Contents lists available atScienceDirect
Industrial Crops & Products
journal homepage:www.elsevier.com/locate/indcropImpact of different extraction solvents and techniques on the biological
activities of Cirsium yildizianum (Asteraceae: Cynareae)
Eulogio J. Llorent-Martínez
a,1, Gokhan Zengin
b,*
,1, Kouadio Ibrahime Sinan
b, Rıdvan Polat
c,
Deniz Canlı
d, Marie Carene Nancy Picot-Allain
e, Mohamad Fawzi Mahomoodally
eaDepartment of Physical and Analytical Chemistry, University of Jaén, Campus Las Lagunillas S/N, E-23071, Jaén, Spain bDepartment of Biology, Science Faculty, Selcuk University, Campus, Konya, Turkey
cDepartment of Landscape Architecture, Faculty of Agriculture, Bingol University, 12000, Bingöl, Turkey dThe Unit of Model University Coordination Center, Bingol Unviersity, 12000, Bingöl, Turkey eDepartment of Health Sciences, Faculty of Science, University of Mauritius, Réduit, Mauritius
A R T I C L E I N F O Keywords: Anatolia Soxhlet Diabetes Phytochemical Phytotherapy A B S T R A C T
Several Cirsium species have been used in folk medicine for the management of human ailments. However, there is a paucity of scientific data regarding their biological activity as in the case of the new species, C. yildizianum, from Anatolia, Turkey. The present study endeavours for the first time to appraise the antioxidant and enzyme inhibitory activity of C. yildizianum. The phytochemical profiles of C. yildizianum extracts obtained using homogeniser-assisted extraction (HAE), ultrasound-assisted extraction (UAE), Soxhlet extraction, maceration, decoction, and infusion, using methanol or water as extraction solvents, was determined by high-performance liquid chromatography with electrospray ionization mass spectrometric detection (HPLC–ESI-MSn) analysis. In all extracts, flavonoids were the most abundant compounds, particularly luteolin and apigenin glycosides. HAE methanol extract presented the highest phenolic (37.10 mg gallic acid equivalent/g) and flavonoid (46.78 mg rutin equivalent/g) contents. Likewise, HAE-methanol extract showed potent radical scavenging (40.76 and 68.13 mg Trolox equivalent [TE]/g, for DPPH and ABTS, respectively) and reducing properties (127.62 and 89.95 mg TE/g, for CUPRAC and FRAP, respectively). HAE-methanol extract showed inhibitory activity against acetylcholinesterase (AChE) (3.57 mg galantamine equivalent [GALAE]/g). UAE-methanol extract was a potent inhibitor of butrylcholinesterase (BChE) (2.72 mg GALAE/g) and tyrosinase (121.06 mg kojic acid equivalent/g) However, poor inhibition was recorded for enzymes targeted in the management of diabetes type II, namely α-amylase and α-glucosidase. On the other hand, potent metal chelating property was observed for water extracts. This study provides comprehensive scientific information on the phytochemical profile of C. yildizianum ex-tracted using different procedures and extraction solvents, which might be considered as valuable baseline data for future bioproducts development.
1. Introduction
Enzyme inhibitors isolated from natural products, particularly medicinal plants, are increasingly being acknowledged as therapeutic tools for the management of several human ailments. For instance, galantamine isolated from Galanthus nivalis is currently used for the management of the most common geriatric neurological complication, Alzheimer’s disease, while metformin, used for the management of diabetes type II, was isolated from Galega officinalis. However, the en-zyme inhibitors currently used in the clinical management of chronic non-communicable diseases, such as Alzheimer’s disease, diabetes type
II, and skin hyperpigmentation problems fail to fully maintain normal metabolic conditions and/or have been associated with side effects. Interestingly, medicinal plants remain the main focus in the quest of novel enzyme inhibitors for the management of chronic non-commu-nicable diseases (Aguilar-Toalá et al., 2019;Dorababu, 2019;Mishra et al., 2019;Sun et al., 2019).
The genus Cirsium Mill. comprises about 250–300 annual or per-ennial plant species mostly distributed in Northern Africa, Europe, Central and Northern America, and Asia (Ghimire et al., 2018). Several Cirsium species have been used in traditional medicine. For instance, C. setidens has been used for the treatment of hemostasis, hypertension,
https://doi.org/10.1016/j.indcrop.2019.112033
Received 19 August 2019; Received in revised form 11 October 2019; Accepted 5 December 2019 ⁎Corresponding author at: Department of Biology, Science Faculty, Selcuk University, Konya, Turkey.
E-mail address:gokhanzengin@selcuk.edu.tr(G. Zengin). 1These Authors contributed equally.
Available online 11 December 2019
0926-6690/ © 2019 Elsevier B.V. All rights reserved.
and hematuria (Jeong et al., 2018); C. japonicum DC has been used against hepatitis, hypertension, uterine cancer, traumatic haemorrhage, liver cancer, and leukaemia (Ma et al., 2019); C. oleraceum has been used in Polish folk medicine as diuretic, hemostatic, anti-inflammatory and astringent; anxiolytic remedies have been prepared from C. rivulare (Nalewajko-Sieliwoniuk et al., 2012). Cirsium has been reported to ex-hibit multiple biological activities, including hepatoprotective, vasor-elaxant, anti-cholesterol, anti-diabetic, anti-oxidant, cardioprotective, anti-glycative, and anti-inflammatory (Jeong et al., 2018;Yang et al., 2018;Zeng et al., 2016). However, C. yildizianum, endemic to Anatolia, Turkey, has not received due scientific attention. Cirsium yildizianum fits the section Epitrachys and is considered as morphologically near to C. macrobotrys and C. turkestanicum s. lato (Arabacı and Dirmenci, 2011). A previous study has assessed the bio-oil production from C. yildizianum through pyrolysis in a fixed-bed reactor (Aysu and Bengü, 2014). However, as far as the scientific literature could ascertain, the in-hibitory activity of C. yildizianum against key enzymes related to Alz-heimer’s disease, diabetes type II, and skin hyperpigmentation condi-tions has not been determined. In addition, the present study will determine the effect of different extraction methods (homogeniser as-sisted extraction, ultrasound-asas-sisted extraction, Soxhlet extraction, decoction, infusion, and maceration) and extraction solvents (methanol and water) on the bioactivity of C. yildizianum. In this regard, this study sets out to evaluate the antioxidant and inhibitory action of C. yildi-zianum extracts on acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase, α-amylase, and α-glucosidase in vitro.
2. Materials and methods
2.1. Plant material and preparation of extracts
Cirsium yildizianum was collected at the Bingol (Kuruca village) in Turkey and was collected in the summer of 2018 (at flowering season). Identification and confirmation of plant material, as well as issuing of voucher specimen, was done by botanist Dr. Ridvan Polat from the Bingol University (Bingol, Turkey). The aerial parts (as mix) of the plant previously were dried naturally (in the shade at room temperature for ten days). The dried plant materials were grounded by a laboratory mill (particle size about 1 mm), and then the powdered plant materials were stored in darkness at room temperature.
In the present work, different extraction methods (decoction, homogenizer assisted extraction (HAE), infusion, maceration (MAC), soxhlet (SE); ultrasonication assisted extraction (UAE) were performed and these methods are summarized inFig. 1. Then, all extracts were filtered and dried. The dried extracts were stored at +4 °C in a re-frigerator.
2.2. Profile of bioactive compounds
The content of two major groups of bioactives (phenols-TPC and flavonoids-TFC) in the obtained extracts was determined spectro-photometrically using appropriate Folin-Ciocalteu and aluminium chloride methods (Uysal et al., 2017). Expression of obtained results was done by equivalents of standards - gallic acid (GAE) (in the case of TPC) and rutin (RE) (in the case of TFC).
2.3. Determination of antioxidant and enzyme inhibitory effects
For the comprehensive insights in bio-potential of the obtained extracts and influence of the extraction techniques on their bioactivity antioxidant, anti-α-amylase, anti-α-glucosidase, anti-cholinesterases, and tyrosinase activities assays were performed. Estimation of anti-enzymatic activity of the extracts was done by in vitro assays previously described by (Uysal et al., 2017). Measurement of extracts potential to be antioxidants and scavengers of free radicals was performed by FRAP (ferric reducing antioxidant power), ABTS
(2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), CUPRAC (cupric reducing an-tioxidant capacity) and DPPH (2,2-diphenyl-1-picrylhydrazyl) tests. A detailed description of applied assays was given previously (Uysal et al., 2017). The obtained results were expressed by using appropriate standard components. More precisely, the following compounds were used: galantamine (GALAE, for cholinesterase), kojic acid (KAE, for tyrosinase), acarbose (ACAE, for amylase and glucosidase), trolox (TE, for ABTS, DPPH, FRAP, CUPRAC and phosphomolybdenum) and ethylenediaminetetraacetic (EDTAE, for metal chelating).
2.4. Instrumentation
Chromatographic analyses were performed with an Agilent Series 1100 HPLC system with a G1315B diode array detector (Agilent Technologies) and an ion trap mass spectrometer (Esquire 6000, Bruker Daltonics) with an electrospray interface operating in negative ioniza-tion mode. The separaioniza-tion was performed in a Luna Omega Polar C18 analytical column (150 × 3.0 mm; 5 μm particle size) with a Polar C18 Security Guard cartridge (4 × 3.0 mm), both purchased from Phenomenex. Chromatographic conditions are detailed in ( Llorent-Martínez et al., 2018). The scan range was at m/z 100–1200 with a speed of 13,000 Da/s. The ESI conditions were: drying gas (N2) flow rate and temperature, 10 ml/min and 365 °C; nebulizer gas (N2) pres-sure, 50 psi; capillary voltage, 4500 V; capillary exit voltage, -117.3 V. We used the auto MSnmode for the acquisition of MSndata, with iso-lation width of 4.0 m/z, and fragmentation amplitude of 0.6 V (MSnup to MS4). Relative Standard Deviations lower than 5 % (n = 3) were observed in all analyses.
2.5. Statistical analysis
The non-parametric Kruskal Wallis or parametric One-way ANOVA (with Tukey’s test) was used to assess the significance of differences (p < 0.05) among the extracts. Principal component analysis (PCA) and Clustered Image Map were applied to explore the similarity between the techniques of extraction. Then linear correlation analysis based on Pearson’s and Spearman correlation coefficients were calculated to find the relationship between the studied biological activities and the quantified phytochemical content. The R (v. 3.5.1.) packages mixOmics and XLSTAT v. 2018 software were used for all calculations.
3. Results and discussion 3.1. HPLC–ESI-MSnanalysis
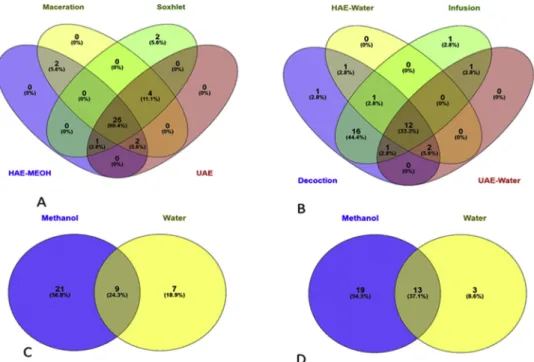
Although there have been few investigations reporting the total phenolic content and some phytochemicals (mainly phenolic acids) in Cirsium species (Kozyra and Glowniak, 2013; Nazaruk et al., 2008; Nazaruk and Jakoniuk, 2005), plants of this genus remain mostly un-explored. In this work, the phenolic profile of C. yildizianum was re-ported for the first time. In addition, the possible difference in the phytochemical profiles of C. yildizianum obtained using different ex-traction solvents (methanol and water) as well as different exex-traction procedures (HAE, UAE, SE, maceration, infusion, and decoction) was assessed. The identification of the compounds (Table 1) was performed using analytical standards (apigenin, chlorogenic acid, ferulic acid, kaempferol, luteolin, neochlorogenic acid, quercetin, and rutin) as well as comparison of the mass spectra with data from the scientific litera-ture. Thirty-four compounds were identified or tentatively character-ized. The extracts that presented the lowest number of compounds were water extracts obtained from HAE and UAE procedures. The number of compounds are also summarized inFig. 2.
3.1.1. Phenolic acids
Compound 4 presented the deprotonated molecular ion at m/z 315 and suffered the neutral loss of 162 Da (hexoside), yielding fragment
ions at m/z 153 and 109, so it was characterized as dihydroxybenzoic acid hexoside. It was detected in all the extracts. Compound 5, [M−H]−at m/z 329, suffered the neutral loss of a hexoside moiety to yield vanillic acid at m/z 167, so it was characterized as vanillic acid-hexoside (Steingass et al., 2015). Compounds 6 and 7 (neochlorogenic acid and chlorogenic acid) were identified by comparison with analy-tical standards. Compound 8, with deprotonated molecular ion at m/z 367 and base peak at m/z 193 was characterized as 3-feruloylquinic acid (Clifford et al., 2003). Compound 10 presented [M−H]−at m/z 355 and, after the loss of 162 Da, yielded ferulic acid at m/z 193 (fragment ions at m/z 149 and 134); it was thus characterized as a feruloyl hexoside.
3.1.2. Flavonoids
Compounds 33, 34, and 37 were identified as luteolin, iso-rhamnetin, and apigenin, respectively, by comparison with analytical standards. Luteolin and isorhamnetin were present in methanol extracts only. Apigenin was absent in HAE methanol and UAE water extracts. Glycosides of these three flavonoids were observed in most of the ex-tracts analysed. The attached moieties were characterised base on the neutral losses of 146 Da (deoxyhexoside), 162 Da (hexoside), 176 Da (glucuronide), and 308 Da (rutinoside) that yielded the aglycones lu-teolin at m/z 285, apigenin at m/z 269, and isorhamnetin at m/z 315. Considering the most common sugars found in flavonoid glycosides, hexosides, and deoxyhexosides were probably glucoses and rhamnoses. Compounds 14 and 15, with [M−H]− at m/z 563, were char-acterized as apigenin-C-hexoside-C-pentoside isomers (probably api-genin-C-arabinoside-C-glucoside) due to the fragment ions observed at [M−H-60]−, [M−H-90]−, [M−H-120]−, [M−H-180]−, and [M−H-210]−, characteristic of di-C-glycoside flavonoids (Han et al., 2008).
Compounds 25 and 32 suffered the neutral loss of 146 Da to yield quercetin at m/z 301 (fragment ions at m/z 179 and 151) and kaemp-ferol at m/z 285 (fragment ion at m/z 255), respectively. They were characterized as quercetin-O-deoxyhexoside and kaempferol-O-deox-yhexoside, respectively. Compounds 28, 30, and 31 were identified as diosmetin-O-glycosides, all of them yielding diosmetin at m/z 299 with a fragment ion at m/z 284 (Fu et al., 2016).
3.1.3. Other compounds
Compound 1 exhibited the neutral loss of a hexoside moiety, and its fragment ions at m/z 179, 161, 143, 119, and 113 are typical of
hexoses, so it was characterized as a disaccharide. Compounds 2 and 3, both with [M−H]−at m/z 191, differed in their fragmentation patterns and were characterized as quinic acid (m/z 173 and 127) and citric acid (base peak a m/z 111), respectively. Compound 9 was tentatively characterized as benzyl alcohol hexose pentose based on evidence from the literature (Bystrom et al., 2008) and was detected in all the extracts. Compound 36, present in all extracts, had deprotonated molecular ion at m/z 387, base peak at m/z 329, and fragment ions at m/z 357, 339, 249, and 193. This fragmentation pattern was consistent with the lignan trachelogenin (Eklund et al., 2008), recently reported as a novel inhibitor of hepatitis C virus (Qian et al., 2016).
Finally, two oxylipins were characterized in all extracts, except HAE methanol extract, compounds 35 and 38 corresponded to oxo-dihy-droxy-octadecenoic acid and trihyoxo-dihy-droxy-octadecenoic acid (Van Hoyweghen et al., 2014), respectively.
3.2. Phenolic quantitation
The quantitation of the most abundant compounds was performed using the same analytical standard, when available, or an analytical standard of the same chemical family. Calibration graphs (0.5−100 μg/ mL; r2> 0.990 in all cases) were prepared using the following stan-dards: apigenin, chlorogenic acid, ferulic acid, isorhamnetin, kaemp-ferol, luteolin, protocatechuic acid, and rutin. Repeatability (n = 10; same day) and intermediate precision (n = 9; 3 consecutive days) were lower than 4 and 6 %, respectively. Flavonoids were quantified at 350 nm, dihydroxybenzoic acids at 280 nm and chlorogenic and ferulic acids at 320 nm, using peak area at the analytical signal in the corre-sponding UV chromatograms. Total individual phenolic content (TIPC) was defined as the sum of the quantified phenolic compounds and the results were presented inTable 2.
All methanolic extracts presented higher recovery yields (higher TIPC) than aqueous extracts. TIPC presented the following order: HAE-MeOH = UAE-MeOH > Soxhlet-MeOH > maceration-MeOH > decoction > HAE-water = infusion = UAE HAE-water. The higher TIPC in methanol extracts was due to the higher solubility of the most abundant compounds (flavonoid glycosides) compared with water. In all extracts, flavonoids were the most abundant compounds, representing at least 87 % of TIPC. Among phenolic acids, the most abundant compound in all extracts was chlorogenic acid. On the other hand, luteolin, apigenin, and their corresponding glycosides were the most abundant flavonoids, making extracts of C. yildizianum an
important source of luteolin and apigenin glycosides. 3.3. Total bioactive compounds and antioxidant properties
The total phenolic and flavonoid contents of C. yildizianum extracts were also determined by spectrophotometric methods as presented in Table 3. The methanol extract of C. yildizianum obtained by HAE pre-sented the highest phenolic content (37.10 mg GAE/g), followed by the Soxhlet methanol extract (36.28 mg GAE/g). Likewise, the
HAE-methanol extract of C. yildizianum (46.78 mg RE/g) possessed the highest flavonoid content. Spectrophotometric determinations of TPC and TFC were in accordance with the data presented inTable 2. HAE-methanol extract, presenting the highest TPC and TFC, was rich in phenolic acids (2.4 mg GAE/g) and flavonoids (25.1 mg RE/g). The lowest TPC and TFC (Table 3) were recorded for HAE-water extract, similarly to the results obtained by chromatography (Table 2). These findings support the reliability of the Folin-Ciocalteu and aluminium chloride assays, respectively used for the determination of phenolic and
Table 1
Characterization of the compounds found in Cirsium yildizianum extracts.
No. tR(min) [M-H]−m/z m/z (% base peak) Assigned identification Occurrence
A B C D E F G H 1 1.7 341 MS2[341]: 179 (100), 161 (20), 143 (14), 119 (16), 113 (17) Disaccharide X X X X X X X
2 1.8 191 MS2[191]: 173 (100), 127 (45), 111 (24) Quinic acid X X X X
3 2.5 191 MS2[191]: 173 (42), 111 (100) Citric acid X X X X
4 3.7 315 MS2[315]: 153 (100), 109 (9), 108 (9) Dihydroxybenzoic acid hexoside X X X X X X X X
5 5.4 329 MS2[329]: 209 (68), 167 (100) Vanillic acid hexoside X X X X X
6 5.7 353 MS2[353]: 191 (100) Neochlorogenic acid X X X
7 9.0 353 MS2[353]: 191 (100), 179 (28) Chlorogenic acid X X X X X X
8 9.2 367 MS2[367]: 193 (100) 3-feruloylquinic acid X X
MS3[367→193]: 149 (34), 134 (100)
9 9.4 401 MS2[401]: 293 (23), 269 (100), 161 (48) Benzyl alcohol hexose pentose X X X X X X X X
10 10.2 355 MS2[355]: 193 (100) Feruloyl hexoside X X X X X X MS3[355→193]: 149 (54), 134 (100) 11 10.6 433 MS2[433]: 385 (100) Unknown X X X X X X X X MS3[433→385]: 223 (100), 205 (51), 161 (34), 153 (31) 12 11.2 565 MS2[565]: 519 (100) Unknown X X X X X X X X MS3[565→519]: 387 (47), 293 (100), 191 (29), 161 (28) MS4[565→519→293]: 149 (100) 13 14.0 415 MS2[415]: 251 (18), 221 (31), 191 (17), 161 (28), 149 (100), 143 (25) Unknown X X X X X X X X MS3[415→149]: 131 (100), 119 (5), 113 (9) 14 15.6 563 MS2[563]: 545 (14), 503 (77), 473 (94), 443 (100), 383 (83), 353 (79) Apigenin-C-hexoside-C-pentoside X X X X X X X X 15 17.1 563 MS2[563]: 503 (30), 473 (100), 443 (52), 383 (56), 353 (62) Apigenin-C-hexoside-C-pentoside X X X X X X 16 17.3 401 MS2[401]: 239 (38), 221 (100), 177 (96), 161 (40), 113 (42) Unknown X X X X X X X 17 17.9 623 MS2[623]: 461 (100), 447 (33), 285 (60) Luteolin-O-hexoside-O-glucuronide X X X X X X MS3[623→461]: 285 (100) MS4[623→461→285]: 241 (100) 18 19.8 609 MS2[609]: 301 (100) Rutin X X X X X X MS3[609→301]: 271 (48), 179 (100), 151 (49) 19 21.2 593 MS2[593]: 447 (100), 285 (95) Luteolin-O-hexoside-O-deoxyhexoside X X 20 21.2 447 MS2[447]: 285 (100) Luteolin-O-hexoside X X X X X X MS3[447→285]: 241 (83), 175 (100) 21 21.4 461 MS2[461]: 285 (100) Luteolin-O-glucuronide X X X X X X MS3[461→285]: 241 (100) 22 21.7 477 MS2[477]: 315 (100) Isorhamnetin-O-hexoside X X X X X X MS3[477→315]: 300 (100) 23 21.9 491 MS2[491]: 315 (100) Isorhamnetin-O-glucuronide X X X X X X MS3[491→315]: 300 (100) 24 24.3 591 MS2[591]: 269 (100) Apigenin-O-deoxyhexoside-glucuronide X X X X X X X X MS3[591→269]: 225 (100) 25 24.7 447 MS2[447]: 301 (100) Quercetin-O-deoxyhexoside X X X X X MS3[447→301]: 179 (100), 151 (90) 26 24.7 577 MS2[577]: 269 (100) Apigenin-O-rutinoside X X X X X X MS3[577→269]: 225 (100) 27 25.3 431 MS2[431]: 269 (100) Apigenin-O-hexoside X X X X X X MS3[431→269]: 225 (100) 28 25.6 607 MS2[607]: 299 (100), 284 (28) Diosmetin-O-rutinoside X X X X X X 29 25.9 445 MS2[445]: 269 (100) Apigenin-O-glucuronide X X X X X X MS3[445→269]: 225 (100) 30 26.1 461 MS2[461]: 299 (100) Diosmetin-O-hexoside X X X X X X MS3[461→299]: 284 (100) 31 26.5 475 MS2[475]: 299 (100), 284 (10) Diosmetin-O-glucuronide X X X X X X 32 28.8 431 MS2[431]: 285 (100) Kaempferol-O-deoxyhexoside X X X X MS3[431→285]: 255 (100) 33 36.2 285 MS2[285]: 241 (100), 175 (35) Luteolin X X X X 34 37.8 315 MS2[315]: 300 (100) Isorhamnetin X X X X MS3[315→300]: 271 (100), 255 (46) 35 39.1 327 MS2[327]: 291 (45), 229 (88), 211 (36), 209 (19), 171 (100) Oxo-dihydroxy-octadecenoic acid X X X X X X X 36 39.3 387 MS2[387]: 357 (25), 339 (41), 329 (100), 249 (30), 193 (10) Trachelogenin X X X X X X X X 37 40.0 269 MS2[269]: 225 (100) Apigenin X X X X X X 38 40.6 329 MS2[329]: 311 (36), 293 (61), 229 (100), 211 (82), 209 (17), 171 (95) Trihydroxy-octadecenoic acid X X X X X X X
A: Decoction; B: HAE methanol; C: HAE water; D: Infusion; E: maceration methanol; F: Soxhlet methanol; G: UAE methanol; H: UAE water.
flavonoid contents. Besides, multiple lines of evidence have claimed the higher antioxidant capacity of extracts rich in phenolics and flavonoids (Mokrani et al., 2019; Olszowy, 2019). Phosphomolybdenum results presented inTable 3clearly indicated that HAE-methanol extract of C. yildizianum possessed the highest total antioxidant capacity and UAE-water extract the lowest potential.
In order to provide a comprehensive understanding of the possible different antioxidant mechanisms of C. yildizianum extracts, a battery of assays, including radical scavenging, reducing potential and metal chelating assays, were employed. DPPH and ABTS assays were used to evaluate radical scavenging abilities, CUPRAC and FRAP were used to
determine the reducing potential and metal chelating potential of C. yildizianum extracts was determined using the iron-ferrozine assay (Table 4). It was found that HAE-methanol extract showed potent ra-dical scavenging (40.76 and 68.13 mg TE/g, for DPPH and ABTS, re-spectively) and reducing properties (127.62 and 89.95 mg TE/g, for CUPRAC and FRAP, respectively). Extracts obtained from maceration and Soxhlet extractions also showed potent antioxidant capacity against radical scavenging and reducing assays (Table 4). Indeed, extracts ob-tained from maceration and Soxhlet extractions conob-tained appreciable amounts of phenolics (Table 3). On the other hand, water extracts of C. yildizianum showed poor radical scavenging and reducing properties.
Fig. 2. Venn diagrams based identified compound numbers in the tested extracts (A: Methanol extracts; B: Water extracts; C: Homogenizer assisted extracts; D:
Ultrasonication assisted extracts).
Table 2
Quantitation of the main compounds found in C. yildizianum extracts.
No. Assigned identification
A B C D E F G H
Phenolic acids
4 Dihydroxybenzoic acid Hex 0.17 ± 0.01b 0.53 ± 0.04a 0.10 ± 0.01c 0.054 ± 0.003d – – – –
5 Vanillic acid Hex 0.28 ± 0.02b 0.42 ± 0.03a – – – 0.29 ± 0.02b 0.39 ± 0.03a –
7 Chlorogenic acid 0.49 ± 0.03c 1.4 ± 0.1a – 0.14 ± 0.01d 1.11 ± 0.06b 1.4 ± 0.1a 1.19 ± 0.07b – 8 3-feruloylquinic acid 0.106 ± 0.007 – – 0.029 ± 0.002 – – – – Total 1.05 ± 0.04c 2.4 ± 0.1a 0.10 ± 0.01d 0.22 ± 0.01d 1.11 ± 0.06c 1.7 ± 0.1b 1.58 ± 0.08b – Flavonoids 14 Apigenin-Hex-Pen 0.37 ± 0.02a 0.35 ± 0.02ab 0.39 ± 0.03a 0.16 ± 0.01d 0.28 ± 0.02c 0.36 ± 0.02a 0.29 ± 0.02bc 0.37 ± 0.03a 15 Apigenin-Hex-Pen 0.22 ± 0.02a 0.15 ± 0.01b 0.23 ± 0.02a 0.134 ± 0.008b 0.142 ± 0.01b – – 0.24 ± 0.02a 17 Luteolin-Hex-Gluc 0.21 ± 0.01a 0.154 ± 0.008b – 0.133 ± 0.009b 0.15 ± 0.01b 0.19 ± 0.01a 0.14 ± 0.01b – 18 Rutin 0.14 ± 0.01c 1.6 ± 0.1a – – 1.18 ± 0.06b 1.04 ± 0.06b 1.5 ± 0.1a – 19-21 Luteolin glycosides 1.01 ± 0.05d 4.2 ± 0.2a – 0.34 ± 0.02e 3.4 ± 0.2c 3.5 ± 0.2bc 4.0 ± 0.3ab – 22 + 23 Isorhamnetin glycosides 0.17 ± 0.01d 0.67 ± 0.04c – 0.066 ± 0.004d 0.95 ± 0.01b 1.3 ± 0.1a 0.65 ± 0.04c – 24 Apigenin-dHex-Gluc 1.8 ± 0.1d 3.2 ± 0.2ab 1.7 ± 0.1de 0.44 ± 0.03f 2.4 ± 0.1c 3.5 ± 0.2a 2.9 ± 0.2b 1.33 ± 0.08e 26 Apigenin-Rut – – – 0.13 ± 0.01 – – – – 27 + 28 Apigenin-Hex + diosmetin-Rut 0.78 ± 0.05c 4.0 ± 0.3ab – 0.29 ± 0.02c 3.6 ± 0.2b 3.8 ± 0.3ab 4.3 ± 0.3a – 29 + 30 Apigenin-Gluc + diosmetin-Hex 0.98 ± 0.04c 2.8 ± 0.2a – 0.41 ± 0.02d 1.23 ± 0.01c 2.3 ± 0.1b 2.6 ± 0.1a – 31 Diosmetin Gluc 1.20 ± 0.06b 1.7 ± 0.1a – 0.36 ± 0.02c 1.07 ± 0.08b 1.6 ± 0.1a 1.26 ± 0.07b – 32 Kaempferol-dHex – 0.30 ± 0.02b – – 0.24 ± 0.02c 0.26 ± 0.02bc 0.37 ± 0.03a – 33 Luteolin – 2.0 ± 0.1b – – 2.1 ± 0.1b 1.6 ± 0.1c 2.5 ± 0.1a – 34 Isorhamnetin – 0.42 ± 0.03b – – 0.37 ± 0.02b 0.28 ± 0.02c 0.49 ± 0.03a – 37 Apigenin 0.20 ± 0.01d 3.5 ± 0.2b 0.24 ± 0.02d 0.13 ± 0.01d 3.3 ± 0.2b 2.9 ± 0.1c 4.6 ± 0.2a – Total 7.1 ± 0.2d 25.1 ± 0.5a 2.6 ± 0.1e 2.59 ± 0.05e 20.4 ± 0.4c 22.6 ± 0.5b 25.6 ± 0.6a 1.94 ± 0.09e TIPC 8.2 ± 0.2d 27.5 ± 0.5a 2.7 ± 0.1e 2.81 ± 0.05e 21.5 ± 0.4c 24.3 ± 0.5b 27.2 ± 0.6a 1.94 ± 0.09e
A: Decoction; B: HAE methanol; C: HAE water; D: Infusion; E: maceration methanol; F: Soxhlet methanol; G: UAE methanol; H: UAE water; Hex: hexoside; Pen: pentoside; Gluc: glucuronide; Rut: rutinoside; dHex: deoxyhexoside; TIPC: Total Individual Phenolic Content (sum of the quantified phenolic compounds).
However, C. yildizianum water extracts showed potent metal chelating properties, UAE-water extract (45.52 mg EDTAE/g) being the most active, followed by C. yildizianum extract obtained by decoction (38.86 mg EDTAE/g) and by infusion (37.71 mg EDTAE/g). Citric acid was identified in all water extracts (Table 1). Citric acid, a weak organic acid with excellent chelating abilities, is readily soluble in water. Citric acid and Fe2+form tridentate mononuclear complexes with two car-boxylic acid groups and the hydroxyl group and such complex forma-tion affect metal mobility and toxicity (Gadd, 1999).
3.4. Enzyme inhibitory effects
The inhibitory potential of C. yildizianum on key enzyme targeted in the management of Alzheimer’s disease, diabetes type II, and pigmen-tation disorders was assessed. In addition, the effect of different ex-traction methods and solvents and summarised inTable 5. As presented inTable 5, the enzyme inhibitory potential of methanol and water ex-tracts differed. For instance, referring to HAE, the methanol extract (3.57 mg GALAE/g) showed inhibitory activity against AChE while the water extract obtained from the same extraction procedure showed no
activity against AChE. It was also observed that the methanol extracts displayed inhibitory activity against AChE ranging from 3.93 to 3.53 mg GALAE/g. These findings suggested that the different extraction procedures investigated exhibited a variable degree of extraction cap-ability. Likewise, a higher inhibitory action of methanol extracts on BChE and tyrosinase was observed (Table 5). Luteolin and isorhamnetin were identified in methanol extracts (Table 1). A group of researchers previously reported the AChE inhibitory activities of isorhamnetin. Molecular docking studies revealed that both luteolin and isorhamnetin bound to AChE, showing docking scores of -8.940 and -8.644, respec-tively, indicating that the ligand-enzyme complex was stable (Zhang et al., 2018). On the other hand, luteolin was reported to reversibly inhibit BChE (Katalinić et al., 2010). FromTable 5, it was also noted that the C. yildizianum extracts prepared by decoction and infusion showed variable inhibitory action against AChE, BChE, as well as tyr-osinase, showing a difference in the biological efficacy of extracts prepared following these traditional preparation methods. C. yildi-zianum extracts obtained using the different extraction methods in-hibited tyrosinase. It was noted that the methanol extracts were more active tyrosinase inhibitors compared with the water extracts. C.
Table 3
Extraction yields (%) and total bioactive compounds and total antioxidant capacity (by phosphomolybdenum assay) of the studied extracts.
Extraction methods/Solvent Extraction yields (%) Total phenolic content (mg GAE/g) Total flavonoid content (mg RE/g) Phosphomolybdenum (mmol TE/g) Decoction 13.6 25.52 ± 0.66ab 9.58 ± 0.24abc 1.10 ± 0.04ab HAE-MeOH 7.90 37.10 ± 0.38a 46.78 ± 0.48a 1.78 ± 0.10a HAE-Water 13.39 22.96 ± 0.46c 4.31 ± 0.13bc 1.11 ± 0.02ab Infusion 13.30 22.95 ± 0.14c 9.39 ± 0.23abc 1.01 ± 0.04b Maceration 8.29 33.72 ± 0.86ab 39.53 ± 0.41abc 1.39 ± 0.03ab SE 13.94 36.28 ± 0.83a 36.02 ± 0.42abc 1.34 ± 0.07ab UAE-MeOH 7.26 31.75 ± 0.70ab 40.43 ± 0.18ab 1.60 ± 0.12a UAE-Water 19.99 23.45 ± 0.41ab 3.79 ± 0.09c 0.99 ± 0.04b
Values expressed are means ± S.D. of three parallel measurements. GAE: Gallic acid equivalent; RE: Rutin equivalent; TE: Trolox equivalent. HAE: Homogenizer assisted extraction; MAC: Maceration; SE: Soxhlet; UAE: Ultrasonication-assisted extraction.
Table 4
Antioxidant properties of the tested extracts.
Extraction methods/Solvent DPPH (mg TE/g) ABTS (mg TE/g) CUPRAC (mg TE/g) FRAP (mg TE/g) Metal chelating (mg EDTAE/g) Decoction 25.78 ± 0.65abc 67.96 ± 2.39a 84.10 ± 0.69ab 62.72 ± 2.70ab 38.86 ± 0.24ab
HAE-MeOH 40.76 ± 1.13ab 68.13 ± 4.99a 127.62 ± 4.62a 89.95 ± 0.49a 30.12 ± 0.61abc
HAE-Water 9.95 ± 1.11c 51.84 ± 0.54d 75.93 ± 0.77b 51.21 ± 0.21b 33.49 ± 0.47abc
Infusion 21.45 ± 1.63abc 63.24 ± 0.92abc 88.47 ± 1.77ab 57.44 ± 0.94ab 37.71 ± 0.73abc
Maceration 40.09 ± 0.33ab 69.37 ± 3.85a 124.12 ± 4.43ab 83.87 ± 2.95ab 22.01 ± 0.50bc
SE 45.08 ± 0.47a 66.57 ± 2.66ab 139.00 ± 7.00a 92.33 ± 7.25a 22.34 ± 2.21c
UAE-MeOH 34.16 ± 0.69abc 59.43 ± 2.04c 119.51 ± 1.50ab 72.90 ± 2.83ab 22.61 ± 1.44bc
UAE-Water 15.59 ± 0.52bc 61.30 ± 0.94bc 75.89 ± 1.38b 50.70 ± 0.81b 45.52 ± 5.31a
Values expressed are means ± S.D. of three parallel measurements. ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid; CUPRAC: cupric reducing anti-oxidant capacity; DPPH: 2,2-diphenyl-1-picrylhydrazyl; FRAP: ferric reducing antianti-oxidant power; TE: Trolox equivalent; EDTAE: EDTA equivalent. HAE: Homogenizer assisted extraction; MAC: Maceration; SE: Soxhlet; UAE: Ultrasonication-assisted extraction.
Table 5
Enzyme inhibitory properties of the tested extracts.
Extraction methods/
Solvent AChE inhibition (mgGALAE/g) BChE inhibition (mgGALAE/g) Tyrosinase inhibition (mgKAE/g) Amylase inhibition (mmolACAE/g) Glucosidase inhibition (mmolACAE/g) Decoction 0.39 ± 0.01abc 0.61 ± 0.14ab 14.65 ± 0.63c 0.10 ± 0.01b na
HAE-MeOH 3.57 ± 0.04abc 1.89 ± 0.56ab 113.34 ± 0.49abc 0.57 ± 0.04a na
HAE-Water na na 39.76 ± 4.09abc 0.19 ± 0.01ab na Infusion 0.13 ± 0.04bc na 43.03 ± 2.76abc 0.09 ± 0.01b na Maceration 3.53 ± 0.11abc 1.80 ± 0.24ab 121.16 ± 1.08a 0.46 ± 0.02ab 0.84 ± 0.10ab SE 3.93 ± 0.04a 2.38 ± 0.31a 118.32 ± 1.96abc 0.48 ± 0.03ab 0.87 ± 0.06ab UAE-MeOH 3.68 ± 0.06ab 2.72 ± 0.46a 121.06 ± 0.77ab 0.60 ± 0.03a 1.16 ± 0.14a UAE-Water 0.33 ± 0.05abc 1.04 ± 0.01ab 37.91 ± 0.87bc 0.10 ± 0.01ab na
Values expressed are means ± S.D. of three parallel measurements. AChE: Acetylcholinesterase; BChE: Butrylcholinesterase; GALAE: galantamine equivalent; KAE: kojic acid equivalent; ACAE: acarbose equivalent; na: not active. HAE: homogenizer assisted extraction; SE: Soxhlet extraction; UAE: Ultrasonication-assisted ex-traction.
yildizianum methanol extracts obtained from maceration and UAE showed the highest tyrosinase inhibitory activity while the water ex-tract prepared by decoction was the least active. Exex-tracts showing po-tent tyrosinase inhibitory activity contained a higher concentration of apigenin (Table 2). A previous study has reported the mixed inhibition of apigenin on tyrosinase (Fan et al., 2017), claiming that apigenin is able to bind to tyrosinase both in the presence and absence of the substrate (Mazzei et al., 2016). Data reported in the present study re-vealed that C. yildizianum extracts were poor inhibitors of α-amylase (0.60-0.09 mmol ACAE/g) and α-glucosidase (1.16-0.84 mmol ACAE/ g).
3.5. Multivariate analysis
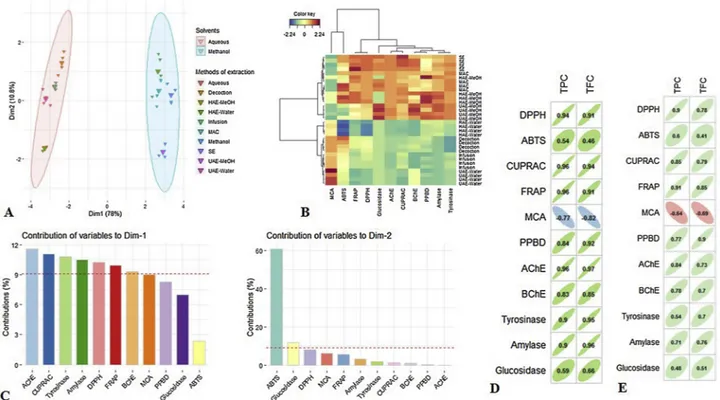
To make the investigation on the similarities between the different extractions methods used in this study, multivariate data analysis namely PCA and HCA were employed on the biological activities da-tasets. By reference to PCA sample plot (Fig. 3), the extraction methods were satisfactorily discriminated. The first component explaining 78 % of the total variance was highly correlated with AChE, CUPRAC, tyr-osinase, amylase, DPPH and FRAP. InFig. 3A, the methanol and water extracts were clearly separated. The second component was 11 %, which was linked with ABTS. Fig. 3B gave the outcome of Clustered Image Map built upon the first two components of PCA, computed using “Euclidean norm” and “Ward linkage”. Two main groups were observed dependent on types of solvent used for the extractions. Otherwise, by visualizing the colour of each block, the methanol extracts proved to be most active in the biological activities, namely methanol was the effi-cient solvent for the extraction of bioactive compounds responsible for these biological activities. Different solvents are commonly employed for the extraction of bioactive compounds of interest from plant ma-terials. This study clearly highlighted the significant effect of solvent used on the biological activities of C. yildizianum. Similar results were noted for different plants by various studies (Abolmaesoomi et al., 2019;Ismail et al., 2019).
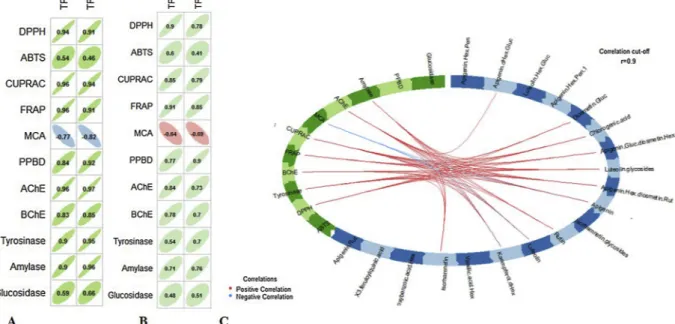
Pearson’s and Spearman correlation analysis were performed
respectively, to explore the role of total bioactive compounds and main compounds on the observed biological activities. Results showed that total polyphenols and total flavonoids content were correlated to an-tioxidant and enzyme inhibitory activities except for ABTS, MCA and glucosidase. Regarding the relationship between the biological activ-ities and the main bioactive compounds, Kaempferol-O-deoxyhexoside, luteolin, isorhamnetin, apigenin and luteolin glycosides correlated with the following biological activities: FRAP, DPPH, CUPRAC, PPBD, AChE, amylase and tyrosinase. Remarkably, as regardsFig. 4, kaempferol-O-deoxyhexoside, luteolin and isorhamnetin were detected only in the methanolic extracts while apigenin and luteolin glycosides were found to be the most abundant compounds in all methanolic extracts (Table 2). Additionally, we reported previously that methanol extracts exhibited the highest FRAP, DPPH, CUPRAC, PPBD, AChE, amylase and tyrosinase activities. Hence, these compounds were likely responsible for the biological activities of C. yildizianum.
4. Conclusion
The present study provided a comprehensive evaluation of the effect of homogeniser-assisted extraction, ultrasound-assisted extraction, Soxhlet extraction, maceration, decoction, and infusion using methanol and water as extractions solvents on the phytochemical profiles, anti-oxidant properties, and enzyme inhibitory activity of C. yildizianum, a Cirsium species which has not received scientific focus. The results showed that methanol was the most effective extracting solvent, showing higher content of phytochemicals and subsequently higher antioxidant capacity. C. yildizianum methanol extracts were also potent inhibitors of AChE, BChE, and tyrosinase. However, it was noted that in general C. yildizianum extracts were poor inhibitors of amylase and α-glucosidase, suggesting that possible anti-diabetic action of C. yildi-zianum might be linked to other glucose-lowering mechanisms. Declaration of Competing Interest
The authors declare no conflicts of interest.
Fig. 3. Multivariate analysis on biological activities datasets of Cirsium yildizianum. A: The PCA sample plots. B: Clustered Image Map (Ward linkage, Euclidean
References
Abolmaesoomi, M., Aziz, A.A., Junit, S.M., Ali, J.M., 2019. Ficus deltoidea: effects of solvent polarity on antioxidant and anti-proliferative activities in breast and colon cancer cells. Eur. J. Integr. Med. 28, 57–67.
Aguilar-Toalá, J.E., Hernández-Mendoza, A., González-Córdova, A.F., Vallejo-Cordoba, B., Liceaga, A.M., 2019. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 122, 170170.
Arabacı, T., Dirmenci, T., 2011. Cirsium yildizianum (Asteraceae: cynareae), a new species from East Anatolia, Turkey. Ann. Bot. Fenn. 48, 503–507.
Aysu, T., Bengü, A.S., 2014. Bio-Oil Production from Cirsium yildizianum through pyr-olysis in a fixed-bed reactor. J. Appl. Sol. Chem. Model. 3, 135.
Bystrom, L.M., Lewis, B.A., Brown, D.L., Rodriguez, E., Obendorf, R.L., 2008. Characterisation of phenolics by LC–UV/Vis, LC–MS/MS and sugars by GC in
Melicoccus bijugatus jacq.‘Montgomery’fruits. Food Chem. 111, 1017–1024.
Clifford, M.N., Johnston, K.L., Knight, S., Kuhnert, N., 2003. Hierarchical scheme for LC-MS n identification of chlorogenic acids. J. Agric. Food Chem. 51 (10), 2900–2911.
Dorababu, A., 2019. Critical evaluation of current Alzheimer′ s Drug Discovery (2018-19) & futuristic Alzheimer drug model approach. Bioorg. Chem. 93, 103299.
Eklund, P.C., Backman, M.J., Kronberg, L.Å., Smeds, A.I., Sjöholm, R.E., 2008. Identification of lignans by liquid chromatography‐electrospray ionization ion‐trap mass spectrometry. J. Mass Spectrom. 43, 97–107.
Fan, M., Zhang, G., Hu, X., Xu, X., Gong, D., 2017. Quercetin as a tyrosinase inhibitor: inhibitory activity, conformational change and mechanism. Food Res. Int. 100, 226–233.
Fu, Q., Zhang, C., Lin, Z., Sun, H., Liang, Y., Jiang, H., Song, Z., Wang, H., Chen, S., 2016. Rapid screening and identification of compounds with DNA-binding activity from Folium Citri Reticulatae using on-line HPLC–DAD–MSn coupled with a post column fluorescence detection system. Food Chem. 192, 250–259.
Gadd, G.M., 1999. Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes. In: Poole, R.K. (Ed.), Advances in Microbial Physiology. Academic Press, pp. 47–92.
Ghimire, B., Suh, G.U., Lee, C.H., Heo, K., Jeong, M.J., 2018. Cypsela morphology of
Cirsium species (Asteraceae) and its taxonomic implications. Flora 249, 40–52.
Han, J., Ye, M., Qiao, X., Xu, M., Wang, B.-r., Guo, D.-A., 2008. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chroma-tography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 47, 516–525.
Ismail, B.B., Pu, Y., Guo, M., Ma, X., Liu, D., 2019. LC-MS/QTOF identification of phy-tochemicals and the effects of solvents on phenolic constituents and antioxidant ac-tivity of baobab (Adansonia digitata) fruit pulp. Food Chem. 277, 279–288.
Jeong, G.H., Park, E.K., Kim, T.H., 2018. New anti-glycative flavonoids from Cirsium
se-tidens with potent radical scavenging activities. Phytochem. Lett. 26, 115–119.
Katalinić, M., Rusak, G., Domaćinović Barović, J., Šinko, G., Jelić, D., Antolović, R., Kovarik, Z., 2010. Structural aspects of flavonoids as inhibitors of human butyr-ylcholinesterase. Eur. J. Med. Chem. 45, 186–192.
Kozyra, M., Glowniak, K., 2013. Phenolic acids in extracts obtained from the flowering herbs of Cirsium vulgare (Savi) ten. Growing in Poland. Acta Soc. Bot. Pol. 82, 325–329.
Llorent-Martínez, E.J., Zengin, G., Lobine, D., Molina-García, L., Mollica, A.,
Mahomoodally, M.F., 2018. Phytochemical characterization, in vitro and in silico approaches for three Hypericum species. New J. Chem. 42, 5204–5214.
Ma, Q., Jiang, J.-G., Yuan, X., Qiu, K., Zhu, W., 2019. Comparative antitumor and anti-inflammatory effects of flavonoids, saponins, polysaccharides, essential oil, coumarin and alkaloids from Cirsium japonicum DC. Food Chem. Toxicol. 125, 422–429.
Mazzei, L., Ciurli, S., Zambelli, B., 2016. Chapter nine - isothermal titration calorimetry to characterize enzymatic reactions. In: Feig, A.L. (Ed.), Methods in Enzymology. Academic Press, pp. 215–236.
Mishra, P., Kumar, A., Panda, G., 2019. Anti-cholinesterase hybrids as multi-target-di-rected ligands against Alzheimer’s disease (1998-2018). Bioorg. Med. Chem. 27, 895–930.
Mokrani, A., Cluzet, S., Madani, K., Pakina, E., Gadzhikurbanov, A., Mesnil, M., Monvoisin, A., Richard, T., 2019. HPLC-DAD-MS/MS profiling of phenolics from different varieties of peach leaves and evaluation of their antioxidant activity: a comparative study. Int. J. Mass Spectrom. 445, 116192.
Nalewajko-Sieliwoniuk, E., Nazaruk, J., Kotowska, J., Kojło, A., 2012. Determination of the flavonoids/antioxidant levels in Cirsium oleraceum and Cirsium rivulare extracts with cerium(IV)–rhodamine 6G chemiluminescence detection. Talanta 96, 216–222.
Nazaruk, J., Czechowska, S.K., Markiewicz, R., Borawska, M.H., 2008. Polyphenolic compounds and in vitro antimicrobial and antioxidant activity of aqueous extracts from leaves of some Cirsium species. Nat. Prod. Res. 22, 1583–1588.
Nazaruk, J., Jakoniuk, P., 2005. Flavonoid composition and antimicrobial activity of
Cirsium rivulare (Jacq.) all. Flowers. J. Ethnopharmacol. 102, 208–212.
Olszowy, M., 2019. What is responsible for antioxidant properties of polyphenolic com-pounds from plants? Plant Physiol. Biochem. 144, 135–143.
Qian, X.-J., Jin, Y.-S., Chen, H.-S., Xu, Q.-Q., Ren, H., Zhu, S.-Y., Tang, H.-L., Wang, Y., Zhao, P., Qi, Z.-T., 2016. Trachelogenin, a novel inhibitor of hepatitis C virus entry through CD81. J. Gen. Virol. 97, 1134–1144.
Steingass, C.B., Glock, M.P., Schweiggert, R.M., Carle, R., 2015. Studies into the phenolic patterns of different tissues of pineapple (Ananas comosus [L.] Merr.) infructescence by HPLC-DAD-ESI-MS n and GC-MS analysis. Anal. Bioanal. Chem. 407, 6463–6479.
Sun, L., Warren, F.J., Gidley, M.J., 2019. Natural products for glycaemic control: poly-phenols as inhibitors of alpha-amylase. Trends Food Sci. Tech. 91, 262–273.
Uysal, S., Zengin, G., Locatelli, M., Bahadori, M.B., Mocan, A., Bellagamba, G., De Luca, E., Mollica, A., Aktumsek, A., 2017. Cytotoxic and enzyme inhibitory potential of twoPotentilla species (P. speciosa L. and P. reptans Willd.) and their chemical com-position. Front. Pharmacol. 8, 290.
Van Hoyweghen, L., De Bosscher, K., Haegeman, G., Deforce, D., Heyerick, A., 2014. In vitro inhibition of the transcription factor NF‐κB and cyclooxygenase by Bamboo extracts. Phytother. Res. 28, 224–230.
Yang, X., Shao, H., Chen, Y., Ding, N., Yang, A., Tian, J., Jiang, Y., Li, G., Jiang, Y., 2018. In renal hypertension, Cirsium japonicum strengthens cardiac function via the inter-medin/nitric oxide pathway. Biomed. Pharmacother. 101, 787–791.
Zeng, Q.-H., Zhao, J.-B., Wang, J.-J., Zhang, X.-W., Jiang, J.-G., 2016. Comparative ex-traction processes, volatile compounds analysis and antioxidant activities of essential oils from Cirsium japonicum Fisch. Ex DC and Cirsium setosum (Willd.) M.BIeb. LWT -Food Sci. Technol. 68, 595–605.
Zhang, L., Li, D., Cao, F., Xiao, W., Zhao, L., Ding, G., 2018. Identification of human acetylcholinesterase inhibitors from the constituents of EGb761 by modeling docking and molecular dynamics simulations. Comb. Chem. High. T. Scr. 21, 41–49.
Fig. 4. The relationship between biological activities and the quantified bioactive compounds of Cirsium yildizianum. A&B: correlation between total bioactive
component (TPC and TFC) and biological activities (A: Pearson’s correlation coefficients. B: Spearman correlation coefficients). C: Circos plot show the correlation (r = 0.9) between the main bioactive compounds and the biological activities.