Hexavalent chromium removal by magnetic particle-loaded micro-sized
chitinous egg shells isolated from ephippia of water
flea
Gulsin Arslan
a, Idris Sargin
a,⁎
, Murat Kaya
ba
Selcuk University, Faculty of Science, Department of Biochemistry, 42075 Konya, Turkey
bAksaray University, Faculty of Science and Letters, Department of Biotechnology and Molecular Biology, 68100, Aksaray, Turkey
a b s t r a c t
a r t i c l e i n f o
Article history:
Received 11 December 2018
Received in revised form 31 December 2018 Accepted 28 January 2019
Available online 5 February 2019
Modified chitin and magnetic particles are two important materials widely used in heavy metal removal studies. Loading of magnetic particles into conventional adsorbents has emerged as a recent convenient way to improve the properties of adsorptive materials. Compared to its deacetylated form chitosan, chitin has very limited use in removal of contaminants because of its insolubility in aqueous environments. This study reports an easy way to produce micro-sized chitin and gives details on loading of magnetic particles into micro-sized chitin to enhance the interaction of chitin with heavy metal contaminant; hexavalent chromium Cr(VI). Removal of Cr(VI) ions from the aquatic environments is of high importance considering its detrimental effects on human health. Chitin microcages that had been isolated from the ephippial eggs of Daphnia longispina (crustacean, waterflea) were incorporated with magnetic particles. Chitin microcages and magnetic particles-loaded chitin microcages were tested in removal of Cr(VI) under different solution and operational parameters; adsorbent dosage, contact time, Cr(VI) ion concentration, pH and temperature. Magnetic particles-loaded chitin microcages yielded higher Cr(VI) removal performance under all the specified conditions; chitin microcages: 0.77 mmol/ g and particles-loaded chitin microcages: 1.29 mmol/ g.
© 2019 Elsevier B.V. All rights reserved.
Keywords: Cr(VI) Ephippium Chitin isolation
1. Introduction
Using natural structural polymers as adsorbents for removal of heavy metal contaminants has gained much recent attention [1]. Among the biological polymers chitin and particularly its deacetylated derivative chitosan has been widely used in environmental research for heavy metal removal [2]. Elimination of acetyl group on the nitrogen atom on the monomer ring from the parent polymer chitin by hydroly-sis gives a deacetylated product called chitosan. Deacetylation is usually carried out by hydrolysis involving very harsh alkaline treatment at elevated temperatures. In hydrolysis process the operational conditions and source of chitin greatly affect the product properties i.e.; deacetylation degree and molecular weight of the product chitosan which in turn greatly affects the physicochemical properties of chitosan. Unlike the parent polymer chitin, free amino groups are formed on the 2′ carbon and presence of amino groups makes chitosan soluble in water and gives cationic nature to chitosan in acidic environments by enhancing its interactions with metal ions. Due to its more cationic na-ture, chitosan is more widely used in removal of oxyanions pollutants like chromate and dichromate. Understandably, many earlier studies
have focused on using chitosan on removal of heavy metal contami-nants including arsenic, cadmium, lead and chromium [3]. However, studies on direct use of chitin in removal of heavy metal ions are fewer and limited.
Chromium is one of the heavy metals that are detrimental to human health. Especially through anthropogenic activities, chromium contam-ination in wastewater and water sources has now an environmental issue demanding immediate intervention [4]. Chromium predomi-nantly occurs in aqueous environments as in two oxidation states; Cr (III) and Cr(VI). While Cr(III) is known to be benign, its oxidized form, Cr(VI), is highly detrimental to human health Cr(VI) is more toxic, mutagenic and carcinogenic than Cr(III). The maximum permissible limit of Cr(VI) in wastewater is very low; WHO recommendation is 0.005 mg L−1[5]. Hexavalent chromium is found in water as oxyanions; CrO42−, Cr2O72−, and their derivatives HCrO4−, HCr2O7−. Many earlier
studies have clearly demonstrated that adsorbents with cationic functional groups on their surfaces are more effective in removal of negatively charged contaminants especially in acidic conditions [6]. At elevated pHs hexavalent chromium is reduced to trivalent chromium and forms precipitate as hydroxides.
Chitin is a structural biopolymer and abundantly found in exoskele-ton of many land and marine organisms including crustaceans, insects or mushrooms. Chitin as a structural polymer also plays a vital role in survival of many planktonic organisms; it is found in the robust shell
⁎ Corresponding author at: Department of Biochemistry, Faculty of Science, Selcuk University, 42075, Konya, Turkey.
E-mail address:idris.sargin@selcuk.edu.tr(I. Sargin).
https://doi.org/10.1016/j.ijbiomac.2019.01.180 0141-8130/© 2019 Elsevier B.V. All rights reserved.
Contents lists available atScienceDirect
International Journal of Biological Macromolecules
of the crustacean resting eggs [7]. Daphnia longispina (Crustacea: Cladocera), waterflea, is planktonic organism living in freshwater. D. longispina is afilter-feeder feeding on organic debris, bacteria, algae and yeast. During unfavourable environmental conditions (e.g., winter, crowding, contamination etc.), this cladoceran species produces resting eggs sexually or parthenogenetically. Resting eggs are sheathed within ephippium, a protective envelopes consisting of mainly chitin and minerals like calcium phosphate or carbonate and magnetite. Resting or ephippial eggs can withstand extreme conditions like freezing, drought and dehydration. They have a crucial role in sur-vival of the Daphnia population. D. longispina switches from production of parthenogenetic eggs to ephippia when the environmental condi-tions deteriorate. In each ephippium rest two eggs of D. longispina. Ephippium serves as a protective case for the eggs inside against harsh environments; therefore, ephippial resting eggs can stay dormant for many years. Earlier studies showed that ephippium is mainly of chitin with pitsfilled with crystalline calcium phosphate and magnetic min-eral and has honeycomb structure [8].
Adsorbents prepared with magnetic particles enhance the removal of hexavalent chromium. Earlier studies reported that magnetite-loaded adsorbents exhibited favourable activities for metal ion contam-inants including Cr(VI) by adsorption/reduction [9–11]. It should be also noted that magnetite loading into adsorbent particles enables separa-tion or collecsepara-tion of used adsorbents by imposing an external magnetic field. In an earlier study [12], our research group successfully isolated the chitinous envelope of ephippium of D. longispina through a line of mineral acid, alkaline and bleaching treatments and the subsequent loading of magnetic particles of iron oxides into the micro-sized chitin-ous envelops was done successfully. In that study loading of magnetic particles enhanced the affinity of chitinous microcages for Cd(II), Cu (II), Ni(II), Cr(III) and Zn(II) ions and we recorded about three-fold adsorption capacity in case of Cd(II), Cu(II) and Ni(II) ions. However, the effect of magnetic particle loading was not that far for trivalent chro-mium and about 20% increase in the removal efficiency was recorded.
In this study, we aimed to see how the presence of magnetic parti-cles would affect the affinity of chitin microcages for another chromium ion species; hexavalent chromium. To make comparison, we prepared two adsorbents; chitin microcages and magnetic particle-loaded chitin microcages, for Cr(VI) removal. Isolation of chitinous microcages from the sheath of resting eggs of a zoo plankton by emptying the egg contents and loading of magnetic particles into the chitin microcages are also detailed in the study.
2. Materials and methods 2.1. Materials
Chitin microcages were isolated from the ephippia of D. longispina, waterflea, a planktonic crustacean. The resting eggs used in this study were from the stock that was used in an earlier study [13]. Collection of ephippial resting eggs was detailed in the same study. Hydrochloric acid solution was used to remove the mineral content of the ephippia. In deproteinization of ephippial eggs sodium hydroxide solution was used. Further purification of chitin microcages and depigmentation of the ephippia was achieved in sodium hypochlorite solution (3%). Iron salts, iron(III) chloride hexahydrate and iron(II) sulphate heptahydrate, ammonia solution, hydrochloric acid were supplied by Merck. Iron salts and ammonia solution was used in production of magnetite particles. Stock solution of Cr(VI) was prepared from potassium dichromate (Sigma-Aldrich).
2.2. Isolation of chitinous micro shells from D. longispina ephippia The scanning electron microscopy image of Daphnia resting egg within the sheath (ephippium) is presented inFig. 1. For isolation of chitinous sheath by removing of organic and inorganic contents of
ephippial resting eggs the procedure reported in a previous study was followed [12]. The details are as follows: In chitinous shell isolation pro-cedure a simple line of chemical treatment was followed; the ephippia (5.0 g) werefirst heated in 250 mL of HCl solution (0.5 M) at 50 °C for 60 min. Then, the sample wasfiltered out using a filter paper and washed with plenty of water to neutrality. The sample was then trans-ferred into 250 mL of sodium hydroxide solution (0.5 M) and heated at 50 °C for 60 min. The sample was collected withfilter paper and ex-tensively washed with water to neutral pH. In bleaching treatment the sample was rested in 200 mL of sodium hypochlorite solution (3%) at 50 °C for 6 min. This bleaching treatment was effective in removal of the pigmentation and the product was white chitin micro shells. Finally, the chitin micro shells (we will call them chitin microcages) were rinsed extensively with water to remove the impurities. Wet chitin micro shells were weighted and one half was dried and the other half (in wet form) was used for magnetic particle loading.
2.3. Preparation of magnetic chitin microcages
Loading of magnetic particles into chitin microcages was performed by using the procedure reported in a previous study [12]. Iron(II) sulphate heptahydrate solution (0.22 g) was dissolved in 200 mL of hot water (70 °C). Chitin microcages in wet form was placed into the so-lution, heated at 70 °C and stirred under constant stirring for 60 min. Then, iron(III) chloride hexahydrate (0.38 g) was added and the mix-ture was heated at the same temperamix-ture and stirred for another 60 min. Finally, 2.0 mL of ammonia solution was added into the mixture under constant stirring and the reaction was heated at 70 °C for another 60 min. Magnetic particle-loaded chitin microcages (we will call them magnetic chitin microcages) were recovered byfiltration and washed with water to remove any loosely bound magnetic particles and the iron salts ions on the surface. The magnetic particles-loaded chitin microcages were left to dry at room temperature for two days. Two SEM images clearly shows the changes in the surface morphology of the chitin micro shells before and after the magnetic particle loading (Figs. 2 and 3).
2.4. Instrumentation
Fourier transform infrared spectra of chitin microcages (pristine and magnetic particle-loaded chitin microcages) was recorded on a Bruker Vertex 70 FTIR spectrometer in range of 4000 and 500 cm−1. Surface features of resting eggs of D. longispina, the isolated chitin microcages
Fig. 1. The scanning electron microscopy image of Daphnia resting egg within the sheath ephippium. The ephippium sometimes encases one resting egg not two as presented in the image.
and the magnetic chitin microcages were examined with a scanning electron microscope (SEM) (EVO LS 10 ZEISS). SEM/Energy Dispersive X-Ray Analysis (EDX) analysis of resting eggs of D. longispina, the iso-lated chitin micro shells and the magnetic-loaded chitin micro shells was also done. Zeta potential measurements were done on the Malvern Zetasizer Nano ZS90). Water was used as dispersant and the samples were placed in a clear disposable capillary cell at 25 °C. The measure-ments were performed 12 runs. Batch-wise Cr(VI) removal experimeasure-ments were done at 200 rpm on a Heidolph Promax 2020 shaker. Aflame atomic absorption spectrometer (ContrAA 300, Analytikjena) was used to determine the chromium ion concentration of the solutions from the sorption experiments.
2.5. Hexavalent chromium removal experiments
Batch-wise sorption experiments at room temperature were done as follows: Chitin microcages or magnetic chitin microcages (0.150 g) was put into Cr(VI) solution (25 mL, 10 mg L−1) and then agitated on the shaker for 4 h. After separating the aqueous phase from the medium byfiltration (Whatman filter paper, No: 42), Cr(VI) ion concentration in the solutions was mesured on the flame atomic absorption spectrometer. Cr(VI) removal experiments were tested under differ-ent operational conditions and solution parameters to ensure the
optimum Cr(VI) removal; adsorbent dosage: 0.05–0.25 g, contact time: 60–480 min, Cr(VI) concentration: 2–12 mg L−1, pH: 1.75,
2.40, 3.60, 4.38 and 5.41, temperature: 25 °C, 35 °C and 45 °C. To see the effect of one parameter on the Cr(VI) removal percentage, the other factors were kept unchanged. pH adjustments were done by dropping dilute hydrochloride or sodium hydroxide solution into the Cr(VI) solution.
The equilibrium removal capacity of Cr(VI) (qe) (mmol/ g) was
calculated using the following equation;
qe¼ Ci−Ce
ð ÞV
W ð1Þ
where Ciand Ceare the initial and equilibrium concentrations of Cr(VI)
in mmol L−1; V is the volume of aqueous solution of Cr(VI) (L); W is the mass of adsorbents; chitin microcages and magnetic chitin microcages (g).
3. Results and discussion
3.1. Characterisation of chitin microcages from ephippial eggs of D. longispina
3.1.1. Surface features of D. longispina resting egg
SEM image of the ephippium of D. longispina is presented inFig. 4. As seen in thefigure, the chitinous structure encases two resting eggs of D. longispina. Two darker regions shows the location of the resting eggs in the ephippium. A close-up SEM image clearly shows the honeycomb structured surface of the ephippium (Fig. 5). Earlier studies demon-strated that these hexagonal structures arefilled with minerals [8]. 3.1.2. Isolation of chitin micro shells from ephippia of the resting eggs of D. longispina
Chitin is a structural biopolymer and it has three allomorphic forms designated asα, β and γ according to alignment of its strands. Of these allomorphsα is the most abundant structure and is mainly found in hard structures like exoskeletons of crustaceans and insects. Unlikeβ andγ allomorphs, in α structure the adjacent chitin strings have oppo-site directions and antiparallel alignment, and this enables the strings to form stronger hydrogen bonds between carbonyl groups and amine groups or side groups (–CH2OH). Therefore,α allomorph has a higher
mechanical strength and more crystalline structure compared toβ and γ allomorphs study [12,14,15].
Fig. 2. The surface morphology of the chitin microcages before magnetic particle loading.
Fig. 3. The surface morphology of the chitin microcages after magnetic particle loading.
Fig. 4. SEM image of the ephippium of D. longispina; two darker regions shows the location of the resting eggs in the ephippium.
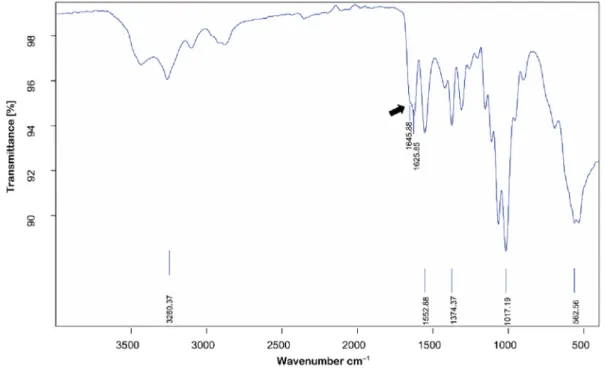
In relation to its structural function,α chitin also occurs in the ephippium. The FT-IR spectrum analysis of chitin microcages isolated from the ephippia of D. longispina revealed that the ephippial chitin has. As presented inFig. 6, the absorption bands that are characteristics ofα chitin allomorph were all recorded and these observations are consistent with FT-IR spectra of ephippial chitin isolated in three earlier reports [12,13,16]. The splitting of amide bond in FTIR spectra of chitin is what distinguishesα crystalline structure from the other two allomorphs;β and γ. In the spectrum of ephippial chitin microcages amide bond was split into two bands at 1645.88 cm−1(amide I) and 1625.85 cm−1(amide II).
Chemical treatment including mineral acid and hot alkaline treatments is commonly used for extraction of chitin from different organisms such as shrimp [18], insect [19] and fungi [20]. Some authors also used an additional depigmentation process using organic solvents to ensure purity of chitin [21,22]. Here, in the extraction
of chitin from the envelope of the resting eggs of D. longispina the demineralization and deproteinization steps were done following the classical chemical treatment but for depigmentation a bleaching agent (sodium hypochlorite solution) was used according to our earlier report [12].
3.1.3. SEM-EDX analysis of chitin microcages
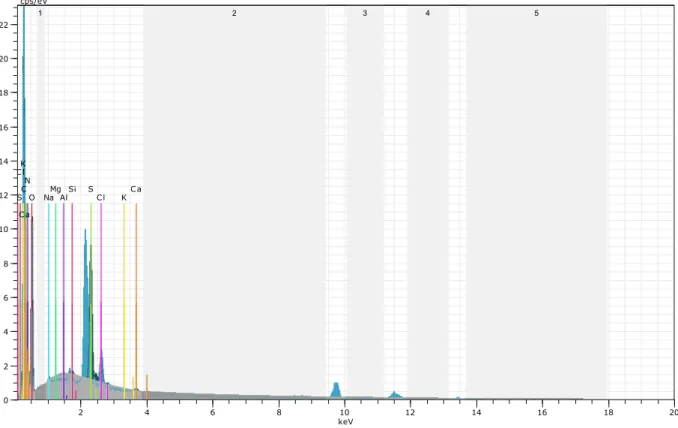
Elemental identification and surface characteristics of the chitin microcages before and after the loading of magnetic particle were examined with SEM-EDX (Figs. 7 and 8). Following the procedure of magnetic particle loading, the Fe peak appeared in the spectrum, indicating the deposition of magnetite particles in the surface of chitin microcages. The reader also should refer toFigs. 2 and 3to make visual comparisons between the pristine and the loaded chitin specimens. As depicted inFigs. 2 and 3, the surface of the chitin was smooth, but magnetic particles made the surface of chitin microcages coarser and rougher. The pristine chitin microcage had some hollow spaces, pits or pores, but the particlesfilled these spaces by giving a more compact structure.
3.1.4. Zeta potential measurements of chitin microcages
Measured zeta potential of chitin microcages and magnetic chitin microcages is presented inFig. 9. Zeta potential of chitin microcages was recorded as−13.1 ± 3.07 mV. After loading of magnetic particles chitin microcages became more negative;−17.1 ± 3.39 mV. Surface becoming more negatively charged upon loading of magnetic particles was also reported in an earlier literature report [23]. In the study in all the sorption experiments a higher performance was recorded with magnetic chitin microcages over chitin microcages, indicating that electrostatic forces did not govern the adsorption process but a more specific interaction via ligand exchange could possibly occurred [24].
3.2. Hexavalent chromium removal by chitin microcages
To see the effect of magnetic particle loading on Cr(VI) removal ef fi-ciency of the chitin microcages, the removal studies were done for both adsorbents under the same conditions. Cr(VI) removal experiments
Fig. 6. FT-IR spectrum of chitin from ephippial eggs of D. longispina. The splitting of the amide bond occurs in the spectrum ofα chitin allomorph. The shoulder at 1645.88 cm−1
corresponds to amide I, indicating the presence ofα crystalline structure of chitin. Fig. 5. A close-up SEM image shows the honeycomb-structured surface of the ephippium of D. longispina.
were repeated for different operational conditions and solution param-eters i.e., adsorbent dosage: 0.05–0.25 g, contact time: 60–480 min, Cr (VI) concentration: 2–12 mg L−1, pH: 1.75, 2.40, 3.60, 4.38 and 5.41,
temperature: 25 °C, 35 °C and 45 °C.
3.2.1. Amount of chitin microcages for the optimum removal of hexavalent chromium
The effect of adsorbent dosage on Cr(VI) removal by chitin microcages and magnetic chitin microcages was studied in a range of 0.05–0.25 g
Fig. 8. SEM-EDX analysis spectrum of the magnetic particles loaded-chitin microcages from the ephippia of D. longispina. Fig. 7. SEM-EDX analysis spectrum of the chitin microcages from the ephippia of D. longispina resting eggs.
(Fig. 10). For both adsorbents the saturation point was recorded at 0.15 g in 25 mL of Cr(VI) solution (10 mg L−1, pH not adjusted) at 25 °C in 4 h. 3.2.2. Minimum contact time for hexavalent chromium removal by chitin microcages
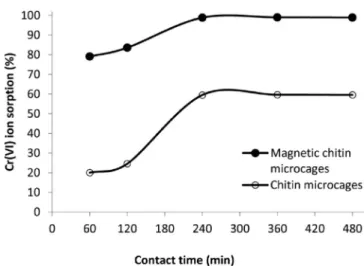
The effect of contact time on Cr(VI) removal by chitin microcages and magnetic chitin microcages was recorded from 60 min to
480 min (Fig. 11) (adsorbent dosage: 0.15 g, Cr(VI) solution param-eters: 25 mL, 10 mg L−1, 25 °C and no pH adjustment). As presented in the figure, 240 min was the optimum contact time for both systems to reach an equilibrium.
Fig. 10. Effect of adsorbent dosage on Cr(VI) removal by chitin microcages and magnetic chitin microcages.
Fig. 11. Effect of contact time on Cr(VI) removal by chitin microcages and magnetic chitin microcages.
3.2.3. Hexavalent chromium removal studies at varying concentrations and isotherm analysis
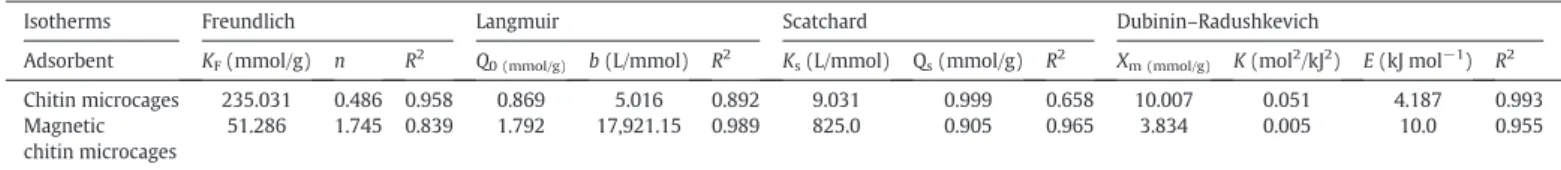
Four common isotherm models, Freundlich [25], Langmuir [26], the Scatchard plot analysis [27] and Dubinin–Radushkevich (D-R) model [28], were employed tofit the experimental data obtained from Cr(VI) ion concentration experiments. The reader should refer to a previous study for the details of the equations, linearized forms and constants of each of these isotherm models [29]. The parameters and correlation coefficients obtained from the plots of Freundlich, Langmuir, Scatchard and D-R are listed inTable 1.
Langmuir model gave betterfit to the experimental data of Cr (VI) removal system with magnetic chitin microcages, demonstrat-ing the homogeneous distribution of binddemonstrat-ing sites on the sorbent. However, experimental data of Cr(VI) removal system with pristine chitin microcages showed better fit to the Freundlich model. Considering high R2obtained in the Scatchard plot analysis of the
system with chitin microcages, it appeared that Cr(VI) adsorption occurred through one type binding site. In the D-R model analysis of the system with magnetic chitin microcages a higher E value was calculated (10.0 kJ mol−1). This showed that Cr(VI) removal by magnetic chitin microcages could occur through chemical ion-exchange rather than physisorption (E values lower than 8 kJ mol−1 can be attributed to physisorption [30]). With lower E value of 4.187 kJ mol−1, Cr(VI) removal by chitin microcages possibly occurred through physical interactions.
3.2.4. pH dependence of hexavalent chromium removal by chitin microcages Cr(VI) removal experiments by chitin microcages and magnetic chitin microcages were repeated at different solution pH (Fig. 12). In the study higher pH values were avoided since chromium ions can precipitate out from the solution as hydroxides. As illustrated in Fig. 12, Cr(VI) removal by both adsorbents was highly pH dependent. As pH was increased, Cr(VI) removal percentage of magnetic chitin microcages dropped from 99% to 65%. In case of chitin microcages, the pH effect was much more evident and Cr(VI) removal percentage was as low as 6%. It should be noted that magnetic particle loading was effective in alleviating this drop in removal percentages. At pH 5.41 Cr (VI) removal percentage was still as high as 65%, which was higher than all the percentage values recorded for chitin microcages. It appeared that at pH values near to the neutrality the affinity of chitin microcages for Cr(VI) ions was very low and chitin particles should be incorporated with magnetic particles to attain favourable removal percentage.
3.2.5. The effect of solution temperature on hexavalent chromium removal by chitin micro shells
Cr(VI) removal studies by chitin microcages and magnetic chitin microcages were repeated at elevated temperatures i.e., 25 °C, 35 °C and 45 °C and the experimental data was subjected to thermodynamic analysis. By plotting the equilibrium constants as a function of temper-ature and using the van't Hoff equation, the changes in Gibbs free energy (ΔG°), entropy (ΔS°) and enthalpy (ΔH°) of the Cr(VI) adsorption pro-cess were calculated [29]. Thermodynamic parameters are listed in Table 2.
Analysis of the thermodynamic parameters in the adsorption system with chitin microcages gave negative values ofΔG°, (ΔH°) and (ΔS°), suggesting that Cr(VI) removal by chitin microcages was spontaneous, exothermic and led to a decrease in the randomness of the system at the specified temperatures. The system with magnetic chitin microcages also gave similar results except for the entropy value of the temperature 25 °C. However, the negative values of Gibbs free energy and enthalpy suggested that the removal of Cr(VI) by magnetic chitin microcages was thermodynamically feasible and exothermic under the specified conditions.
Magnetic particles-loaded chitin microcages showed higher Cr(VI) removal performance; chitin microcages: about 40 mg g−1and magnetic particles-loaded chitin microcages: 67 mg g−1. In literature hexavalent chromium removal with chitin is too limited. However, deacetylated form of chitin, i.e., chitosan, has been widely acknowledged as an effec-tive adsorbent for metal contaminants due to its hydrophilicity,flexible structure of polymer chain and large number of free amino groups [31]. Many researchers reported high Cr(VI) adsorption capacities
Table 1
The parameters of Freundlich, Langmuir, Scatchard and D-R isotherms for removal of Cr(VI) by chitin microcages and magnetic chitin microcages.
Isotherms Freundlich Langmuir Scatchard Dubinin–Radushkevich
Adsorbent KF(mmol/g) n R2 Q0 (mmol/g) b (L/mmol) R2 Ks(L/mmol) Qs(mmol/g) R2 Xm (mmol/g) K (mol2/kJ2) E (kJ mol−1) R2
Chitin microcages 235.031 0.486 0.958 0.869 5.016 0.892 9.031 0.999 0.658 10.007 0.051 4.187 0.993 Magnetic
chitin microcages
51.286 1.745 0.839 1.792 17,921.15 0.989 825.0 0.905 0.965 3.834 0.005 10.0 0.955
Fig. 12. Effect of pH on Cr(VI) removal by chitin microcages and magnetic chitin microcages.
Table 2
Thermodynamic parameters for the sorption of Cr(VI) by chitin microcages and magnetic chitin microcages.
Adsorbents ΔH° (J mol−1) ΔS° (J K−1mol−1) ΔG° (J mol−1) T = 298.15 (K) T = 308.15 (K) T = 318.15 (K)
Cr(VI) Chitin microcages −12,001.487 −30.841 −2403.098 −2155.972 −1982.461
for modified chitosan-based adsorbents; chitosan-based polymeric surfactants: 180 mg g−1[32], chitosan beads: 625 mg g−1andflakes: 256 mg g−1[33], quaternary chitosan salt: 625 mg g−1[34].
4. Conclusions
The study clearly demonstrated that loading of magnetic particles greatly enhanced the interaction of chitin with hexavalent chromium ion even at high pH values. The study also showed that chitin microcages could be isolated from the ephippia of D. longispina resting eggs and subsequent magnetic particle incorporation into chitin microcages can be achieved by employing a simple chemical treatment. Chemical struc-ture analysis of the chitin specimen from the ephippia demonstrated that chitin occurs in the ephippium asα allomorph, confirming the earlier literature reports on the ephippial chitin. In further studies mag-netic particles-loaded chitin isolates can be tested in removal of heavy metal contaminants occurring as oxyanions in aquatic environments. Conflict of interest
The author declares no conflict of interest. References
[1] D. Mohan, C.U. Pittman Jr., J. Hazard. Mater. 137 (2006) 762–811. [2] J. Wang, C. Chen, Biotechnol. Adv. 27 (2009) 195–226.
[3] W.W. Ngah, L. Teong, M. Hanafiah, Carbohydr. Polym. 83 (2011) 1446–1456. [4] A. Gupta, C. Balomajumder, J. Environ. Chem. Eng. 3 (2015) 785–796.
[5] D.M. Hausladen, A. Alexander-Ozinskas, C. McClain, S. Fendorf, Environ. Sci. Technol. 52 (2018) 8242–8251.
[6] F. Fu, Q. Wang, J. Environ. Manag. 92 (2011) 407–418. [7] V. Alekseev, W. Lampert, Nature 414 (2001) 899.
[8] T. Kawasaki, H. Yoshimura, T. Shibue, Y. Ikeuchi, M. Sakata, K. Igarashi, H. Takada, K. Hoshino, K. Kohn, H. Namiki, Zool. Sci. 21 (2004) 63–67.
[9] P. Yuan, M. Fan, D. Yang, H. He, D. Liu, A. Yuan, J. Zhu, T. Chen, J. Hazard. Mater. 166 (2009) 821–829.
[10]P. Yuan, D. Liu, M. Fan, D. Yang, R. Zhu, F. Ge, J. Zhu, H. He, J. Hazard. Mater. 173 (2010) 614–621.
[11] K.S. Padmavathy, G. Madhu, P.V. Haseena, Procedia Technol. 24 (2016) 585–594. [12] I. Sargin, G. Arslan, M. Kaya, Carbohydr. Polym. 207 (2019) 200–210.
[13] M. Kaya, Y.S. Cakmak, T. Baran, M. Asan-Ozusaglam, A. Mentes, K.O. Tozak, Biotechnol. Bioprocess Eng. 19 (2014) 58–69.
[14] M.-K. Jang, B.-G. Kong, Y.-I. Jeong, C.H. Lee, J.-W. Nah, J. Polym. Sci. A Polym. Chem. 42 (2004) 3423–3432.
[15] M. Kaya, M. Mujtaba, H. Ehrlich, A.M. Salaberria, T. Baran, C.T. Amemiya, R. Galli, L. Akyuz, I. Sargin, J. Labidi, Carbohydr. Polym. 176 (2017) 177–186.
[16] M. Kaya, I. Sargin, K.Ö. Tozak, T. Baran, S. Erdogan, G. Sezen, Int. J. Biol. Macromol. 61 (2013) 459–464.
[18] R.M. Abdel-Rahman, R. Hrdina, A.M. Abdel-Mohsen, M.M.G. Fouda, A.Y. Soliman, F.K. Mohamed, K. Mohsin, T.D. Pinto, Int. J. Biol. Macromol. 80 (2015) 107–120. [19] A.T. Paulino, J.I. Simionato, J.C. Garcia, J. Nozaki, Carbohydr. Polym. 64 (2006) 98–103. [20] T. Wu, S. Zivanovic, F.A. Draughon, W.S. Conway, C.E. Sams, J. Agric. Food Chem. 53
(2005) 3888–3894.
[21] N.H. Marei, E.A. El-Samie, T. Salah, G.R. Saad, A.H.M. Elwahy, Int. J. Biol. Macromol. 82 (2016) 871–877.
[22] C.M.d. Moura, J.M.D. Moura, N.M. Soares, L.A.d.A. Pinto, Chem. Eng. Process. Process Intensif. 50 (2011) 351–355.
[23] M. Bayrakcı, E. Maltaş, Ş. Yiğiter, M. Özmen, Macromol. Res. 21 (2013) 1029–1035. [24] J. Hu, I.M.C. Lo, G. Chen, Sep. Purif. Technol. 56 (2007) 249–256.
[25] H.M.F. Freundlich, Z. Phys. Chem. 57 (1906) 385–471. [26] I. Langmuir, J. Am. Chem. Soc. 40 (1918) 1361–1403.
[27] R.H. Crist, J.R. Martin, D. Carr, J.R. Watson, H.J. Clarke, D.L.R. Crist, Environ. Sci. Technol. 28 (1994) 1859–1866.
[28] M.M. Dubinin, L.V. Radushkevich, Chem. Zentralbl. 1 (1947) 875–889. [29] İ. Sargın, G. Arslan, M. Kaya, Carbohydr. Polym. 138 (2016) 201–209. [30] M. Tuzen, A. Sarı, Chem. Eng. J. 158 (2010) 200–206.
[31] M. Owlad, M.K. Aroua, W.A.W. Daud, S. Baroutian, Water Air Soil Pollut. 200 (2009) 59–77.
[32] M.Y. Lee, K.J. Hong, Y. Shin-Ya, T. Kajiuchi, J. Appl. Polym. Sci. 96 (2005) 44–50. [33] N. Sankararamakrishnan, A. Dixit, L. Iyengar, R. Sanghi, Bioresour. Technol. 97
(2006) 2377–2382.