and %14 migration rate of complications have been reported. Migration can be rarely observed in the spinal subarachnoid space or even in the intracranial area. We report a case where the LP shunt migrated upward to the cerebellomedullary cisterns.
Case Descrıptıon: A 37-year-old female patient underwent lumboperitonel shunt surgery for pseudotumor cer-ebri. After the LP shunt surgery, the patient's complaints of headache and blurred vision disappeared. The patient admitted to polycyclic at the postoperative first month with neck pain and neck sucking sensation. Cerebellomedullar cysterna shunt tip was seen in brain CT. The patient was operated again and the proximal end of the shunt was lowered to Lumbar 1 level under C-Arm Scope device control. Then, we fixed the subcutaneous tissue of the shunt of the LP shunt with the help of a non-resorbable suture and stitches.
Conclusıons: LP shunt distal migration is more common, such proximal migrations are rarely reported in the literature. LP shunt displacement must be due to defective or insufficient anchoring devices of the LP shunt tube. We think that great care must be taken to fix the LP shunt properly with the help of suture collars with an unresorbable suture at three places to the subcutaneous tissue.
1. Introductıon
Lumboperitoneal (LP) shunt is a type of treatment commonly used in the surgical treatment of pseudotumor cerebri, comorbid hydro-cephalus, cerebrospinal fluid fistula (CSF) [1–3]. Shunt-related com-plications can be seen anywhere between the spinal region and the abdominal end of the peritoneal region as well as in the lumen struc-tures such as the thorax, abdominal solid organs or intestine [4–6]. Despite the promising results of the LP shunt blockage of %7 and %14 migration rate of complications and infections have been reported[4]. LP shunt is generally designed as a one-piece model in the form of silicone elastomeric catheter approximately 80 cm long. The proximal lumbar tip and the distal peritoneal tip can be fixed by the suture rings that secure the tube to the subcutaneous tissue[7,8]. However, there are some complications from subarachnoid hemorrhage, chronic sub-dural hematoma, hyperlordosis, radiculopathy, myelopathy and mi-grations, despite a lower incidence of complications compared to the ventriculoperitoneal shunt of the LP shunt[7–9]. A one-piece LP shunt system without partition can be moved and migrated when anchoring
or loosening anchor sutures[10]. In such cases, very rare distal mi-gration may occur in the peritoneal cavity. Rarely, proximal mimi-gration can be observed in the spinal subarachnoid space or even in the in-tracranial area[8–11]. We report a case where the LP shunt migrated upward to the cerebellomedullary cisterns.
2. Case report
A 37-year-old female patient was followed with pseudotumor cer-ebri for 7 years and she was on acetazolamide therapy. Lumbar punc-ture was planned because of blurred vision and headache. After the lumbar puncture, the pressure was 30 mmHg. Lumboperitoneal shunt (DESU from Turkey) surgery was recommended to the patient with bilateral papilledema. We allowed 10 cm in intrathecal placement in the first surgery. We fixated LP shunt with silk sutures in the first place. After the LP shunt surgery, the patient's complaints of headache and blurred vision disappeared (Fig. 1). On the post op 15th day, the patient was seen to have a clean wound. The patient who suffered from back pain and headache was taken to the brain and lumbar computer
https://doi.org/10.1016/j.inat.2019.100604
Received 6 August 2019; Received in revised form 12 October 2019; Accepted 13 October 2019 ⁎Corresponding author.
E-mail address:dr.burakgezer@gmail.com(B. Gezer).
Interdisciplinary Neurosurgery 19 (2020) 100604
2214-7519/ © 2019 Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
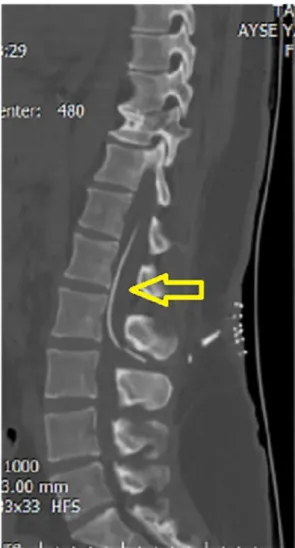
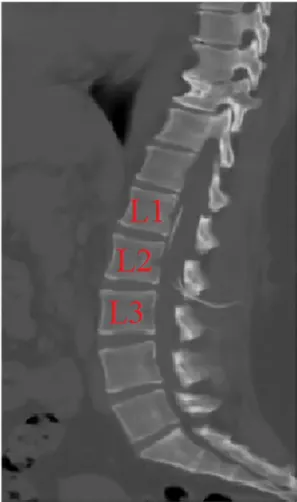
tomography (CT). It was seen that the tip of the shunt was distal to the abdomen at the proximal thoracic 12 level (Fig. 2). No pathology was observed in brain CT. Abdominal ultrasound revealed free CSF in the abdomen. The patient was given analgesia treatment for 3 days hospi-talized. After the pain, he was discharged with recommendations. The patient readmitted to polycyclic at the postoperative first month with neck pain and neck sucking sensation. Cerebellomedullar cysterna shunt tip was seen in brain CT. Thereafter, cervical, thoracic and lumbar CT were seen (Fig. 3). The patient was operated again and the proximal end of the shunt was lowered to lumbar 1 level under C-Arm Scope device control (Fig. 4). Then, we fixed the subcutaneous tissue of the shunt of the LP shunt with the help of a non-resorbable suture and stitches.
3. Dıscussıon
The reasons for failure of the LP shunt include shunt obstruction, fracture, infection, distal tip out of the peritoneal cavity, or removal of
the proximal end from the spinal canal; this occurs by the migration of the shunt tube in the distal direction (peritoneal cavity) or in the proximal direction (spinal subarachnoid space or even intracranial area)[11–14]. Although distal migration is more common, such prox-imal migrations are rarely reported in the literature[7,8,10]. In this case, although the lumbar part of the shunt and the abdominal part of the shunt were locked by the locking mechanism, the shunt migration was not prevented in the first operation of the patient (Fig. 1). LP shunt displacement must be due to defective or insufficient anchoring devices of the LP shunt tube[10,11]. Various modifications are proposed in the literature for the improvement of the fixation of the shunt tube to the subcutaneous tissue, among others: to incorporate a reservoir or valve, to place a connector between the peritoneal and lumbar shunt tube or to use T-tube LP shunt[8,15]. The mechanism for proximal shunt mi-gration is obscure and some possible explanations have been described. It may be a consequence of rotational and lateral movements of the lumbar spine as well as flexion and extension of the head and neck, both causing slow but steady upward migration of the catheter tube[16]. Another possibility is the intraspinal CSF flow, which acts in combi-nation with the respiratory and heart rate of the CSF and forces the rope in the rostral direction. In addition, pressure changes in the dural sac
Fig. 1. The blue arrow indicates the mechanism where the lumbar part of the
shunt and abdominal part of the shunt are locked together. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2. The tip of the shunt is shown at the proximal thoracic 12 level in sagittal
section of toracolumbar CT.
B. Gezer, et al. Interdisciplinary Neurosurgery 19 (2020) 100604
associated with respiratory and abdominal pressure changes may act as distorted and expanding forces, such as moving the catheter upward [12,16,17]. The most important contributing factors are the use of anchoring seams for fixing the catheter to the periosteum and the de-sign of the catheter tube (flat catheters without a wash system). All of these factors can move simultaneously, causing shunt migration. In our case, we thought that many factors had an effect. Proximal migration of
shunt catheters in LP shunts might be breakage of the LP shunt at the lumber level. But 3D abdominal CT showed no shunt breakage (Fig. 5). In addition, LP shunt was found to be continuous on cervical, thoracic and lumbar tomography (Fig. 3).
In a case report, they did not remove the shunt from the intradural area because the proximal of the shunt did not cause any signs or symptoms; and the removal of the shunt was too risky for the structures within and around the brain stem [18]. However, in our case, the proximal shunt was carefully removed due to the lack of brain stem and vital organs.
4. Conclusions
We think that great care must be taken to fix the LP shunt properly with the help of suture collars with an unresorbable suture at three places to the subcutaneous tissue: first, at the point when lumbar por-tion comes out of the thoracolumbar fascia, then at the central porpor-tion at flank, and finally, at the abdominal incision site. This is especially necessary for one-piece LP systems.
In addition, we think that the suture harnesses coming out of the shunt system and preventing the shunt from slipping by fixing the shunt in the lumbar region should be shunt-proof. We recommend that a patient who has undergone LP shunt operation should be considered in the complication of shunt migration when referring to headache, neck or back pain.
Fig. 3. (A) The yellow arrow indicates
mi-gration of the tip of shunt at the cervical level in sagittal section of toracolumbar CT. (B) The yellow arrow indicates migration of the tip of shunt at the craniocervical junc-tion in sagittal secjunc-tion of toracolumbar CT. (C) The yellow arrow indicates migration of the tip of shunt at the level of cerebello-medullar cysterna in sagittal section of tor-acolumbar CT. (D–F) The yellow arrow in-dicates migration of the tip of shunt at the thoracic level in sagittal section of tor-acolumbar. (For interpretation of the refer-ences to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4. (A) First postoperative 3-D CT of ventriculoperitoneal shunt in
in-traabdominal area. (B) 3-D abdominal tomography after proximal migration of ventriculoperitoneal shunt.
B. Gezer, et al. Interdisciplinary Neurosurgery 19 (2020) 100604
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influ-ence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps:// doi.org/10.1016/j.inat.2019.100604.
References
[1] P. Bret, F. Hor, J. Huppert, C. Lapras, G. Fischer, Treatment of cerebrospinal fluid rhinorhea by percutaneus lumboperitoneal shunting: review of 15 cases, Neurosurgery 16 (1985) 44–47.
[2] P.D. Chumas, A.V. Kulkarni, J.M. Drake, H.J. Hoffman, R.P. Humpreys, J.T. Rutka, Lumboperitoneal shunting: a retrospective study in the pediatric population, Neurosurgery 32 (1993) 376–383.
[3] V.Y. Wang, N.M. Barbaro, M.T. Lawton, S. Kunwar, N. Gupta, M.W. Mcdermott, Complications of lumboperitoneal shunts, Neurosurgery 60 (6) (2007) 1045–1048. [4] S.E. Kelman, R.C. Sergot, G.A. Cioffi, P.J. Savino, T.M. Bosley, M.J. Elman, Modified
optic nerve seath decompression in patients with functioning lumboperitoneal shunt and progressive visual loss, Ophtalmology 98 (1991) 1449–1453. [5] A. Fukamachi, H. Wada, O. Toyoda, T. Wakao, J. Kawafuchi, Migration or extrusion
of shunt catheters, Acta Neurochir. 64 (1982) 160–166.
[6] H. Touho, M. Nakauchi, T. Tasawa, J. Nakagawa, J. Karasawa, intrahepaticmigra-tion of a peritoneal shunt catheter: case report, Neurosurgery 21 (1987) 258–259. [7] I. Solaroglu, O. Okutan, E. Beskonakli, Foraminal migration of a lumboperitoneal
shunt catheter tip, J. Clin. Neurosci. 12 (2005) 956–958.
[8] D. Rodrigues, R. Nannapaneni, S. Behari, M. Prasad, A. Herwadkar, C.J. Gerber, P. Mitchell, Proximal migration of a lumboperitoneal unishunt system, J. Clin. Neurosci. 12 (2005) 838–841.
[9] N. Aoki, Lumboperitoneal shunt: clinical applications, complications, and com-parison with ventriculoperitoneal shunt, Neurosurgery 26 (1990) 998–1004. [10] S. Yoshida, S. Masunaga, M. Hayase, Y. Oda, Migration of the shunt tube after
lumboperitoneal shunt – two case reports, Neurol. Med. Chir. (Tokyo) 40 (2000) 594–596.
[11] T.A. Caroll, J. Jakubowski, Intrathecal migration of a lumboperitoneal shunt, Br. J. Neurosurg. 14 (2000) 496–497.
[12] T. Satow, Y. Motoyama, N. Yamazoe, F. Isaka, K. Higuchi, S. Nabeshima, Migration of a lumboperitoneal shunt catheter into the spinal canal – case report, Neurol. Med. Chir. (Tokyo) 41 (2001) 97–99.
[13] R.A. Burgett, V.A. Purvin, A. Kawasaki, Lumboperitoneal shunting for pseudotu-mour cerebri, Neurology 49 (1997) 734–739.
[14] P.D. Chumas, A.V. Kulkarni, J.M. Drake, H.J. Hoffman, R.P. Humphreys, J.T. Rutka, Lumbopritoneal shunting: a retrospective study in the pediatric population, Neurosurgery 32 (1993) 376–383.
[15] J.F. Martinez-Lage, M. Poza, H.A. Esteban, Mechanical complications of the re-servoirs and flushing devices in the ventricular shunt systems, Br. J. Neurosurg. 6 (1992) 321–325.
[16] F. Villarejo, C. Alvarez-Sastre, D. Gimenez, C. Gonzalez, Migration of an entire one-piece shunt into the ventricle, Neurochirurgia (Stuttg) 22 (1979) 196–198. [17] R. Acharya, A. Bhutani, H. Saxena, V.S. Madan, Complete migration of
ven-triculoperitoneal shunt into the ventricle, Neurol. Sci. 23 (2002) 75–77. [18] Gorazd Bunc, Matjaz Vorsic, Janez Ravnik, Tomaz Velnar, Proximal migration of a
lumboperitoneal shunt into the prepontine and ambiens cisterns, Clin. Neurol. Neurosurg. 113 (2011) 75–77.
Fig. 5. Sagittal section of toracolumbar CT of ventriculoperitoneal shunt after
the second operation.
B. Gezer, et al. Interdisciplinary Neurosurgery 19 (2020) 100604