Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=ijds20
ISSN: 1939-0211 (Print) 1939-022X (Online) Journal homepage: https://www.tandfonline.com/loi/ijds20
Beta-Hydroxy-Beta-Methyl-Butyrate, L-glutamine,
and L-arginine Supplementation Improves
Radiation-Induce Acute Intestinal Toxicity
Cagdas Yavas, Guler Yavas, Esin Celik, Ahmet Buyukyoruk, Cennet
Buyukyoruk, Deniz Yuce & Ozlem Ata
To cite this article: Cagdas Yavas, Guler Yavas, Esin Celik, Ahmet Buyukyoruk, Cennet
Buyukyoruk, Deniz Yuce & Ozlem Ata (2019) Beta-Hydroxy-Beta-Methyl-Butyrate, L-glutamine, and L-arginine Supplementation Improves Radiation-Induce Acute Intestinal Toxicity, Journal of Dietary Supplements, 16:5, 576-591, DOI: 10.1080/19390211.2018.1472709
To link to this article: https://doi.org/10.1080/19390211.2018.1472709
Published online: 03 Jul 2018.
Submit your article to this journal
Article views: 150
View related articles
View Crossmark data
https://doi.org/./..
ARTICLE
Beta-Hydroxy-Beta-Methyl-Butyrate, L-glutamine, and
L-arginine Supplementation Improves Radiation-Induce Acute
Intestinal Toxicity
Cagdas Yavasa, Guler Yavasa, Esin Celikb, Ahmet Buyukyorukc, Cennet Buyukyorukd,
Deniz Yucee, and Ozlem Ataf
aSelcuk University, Department of Radiation Oncology, Konya, Turkey;bSelcuk University, Department of Pathology, Konya, Turkey;cKonya Training and Research Hospital, Department of Radiation Oncology, Konya, Turkey;dNecmettin Erbakan University, Department of Family Medicine, Konya, Turkey;eHacettepe University, Department of Preventive Oncology, Ankara, Turkey;fSelcuk University, Department of Medical Oncology, Konya, Turkey KEYWORDS intestine; L-arginine; L-glutamine; radiation-enteropathy; β-hydroxy-β-methylbutyrate ABSTRACT
We aimed to evaluate effects of β-hydroxy-β-methylbutyrate, L-glutamine, and L-arginine (HMB/GLN/ARG) on radiation-induced acute intestinal toxicity. Forty rats were divided into four groups: group (G) 1 was defined as control group, and G2 was radiation therapy (RT) control group. G3 and G4 were HMB/GLN/ARG control and RT plus HMB/GLN/ARG groups, respectively. HMB/GLN/ARG started from day of RT and continued until the animals were sacrificed 10 days after RT. The extent of surface epithelium smoothing, villous atrophy, lamina propria inflammation, cryptitis, crypt distortion, regenerative atypia, vascular dilatation and congestion, and fibrosis were quantified on histological sections of intestinal mucosa. Statistical analyses were performed using the analysis of variance (ANOVA) test. There were significant differences between study groups regarding extent of surface epithelium smooth-ing, villous atrophy, lamina propria inflammation, cryptitis and crypt distortion, regenerative atypia, vascular dilatation and congestion, and fibrosis (p values were 0.019 for fibrosis,<.001 for the others). Pair-wise comparisons revealed significant differences regarding surface epithelium smoothing, villous atrophy, lamina propria inflammation, cryptitis, vascular dilatation, and congestion between G2 and G4 (p values were<.001, .033, <.001, .007, and <.001, respectively). Fibrosis score was significantly different only between G1 and G2 (p= .015). Immunohistochemical TGF-β score of G2 was significantly higher than G1 and G3 (p values were .006 and .017, respectively). There was no differ-ence between TGF-β staining scores of G2 and G4. Concomitant use of HMB/GLN/ARG appears to ameliorate radiation-induced acute intestinal toxicity; however, this finding should be clarified with further studies.
Introduction
Radiation induces an important inflammatory response in the irradiated organs,
charac-terized mainly by leukocyte infiltration and vascular changes (Molla & Panes,2007). Most
CONTACT Guler Yavas, MD guler.aydinyavas@gmail.com Selcuk University, Faculty of Medicine, Department of Radiation Oncology, Konya, Turkey, .
Color versions of one or more of the figures in the article can be found online atwww.tandfonline.com/ijds.
© Taylor & Francis Group, LLC 2019, VOL. 16, NO. 5, 576–591
patients with diagnosis of abdominal tumors, including stomach cancer, pancreatic cancer, and hepatobiliary tumors, need radiotherapy (RT) during their treatment, for either curative or palliative intent, and develop induced intestinal toxicity. Therefore, radiation-induced intestinal toxicity is an important dose-limiting factor during abdominal irradiation
(Dincbas et al.,2009)
The gastrointestinal epithelium has a high proliferative rate, making it susceptible to injury from irradiation. Although the pathogenesis of radiation-induced intestinal toxicity is not clear, it is presumed to be an inflammatory process. The primary effect of radiation is on mucosal stem cells within the crypts. The initial histologic evidence of damage is seen within hours of irradiation. This is followed by an infiltration of leukocytes with crypt abscess for-mation within two to four weeks; ulceration may also occur. Subsequent changes include a progressive occlusive vasculitis with foam cell invasion of the intima and hyaline thickening of the arteriolar walls, as well as collagen deposition and fibrosis, often in the submucosal layer
(Brian, Meyer, & Willett,2017; Hasleton, Carr, & Schofield,1985).
Gastrointestinal symptoms induced by pelvic RT can cause morbidity and distress in the acute phase during treatment and can develop into a chronic, intractable form months or
years after the cessation of treatment (Denton, Forbes, Andreyev, & Maher,2002; McGough,
Baldwin, Frost, & Andreyev,2004). There are many clinical and preclinical studies
investigat-ing different strategies and agents for improvinvestigat-ing radiation-induced intestinal toxicity; how-ever, their results are conflicting. Some of them demonstrate that there is no evidence base for the use of nutritional interventions to prevent or manage bowel symptoms attributable to
RT (McGough et al.,2004); others indicate that supporting patients’ nutritional status may
improve radiation-induced intestinal toxicity and quality of life (Caro et al.,2007; Ravasco
et al.,2003; Unsal et al.,2006).
An oral supplement containingβ-hydroxy-β-methylbutyrate, L-glutamine, and L-arginine
(HMB/GLN/ARG) has been shown to restore muscle mass in cachexia due to cancer, AIDS,
and critically illness (Berk et al.,2008; Clark et al.,2000; Kuhls et al.,2007; Marcora, Lemmey,
& Maddison,2005; May, Barber, D’Olimpio, Hourihane, & Abumrad,2002). Although each
component of the supplement has been shown to slow muscle proteolysis, HMB, the naturally occurring metabolite of the essential amino acid leucine, is the most active ingredient in the
mixture and has been shown to stimulate protein synthesis (Eley et al.,2007), prevent
prote-olysis (Smith, Wyke, & Tisdale,2004), and improve nitrogen balance in critically ill patients
(Kuhls et al.,2007).
GLN, traditionally considered to be a nonessential amino acid, is now regarded as “con-ditionally essential” during inflammatory response and the hypermetabolic state (Fan et al.,
2015). It is well known that GLN is an important energy source for the intestinal
epithe-lium, and its supplementation protects intestinal epithelial cells by induction of glutathione
(Venoji et al.,2015). GLN supplementation has shown protection against intestinal injury
by ischemia–reperfusion, bacterial overgrowth, or surgical stress (Fan et al.,2015; Thomas,
Prabhu, & Balasubramanian, 2005). Numerous published data have demonstrated that
GLN can improve gut-barrier function, modulate inflammatory response, and stimulate
immune function (Bollhalder, Pfeil, Tomonaga, & Schwenkglenks,2013; Cavalcante et al.,
2012; Demirkan, Savas¸, & Melli, 2010; Fan et al., 2015). In a recent study, Gong, Yuan,
Dong, and Peng (2017) showed that GLN with probiotics attenuates intestinal inflammation
and oxidative stress in a rat burn injury model. Moreover ,GLN, which is a regulator of muscle turnover, protects immune and gut-barrier function during radiochemotherapy in
patients with advanced cancer (Boza et al.,2001; Yoshida et al.,2001). ARG and GLN have
been reported to increase collagen synthesis and protein synthesis (Witte, & Barbul,2003;
Table .Abbreviations used for the study groups.
Group (G)
G Sham-irradiated control group G Radiotherapy control group
G HMB/GLN/ARG control group
G RT+ HMB/GLN/ARG control group HMB= β-hydroxy-β-methylbutyrate; GLN = glutamine; ARG = arginine.
exert anticatabolic effects in cancer patients and reduce atrophy (Ham, Caldow, Lynch, &
Koopman,2014). In a recent study, daily ARG supplementation reduced mucositis levels
in the small intestine after 5-FU chemotherapy (Balmant et al., 2018). Considering these
findings, we hypothesized that oral supplementation with HMB/GLN/ARG may attenuate radiation-induced acute intestinal toxicity.
Materials and methods
Study design
The study included 40 adult female Wistar albino rats (250–300 g), the use of which was approved by Necmettin Erbakan University Kombassan Animal Care and Use Committee. Animals were housed four per cage in a controlled animal holding room with a 12-/12-hour light/dark cycle; temperature and relative humidity were continually monitored to provide standard laboratory conditions. Animals had free access to standard rat chow diet. All exper-iments were carried out in compliance with the regulations of our institution and the 3R (reduction, replacement, refinement) ethical guidelines. Rats were randomly divided into four groups (G) composed of 10 animals. G1 was defined as control group, and G2 was the radi-ation therapy (RT) group. G3 and G4 were HMB/GLN/ARG control and 12.5 Gy RT plus
HMB/GLN/ARG groups, respectively (Table 1). As an end point, the extent of smoothing
in the surface epithelium, atrophy of the villus, inflammation in the lamina propria, crypti-tis and distortion of the crypt, regenerative atypia, vascular dilatation, congestion and fibro-sis for each rat were quantified with image analyfibro-sis of histological sections of the intestinal mucosa.
Irradiation protocol
RT was applied under general anesthesia with intraperitoneally administered 90 mg/kg ketamine hydrochloride (Ketalar, EWL Eczacibasi Warner Lambert Ilaç Sanayi ve Ticaret A.S., Istanbul, Turkey) and 10 mg/kg xylazine (Rompun 2%, Bayer Kimya San. Ltd. Sti., Istanbul, Turkey). A single dose of 12.5 Gy, which has been shown to lead to intestinal mucosal injury
(Yoon et al.,2012), with 6-MV photon beams, was applied to the whole abdominal region via
anterior 5× 5 cm single portal field with source-axis distant technique. The distance from a
source center to abdomen cavity was 100 cm, and a 1 cm bolus was used to build up the radia-tion dose on the abdomen. After irradiaradia-tion, the animals were closely observed until recovery from anesthesia. The rats of G1 received an equal field sham irradiation.
B-Hydroxy-β-methylbutyrate, L-glutamine, and L-arginine protocols
The active supplement consisting of 5.2 g of HMB, 29.6 g arginine, and 29.6 g of glutamine (Abound, Abbott Park, IL, USA), which was equivalent to 60 kg adult dose, was calculated for
each rat and administrated orally with drinking water. HMB/GLN/ARG started from the day of RT and continued until the animals were sacrificed 10 days after the RT.
Morphologic study and light microscopy
The animals were anesthetized with 90 mg/kg ketamine hydrochloride and 10 mg/kg xyalazine and sacrificed by cervical dislocation 10 days after the start of irradiation. The intestinal samples obtained from each rat were excised and fixed in 10% neutral buffered formaldehyde, and representative parts were embedded in paraffin after routine processing of the tissues. Five-micrometer-thick sections were obtained from each paraffin block. After deparaffinizing and rehydrating, the sections were stained with hematoxylin-eosin (H&E) to evaluate the histopathological changes and with histochemical Masson trichrome staining to identify the fibrosis; they were examined under the light microscope (Olympus; BX51). Dig-ital images (Olympus; DP72) were recorded for each sample.
The extent of smoothing in the surface epithelium, atrophy of the villus, inflammation in the lamina propria, cryptitis and distortion of the crypt, regenerative atypia, vascular dilata-tion and congesdilata-tion, and fibrosis for each rat was quantified on each histological secdilata-tion of the
intestinal mucosa. All the parameters were scored between 0 and 3 (0= none; 1 = minimal;
2= moderate; 3 = severe). The pathologist was not aware of the treatment groups at the time
of the histological examination of the specimens.
Immunohistochemistry staining and scoring procedure
Paraffin-embedded tissues of chosen slides were collected, and 4 µm thick sections were prepared for immunohistochemistry. The sections were deparaffinized at 37ºC in the oven overnight. Immunohistochemical staining was performed using an automatic staining machine (Ventana, Benchmark XT). The sections were boiled in sodium citrate buffer at
95ºC for 60 min and then incubated with primary antibody anti TGF-β rabbit polyclonal
antibody (ABCAM, ab92486, Cambridge, UK) at a dilution of 1:100 for 52 minutes. The sections were incubated with the secondary antibody for 20 min at room temperature, incubated with Ultra iView detection kit, and counterstained with hematoxylin for 8 min.
The immunohistochemical TGF-β stained slides were evaluated and scored by a single
pathologist blinded to patients’ data. On light microscopic evaluation of each 4 µm thick
section, 10 different fields magnified 100× were reviewed. Immunoreactivity scoring
sys-tem (IRS), which we described in our previous study (Celik et al.,2017), was used to
deter-mine TGF-β expression levels. This system depends on multiplication of staining intensity
and TGF-β positive intestinal epithelial cell percentage. The percentage of positive cells was
scored as 0= negative; 1 = 1%–25%; 2 = 26%–50%; 3 = 51%–75%; 4 = 76%–100%; staining
intensity was scored as 0= (−), 1 = (+), 2 = (++), 3 = (+++).
Statistical analysis
Statistical analyses were performed using SPSS version 16.0 (SPSS Inc, Chicago, IL). Values
were expressed as mean± standard error of the mean. Groups of data were compared with
an analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests; p< .05 was
Table .Body-weight changes of the animals of all groups at the beginning of the study and on the day of sacrifice.
Group At the beginning of the study On day of sacrifice G – (mean: .) – (mean: .) G – (mean: .) – (mean: .) G – (mean: .) – (mean: .) G – (mean: ) – (mean: .)
Results
No mortality was observed in any group. Histopathological and immunohistochemical anal-yses were made on 40 rats. All animals were evaluated using the same microscopic procedures by the blinded pathologist. We did not observe any difference with respect to the body weights of the animals between groups both at the beginning of the study and on the day of sacrifice (Table 2).
Histopathological findings
In G1, a normal epithelial and glandular structure was observed in regular morphology. In G2, radiation-induced mucosal damage was observed with respect to all parameters, includ-ing the extent of smoothinclud-ing in the surface epithelium, atrophy of the villus, inflammation in the lamina propria, cryptitis, distortion of the crypt, regenerative atypia, vascular dilatation,
congestion, and fibrosis (Figure 1). The findings of G3 were similar to those of the control
group. In G4, the histopathological findings were more severe than in G1 and G3 but less
severe than in G2.Table 3shows the mean scores of all parameters from the corresponding
groups.
There were significant differences between the study groups with respect to the extent of smoothing in the surface epithelium, atrophy of the villus, inflammation in the lamina pro-pria, cryptitis and distortion of the crypt, regenerative atypia, vascular dilatation, congestion,
and fibrosis (p values were .019 for fibrosis,< .001 for the others). The rats from the RT groups
had higher scores for all parameters. The scores of G3, HMB/GLN/ARG control group, were
Figure .Light microscopic appearance with hematoxylin-eosin staining of intestinal samples showing (a) surface epithelial erosion, villous atrophy, and crypt distortion; and (b) severe regenerative atypia of crypt epithelium in radiation therapy (RT) only group (HEX).
Ta b le . Th e semiquantita tiv e sc oring of the ex tent o fs moothing in the sur fac e epithelium, at ro ph y o ft he villous ,inflamma tion in the lamina pr opria, cr ypti tis and dist or tion of the cr ypt ,r egenera tiv e at ypia, vascular d ila ta tion and co ngestion, and fi br osis in the int estinal samples of the ra ts . Gr oup SSE mean ± SD V A mean ± SD LPI m ean ± SD C m ean ± SD CD mean ± SD RA mean ± SD VDC m ean ± SD F m ean ± SD G . ± . . ± . . ± . . ± . . ± . . ± . . ± . . ± . G . ± . . ± . . ± . . ± . . ± . . ± . . ± . . ± . G . ± . . ± . . ± . . ± . . ± . . ± . . ± . . ± . G . ± . . ± . . ± . . ± . . ± . . ± . . ± . . ± . SD = standar d devia tion; SSE = sur fac e epithelium smoothing; V A = villous at ro ph y; LPI = lamina pr opria inflamma tion; C = cr yptitis; CD = cr ypt d ist or tion; R A = re g en er at ive at yp ia ;V D C = vascular dila ta tion and congestion; F = fibr osis .
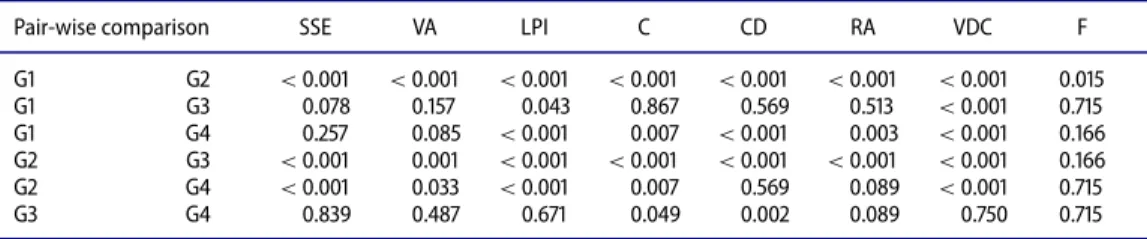
Table .Pair-wise comparisons of study groups with respect to the extent of smoothing in the surface epithelium atrophy of the villous, inflammation in the lamina propria, cryptitis and distortion of the crypt, regenerative atypia, vascular dilatation and congestion, and fibrosis.
Pair-wise comparison SSE VA LPI C CD RA VDC F
G G < . < . < . < . < . < . < . . G G . . . . . . < . . G G . . < . . < . . < . . G G < . . < . < . < . < . < . . G G < . . < . . . . < . . G G . . . . . . . . SSE= surface epithelium smoothening; VA = villous atrophy; LPI = lamina propria inflammation; C = cryptitis; CD = crypt
distortion; RA= regenerative atypia; VDC = vascular dilatation and congestion; F = fibrosis.
similar to the scores of G1 except for lamina propria inflammation score and vascular dilata-tion and congesdilata-tion score. Both scores were significantly higher in G3 than in G1 (p values
were.043 and< .001, respectively). G2 had significantly higher scores than G4 for all
param-eters except for the crypt distortion, regenerative atypia, and fibrosis scores (p values were
.569, .089, and .715, respectively) (Table 4,Figures 2and3). The fibrosis scores were
simi-lar between the study groups. The only differences regarding fibrosis scores were observed
between G1 and G2 (p= .015) (Table 4). Masson trichrome staining revealed that, in the
G2, collagen fibers significantly increased at the lamina propria and submucosa compared to
G1 (p= .015). In G4, the amount of the collagen fibers was lower than in G2; however, the
difference was not statistically significant (p= .715) (Figure 3).
Last, the lamina propria inflammation, cryptitis, crypt distortion, regenerative atypia, and vascular dilatation and congestion scores were significantly higher in G4 when compared to
G1 (p values were<.001, 0.007, <.001, .003, and <.001, respectively) (Table 4).
Immunohistochemical findings
Table 5shows immunohistochemical TGF-β staining scores of the intestinal epithelial cells of
the rats. The TGF-β scores were significantly different between the study groups (p = .004).
The pair-wise comparisons revealed that the TGF-β score of G2 was significantly higher than
Figure .Light microscopic appearance of intestinal mucosa samples from (a) RT-only group and (b) RT+Abound group. (a) Severe (score ) inflammation of intestinal mucosa in RT-only group (HEX); (b) minimal (score ) inflammation, preservation of villous structures of intestinal mucosa in RT+Abound group. RT= radiation therapy.
Figure .Immunohistochemical Masson trichrome staining of intestinal mucosa samples from (a)
RT-only group and (b) RT+Aboud group. (a) Moderate (score ) fibrosis of submucosa in RT group (Masson
trichromeX); (b) mild (score ) fibrosis of submucosa in RT+Abound group (Masson trichromeX).
RT= radiation therapy.
those of G1 and G3 (p values were .006 and .017, respectively). There was no difference with
respect to TGF-β staining scores of G2 and G4 (p = .409).
Discussion
The present study sought to evaluate the impact of HMB/GLN/ARG supplementation on radiation-induced acute intestinal toxicity. Our results suggest that HMB/GLN/ARG sup-plementation ameliorated radiation-induced intestinal toxicity by improving the surface epithelium smoothing, villous atrophy, lamina propria inflammation, cryptitis, and vascular dilatation and congestion in the intestinal epithelium.
Abdominal or pelvic RT is now commonly used for primary or adjunctive treatment of gynecologic, genitourinary, and colorectal malignancies. Unfortunately, radiation-induced intestinal toxicity, or radiation enteritis, remains a problem during abdominal/pelvic
irra-diation (Waddell, Rodriguez-Bigas, Lee, Weber, & Petrelli,1999). The gastrointestinal tract
is the second-most sensitive organ to radiation, after bone marrow (Grammaticos et al.,
2013). One-half to three-quarters of patients given RT develop acute symptoms of radiation
enteritis (Becciolini et al.,1997). Radiation enteritis can limit the maximum tolerated dose of
RT and chemotherapy and thus may limit the efficacy of treatment. Abdominal irradiation causes mucosal damage in the gastrointestinal epithelium by activating inflammatory cells. Table .The semiquantitative scoring of TGF-β staining in the intestinal samples of the rats.
Group TGF-β staining score (mean ± SD) p value Pair-wise comparison p value
G .± . .∗ G–G .∗ G .± . G–G . G .± . G–G . G .± . G–G .∗ G–G . G–G . SD= standard deviation. ∗p< ..
Radiation-induced mucosal injury in the intestine is characterized by destruction of crypt cells, decrease in villous height and number, ulceration, and necrosis of the gastrointestinal
epithelium (Erbil et al.,2005; Hwang et al.,2003; Maj et al.,2003). In the clinical practice,
radiation enteritis can be associated with morbidity and can lead to discomfort not only dur-ing the acute phase but also months or even years after treatment has ceased (Hasleton et al.,
1985; Andreyev,2005; Galland, & Spencer,1985).
The initial histologic findings of radiation-induced acute intestinal injury are seen follow-ing hours of irradiation. After this, there is infiltration of leukocytes with crypt abscess for-mation within two to four weeks; ulceration may be seen. Stem cell damage, either acutely as a direct consequence of radiation or subsequently as a result of microvascular damage, leads to a decrease in cellular reserves for the intestinal villi. This results in mucosal denudation with associated intestinal inflammation, edema, shortened villi, and decreased absorptive area
(Brian et al.,2017). Progressive occlusive vasculitis with foam cell invasion of the intima and
hyaline thickening of the arteriolar walls, as well as collagen deposition and fibrosis, may be
observed (Brian et al.,2017; Hasleton et al.,1985). In the clinical practice, mucosal ulcerations
may lead to perforation, fistulas, or abscess formation after irradiation. There can be fibrosis during the healing of ulcers that causes narrowing of the lumen of the intestine and stric-ture formation or even obstruction, which severely deteriorates the quality of life in cancer patients. Stasis can lead to small intestinal bacterial overgrowth. Even if the intestine appears
normal, patients are at risk of spontaneous perforation (Brian et al.,2017; Galland, & Spencer,
1985).
The amino acid leucine has been shown to stimulate skeletal muscle protein synthesis and attenuate muscle proteolysis. HMB, a leucine metabolite, has been demonstrated to be
anti-catabolic and effective at attenuating muscle atrophy during exercise stress (Nissen et al.,1996;
Pimentel et al.,2011) and in models of cancer (Nunes et al.,2008), congestive heart failure,
sepsis, and HIV (Eley, Russel, & Tisdale,2008).
L-Glutamine (GLN) is considered a nonessential amino acid, although it has many pri-mary biochemical roles. It is the preferred energy source for cells with rapid turnover such as lymphocytes, enterocytes, and malignant cells while also being indirectly involved in the regu-lation of protein synthesis. GLN is also a precursor for DNA and glutathione (GSH) synthesis. It is an essential energy source in the maintenance and restoration of the gastrointestinal (GI)
tract (Eley et al.,2008). Mucositis affects the rapidly dividing mucosal cells of the entire GI
tract. These GI cells normally have a short life span of three to four days and use GLN as an oxidative fuel source. Oncological therapies such as RT can destroy these cells quickly (Noé,
2009; Skubitz, & Anderson,1996). If these cells are not replaced right away, an inflammatory
process begins that creates mucositis.
There are many clinical and preclinical studies in the literature investigating the role of GLN in radiation-induced intestinal toxicity; however, their results are conflicting. Richards
et al. (1992) investigated the effect of GLN (21 g/d) radiation-induced intestinal toxicity in
prostate cancer patients (Vidal-Casariego, Calleja-Fernández, Cano-Rodríguez, Cordido, &
Ballesteros-Pomar,2015). Their results suggested that the use of GLN was associated with less
tissue damage in the rectum, but there was no difference in stool frequency. Vidal-Casariego
et al. (2014) found that the administration of oral GLN (30 g/d) during abdominal or pelvic
RT was not useful for the prevention of acute enteritis and could have favored the develop-ment of diarrhea. In a study that mainly evaluated the patients with gynecological tumors,
GLN (30 g/d) ameliorated the acute radiation-induced intestinal toxicity (Costa et al.,2003).
These conflicting results may be attributed to different doses of GLN as well as different treat-ment sites and radiation doses. In the current study, we used the irradiation dose that has
been shown to induce radiation-induced acute intestinal toxicity in rat models (Alsan Cetin
et al.,2015; Costa et al.,2003; Vidal-Casariego et al.,2014). In addition, we used the active
supplement consisting of 5.2 g of HMB, 29.6 g GLN, and 29.6 g of ARG, which was equivalent to a 60 kg adult dose in this study.
Among the immunonutrients that are often used in the composition of these immunomod-ulating formulas, L-arginine (ARG) is the best-studied one as a single agent, and evidence
suggests that it has beneficial effects in wound healing (Chow, & Barbul,2014). Its various
combinations in different nutrition supplements have been shown to reduce anastomotic leaks
(Waitzberg et al.,2006), increase collagen deposition (Williams et al.,2002), and enhance the
healing of pressure ulcers (Cereda, Klersy, Serioli, Crespi, & D’Andrea,2015; van Anholt et al.,
2010). Dietary ARG supplementation, above the amounts of optimal growth, nitrogen
bal-ance, or health, increases wound collagen accumulation in healthy rodents and humans (Kirk
et al.,1993; Seifter, Rettura, Barbul, & Levenson,1978). L-arginine–containing supplements
were also studied in radiation-induced intestinal toxicity. Atasoy et al. (2010) demonstrated
that prophylactic administration of an ARG-rich diet protected against chemoradiation-induced gastrointestinal injury. In an experimental study, Gurbuz, Kunzelman, and Ratzer
(1998) examined the effect of 2% ARG, 4% ARG, and 4% glycine on radiation-induced acute
enteritis in male Sprague-Dawley rats. They used the dose of 11 Gy to the abdominal area and investigated ARG levels in blood in addition to quantitative aerobic and anaerobic cultures and mesenteric lymph nodes. They also performed morphometric examination including villous height, number of villi per centimeter of intestine, and the number of mucous cells per villous in jejunum and ileum segments. Their results suggested that dietary 4% ARG supplementation enhanced bacterial clearance from mesenteric lymph nodes compared to both 2% ARG and 4% glycine supplementation following acute radiation enteritis. Moreover, they showed that 4% ARG resulted in clear improvement in intestinal mucosal recovery when compared to 2% ARG and 4% glycine after abdominal irradiation in rats.
Recent reports have suggested that the combination of these amino acids, HMB/GLN/ ARG, increases collagen accumulation in the tissues and preserves muscle mass in elderly
individuals (Williams et al.,2002) as well as in cachexia due to cancer, rheumatoid arthritis,
and AIDS (Berk et al.,2008; Clark et al.,2000; Marcora et al.,2005) and may have a positive
influence on the healing of some diabetic foot ulcers (Armstrong et al.,2014; Tatti, Barber, di
Mauro, & Massel,2010). In our previous experimental study, we investigated the impact of
HMB/GLN/ARG on radiation-induced acute inflammation and mucosal atrophy in the oral
mucosa (Yavas et al.,2013). Our results suggested that the use of HMB/GLN/ARG
supple-mentation was effective in preventing radiation-induced acute mucositis in rats. Although the exact mechanisms of both radiation-induced oral mucositis and radiation enteritis have not been clearly demonstrated, since the mucosal linings of both tissues have similar characteris-tics, findings in the oral mucosa are expected to be seen in the intestinal mucosa. Therefore, we aimed to investigate the effect of HMB/GLN/ARG on radiation-induced intestinal toxicity using an experimental rat model. We demonstrated that HMB/GLN/ARG supplementation ameliorated radiation enteritis by decreasing smoothing in the surface epithelium, atrophy of the intestinal villi, inflammation in the lamina propria, and vascular congestion and dilatation.
Alsan Cetin et al. (2015) evaluated the effects of HMB/GLN/ARG on
chemoradiation-induced intestinal toxicity in a rat model. They assessed the mucosal thickness (from the base of the muscularis mucosa to the villus tip), villus height, crypt height, and
number of villi/mm2 during histomorphological examination. Their results suggested that
HMB/GLN/ARG supplementation effectively prevents chemoradiation-induced acute gas-trointestinal injury. Our results were consistent with their findings. Moreover, in the current
study, we evaluated the extent of surface epithelium smoothing, lamina propria inflammation, regenerative atypia, vascular dilatation and congestion, and fibrosis in addition to changes in villus and crypt morphology.
In our previous study we demonstrated that HMB/GLN/ARG supplementation led to epithelial hypertrophy in the oral mucosa. In addition, when used with RT, HMB/GLN/ARG supplementation reversed RT-induced atrophy in the epithelial layer of the oral mucosa (Yavas
et al., 2013). Similarly, in the current study, HMB/GLN/ARG supplementation improved
villous atrophy in intestinal mucosa. However, in the current study, HMB/GLN/ARG sup-plementation deteriorated the lamina propria inflammation and vascular dilatation and congestion when compared to control group. We think that the HMB/GLN/ARG supple-mentation itself might have stimulated the inflammatory cytokines and resulted in lamina propria inflammation and vascular dilatation and congestion.
In the current study, we demonstrated a significant difference in terms of the fibrosis
scores only between G1 and G2 with Masson trichrome staining. On the other hand, TGF-β
immunohistochemical staining showed significant differences between G2 and G3 in addition
to between G1 and G2. We believe that the TGF-β staining method we used in the current
study was more quantitative than the histochemical Masson trichrome staining; therefore,
the TGF-β scoring system might have been more sensitive. Nevertheless, both scoring
sys-tems failed to show any difference between G2 and G4. However we aimed to determine acute effects of radiation on intestinal epithelial cells and, fibrosis is a chronic effect of irradiation (fibrosis is not acute effect of radiation ttherefore we could not show any difference).
The irradiation doses used in routine clinical practice are different from those used in ani-mal studies. Most patients in routine practice are treated with conventional fractionation to a total dose of 50–70 Gy, and it is given in 25–35 fractions. In animal studies, it is not prac-tical to use the fractionated schemes. Therefore, the biologically equivalent dose of the same total dose in one fraction should be calculated. According to the linear quadratic model, the 12.5 Gy single dose of radiation, as used in the current study, corresponds to approximately 45 Gy. In experimental studies evaluating the acute radiation enteritis, the chosen irradiation
doses were 10–20 Gy (Gurbuz et al.,1998; Yoon et al.,2012; Alsan-Cetin et al.,2015; Nunes
et al.,2017; Tas, Ozkul, Arik, Kiraz, & Vural,2016). Therefore, in the current study, we used
a single dose of 12.5 Gy, which has been shown to induce radiation enteritis.
The intestinal microbiota have an important role in the pathogenesis of mucositis via (1) modification of intestinal barrier function, (2) innate immunity mechanisms, and (3)
intestinal repair mechanisms (Touchefeu et al.,2014). It has been postulated that bacterial
translocation plays a central role in the development of radiation enteropathies due to the dis-ruption of the intestinal mucosal barrier, stimulation of bacterial overgrowth, and decreased
peristaltic clearance (Guzman-Stein, Bonsack, Liberty, & Delaney,1989). Dysbiosis might
play a part in radiation enteropathy. The gut flora is heterogeneous, and different
microor-ganisms have various radiosensitivities (Ferreira, Muls, Dearnaley, & Andreyev, 2014).
Gerassy-Vainberg et al. (2018) demonstrated that the most significant shift in microbial
com-position was specifically noted in the inflamed colon six weeks after irradiation, with reduced Firmicutes abundance and increased abundance of Proteobacteria. Transmission of the radiation-altered microbiota rendered susceptibility to radiation injury. In a recent study it was found that HMB/GLU/ARG supplementation before and during chemoradiation reduced
bacterial counts in both the cecum and mesenteric lymph nodes (Alsan Cetin et al.,2015).
We have some limitations in the current study that are worth mentioning as well. First, we used only light microscopy and put out only histomorphological changes. The ultrastructural changes might be assessed using electron microscopy. Second, we could not demonstrate any
significant differences with respect to fibrosis scores between RT and RT+ HMB/GLN/ARG groups, as we evaluated only acute phase. Third, we did not perform any microbial analyses. However, we are planning a new study evaluating the changes in microbial environment after irradiation as well as the effect of cytokines during this process.
In conclusion, our experimental study supports that the use of HMB/GLN/ARG supple-mentation is effective in ameliorating radiation-induced intestinal toxicity. Our results were encouraging; however, these findings should be confirmed with clinical studies with long-term follow-up.
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding
This work was supported by Selcuk University (Project number: 14401052). There is no role of study sponsors in the study design, in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
About the authors
Cagdas Yavashas a MD in Radiation Oncology. He is an associate professor in Selcuk University. He is the chair of Selcuk University, Faculty of Medicine, and Department of Radiation Oncology. His research interests are prostate cancer, lung cancer and central nervous system tumors.
Guler Yavashas a MD in Radiation Oncology. She is an associate professor in Selcuk University. Her research interests are gynecological cancer, breast cancer, pediatric tumors and head and neck cancer. Esin Celikhas a MD in Pathology. She is an assisted professor in Selcuk University. Her research inter-ests are genitourinary tumors, sarcomas, lymphoma and head and neck cancer.
Ahmet Buyukyorukhas a MD in Radiation Oncology. He is working in Konya Research and Training Hospital. His research interests are lung cancer and head and neck cancer.
Cennet Buyukyorukhas a MD in Family Medicine. She is working in Necmettin Erbakan University, Department of Family Medicine.
Deniz Yucehas a MD in Medicine and, a PhD in Epidemiology. He is working in Hacettepe Univer-sity, Faculty of Medicine, Department of Preventive Oncology. His research interests are epidemiology, biostatistical analysis and preventive oncology.
Ozlem Atais a Professor of Internal medicine and Medical Oncology She is working in Selcuk Uni-versity. Her research interests are gynecological cancer, breast cancer, lung cancer and gastrointestinal system tumors.
References
Alsan Cetin, Atasoy BM, Cilaker S, Alicikus LZ, Karaman M, Ersoy N, Demiral AN, Yilmaz
O. 2015. A Diet Containing Beta-Hydroxy-Beta-Methylbutyrate, L-glutamine and L-arginine
ameliorates chemoradiation-induced gastrointestinal injury in rats. Radiat Res. 184(4):411–21. doi:10.1667/RR14088.1. PMID:26430821.
Andreyev J.2005. Gastrointestinal complications of pelvic radiotherapy: are they of any importance?
Armstrong DG, Hanft JR, Driver VR, Smith AP, Lazaro-Martinez JL, Reyzelman AM, Furst
GJ, Vayser DJ, Cervantes HL, Snyder RJ, et al. 2014. Diabetic Foot Nutrition Study Group.
Effect of oral nutritional supplementation on wound healing in diabetic foot ulcers: a
prospective randomized controlled trial. Diabet Med. 31(9):1069–77. doi:10.1111/dme.12509.
PMID:24867069.
Atasoy BM, Deniz M, Dane F, Özen Z, Turan P, Ercan F, Çerikçio˘glu N, Aral C, Akgün Z, Abacio˘glu U,
et al.2010. Prophylactic feeding with immune-enhanced diet ameliorates chemoradiation-induced
gastrointestinal injury in rats. Int J Radiat Biol. 86(10):867–79. doi:10.3109/09553002.2010.487026. PMID:20653343.
Balmant BD, Araújo EON, Yabuki D, Novais AB, Genaro SC, Laposy CB, Goiozo PFI, Chacur
MGM, Giuffrida R, Reis LSLS.2018. Effects of L-arginine supplementation on leukogram,
inflam-matory bowel infiltrates and immunoglobulins with 5-FU use in rats. Nutr Cancer. 70:249–56. doi:10.1080/01635581.2018.1424346. PMID:29345500.
Becciolini A, Balzi M, Fabbrica D, Potten CS.1997. The effects of irradiation at different times of the day on rat intestinal goblet cells. Cell Proliferation. 30:161–70. doi:10.1111/j.1365-2184.1997.tb00932.x. PMID:9375028.
Berk L, James J, Schwartz A, Hug E, Mahadevan A, Samuels M, Kachnic L.2008. A randomized,
double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer. 16:1179–88. doi:10.1007/s00520-008-0403-7. PMID:18293016.
Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M.2013. A systematic literature review and
meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr. 32:213–
23. doi:10.1016/j.clnu.2012.11.003. PMID:23196117.
Boza JJ, Turini M, Moënnoz D, Montigon F, Vuichoud J, Gueissaz N, Gremaud G, Pouteau E, Piguet-Welsch C, Finot PA, et al.2001. Effect of glutamine supplementation of the diet on tissue protein syn-thesis rate of glucocorticoid-treated rats. Nutrition. 17:35–40. doi:10.1016/S0899-9007(00)00505-0. PMID:11165886.
Brian GC, Meyer JJ, Willett CG. 2017. Overview of gastrointestinal toxicity of radiation therapy.
Up to date 2017. Available online: https://www.uptodate.com/contents/overview-of-gastroin
testinal-toxicity-of-radiation-therapy?source=search_result&search=radiation%20induced%20 intestinal%20toxicity&selectedTitle=1∼150.
Caro MM, Laviano A, Pichard C, Candela CG.2007. Relationship between nutritional intervention and
quality of life in cancer patients. Nutr Hosp. 22(3):337–50. PMID:17612376.
Cavalcante AA, Campelo MW, de Vasconcelos MP, Ferreira CM, Guimarães SB, Garcia JH, de
Vasconcelos PR. 2012. Enteral nutrition supplemented with L- ∼ glutamine in patients with
systemic inflammatory response syndrome due to pulmonary infection. Nutrition. 28:397–402. doi:10.1016/j.nut.2011.07.011. PMID:22055478.
Celik ZE, Kaynar M, Karabagli P, Gergerlioglu N, Goktas S.2017. The relation between Ring
Box-1 protein overexpression and tumor grade and stage in bladder urothelial cell carcinoma. Cancer
Biomark. 20(4):389–94. doi:10.3233/CBM-170002. PMID:28946546.
Cereda E, Klersy C, Serioli M, Crespi A, D’Andrea F; for the OligoElement Sore Trial Study Group.
2015. A nutritional formula enriched with arginine, zinc, and antioxidants for the healing of
pressure ulcers: a randomized trial. Ann Intern Med. 162(3):167–74. doi:10.7326/M14-0696.
PMID:25643304.
Chow O, Barbul A.2014. Immunonutrition: role in wound healing and tissue regeneration. Adv Wound
Care (New Rochelle). 3(1):46–53. doi:10.1089/wound.2012.0415. PMID:24761344.
Clark RH, Feleke G, Din M, Yasmin T, Singh G, Khan FA, Rathmacher JA.2000. Nutritional treatment
for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. JPEN J Parenter
Enter Nutr. 24:133–9. doi:10.1177/0148607100024003133.
Costa F, Mumolo MG, Bellini M, Romano MR, Ceccarelli L, Arpe P, Sterpi C, Marchi S, Maltinti G. 2003. Role of calprotectin as non-invasive marker of intestinal inflammation. Dig Liv Dis. 35:642–7. doi:10.1016/S1590-8658(03)00381-5.
Demirkan A, Savas¸ B, Melli M. 2010. Endotoxin level in ischemia-reperfusion injury in rats:
effect of glutamine pretreatment on endotoxin levels and gut morphology. Nutrition. 26:106–11. doi:10.1016/j.nut.2009.04.010. PMID:19596185.
Denton A, Forbes A, Andreyev J, Maher EJ.2002. Non surgical interventions for late radiation proc-titis in patients who have received radical radiotherapy to the pelvis. Cochrane Database Syst Rev. 1:CD003455.
Dincbas FO, Oksüz DC, Atalar B, Altug T, Ilvan S, Gedik N, Ozel S, Koca S. 2009. The role
of amifostine on late normal tissue damage induced by pelvic radiotherapy with
concomi-tant gemcitabine: an in vivo study. Med Oncol. 26:402–8. doi:10.1007/s12032-008-9136-1.
PMID:19043677.
Eley HL, Russel ST, Tisdale MJ.2008. Attenuation of depression of muscle protein synthesis induced by
lipopolysaccharide, tumor necrosis factor and angiotensin II by beta-hydroxy-beta-methylbutyrate.
Am J Physiol Endocr Metab. 295:1409–16. doi:10.1152/ajpendo.90530.2008.
Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. 2007. Signaling pathways initiated by
beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skele-tal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab. 293:E923–31. doi:10.1152/ajpendo.00314.2007. PMID:17609254.
Erbil Y, Oztezcan S, Giri¸s M, Barbaros U, Olgaç V, Bilge H, Küçücük H, Toker G.2005. The effect of
glutamine on radiation-induced organ damage. Life Sci. 78:376–82. doi:10.1016/j.lfs.2005.04.068.
PMID:16129454
Fan J, Li G, Wu L, Tao S, Wang W, Sheng Z, Meng Q.2015. Parenteral glutamine supplementation in
combination with enteral nutrition improves intestinal immunity in septic rats. Nutrition. 31:766–
74. doi:10.1016/j.nut.2014.11.021. PMID:25837225.
Ferreira MR, Muls A, Dearnaley DP, Andreyev HJ.2014. Microbiota and radiation-induced bowel
tox-icity: lessons from inflammatory bowel disease for the radiation oncologist. Lancet Oncol. 15:e139–
47. doi:10.1016/S1470-2045(13)70504-7. PMID:24599929.
Galland RB, Spencer J.1985. Spontaneous postoperative perforation of previously asymptomatic
irra-diated bowel. Br J Surg. 72:285. doi:10.1002/bjs.1800720412. PMID:3986478.
Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, Gershovich K, Sabo E, Nevelsky A, Daniel S, Dahan A,
Ziv O, Dheer R, et al.2018. Radiation induces proinflammatory dysbiosis: transmission of
inflam-matory susceptibility by host cytokine induction. Gut. 67:97–107. doi:10.1136/gutjnl-2017-313789.
PMID:28438965.
Gong ZY, Yuan ZQ, Dong ZW, Peng YZ.2017. Glutamine with probiotics attenuates intestinal
inflam-mation and oxidative stress in a rat burn injury model through altered iNOS gene aberrant methy-lation. Am J Transl Res. 9:2535–47. PMID:28560003.
Grammaticos P, Giannoula E, Fountos GP.2013. Acute radiation syndrome and chronic radiation
syn-drome. Hell J Nucl Med. 16(1):56–9. PMID:23570025.
Gurbuz AT, Kunzelman J, Ratzer EE.1998. Supplemental dietary arginine accelerates intestinal mucosal
regeneration and enhances bacterial clearance following radiation enteritis in rats. J Surg Res.
74(2):149–54. doi:10.1006/jsre.1997.5231. PMID:9587353.
Guzman-Stein G, Bonsack M, Liberty J, Delaney JP.1989. Abdominal radiation causes bacterial
translo-cation. J Surg Res. 46:104–7. doi:10.1016/0022-4804(89)90211-4. PMID:2918713.
Ham DJ, Caldow MK, Lynch GS, Koopman R.2014. Leucine as a treatment for muscle wasting: a critical
review. Clin Nutr. 33(6):937–45. doi:10.1016/j.clnu.2014.09.016. PMID:25444557.
Hasleton PS, Carr N, Schofield PF.1985. Vascular changes in radiation bowel disease. Histopathology.
9:517. doi:10.1111/j.1365-2559.1985.tb02833.x. PMID:4007790.
Hwang JM, Chan DC, Chang TM, Tsao TY, Tsou SS, Lu RH, Tsai LM.2003. Effects of oral arginine and
glutamine on radiation-induced injury in the rat. J Surg Res. 109(2):149–54. doi:
10.1016/S0022-4804(02)00096-3. PMID:12643857.
Kirk SJ, Hurson M, Regan MC, Holt DR, Wasserkrug HL, Barbul A. 1993. Arginine
stimu-lates wound healing and immune function in elderly human beings. Surgery. 114:155–60. PMID:8342121.
Kuhls DA, Rathmacher JA, Musngi MD, Frisch DA, Nielson J, Barber A, MacIntyre AD, Coates JE, Fildes JJ.2007. Betahydroxy- beta-methylbutyrate supplementation in critically ill trauma patients.
J Trauma. 62(1):125–32. doi:10.1097/TA.0b013e31802dca93. PMID:17215743.
Kuhn KS, Muscaritoli M, Wischmeyer P, Stehle P.2010. Glutamine as indispensable nutrient in
oncol-ogy: experimental and clinical evidence. Eur J Nutr. 49:197–210. doi:10.1007/s00394-009-0082-2.
Maj J.G, Paris F, Haimovitz-Friedman A, Venkatraman E, Kolesnick R, Fuks Z. 2003. Microvas-cular function regulates intestinal crypt response to radiation. Cancer Res. 63(15):4338–41. PMID:12907601.
Marcora S, Lemmey A, Maddison P. 2005. Dietary treatment of rheumatoid cachexia with
beta-hydroxy-beta-methylbutyrate, glutamine and arginine: a randomized controlled trial. Clin Nutr.
24:442–54. doi:10.1016/j.clnu.2005.01.006. PMID:15896432.
May PE, Barber A, D’Olimpio JT, Hourihane A, Abumrad NN.2002. Reversal of cancer-related
wast-ing uswast-ing oral supplementation with a combination of [beta]-hydroxy-[beta]-methylbutyrate,
arginine, and glutamine. Am J Surg. 183:471–9. doi:10.1016/S0002-9610(02)00823-1.
PMID:11975938.
McGough C, Baldwin C, Frost G, Andreyev HJ. 2004. Role of nutritional intervention in
patients treated with radiotherapy for pelvic malignancy. Br J Cancer. 14;90(12):2278–87. doi:10.1038/sj.bjc.6601868. PMID:15162154.
Molla M, Panes J. 2007. Radiation-induced intestinal inflammation. World J Gastroenterol.
13(22):3043–6. doi:10.3748/wjg.v13.i22.3043. PMID:17589918.
Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC Jr, Connelly AS, Abumrad N.1996. Effect
of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physion. 81:2095–104. doi:10.1152/jappl.1996.81.5.2095.
Noé JE. 2009. L-Glutamine use in the treatment and prevention of mucositis and cachexia:
a naturopathic perspective. Integr Cancer Ther. 8(4):409–15. doi:10.1177/1534735409348865.
PMID:19942578.
Nunes EA, Kuczera D, Brito GA, Bonatto SJ, Yamazaki RK, Tanhoffer RA, Mund RC, Kryczyk M,
Fer-nandes LC.2008. Beta-hydroxy-beta-methylbutyrate supplementation reduces tumor growth and
tumor cell proliferation ex vivo and prevents cachexia in Walker 256 tumor-bearing rats by
mod-ifying nuclear factor-kappaB expression. Nutr Res. 28:487–93. doi:10.1016/j.nutres.2008.04.006.
PMID:19083450.
Nunes VRT, Vidigal PVT, Pereira MT, Ladeira LCD, Barbuto RC, Duval-Araujo I.2017.
Develop-ment of a new model of actinic enteritis in rats using a cobalt-60 open source and a
protec-tion device as a collimator. Acta Cir Bras. 32:319–24. doi:10.1590/s0102-865020170040000007.
PMID:28538806.
Pimentel GD, Rosa JC, Lira FS, Zanchi NE, Ropelle ER, Oyama LM, Oller do Nascimento CM, de Mello
MT, Tufik S, Santos RV.2011.β-Hydroxy-β-methylbutyrate (HMB) supplementation stimulates
skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr Metab. 8:11–18. doi:
10.1186/1743-7075-8-11.
Richards EW, Long CL, Pinkston JA, Ellis V, Mostaghimi M, Gandy RE.1992. The role of oral glutamine
supplementation in the prevention of radiation-induced enterocolitis in prostate cancer patients [abstract]. FASEB J. 6:A1680.
Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME.2003. Nutritional deterioration in cancer: the role
of disease and diet. Clin Oncol (R Coll Radiol). 15(8):443–50. doi:10.1016/S0936-6555(03)00155-9.
PMID:14689999.
Seifter E, Rettura G, Barbul A, Levenson SM.1978. Arginine: an essential amino acid for injured rats.
Surgery. 84:224–30 PMID:684614.
Skubitz KM, Anderson PM.1996. Oral glutamine to prevent chemotherapy induced stomatitis: a pilot
study. J Lab Clin Med. 127:223–8. doi:10.1016/S0022-2143(96)90082-7. PMID:8636652.
Smith HJ, Wyke SM, Tisdale MJ.2004. Mechanism of the attenuation of proteolysis-inducing
fac-tor stimulated protein degradation in muscle by beta-hydroxy-beta-methylbutyrate. Cancer Res.
64:8731–5. doi:10.1158/0008-5472.CAN-04-1760. PMID:15574784.
Tas S, Ozkul F, Arik MK, Kiraz A, Vural A.2016. The effect of amifostine on bacterial
translo-cation after radiation ınduced acute enteritis. Acta Cir Bras. 31:156–60. doi:
10.1590/S0102-865020160030000002. PMID:27050785.
Tatti P, Barber AE, di Mauro P, Massel M.2010. Nutritional supplement is associated with a reduction
in healing time and improvement of fat free body mass in patients with diabetic foot ulcers. EWMA J. 10(3):13–17.
Thomas S, Prabhu R, Balasubramanian KA. 2005. Surgical manipulation of the intestine and
distant organ damage-protection by oral glutamine supplementation. Surgery. 137:48–55. doi:10.1016/j.surg.2004.04.038. PMID:15614281.
Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de
La Cochetière MF.2014. Systematic review: the role of the gut microbiota in chemotherapy- or
radiation-induced gastrointestinal mucositis – current evidence and potential clinical applications. Aliment Pharmacol Ther. 40:409–21. PMID:25040088.
Unsal D, Mentes B, Akmansu M, Uner A, Oguz M, Pak Y.2006. Evaluation of nutritional status
in cancer patients receiving radiotherapy: a prospective study. Am J Clin Oncol. 29(2):183–8. doi:10.1097/01.coc.0000198745.94757.ee. PMID:16601440.
van Anholt RD, Sobotka L, Meijer EP, Heyman H, Groen HW, Topinková E, van Leen M, Schols
JM.2010. Specific nutritional support accelerates pressure ulcer healing and reduces wound care
intensity in non-malnourished patients. Nutrition. 26(9):867–72. doi:10.1016/j.nut.2010.05.009.
PMID:20598855.
Venoji R, Amirtharaj GJ, Kini A, Vanaparthi S, Venkatraman A, Ramachandran A.2015. Enteral
tamine differentially regulates Nrf 2 along the villus-crypt axis of the intestine to enhance
glu-tathione levels. J Gastroenterol Hepatol. 30:1740–7. doi:10.1111/jgh.13019. PMID:26095579.
Vidal-Casariego A, Calleja-Fernández A, Cano-Rodríguez I, Cordido F, Ballesteros-Pomar MD.2015.
Effects of oral glutamine during abdominal radiotherapy on chronic radiation enteritis: a random-ized controlled trial. Nutrition. 31(1):200–4. doi:10.1016/j.nut.2014.08.003. PMID:25466666. Vidal-Casariego A, Calleja-Fernández A, de Urbina-González JJ, Cano-Rodríguez I, Cordido
F, Ballesteros-Pomar MD. 2014. Efficacy of glutamine in the prevention of acute
radia-tion enteritis: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 38(2):205–13. doi:10.1177/0148607113478191. PMID:23471208.
Waddell BE, Rodriguez-Bigas MA, Lee RJ, Weber TK, Petrelli NJ.1999. Prevention of chronic radiation
enteritis. J Am Coll Surg. 189(6):611–24. doi:10.1016/S1072-7515(99)00199-4. PMID:10589598.
Waitzberg DL, Saito H, Plank LD, Jamieson GG, Jagannath P, Hwang TL, Mijares JM, Bihari D.
2006. Postsurgical infections are reduced with specialized nutrition support. World J Surg. 30(8):
1592–604. doi:10.1007/s00268-005-0657-x. PMID:16794908.
Williams JZ, Abumrad N, Barbul A.2002. Effect of a specialized amino acid mixture on human collagen
deposition. Ann Surg. 236(3):369–74. doi:10.1097/00000658-200209000-00013. PMID: 12192323.
Witte MB, Barbul A.2003. Arginine physiology and its implication for wound healing. Wound Repair
Regen. 11:419–23. doi:10.1046/j.1524-475X.2003.11605.x. PMID:14617280.
Yavas C, Yavas G, Acar H, Toy H, Yuce D, Akyurek S, Ata O. 2013. Amelioration of
radiation-induced acute inflammation and mucosal atrophy by beta-hydroxy-beta-methylbutyrate, L-glutamıne, and L-argınıne: results of an experimental study. Support Care Cancer. 21(3):883–8. doi:10.1007/s00520-012-1601-x. PMID:22993027.
Yoon WS, Kim CY, Yang DS, Park YJ, Park W, Ahn YC, Kim SH, Kwon GY.2012. Protective effect of
triphala on radiation induced acute intestinal mucosal damage in Sprague Dawley rats. Indian J Exp Biol. 50:195–200. PMID:22439434.
Yoshida S, Kaibara A, Ishibashi N, Shirouzu K.2001. Glutamine supplementation in cancer patients.