Europ.Poult.Sci., 79. 2015, ISSN 1612-9199, © Verlag Eugen Ulmer, Stuttgart. DOI: 10.1399/eps.2015.102
Effects of combined in ovo injection of dried whey and Enterococcus faecium
on performance, ileal histomorphology, erythrocyte morphology and ileal
microbiota of broiler chickens
Einfluss der kombinierten in ovo-Injektion von getrockneter Molke und Enterococcus feacium
auf die Leistung, die Dünndarmmorphologie, die Erythrozyten-Morphologie und die ileale
Mikroflora von Broilern
I. Coskun2, E. Tahtabicen1, F. Koç1, A.A. Okur1, K. Yilmaz1, M. Kanter3, C. Aktas4, M. Erboga4 and H.E. Samli1*,
1 Namık Kemal University, Agricultural Faculty, Dept. of Animal Science, Tekirdağ. Turkey 2 Ahi Evran University, Agricultural Faculty, Dept. of Animal Science, Kirsehir, Turkey
3 Medeniyet University, Faculty of Medical Sciences, Dept. of Histology and Embryology, Istanbul, Turkey 4 Namık Kemal University, Faculty of Medical Sciences, Dept. of Histology and Embryology, Tekirdağ, Turkey
Correspondence: esamli@nku.edu.tr
Manuscript received 25 February 2015, accepted 19 June 2015
Introduction
Probiotics, prebiotics and mixtures are used as competitive exclusion culture (CEC) in poultry nutrition. The term of CEC is used to describe beneficial bacteria preventing proliferation of pathogenic bacteria such as E. coli, Enterobacteriaceae, Salmonella, etc. in the digestive tract. Competitive exclusion culture entails the prevention of pathogenic bacteria and the replenishment of beneficial bacteria populations in the digestive tract. Before hatching intestines are sterile, but after hatching, intestinal microflora is created in the digestive tract after ingestion of microorganisms by the bird. Early introduction of CEC in the chicken digestive tract by supplementing lactic acid bacteria to the diet has been shown to prevent growth of of pathogen microorganisms in broiler chickens. CEC facilitates a barrier between the intestinal wall and the lumen of gut for the pathogenic bacteria. CEC in a bird’s digestive tract increases the production of volatile fatty acids which in turn decrease the pH level in the digestive tract. Lowering the pH level and decreasing high volatile fatty acid create an unfavourable environment for pathogens. Enterococcus faecium (EF) is one of the probiotic bacteria used as a competitive exclusion culture in poultry nutrition. CAPCAROVA et al. (2010) reported that the inclusion of Enterococcus faecium to drinking water
increased broiler growth performance. AWAD et al. (2008, 2009) reported that a mixture of Enterococcus faecium and
oligosaccharide increased growth performance of broiler chickens. SAMLI et al. (2007, 2010) used dried whey (DW) as a prebiotic. Dried whey is a by-product of cheese making and contains 80% lactose. VAN DOOREN (2001) reported that lactose prevents the proliferation of pathogens and increases the number of beneficial bacteria by acting as a prebiotic. SAMLI et al. (2007, 2010) reported that the addition of DW and EF to the feed increased weight gain and
improved feed conversion ratio (FCR) of broilers. Although the efficacy of using dried whey and Enterococcus faecium in the feed as a symbiotic or CEC together has been clearly demonstrated by SAMLI et al. (2007, 2010) there is no study on in ovo injection of dried whey and Enterococcus faecium into fertile eggs and it is not known whether dried whey and Enterococcus faecium in ovo injection into the fertile eggs can enhance broiler performance or affects broiler ileal microbiota and ileal histomorphology. SCHNEITZ et al. (2002) reported that in ovo injection of CEC derived from an adult hen in the air cell of eggs was beneficial and did not decrease hatchability. Therefore the aim of this study was to determine the effect of an in ovo injection of dried whey and Enterococcus faecium to fertile Ross 308 eggs on performance, gut microbiota, ileal histomorphology and erythrocyte morphology.
Materials and methods
Egg injection and incubation
This study was approved by the ethical committee of Namik Kemal University. A total of 180 fertile eggs provided from a breeder flock at 40 weeks of age (Ross 308) were obtained from the hatchery of the Banvit Poultry Company. The eggs were incubated under optimal conditions at the Banvit Poultry Company in Bandirma. After unfertilised or eggs with dead embryos were discarded through illumination after 12 days of incubation at 18 days of incubation, fertile eggs were weighed and divided into 4 equal weight eggs groups (control, in ovo injection of dried whey 4% into distilled water, in ovo injection of Enterococcus faecium 1.75 g/l into distilled water, in ovo injection of 4% dried whey and Enterococcus faecium 1.75 g/l into distilled water). Each group was then injected with appropriate in ovo treatment solutions to the air sac of the eggs (0.2 ml/egg) with INUTECH Automatic Hatchery Systems.
Animals and housing
The experiment consisted of one dietary treatment in a 2×2 factorial design. During hatching the chicks that hatched during the first 6 h were discarded from the study to ensure equal hatching time. After hatching 120 day-old mixed sex healthy chicks were housed in three-tier battery cages per treatment with 6 replications and 5 chicks per replicate for 21 days. Battery cages were equipped with wire mesh, dropping trays, nipple drinkers and trough feeders. The battery cages were placed in an environmentally controlled room with windows. Chicks were given mashed feed and watered ad libitum with 1D:23L illumination by fluorescent lamps. Birds were weighed weekly and feed intake, feed conversion ratio and weight gain was calculated. Two birds (1 female and 1 male) chosen randomly from each replication (12 birds per treatment) were slaughtered at day 21 to determine proventriculus, gizzard, liver, duodenum, jejunum and ileum weight. Weights of edible inner organs and duodenum, jejunum, ileum weights (gizzard, heart and liver) were recorded as g/100 g body weight.
Dried whey and Enterococcus faecium
In the study, Enterococcus faecium was used as a probiotic (Cylactin1 LBC ME10 deposition number NCIMB 10415, Nutritional Products Ltd., Birsfelden, Switzerland) and dried whey containing 80% lactose as a by-product of cheese manufacturing were used. Dosages of DW and EF used in the study were calculated based on the live weight of animals based on the previous studies of SAMLI et al. (2007, 2010).
Diet
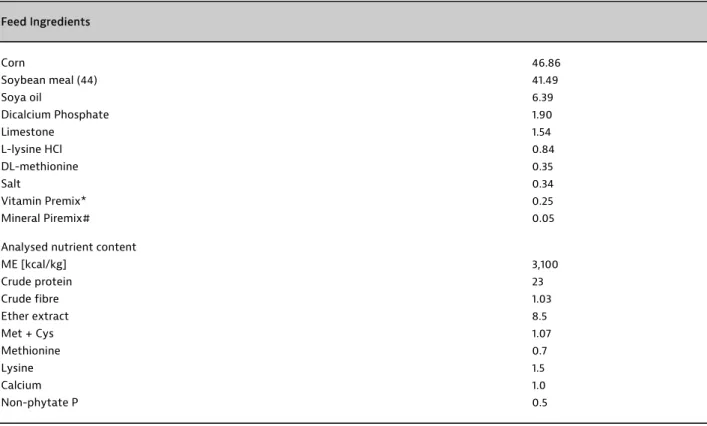
The experimental diet was prepared according to the NRC recommendations (NRC, 1994). Soybean meal and maize-based diets were prepared at Namik Kemal University facility holding 23% CP and 3100 kcal ME/kg (Table 1).
Table 1. Composition and analyzed content of nutrients of the experimental diet (%, DM basis) Zusammensetzung und analysierte Nährstoffgehalte der Versuchsration (% der Trockenmasse)
Feed Ingredients Corn 46.86 Soybean meal (44) 41.49 Soya oil 6.39 Dicalcium Phosphate 1.90 Limestone 1.54 L-lysine HCl 0.84 DL-methionine 0.35 Salt 0.34 Vitamin Premix* 0.25 Mineral Piremix# 0.05
Analysed nutrient content
ME [kcal/kg] 3,100 Crude protein 23 Crude fibre 1.03 Ether extract 8.5 Met + Cys 1.07 Methionine 0.7 Lysine 1.5 Calcium 1.0 Non-phytate P 0.5
* Vitamin D3 5,000 IU, Vit A 14,000 IU, Vit K 34 mg, Vit E 50 mg, Vit B1 3 mg, Vit B6 4 mg, Vit B2 8 mg, Vit B12 16 mg, niacin 20 mg, folic acid 2 mg, biotin 150 mg, pantothenic acid 20 mg, choline 1,800 mg, #Cu 5 mg, Fe 80 mg, Zn 80 mg, Mn 100 mg, Se 150 mg for per kg of diet
Gut histomorphology
At the 21st day of the trial two chicks were removed randomly from each replicate (12 chicks per treatment), weighed individually and killed. The ileum was removed, purified with formalin solution and cut into 0.5 cm pieces and placed into 10% formalin for further processing. Tissue sections were placed into tissue cassettes for dehydration and were embedded in paraffin blocks, and subsequently cut in 5 μm thick slices and placed on a slide. Each ileal histomorphologic tissue sample was prepared and stained with haematoxylin and eosin solution using standard paraffin-embedding methods (XU et al., 2003). After the embedding process, the thickness of the lamina muscularis
mucosae, crypt depth, villus height and villus width were photographed and evaluated by using an image processing and analysis system (Motic Images Plus 2.0).
Microbiology of the small intestine
Samples of the gastrointestinal tract content were collected post mortem. All of the gastrointestinal tract content was transferred under aseptic conditions into sterile glass tubes and kept on ice until subsequent inoculation into agars. MRS agar (Merck, Darmstadt, Germany, 1.10660) was used for the enumeration of lactic acid bacteria (LAB) at 37°C for a 3-day incubation period and malt extract agar (Merck, Darmstadt, Germany, 1.05398) was used for the enumeration of yeast at 25°C for a 3-day incubation period. Violet Red Bile (VRB) (Merck, Darmstadt, Germany, 1.01406) agar was used for enumeration of Enterobacteriaceae at 37°C for an 18–20 hour incubation period.
Bacterial colonies were counted and the average number of live bacteria was calculated based on weight (g) of ileal content. The LAB, yeast and Enterobacteriaceae counts of the samples were converted into log colony forming units (cfu g–1).
Slide preparation and staining
Slide preparation and staining were based on the SAMLI et al. (2010) study. A drop of blood was smeared over a slide
and air-dried. The smears were fixed in methanol and stained using the Giemsa azur eosin methylene blue solution (Merck, Darmstadt, Germany, 1.09204) method. Blood smears were observed under a microscope (BX 51, Olympus, Japan) at 40× magnification. Erythrocyte length and width were determined using an image processing and analysis system (Motic Images Plus 2.0, China).
Statistical analyses
Collected data were recorded on a weekly basis and statistically subjected to ANOVA using a statistical package program (SAS Institute, 1994). Differences between group means were separated by Duncan’s multiple range tests. Results and discussion
Performance parameters
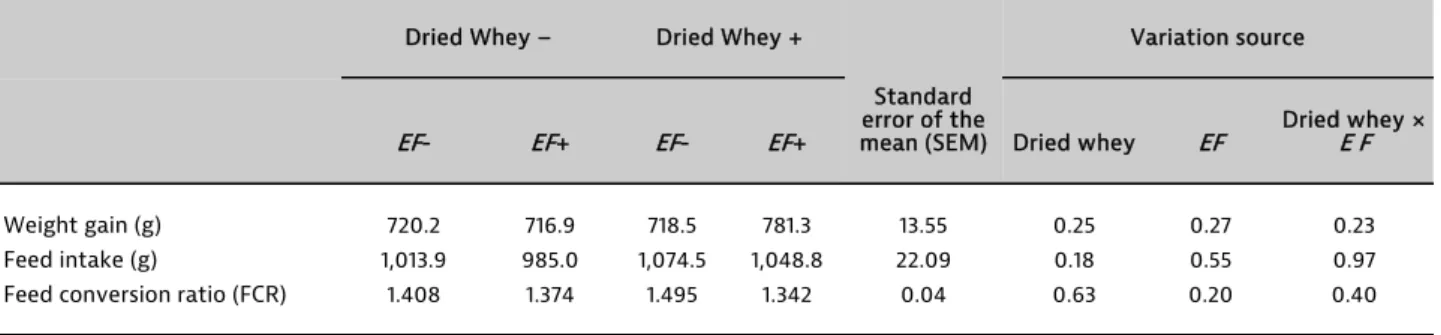
Hatchability was similar among the groups (between 94% and 96%). The effects of in ovo injection of dried whey (DW) and Enterococcus faecium (EF) to fertile chicken eggs on performance parameters are presented in Table 2. In ovo injection of DW and EF bacteria to fertile chicken eggs at the 18th day of incubation did not affect weight gain, food intake and feed conversion ratio (FCR) of broilers (P> 0.05). In the trial, DW and EF were used as a symbiotic or competitive exclusion culture (CEC) for in ovo nutrition for broilers. Injection of DW and EF to fertile chicken eggs at the 18th day of hatch did not have any effect on broiler performance or hatchability. Our results were consistent with the results of MAIORANO et al. (2012) showing that the injection of a prebiotic and a symbiotic to fertile broiler eggs
have no effect on growth performance. However, they found that FCR increased with prebiotic and symbiotic injections. Several studies have demonstrated benefits of adding a probiotic and a prebiotic as a symbiotic to broiler diets. SAMLI et al. (2007, 2010) reported that adding DW and EF to the feed increased weight gain and improved feed
conversation ratio of broilers. AWAD et al. (2008) reported that adding a symbiotic including EF and oligosaccharide to broiler feed improved the performance of broilers compared to the controls. AWAD et al. (2009) also reported that adding a probiotic and a symbiotic to the feed increased performance of broilers significantly (P<0.05) in comparison to the control group. However, MOUNTZOURIS et al. (2007), KARAOĞLU and DURDAG (2005), ARSLAN (2004), CELIK et al.
(2007) and AKSU and BOZKURT (2009) reported no positive effect of inclusion of a probiotic or a symbiotic on broiler performance when added to broiler diets. These results show that broiler performance depends on multiple factors such as genetic, environmental or different stress factors as reported by OZTURK and YILDIRIM (2004). Although there
were no difference on the chick growth performance among the groups, DW and EF interaction as a symbiotic or CEC increased performance by approximately 8.5% compared to the other groups.
Table 2. Effects of in ovo injection of dried whey and Enterococcus faecium to fertile chicken eggs on performance Einfluss der In-ovo-Injektion von getrockneter Molke und in befruchtete Bruteier auf die Leistung
Dried Whey – Dried Whey +
Standard error of the mean (SEM)
Variation source
EF- EF+ EF- EF+ Dried whey EF Dried whey × E F
Weight gain (g) 720.2 716.9 718.5 781.3 13.55 0.25 0.27 0.23
Feed intake (g) 1,013.9 985.0 1,074.5 1,048.8 22.09 0.18 0.55 0.97
Feed conversion ratio (FCR) 1.408 1.374 1.495 1.342 0.04 0.63 0.20 0.40
Table 4. Effects of in ovo injection of dried whey and Enterococcus faecium to fertile chicken eggs on gut microbiota and organ weights (gr/100 gr Live weight)
Einfluss der In-ovo-Injektion von getrockneter Molke und Enterococcus faecium in befruchtete Bruteier auf die Zusammensetzung der Dünndarmflora und auf die Organgewichte (g/100 g Lebendmasse)
Dried Whey – Dried Whey +
Standard error of the mean (SEM)
Variation source
EF- EF+ EF- EF+ Dried whey EF Dried whey × E F
Proventriculus 0.53 a 0.42 b 0.50 ab 0.46ab 0.016 0.80 0.02 0.22 Gizzard 3.28 3.22 3.43 3.26 0.147 0.77 0.70 0.86 Liver 2.25 ab 2.26 ab 2.12ab 2.41 a 0.048 0.88 0.12 0.13 Duodenum 1.00 1.09 1,11 1.15 0.034 0.23 0.36 0.97 Jejunum 2.61 b 2.96 ab 2.60 b 3.53a 0.131 0.23 0.01 0.21 Ileum 1.33 b 1.70 ab 1.77 a 2.04a 0.082 0.01 0.03 0.73
Lactic acid bacteria (106) 3.68 d 4.81 b 4.04 c 5.92a 0.180 < 0.001 < 0.001 < 0.001
Yeast (107) 3.64 c 3.84 ab 3.71 b 4.01a 0.056 0.25 0.02 0.60
Enterobacteriaceae (107) 1.77 1.97 1.58 1.58 0.341 0.26 0.39 0.39
Abc Means in the same column with no common superscripts differ (P < 0.05). EF= Enterococcus faecium
The effects of in ovo injection of DW and EF to fertile chicken eggs at the 18th day of incubation on ileal microbiota of broilers are presented in Table 4. The effect of DW × EF interaction on LAB colonisation was significant. An injection of DW and EF enhanced the LAB colonisation in the gut compared to the control group. Although there was no effect of DW × EF interaction on yeast colonisation, EF injection increased yeast count compared to the other groups. There was no difference in the enterobacteriaceae count among the groups.
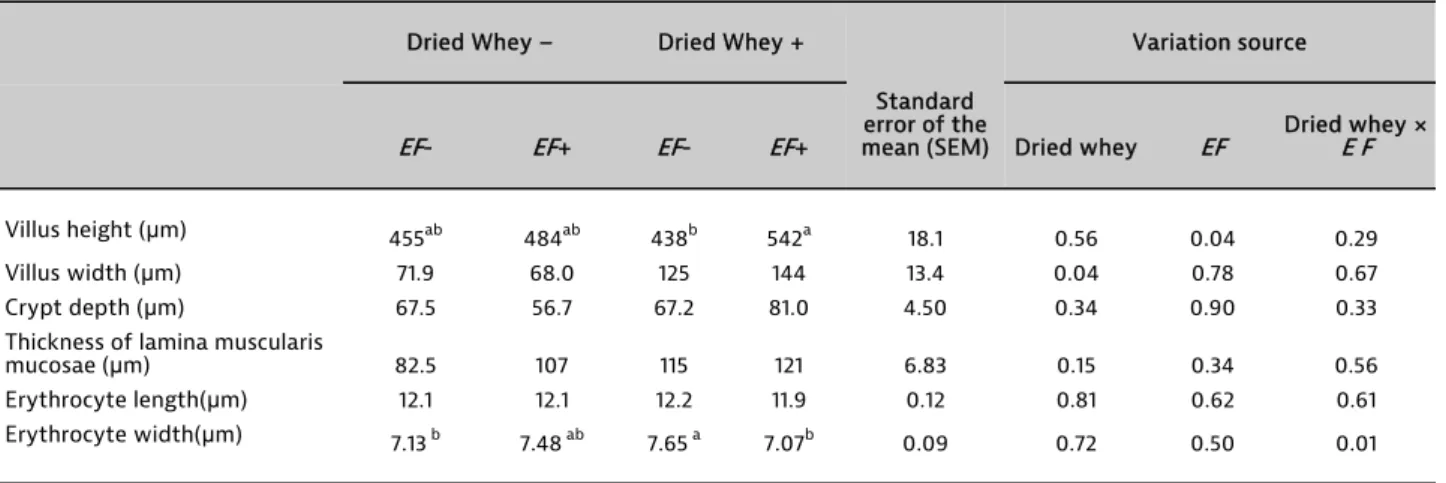
The effects of DW and EF to fertile chicken eggs on ileal histomorphology parameters are presented in Table 3. At 21 days of age, there was no interaction effect on ileal histomorphology. Only an EF injection to fertile eggs increased villus height with regard to a DW injection. Except for villus height, other ileal histormorphological parameters were not affected by the injection. Results show that a combined injection of DW and EF as a symbiotic improved ileal microbiota. These results were consistent with the findings of UNI and FERKET (2004), TAKO et al. (2004) and SMIRNOV
et al. (2006). These researchers showed that early colonisation of the intestinal tract of chickens with a bacterial culture known as competitive exclusion.
Table 3. Effects of in ovo injection of dried whey and Enterococcus faecium to fertile chicken eggs on ileal histomorphology and erythrocyte morphology
Einfluss der In-ovo-Injektion von getrockneter Molke und Enterococcus faecium in befruchtete Bruteier auf die Dünndarm- und die Erythrotzyten-Morphologie
Dried Whey – Dried Whey +
Standard error of the mean (SEM)
Variation source
EF- EF+ EF- EF+ Dried whey EF Dried whey × E F
Villus height (μm) 455ab 484ab 438b 542a 18.1 0.56 0.04 0.29
Villus width (μm) 71.9 68.0 125 144 13.4 0.04 0.78 0.67
Crypt depth (μm) 67.5 56.7 67.2 81.0 4.50 0.34 0.90 0.33
Thickness of lamina muscularis
mucosae (μm) 82.5 107 115 121 6.83 0.15 0.34 0.56
Erythrocyte length(μm) 12.1 12.1 12.2 11.9 0.12 0.81 0.62 0.61
Erythrocyte width(μm) 7.13 b 7.48 ab 7.65 a 7.07b 0.09 0.72 0.50 0.01
ab Means in the same column with no common superscripts differ (P < 0.05). EF= Enterococcus faecium.
The present results show that combined injection of DW and EF as a symbiotic has several advantages on broiler performance such as increasing lactic acid bacteria in the gut, improving villus height and enhancing ileum and jejunum weight. These results are consistent with the findings of AWAD et al. (2008, 2009). AWAD et al. (2008)
reported that addition of a symbiotic including EF increased (P< 0.01) villus height/crypt depth ratio and villus height in ileum. Also, AWAD et al. (2009) reported that addition of either a probiotic or a symbiotic increased (P< 0.05) villus height/crypt depth ratio and villus height in both the duodenum and ileum. Erythrocyte length was not affected by the injection (Table 2). Dried whey injection to fertile chicken eggs increased erythrocyte width. Dried whey × EF interaction on erythrocyte width was significant. HARTMAN and LESSLER (1963) reported that erythrocyte size is
related to general metabolic activity. SAMLI et al. (2010) demonstrated that erythrocyte size can be affected when birds are housed in different environments or by a varying metabolic rate when displaying greater feeding, mixing of floor material (playing) and walking behaviours, as reported by FOUAD et al. (2008). Growth performance results
were not reflected by the increased ileal villus height and ileal LAB colonisation. An increase in performance was expected with the increase in ileal villi height and an increase of ileal LAB colonisation. This study was conducted in environmentally controlled room and the summer season in Tekirdağ province and there were no negative environmental and dietary factors during the period of study. Growth performance may be unaffected for these reasons.
Although not statistically significant the inclusion of EF and DW through the air cell of eggs on the 18th day of incubation did tended to increase performance to day 21 by about 8.5% compared to the other groups. However, a combined injection of EF and DW increased LAB and villus height in the gut and increased jejunum and ileum weights. To conclude, an in ovo injection of DW and EF can be used to improve villi morphology and to develop LAB colonisation in ileum. However, further studies should be conducted to determine the effects of in ovo injection of different probiotic and/or symbiotic microorganisms and different stress factors on broiler performance.
Summary
The aim of this experiment was to determine the effects of in ovo injection of dried whey and Enterococcus faecium to fertile Ross 308 chicken eggs on performance, ileal histomorphology, ileum microbiota and edible viscera weights. A 2×2 factorial design was used. Fertile Ross 308 eggs were injected with 4 different solutions: A) control solution
At the end of the experiment, lactic acid bacteria (LAB) colonisation in ileum were 3.68, 4.81, 4.04 and 5.92 cfu/g respectively and the highest LAB colonisation was in the combined injection group. At the end of the experiment, villus height was 455, 484, 438 and 542 μm respectively and the highest villus height was found in the combined injection group. Although broiler performance was not different among the groups, a combined injection of Enterococcus faecium and dried whey provided better LAB colonisation and increased villus height in ileum. In conclusion, a combined injection of Enterococcus faecium and dried whey has a symbiotic effect on ileal histomorphology and gut microbiota. However, further studies should be conducted to determine the effects of in ovo injection of different probiotic and/or symbiotic microorganisms and different stress factors on broiler performance.
Key words
Broiler, in ovo injection, dried whey, Enterococcus faecium, intestinal morphology, ileal microflora Zusammenfassung
Einfluss der kombinierten in ovo-Injektion von getrockneter Molke und Enterococcus feacium auf die Leistung, die Dünndarmmorphologie, die Erythrozyten-Morphologie und die ileale Mikroflora von Broilern
Die Studie hatte die Untersuchung des Einflusses einer in-ovo-Injektion von getrockneter Molke und Enterococcus faecium in befruchtete Ross 308 Bruteier auf die Leistung, die Dünndarmmorphologie, die Zusammensetzung der Mikroflora im Dünndarm und auf die Gewichte der essbaren Organe zum Ziel. Der Versuch war 2x2-faktoriell angelegt. In die befruchteten Ross 308 Bruteier wurden vier unterschiedlichen Lösungen injiziert: A) Kontrolle (destilliertes Wasser), B) destilliertes Wasser + 4% getrocknete Molke, C) destilliertes Wasser + Enterococcus faecium, D) destilliertes Wasser + 4% getrocknete Molke + Enterococcus faecium. Am 21. Versuchstag wurden die Lebendmassen, die Futteraufnahme, die Futterverwertung, die Dünndarm-Morphologie, die Zusammensetzung der Mikroflora im Dünndarm und die Organgewichte bestimmt.
Am Versuchsende konnten zwischen den Behandlungsgruppen keine Unterschiede für die Lebendmassezunahme, die Futteraufnahme und die Futterverwertung ermittelt werden (P > 0,05). Im Ileum wurden für die vier Behandlungsgruppen 3,68, 4,81, 4,04 bzw. 5,92 log cfu Milchsäure-bildende Bakterien (LAB)/g Darminhalt ermittelt. Die höchste Kolonisation mit LAB wurde für die kombinierte Injektionsgruppe vorgefunden. Am Versuchsende betrug die Höhe der Villi in den vier Versuchsgruppen 455, 484, 438 bzw. 542 μm und die höchste Höhe der Villi lag in der kombinierten Injektionsgruppe vor (P < 0,05). Obwohl sich die Gruppen nicht in der Leistung unterschieden, führte die kombinierte Injektion von getrockneter Molke und Enterococcus faecium zu einer besseren Besiedelung mit LAB und zu einer höheren Villushöhe im Ileum. Es wurde daher der Schluss gezogen, dass die kombinierte Injektion von getrockneter Molke und Enterococcus feacium einen synbiotischen Effekt auf die Dünndarmmorphologie und die Zusammensetzung der Dünndarm-Mikroflora hat. Es erscheinen allerdings noch weitere Untersuchungen erforderlich, um die möglichen Effekte der in-ovo-Injektion von unterschiedlichen, probiotischen Mikroorganismen bzw. synergistischen Effekten sowie den Einfluss unterschiedlicher Stressfaktoren auf die Leistung der Broiler bewerten zu können.
Stichworte
Broiler, in ovo-Injektion, Molke, Enterococcus feacium, Darmmorphologie, intestinale Mikroflora References
AKSU, T., A.S. BOZKURT, 2009: Effect of dietary essential oils and/or humic acids on broiler performance, microbial population of intestinal content and antibody titres in the summer season. Kafkas. Univ. Vet. Fak. Derg. 15, 185-190.
ARSLAN, C., 2004: Effect of dietary probiotic supplementation on growth performance in the rock partridge (alectoris graeca). Turk. J. Vet. Anim. Sci. 28, 887-891.
AWAD, W.A., K, GHAREEB. S, ABDEL-RAHEEM, J. BÖHM, 2009: Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 88, 49-55.
AWAD, W., K, GHAREEB, J. BÖHM, 2008: Intestinal structure and function of broiler chickens on diets supplemented with a synbiotic containing Enterococcus faecium and oligosaccharides. Int. J. Mol. Sci 9, 2205-2216.
BAILEY, J.S, E. LINE, 2001: In Ovo Gentamicin and Mucosal Starter Culture to Control Salmonella in Broiler Production. J. Appl. Poult. Res. 10, 376-379.
CAPCAROVA, M., J. WEISS, C. HRNCAR, A. KOLESAROVA, G. PAL, 2010: Effect of Lactobacillus fermentum and Enterococcus faecium strains on internal milieu, antioxidant status and body weight of broiler chickens. J. Anim. Phys and Anim. Nutr. 94, Issue 5, pages e215–e224, DOI: 10.1111/j.1439-0396.2010.01010.x.
CELIK, K., M. MUTLUAY, A. UZATICI, 2007: Effects of probiotic and organic acid on performance and some tissue in broiler chicks. 6th Symposium of Animal, Biology and Nutrition, 46-51, Cartea Universitara Archiva Zootechnica. FOUAD, M.A., A.H. ABDEL RAZEK, B.M. EL SAYED, 2008: Broilers welfare and economics under two management
alternatives on commercial scale. Int. J. Poult. Sci. 7, 1167-1173.
KARAOĞLU, M., H. DURDAG, 2005: The influence of dietary probiotic (saccharomyces cerevisiae) supplementation and different slaughter age on the performance, slaughter and carcass properties of broilers. Int. J. Poult. Sci. 4, 309- 316.
HARTMAN, F.A., M.A. LESSLER, 1963: Erythrocyte measurements in birds. Auk. 80, 467-473.
MAIORANO, G., A. SOBOLEWSKA, D. CIANCIULLO, K. WALASIK, G. ELMINOWSKA-WENDA, A. SŁAWIŃSKA, S. TAVANIELLO, J. ŻYLIŃSKA, J. BARDOWSKI, M. BEDNARCZYK, 2012: Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 91, 2963-2969.
MEIJERHOF, R., R.M. HULET, 1997: In ovo injection of competitive exclusion culture in broiler hatching eggs. J. Appl. Poult. Res. 6, 260- 266.
MOUNTZOURIS, K.C., P. TSIRTSIKOS, E. KALAMARA, S. NITSCH, G. SCHATZMAYR, K. FEGEROS, 2007: Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating caecal microflora composition and metabolic activities. Poult. Sci 86, 309- 317.
NRC, 1994: Nutrient Requirements of Poultry. 9th ed. National Academy Press, Washington, DC.
NURMI, E.V., M. RANTALA, 1973: New aspect of Salmonella infection in broiler production. Nature 241, 210- 211. OZTURK, E., A. YILDIRIM, 2004: Probiyotiklerin etlik piliçlerin performansı ve bağırsak mikrobiyolojik özelliklerine
etkileri. Ulusal Zootekni Bilim Kongresi, Cilt 2. 152-156, ISPARTA.
SAMLI, H.E., N. SENKOYLU, F. KOC, M. KANTER, A. AGMA, 2007: Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota. Arch. Anim. Nutr. 61, 42- 49.
SAMLI, H.E., S. DEZCAN, F. KOC, M.L.OZDUVEN, A.O. AGMA, N. SENKOYLU, 2010: Effects of Enterococcus faecium supplementation and floor type on performance, morphology of erythrocytes and intestinal microbiota in broiler chickens. Br. Poult. Sci. 51, 564- 568.
SCHNEITZ, C.E., E.V. NURMI, P.M.L. VEIJALAINEN, 2002: Preventing of gastrointestinal disorders due to salmonella, campylobacter, escherichia and/or clostridium by injecting vaccines into poultry eggs during incubation. Patent no: US 6,491,910 B1.
SMIRNOV, A., E. TAKO, P.R. FERKET, Z. UNI, 2006: Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult. Sci. 85, 669- 673.
TAKO, E., P.R. FERKET, Z. UNI, 2004: Effects of in ovo feeding of carbohydrates and beta-hydroxy-beta-methylbutyrate on the development of chicken intestine. Poult. Sci. 83, 2023- 2028.
VAN DOOREN, P., 2001: The use of milk products in young poultry nutrition. International. Poultry. Production. 9, 24 – 25.