REPUBLIC of TURKEY SELÇUK UNIVERSITY HEALTH SCIENCES INSTITUTE
In vitro correction of HBB V6G mutation in lymphocyte cells of patients with sickle cell anemia using genome-editing CRISPR / Cas9 technique
Hasanain Akram Zainalbden ZAINALBDEN
MASTER THESIS
DEPARTMENT OF MEDICAL GENETISCS
SUPERVISOR
Assist. Prof. Dr. Nadir KOÇAK
ii REPUBLIC of TURKEY
SELÇUK UNIVERSITY HEALTH SCIENCES INSTITUTE
In vitro correction of HBB V6G mutation in lymphocyte cells of patients with sickle cell anemia using genome-editing CRISPR / Cas9 technique
Hasanain Akram Zainalbden ZAINALBDEN
MASTER THESIS
DEPARTMENT OF MEDICAL GENETISCS
SUPERVISOR
Assist. Prof.Dr.Nadir KOÇAK
Bu araştırma Selçuk Üniversitesi Bilimsel Araştırma Projeleri Koordinatörlüğü tarafından proje(17202004) numarası ile desteklenmiştir
i PREFACE
This project has been sponsored by Selçuk University Scientific Research Projects Coordination Unit (BAP). I would like to thank this institution and the staff for their academic support,
To my lecturers who did not spare their help and assistances, Assist. Prof. Dr.Nadir KOÇAK, Prof. Dr. Tülin ÇORA, Prof. Dr. Hasan ACAR and Spec. Dr. Süleyman NERGİZ.
Special thanks to my beloved wife( Dilek BEYATLI) who supported me financially and morally throughout the study period.
I dedicate this thesis to my father, my mother, sisters and my brothers in law, to whom I will always be indebted. I would also like to particularly thank to Ali Kemal BEYATLI, Tuğçe DURAN, Mustafa Ugur AVŞAR , Faten ÖZTÜRK (My patient) and the other researchers who were involved in different stages of the project.
ii CONTENTS
TABLE AND FIGURE LIST ... iv
ABBREVİATİONS ... vi
SUMMARY ... vii
ÖZET ... ix
1. INTRODUCTION ... 1
1.1. Sickle cell anemia ... 1
1.2. Frequencies ... 2
1.3. HBB gene ... 3
1.3. Studies on sickle cell anemia ... 4
1.4. Genome editing ... 5
1.5. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat) ... 6
1.6. CRISPR-Case System Types ... 10
1.7. Genetic engineering applications of CRISPR-Cas system ... 11
1.8. Genome editing applications in embryo ... 14
1.9. In vivo genome editing applications ... 15
1.10. Ex vivo genom editing applications ... 17
2. MATERIALS AND METHODS ... 20
2.1 collecting Blood samples from patients ... 20
2.2. Cell Culture ... 20
2.3. CRISPR gRNA design ... 20
2.4. HR SSODN templates design for CRISPR-directed HR ... 27
2.5. Primer Design For The HBB Gene ... 32
2.6. Culture of Bacteria ... 33
2.7. Bacterial Colonies ... 33
2.8. Lentiviral vector digestion, oligo annealing, and cloning into digested vector ... 34
2.9. Agarose Gel Electrophoresis(1% Agarose Gel) ... 34
2.10. Loading Samples and Running an Agarose Gel ... 35
2.11. Making Calcium Competent Cells ... 35
2.12. Bacterial Transformation (Heat shock transfecting methods) ... 36
2.13. Colony selection and culturing ... 37
iii
2.15. 1 mg/ml Polyethylenimine in ddH2O(preparation) ... 38
2.16. Transfecting CRISPRs into cells (lymphocytes) ... 38
2.17. DNA isolation from lymphocytes ... 38
2.18. Gene expression studies (RTqPCR) ... 39
3. RESULTS ... 40
3.1 Vector plasmid transformation to DH5-Alpha E.Coli bacteria analysis ... 40
3.2. Agarose gel electrophoresis analysis ... 40
3.3. Lentiviral vector digestion analysis ... 41
3.4. Transformation of ligation product to DH5-Alpha E.Coli analysis ... 41
3.5. Transformation of CRISPR gRNA to cells of interest analysis ... 42
3.6. Verify the segment of the CRISPR / Cas9 system(RT qPCR analysis): ... 44
4.DISCUSSION ... 48
5.CONCLUSION AND RECOMMENDATIONS ... 53
6.REFERENCES ... 54
iv TABLE AND FIGURE LIST
Figure 1.1. Asingle nucleotide change (mutation) in the DNA encoding the beta globin
gene.
Figure 1.2. HBB Gene, Molecular Location. (Homo sapiens Annotation Release 109,
GRCh38.p12) (NCBI).
Figure 1.3. Structure of a CRISPR region on a bacterial chromosome.
Figure 1.4. CRISPR-Cas 3-step working mechanism.
Figure 1.5. Mechanism of crRNA Biogenesis and Targeting in the Three Types of
CRISPR-Cas Systems.
Figure 1.6. Mechanism of DNA repair with NHEJ and HR in the cell by cutting
double-stranded DNA.
Figure 2.1. Image Show how to beginning CRISPR gRNA design.
Figure 2.2. Image Show page of selecting the gene of interest (HBB gene). Figure 2.3. Image Show guide parameters for gRNA design.
Figure 2.4. Image Show annotations tab for selecting exon on amino acids.
Figure 2.5. Image Show how to set the target region on selected Exon. Figure 2.6.Image Show how to set off-target and on-target scores.
Figure 2.7. Image Show how selecting gRNAs with high off-target and on target scores.
Figure 2.8. Image Show the precense of three selecting gRNAs on sequence map.
Figure 2.9. Image Show how to save the selected gRNAs .
Figure 2.10. Image Show one of the selected gRNAs which saved in clipboard. Figure 2.11. Image Show how to creat the Reverse complement for each guide. Figure 2.12. Image Show choosing gRNA from the list for designing SSODN.
Figure 2.13. Image Show the beginning for HR Template(SSODN) design.
Figure 2.14. Image Show creation a copy of HR template sequence after selecting
(Genome and PAM type).
Figure 2.15. Image Show use of annotation tab to select Exon.
v Figure 2.17. Image Show changing of Glutamic Acide amino acide to Valin.
Figure 2.18. Image Show how to adjust HR arms.
Figure 2.19. Image show list of possible silent mutations in HR Template to change the target site.
Figure 2.20. Image show summary of designed HR template.
Figure 2.21. Full Sequence Map for pCAG-eCas9-GFP-U6-gRNA(addgene). Figure 3.1. Image of transformed bacteria growth on LB agar plate.
Figure 3.2. Images of agarose gel electrophoresis under UV-transilluminators (M:10 KB
Marker, A: Vector plasmid (pCAG-eCas9-GFP-U6-gRNA).
Figure 3.3. Image of agarose gel electrophoresis under UV-transilluminators (A:Plasmid
befor digestion with BsmBl (Supercoiled), M:10Kb Marker, B: Plasmid after digestion with BsmBl(Linearized).
Figure 3.4. Image of agarose gel electrophoresis under UV-transilluminators (M: 10Kb Marker, 1:ligation product with CRISPR gRNA 1, 2: ligation product with CRISPR gRNA 2, 3: ligation product with CRISPR gRNA 3).
Figure 3.5. Images of normal lymphocytes under inverted microscope (Eclipse TC100-F -
Nikon, Japan) .
Figure 3.6. Image of lymphocytes after transformation of CRISPR gRNA by( PEI) under inverted microscope (Eclipse TC100-F - Nikon, Japan) .
Figure 3.7. Images of RTqPCR (Melting and Ampilification curve) for sickle cell patient
after CRISPR application, Melting curve seen in (84).
Figure 3.8. Images of RTqPCR (Melting and Ampilification curve) for normal person.
melting curve seen in( 84).
Figure 3.9. Images of RTqPCR (Melting and Ampilification curve) for sickle cell patient
befor CRISPR application, Melting curve seen in( 86).
Figure 3.10. Image of RTqPCR (Melting curve) for sickle cell patient after(Melting curve
vi ABBREVĠATĠONS
SCD Sickle cell disease
SCA Sickle Cell Anemia
HbS hemoglobin S
FBS Fetal Bovine Serum
PBS Phosphate Buffered Saline
HSC hematopoietic stem cell
HbF Fetal hemoglobin
HbA Adult hemoglobin
SIN Self-inactivating
HDR homology-directed repair
DNase Deoxyribonucleas
NHEJ Nonhomologous end-joining ZFNs Zinc Finger Nucleases
TALENs Transcription Activator-Like Effector Nucleases
CRISPR Clustered Regularly Interspaced Short Palindromic Repeat pre-crRNA precursor CRISPR RNAs
crRNA CRISPR RNA
PAM protospacer adjacent motif tracrRNA trans-activating crRNA
FAH fumarylate acetoacetate hydrolase iPSCs pluripotent stem cells
vii SUMMARY
REPUBLIC of TURKEY
SELÇUK UNIVERSITY HEALTH SCIENCES INSTITUTE
In vitro correction of HBB V6G mutation in lymphocyte cells of patients with sickle cell anemia using genome-editing CRISPR / Cas9 technique
Hasanain Akram Zainalbden ZAINALBDEN
Department of Medical Genetics
MASTER THESIS / KONYA-2018
Sickle-cell disease (SCD), also known as sickle-cell anemia, is a hereditary blood disorder characterized by the presence of abnormal hemoglobin, the oxygen-carrying protein found in red blood cells. Sickle-cell anemia is caused by a mutant form of hemoglobin, the protein that transports oxygen from the lungs to cells in the body. Hemoglobin is a composite molecule made up of two different polypeptides, a-globin and b globin, each encoded by a different gene. Each functional hemoglobin molecule contains two a-globin and two b globin chains. In sickle-cell anemia, a mutation in the gene encoding b-globin causes an amino acid substitution in 1 of the 146 amino acids in the protein. Notice that the mutation in sickle-cell anemia consists of a change in one DNA nucleotide, which leads to a change in codon 6 in mRNA from GAG to GUG, which in turn changes amino acid number 6 in b-globin from glutamic acid to valine. The other 145 amino acids in the protein are not changed by this mutation. This devastating hematologic disease affects millions of children worldwide.
Recent advances in genome-editing techniques have made it possible to modify any desired DNA sequence by employing programmable nucleases. These next-generation genome-modifying tools are the ideal candidates for therapeutic applications, especially for the treatment of genetic disorders like sickle cell disease (SCD). Substantial success has been achieved in the development of supportive therapeutic strategies for SCD, but unfortunately there is still a lack of long-term universal cure. The only existing curative treatment is based on allogeneic stem cell transplantation from healthy donors; however, this treatment is applicable to a limited number of patients only.
Genome editing technologies have improved significantly in the last few years. Currently, The commonly applied genome editing technologies are Zinc Finger Nucleases (ZFNs), Transcription Activator-Like
viii Effector Nucleases (TALENs), and the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR). ZFNs and TALENs consist of endonucleases joined to a DNA-binding domain while the CRISPR utilizes guide RNAs and Cas9 to targeted the genomic location. The double-strand breaks produced by these endonucleases are repaired in the cells either by homology directed repair (HDR) or non-homologous end joining, resulting in insertions/deletions. These technologies utilize normal DNA repair mechanisms of the cell for correctionof the targeted genome site and have been applied to manipulate the genome in different genetic disorder. These genome editing tools can be also utilized to analyze gene function, to identify new therapeutic targets, and to explore new pathway.
By using the CRISPR / Cas9 technique in our project, we aimed to knock out the V6G mutation that causes sickle cell anemia. In our project, we used the lymphocyte containing the V6G mutation obtained in the sickle cell anemia. For this, we first designed the region-specific guide RNA we are targeting. After transferring this guide RNA sequence (gRNA) to the vector, we propagated the vector in the E. coli bacterial cell. After plasmid isolation from bacterial culture, we transfect the vector by Polyethylenimine (PEI) transfection methods into the lymphocyte cells. The cell culture continued for one or two more weeks in sterile conditions. DNA isolation from the cells followed by Real-time PCR analysis performed to confirm the knockout.
ix
ÖZET
T.C.
SELÇUK ÜNĠVERSĠTESĠ
SAĞLIK BĠLĠMLERĠ ENSTĠTÜSÜ
Orak hücre anemisi hastalarının lenfosit hücrelerinde HBB V6G mutasyonunun in vitro olarak genom düzenleme CRISPR / Cas9 tekniği kullanılarak düzeltilmesi
Hasanain Akram Zainalbden ZAINALBDEN
Tibbi Genetik Anabilim Dalı YÜKSEK LĠSANS TEZĠ / KONYA-2018
Orak hücre hastalığı OHA kırmızı kan hücrelerinde oksijen taşıyan bir protein olan hemoglobinin anormal oluşumu ile karakterize bir kalıtsal kan hastalığıdır. Orak hücreli anemi akciğerden vücuttaki hücrelere oksije taşıyan hemoglobinin mutant formu tarafından meydana getirilmektedir. Hemoglobin farklı genler tarafından kodlanan a-globin ve b-globin olamak üzere iki polipeptiden oluşmuştur. Herbir fonksiyonel hemoglobin molekülü iki a-globin ve iki b-globini içerir. Orak hücreli anemide b-globini kodlayan gendeki bir mutasyon proteindeki 146 aminoasitten birinde bir aminoasit yer değişikliğine yolaçmaktadır. Bakıldığında orak hücreli anemide ki mutasyon bir DNA nükleotinde değişikliği olup mRNA‟da kodon 6 daki GAG dizisini GUG şeklinde dönüştürmekte, buda b-globinde glutamik asit yerine valin gelmesinde yolaçmaktadır. Proteindeki diğer aminoasitler bu mutasyondan etkilenmemektedir. Bu ciddi hastalık dünya çapında milyonlarca çocuğu etkilemektedir.
Genom düzenleme tekniklerindeki son gelişmeler programlanabilir nükleazlar kullanılarak istenen herhangi bir DNA dizisini değiştirmek mümkün hale getirmiştir. Bu yeni nesil genom değiştirici araçlar özellikle orak hücre hastalık OHA gibi hastalıkların tedavesine yönelik terapötik uygulamalar için ideal adaylardır. OHA için destekleyici tedavi stratejilerinin geliştirilmesinde önemli bir başarı elde edilmiştir, ama ne yazık ki hala uzun vadeli evrensel tedavi eksikliği vardır. Günümüzde mevcut küratif tedavi sağlıklı vericilerden allojenik kök hücre transplantasyonu dayanmaktadır; Bununla birlikte, bu tedavi, sadece sınırlı sayıda hastalara uygulanabilmektedir.
Genome editing teknolojilerinde son birkaç yıl içerisinde önemli gelişmeler sağlandı. Günümüzde yaygın olarak kullanılan genom editing teknoljileri ZFN (Zinc Finger Nucleases), TALEN (Transc ription Activator-Like Effector Nucleases) ve CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)
x teknolojileridir. ZFN and TALEN genomik bölgeyi hedfelemek için DNA binding domaine eklenen endonükleazlardan oluşturulurken, CRISPR`da bu guide RNA ve Cas9 aracığıyla gerçekleşmektedir. Bu endonükleazlar aracılığıyla oluşturulan çift DNA zincir kırıkları homolog direkt tamir veya homolog olmayan uç birleştirmeleri yoluyla tamir edilmekte ve buda insersiyon veya delesyonlarla sonuçlanmaktadır. Bu teknolojiler hedef genom bölgelerinin düzeltilmesinde hücrenin normal DNA tamir mekanizmlarını kullanmakta ve değişik genetik hastalıklarda genomun manupülasyonu amacıyla uygulanmaktadır. Bu genom editing araçları ayrıca gen fonksiyon analizi, yeni tedavi hedeflerinin bulunması ve yeni yolakların araştırılmasında kullanılabilmektedir.
Projemizde CRISPR/Cas9 tekniği kullanılarak sickle cell anemia neden olan V6 G mutasyonunu yok etmeyi amaçlandı. Projemizde V6G mutasyonunu içeren orak hücrel anemili vakalardan elde edilen lenfosit hücreleri kullandık. Bunun için öncelikle hedeflediğimiz bölgeye özgü rehber RNA'yı dizayn ettik. Bu rehber RNA sekansını (gRNA) vektöre aktardıktan sonra vektörü E. coli bakteri hücresinde çoğaltik. Bakteri kültüründen plazmid izolasyonundan sonra, vektörü Polyethylenimine (PEI) transfeksiyon yoluyla lenfosit hücrelerine transfekte ettik. Hücre kültürü, sterile koşullarda bir veya iki hafta daha devam edildi. Ardından DNA izolasyonunu takiben Knockout„u doğrulamak için real-time PCR analizi yapıldı.
1 1. INTRODUCTION
1.1. Sickle cell anemia
Sickle cell anemia (SCA) is one of the most common human genetic diseasea(Weatherall and Clegg 2001). The Sickle Cell Anemia (SCA) was first reported by Herrick in 1910. The first important information about the molecular etiology of SCA was obtained after Herrick's first report (Modell and Darlison 2008).
The molecular basis for sickle cell anemia as in figure 1.1 is an A to T transversion in the 6th codon of the human β-globin gene. This simple transversion changes a polar glutamic acid residue to a non-polar valine in the βS-globin chain on the surface of HbS (α2βS2) tetramers. The valine creates a hydrophobic projection that fits into a natural hydrophobic pocket formed on Hb tetramers after deoxygenation(Padlan and Love 1985; Wishner et al. 1975).
Adult hemoglobin (HbA) consists of four globin chains, two alpha and two beta (α2β2) with oxygen carrying capacity in erythrocytes. Globin proteins are derived from beta-globin genes found on chromosome 11, And alpha-globin genes found on chromosome 16. A single nucleotide change in the sixth codon of the SCD beta-globin gene is the resultant event. This mutation results in the formation of hemoglobin S (HbS) under low oxygen conditions, which in turn impairs erythrocytes, resulting in the clogging of small blood vessels and the degradation of oxygen transport to tissues. These vaso-occlusive events ultimately lead to functional asplenia and irreversible organ damage, particularly acute and painful crises and anemia leading to them(Modell and Darlison 2008).
Figure 1.1 A single nucleotide change (mutation) in the DNA encoding the beta-globin gene (CTC -> CAC) leads to an altered mRNA codon (GAG -> GUG). The resulting change in the amino
2 acid (Glu -> Val) produces a mutant beta-globin, causing sickle cell anemia.(http://bio3400.nicerweb.com/Locked/media/ch01/01_12-sickle_cell_mutation.jpg)
The reason for SCA is the presence of a mutation causing hemoglobin S (HbS) variant in at least one of two abnormal β-globin (HBB) genes. The presence of a mutant variant in two copies of the HbS gene (HBSS) results in a more severe form of the disease according to the heterozygous form(Frenette and Atweh 2007).
1.2. Frequencies
Sickle cell disease (SCD) affects millions of people throughout the world and is particularly common among those whose ancestors came from sub-Saharan Africa; Spanish-speaking regions in the Western Hemisphere (South America, the Caribbean, and Central America); Saudi Arabia; India; and Mediterranean countries such as Turkey, Greece, and Italy. It is estimated that:
SCD affects approximately 100,000 Americans.
SCD occurs among about 1 out of every 365 Black or African-American births. SCD occurs among about 1 out of every 16,300 Hispanic-American births.
About 1 in 13 Black or African-American babies is born with sickle cell trait (SCT)(Barrangou 2012).
Hemoglobinopathies are the commonest autosomal recessive disorders worldwide(Modell and Darlison 2008; Weatherall and Clegg 2001). Inherited hemoglobin disorders are characteristics of the tropics and subtropics and also prevalent in Mediterranean basin. Sickle cell anemia as the commonest form of hemoglobinopathies mainly occurs in sub-Saharan Africa; the data suggest that each year almost 180 000 babies are born with sickle cell anemia.
Hemoglobinopathies are very important health problems in Turkey. The incidence of the beta-thalassemia trait ranged from 1.1% to 13.1% and the incidence of the SCA trait ranged from 0.5% to 44.2%.(Çavdar and Arcasoy 1971; Gali et al. 1999).The prevalence of the beta-thalassemia and SCA traits was high in the southern part (Mediterranean region) of Turkey.(Gali et al. 1999; Çavdar and Arcasoy 1971)The highest prevalence of the Hb S trait (10%) was in the Cukurova region(Canatan et al. 2006).
3
Sickle cell anemia (SCA) affects 70,000 to 100,000 people every year in the United States (Hassell 2010). For this reason it is accepted as one of the most common monogenic diseases. The average lifespan of SCA patients in the United States is 42 years for women and 38 years for men(Lanzkron, Carroll, and Haywood Jr 2013).
Sickle cell disease (SCD) is an, autosomal recessive disorder that affects a significant proportion (approximately 1 in 500 individuals) of the African-American population. Hispanic, Arabic, Mediterranean and some Asian populations are also affected. Estimates from gene frequencies worldwide suggest that approximately 250,000 children are born each year with SCD(Ingram 1956).
Moreover, sickle cell disease is designated by the World Health Organization as a public health priority, with 300,000 births yearly, and it is estimated that 10 million African, Arab, and Indian individuals will be living with this disease in the future(Piel et al. 2013).
1.3. HBB gene
The HBB gene supply instructions for building a protein which called beta-globin. This protein is a subunit of a larger protein called hemoglobin, That located inside erythrocyte. Normally In adults, hemoglobin consists of four protein subunits: two beta-globin and two alpha-beta-globin subunits. Globin proteins are derived from beta-beta-globin genes found on chromosome 11, And alpha-globin genes found on chromosome 16(Modell and Darlison 2008). Each of these protein subunits is bonded to an iron-containing molecule called heme, Each heme contains an iron molecule in its center that can bind to one oxygen molecule. In the lungs hemoglobin inside red blood cells binds to oxygen molecules. These red blood cells then travel throughly the bloodstream and transfer oxygen
to tissues throughout the body.
Figure1.2. HBB Gene, Molecular Location. (Homo sapiens Annotation Release 109, GRCh38.p12) (NCBI).
4 1.3. Studies on sickle cell anemia
Although SCA is a major health problem worldwide, unfortunately, there is no effective treatment except for bone marrow transplantation(Weatherall and Clegg 2001).
Supportive therapies are important complications, although they help to reduce the disease. In addition to preventive treatment approaches such as blood transfusion, penicillin prophylaxis, and pneumococcal vaccination, they are being used in treatments aimed at reducing HbS polymerization by raising fetal hemoglobin (HbF) levels(Aliyu, Tumblin, and Kato 2006).
However, despite all these applications, allogeneic hematopoietic stem cell transplantation (HSC) has been the only curative treatment approach available for sickle cell disease so far(Shenoy 2011).
Although hematopoietic stem cell transplantation and proper donor coverage is a promising treatment strategy with 85-90% success rate, it is not always possible to find a fully compatible donor(Locatelli and Pagliara 2012).
In principle, autologous transplantation of patient-derived stem cells after an in vitro amelioration of the mutation in hematopoietic stem cells from patients in SCA may offer an invaluable solution for patients without a compatible donor.
Recently, gene therapy has become an important approach in the treatment of various hemoglobinopathies including SCA(Pawliuk et al. 2001). provided long-term (10 months) normal Beta globin expression in adult erythrocytes by transferring lenti vectors containing the normal beta-globin gene to hematopoietic stem cells of SCA mice.
Hematopoietic stem cells transplanted in vitro were transferred ex vivo to mice and it was determined that 50% of the total hemoglobin was generated from normal hemoglobin. Two of the SCA mice used here have improved hematologic parameters and other pathological findings (splenomegaly and renal findings) of SCA have been observed(Pawliuk et al. 2001).
Although the sickle cell anabolic molecular structure is the first inherited hereditary disease, there has been no long-term effective treatment except for a limited group of patients at the treatment point. Although allogeneic bone marrow transplantation is currently the longest effective treatment available, this treatment can only be performed on a donor with complete tissue maturation, which reduces the feasibility of treatment. For
5
this reason, it has become essential to develop gene therapy protocols that can be applied to humans after they are developed in animal models.
For this purpose, SIN (self-inactivating) lentiviruses with normal beta-globin gene delivery were developed and transferred to hematopoietic stem cells from patients with SCA. Later, when these stem cells were transplanted into the in vitro propagated disease, it was observed that the healing of the hematolytic anemina and disease-specific pathologies(Levasseur et al. 2003).
(Sadelain et al. 2004) designed a model in which a β-globin expression was serially-specific by placing transcriptional control elements in front of the β-globin gene that they cloned into lentiviral vectors. When transfection of mice with beta thalassemia with this vector, they observed that pathologic findings of beta thalassemia in large part of the mice were greatly reduced.
In this way, a β-globin expression is provided in the desired series in bone marrow, and oncogenic activities, which can be observed as a consequence of ineffective gene transduction in hematopoietic precursor cells, are prevented by gene transfer. This development opened the way for the use of controllable vectors in gene therapy(Sadelain et al. 2004).
In gene therapy, it is necessary to transfer the gene through viral vectors in order to produce stabilized protein. Viral gene transfer has drawbacks such as the fact that the gene integrates into undesired regions, resulting in carcinogenesis(Check 2002).
In this new approach, The high-level erythrocyte-specific β-globin expression could be achieved following the administration of only 1 to 2 vector copies per cell, and ineffective erythropoiesis and hemolytic anemia could be corrected in mouse models(Baum et al. 2003). Due to these drawbacks in gene therapy approaches based on viral gene transfer, the gene therapy approaches have begun to gain importance from the exogenous gene transfer to the highly mutated regulatory approaches. It is thought that such an approach would make a safer approach than the exogenous gene transfer.
1.4. Genome editing
The concept of genomic editing came to the fore with the understanding that double-stranded DNA breaks stimulate cell-DNA repair mechanisms.
6
As is well known, DNA fractures are generally repaired by mechanisms based on homology-directed repair (HDR) and nonhomologous end-joining (NHEJ)(Takata et al. 1998). HDR is based on the template-dependent repair of the fracture site using a homologous chain (Szostak et al. 1983).
The fact that cells have complex DNA repair mechanisms has opened the way to genomic alterations and manipulations in a specific way to the region(Ousterout et al. 2015). Gene editing based on double chain breaks has universal qualities for any cell type since the cell is based on its own endogenous repair mechanisms. Despite this advantage, the main problem in editing processes is the creation of double strand breaks (Ousterout et al. 2015).
For this purpose, methods have been developed that can produce targeted double-stranded DNA breaks in the near future: zinc finger nucleases (ZFNs), TALE-nuclease (TALENs), meganuclease, and also the CRISPR / Cas system.
If each of these approaches has its own advantages and disadvantages, the recently developed CRISPR / Cas system is at the forefront of the targeted effectiveness and specificity rates(Maeder and Gersbach 2016).
1.5. CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat)
Is based on 1987 (Ishino et al 1987) noted that 29 nucleotide repeat sequences in the E. coli genome were sequenced at 32 nucleotide intervals, investigating how alkaline phosphatase isoenzyme transformations were performed in Escherichia coli.
In the following process, a number of such similar repeat sequences have been identified in different bacteria and backgrounds. According to Mojica et al(Mojica, García-Martínez, and Soria 2005). These short repeat sequences are present in about 40% of the bacteria and 90% of the archaea.
These short sequences were identified as (Clustered Regularly Interspaced Short Palindromic Repeat) in 2002, and since then they have been shortly called CRISPR (Jansen et al. 2002). Later clustering of Cas genes near the CRISPR regions was found in prokaryotes (Schouls et al. 2003).
Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) and related proteins (Cas), which make up the CRISPR-Cas system, provide adaptive immunity against foreign substances in many bacteria and many Archaea(Barrangou and Marraffini 2014).
7
CRISPR-Cas systems include CRISPR-related (Cas) genes and corresponding CRISPR sequences(Nishimasu et al. 2014).
For CRISPR activity, the presence of CRISPR related (Cas) genes adjacent to CRISPR sequences and encoding proteins for immunological response are needed(Rath et al. 2015).
These characteristic CRISPR sequences are formed by the insertion of non-repeated sequences derived from short segments of foreign genetic material into repetitive sequences(Nishimasu et al. 2014).
That is, certain parts of the bacterium-infecting virus DNA are re-inserted into the CRISPR region along with their genes (Figure1.3). Another feature associated with CRISPR regions is the presence of conserved sequences, called leaders, behind CRISPR according to the transcription direction.
Although the presence of these leaders sequences was initially observed only in
Methanocaldococcus jannaschii, Archaeoglobus fulgidus and Methanothermobacter thermautotrophicus, it was found in many species of bacteria in later studies(Rath et al.
2015).
Figur1.3 Structure of a CRISPR region on a bacterial chromosome(Anonim)
The sequence and length of repeat regions and the length of the interval regions are well preserved in the CRISPR region, but these CRISPR regions may differ in the same or different genomes. The repeat sequences are between 21-48 base pairs (bp) while the spacing ranges between 26-72 bp. The spacing in the CRISPR region varies widely from several to several hundred.
The genome may contain single or multiple CRISPR regions and, in some species, these regions may constitute an important part of the chromosome.
8
For example, Methanocaldococcus sp. The CRISPR regions in FS406-22 (including 18 CRISPR and 191 spacing) and Sulfolobus tokodaii 7 (containing five CRISPR and 458 spacing) constitute 1% of the genome(Rath et al. 2015).
The CRISPR-Cas immune system performs immunity in the cell in three steps (Figure 1.2). The first step is an adaptation in which the cleavage fractions obtained from the exogenous nucleic acid are placed in the CRISPR region(Barrangou and Marraffini 2014). In this step, the selection of proto intervals is determined by the specific recognition of the protospacer adjacent motif-PAM in the invading plasmid and phage genomes(Jiang and Doudna 2015). PAMs are highly conserved sequence motifs composed of 2-5 nucleotides(Barrangou and Marraffini 2014).
The foreign DNA spacer portion with the PAM sequence is inserted into the CRISPR region along with the repetitive genes. Since the lack of the PAM recognition sequences in the repetitions of the bacterial CRISPR region precludes the possibility of self-targeting and self-splicing of the CRISPR-Cas systems, Mutations in the PAM sequence allow the phage to escape the CRISPR immunity(Jiang and Doudna 2015).
In the second step, the region inserted into the CRISPR locus of the target sequence in the invasive DNA is transcribed into precursor CRISPR RNAs (pre-crRNA) and the resulting pre-crRNA transcripts are converted into small crRNAs that express Watson-Crick base pairing with exogenous DNA target sequences by Cas endoribonuclease (The sequence of the crRNA corresponds to the sequence of the invading DNA)(Nishimasu et al. 2014; Barrangou and Marraffini 2014; Savić and Schwank 2016).
The final step is targeting, targeting invasive nucleic acids using crRNA and Watson-Crick base pairing, and by cutting homologous sequences with Cas nuclease to prevent the proliferation of viruses and plasmids(Nishimasu et al. 2014).
9 Figure 1.4 CRISPR-Cas 3-step working mechanism (1) the PAM-bearing DNA region of
the bacterium-infecting virus is introduced into the CRISPR locus along with the repetitive regions, (2) CRISPR RNA (crRNA) biogenesis, in which CRISPR sequences are expressed and processed to form small crRNA sequences, (3) Combination of the crRNA corresponding to the virus DNA with the Cas proteins to target the virus DNA and cutting off the virus DNA(Anonim).
Although many CRISPR loci were identified afterwards, the biological significance of this was not understood until 2005. At this time, three independent researchers identified CRISPR as containing phage and plasmid-based spacer sequences(Mojica, García-Martínez, and Soria 2005; Bolotin et al. 2005).
As a result of further studies, it was concluded in 2007 that CRISPR is actually an adaptive immune response mechanism that protects against the bacterial genome, phage and plasmids(Barrangou et al. 2007).
Subsequent studies have revealed that CRISPR has a detailed structure that will actually form a system, by identifying Cas gene, Cas protein, PAM motif (protospacer adjacent motif), crRNA (CRISPR RNA) and tracrRNA (trans-activating crRNA)(Bolotin et al. 2005; Brouns et al. 2008; Haurwitz et al. 2010).
10
Although all aspects of the CRISPR system have not yet been fully elucidated, the general functions and processes of formation have been determined in a significant way.
1.6. CRISPR-Case System Types
Cas proteins are very diverse and interact with nucleic acids such as nucleases, helicases and RNA binding proteins. Cas1 and Cas2 proteins play a role in adaptation and these proteins are present in all CRISPR-Cas systems. Other Cas proteins are only associated with certain types of CRISPR-Cas systems.
The diversity of Cas proteins, the presence of multiple CRISPR regions, and the transition between living beings make it difficult to classify CRISPR-Cas systems(Rath et al. 2015). According to the organization of the CRISPR region and the content of the Cas genes, CRISPR-Cas systems are classified into three main types(I, II and III) and 11 subtypes(I-A to I-F, II-A to II-C, III-A to III-B)(Jiang and Doudna 2015).
The CRISPR system has been divided into three categories as type I, type II and type III according to homology between Cas proteins. While only one Cas protein is sufficient for the identification and segmentation of target regions of Type II, Cas protein sets are required for Type I and III to function(Brouns et al. 2008; Makarova et al. 2011; Wiedenheft et al. 2011).
Since the Cas proteins are responsible for the biogenesis of crRNA and recognition and degradation of invasive nucleic acids, the molecular mechanism of each CRISPR type is specific(Barrangou and Marraffini 2014).
In Type I and Type III systems, the endoribonucleolytic cleavage of the repeated sequences of pre-crRNAs to produce small mature crRNAs are based on the Cas6 nuclease family(Jiang and Doudna 2015). In the Type I system, the resulting crRNA molecule combines with Cascade and Cas3 proteins to cut foreign DNA, but In the type III system, the crRNA that forming from pre-crRNA is complexed with Cmr / Cas10 or Csm / Cas10 proteins, and the complex Cas proteins cut foreign DNA.
The Type II system is the system that works best among these systems and is the best-illuminated system. In the type II system, a ribonucleoprotein complex with a non-coding RNA (trans-activating CRISPR RNA (tracrRNA)), crRNA, endonuclease Cas9 is complexed and recognizes and intercepts invasive DNA(Savić and Schwank 2016).
11
There may be different types of CRISPR-Cas systems in a single organism(Rath et al. 2015). In Figure1.5, The CRISPR-Cas systems are given the crRNA biogenesis and targeting mechanisms.
CRISPR type II properties have made it a target for genome editing studies. The presence of Cas9-crRNA complexes, which function as RNA-guided endonucleases in
Streptococcus thermophilus and Streptococcus pyogenes, in 2012, has also led to the
potential existence of the CRISPR system for genome editing.
Cas9-crRNA constitutes a complex of Guide RNA that recognizes target sequences with the Cas9 protein that cuts DNA from specific regions(Jinek et al. 2012).
All these Data suggest that the Cas9-crRNA complex may be a valuable tool for genome editing. The fact that the CRISPR / Cas9 system is open to modification has opened up new approaches and this technology has been accepted as a new era in targeted technologies(Makarova et al. 2011).
Figure1.5. Mechanism of crRNA Biogenesis and Targeting in the Three Types of CRISPR-Cas Systems(Barrangou and Marraffini 2014).
1.7. Genetic engineering applications of CRISPR-Cas system
CRISPR systems, such as restriction enzymes, are also prokaryotic immune systems that allow specific DNA sequences to be cleaved. while binding enzymes typically bind 4-8 bp double-stranded DNA molecules and cut the DNA molecule, CRISPR systems
12
that vary widely can be programmed to easily target any DNA or RNA molecule(Wilkinson and Wiedenheft 2014).
Following the discovery of the CRISPR adaptive immune system in the Streptococcus thermophilus bacterium used as starter culture in the dairy industry, the CRISPR system has been used to obtain more efficient, long-lived and phage-resistant mutants(Barrangou 2012).
Beyond viral resistance, the CRISPR adaptive immune system has the potential to vaccinate bacterial strains against ingestion and spread of undesirable genetic elements, such as plasmids carrying antibiotic resistance genes or disease-causing properties(Barrangou and Marraffini 2014).
The CRISPR immune system, particularly the Cas9-based Type II system, has been extensively characterized and potentially discovered in the genomic engineering of the Cas9 enzyme(Barrangou and Marraffini 2014). Type I and Type III CRISPR cas systems have RNA-targeted nuclease activity, but do so with a large multimeric crRNA-Cas ribonucleoprotein complex. This makes it difficult to develop these systems as a molecular tool for gene regulation.
In contrast to Type I and Type III, the Type II system is based on a single endonuclease activity that interrupts the target DNA molecule(Sapranauskas et al. 2011).
In 2013 there has been a tremendous increase in the number of scientific studies and publications in which the RNA-targeted Cas9 system has been used for genomic regulation, genetic screening and gene expression regulation(Pennisi 2013).
Within the cell, the Cas9 system requires the RNase III enzyme with crRNA and tracrRNA to intercept the target DNA(Barrangou and Marraffini 2014). All these requirements can be abolished by the creation of a single-guided RNA-sgRNA, known as the single-guided RNA-sgRNA, which is generated by the assembly of tracRRNA and crRNA.
The crRNA in the sgRNA that is generated to maintains its guiding function and secondary structure of the sgRNA is designed to be appropriate for the truncation of the Cas9 target region(Jinek et al. 2012).
This technological advance has enabled Cas9 to use an ideal RNA-targeted double-stranded DNA (dsDNA) as a basis for the nuclease, and many researchers have used Cas9 as a genetic engineering tool, as was the case with ZFN and TALEN enzymes in the past(Wood et al. 2011).
13
In contrast to ZFN and TALEN, the CRISPR-Cas9 system also cuts methylated DNA in human cells, Allowing genetic modification that can not be done with other nucleases(Bortesi and Fischer 2015).
Cas9 possesses the alpha helical lobe that interacts with the HNH and RUVC nuclease domains and sgRNA required to cut double-stranded DNA(Peters et al. 2015).
In addition to the sgRNA consisting of 20 nucleotides, the PAM sequence is required for the Cas9 protein to recognize the target DNA. The Cas9-sgRNA complex is highly stable when it binds to DNA and has been shown to be nearly irreversible for long time binding between the Cas9-sgRNA complex and the target DNA by in vitro binding kinetic studies.
In gene regulation, the most important feature of the Cas9 based CRISPR system is its programmability. The Cas9 enzyme can modify the base sequence of the sgRNA and direct it to any region of the PAM-sequence.
The second important feature of this system is its versatility. In addition to using Cas9 nucleases in genomic regulation, they are also used to activate or suppress dCas9 (dead Cas9) enzyme gene expression, which is not possible to break down catalytically inactive, double-stranded DNA(Peters et al. 2015).
The CRISPR-Cas9 complex is based on the principle of genomic rearrangement of cellular DNA repair with double-strand breaks in the target DNA sequence. The dsDNA breaks generated by nuclease are repaired by non-homologous end-joining (NHEJ) or homologous recombination (HR).
NHEJ is error-prone and often causes nucleotide insertion or deletion resulting in frame shift mutations that result in genetic knockout at the target site, or insertion of stop codons. Repair of double-stranded DNA breaks by HR is dependent on the presence of template DNA containing sequences specific to the target region.
When dsDNA is a template DNA molecule with sequence homology around the breaks, DNA damage can be repaired by HR and this mechanism can be used to sensitively modify the gene or obtain gene insertion(Wilkinson and Wiedenheft 2014).
In eukaryotic cells, repair of double-stranded DNA breaks generated by nuclease by NHEJ and HR mechanisms is shown in(Figure1.6).
14
Figure 1.6. Mechanism of DNA repair with NHEJ and HR in the cell by cutting double-stranded DNA(Bortesi and Fischer 2015).
1.8. Genome editing applications in embryo
Recently, the transfer of CRISPR / Cas9 integrants to animal embryos has been able to produce the desired genomic changes throughout the organism(Wu et al. 2013; Niu et al. 2014; Liang et al. 2015).
It is also possible to transfer these changes to subsequent generations when the organism covers all the cells.(Wu et al. 2013)use mouse embryos to drive mutations in the Crygc gene that result in cataracts and loss of vision in these organisms. By targeting the Crygc mutant allele through CRISPR / Cas9, this allele corrected through the normal allele in the homologous chromosome.
In another study using the CRISPR / Cas9 system on mouse embryos, the mutant allele of Duchenne muscular dystrophy induced dystrophin was corrected by this system(Long et al. 2014). Here we used the Cas9 mRNA and sgRNA as well as normal allele compatible exogenous oligonucleotides as templet for DNA editing. In this study, the editing process was performed after the zygot step, so the editing was performed as a mosaic in the organism. However, the majority of the muscle cells in the desired regulation could prevent the emergence of disease symptoms.
Very recently,(Liang et al. 2015) showed that despite the ethical problems in this regard, human embryos could also be edited via CRISPR / Cas9. Three pronuclear zygotes were used in this study. Using these zygotes, the mutant allele in the beta hemoglobin responsible for beta thalassemia was studied to be corrected by CRISPR / Cas9. However, this study has shown that, in addition to the desired allelic improvement, a large number of
15
unwanted non-target changes occur. This result shows that editing technologies can present undesirable results in human embryos as they are.
1.9. In vivo genome editing applications
The first postnatal application of CRISPR / Cas9 technology was performed by Yin et al In mouse models of type I tyrosinemia(Yin et al. 2014).
The hereditary type I tyrosinemia fumarylateacetoacetate hydrolase (FAH) is a deficiency in the enzyme, and cytotoxic metabolites resulting from enzyme deficiency are involved in the liver leading to the death of heptocytes. The researchers transferred the oligonucleotides used as Cas9, sgRNA and templet to the mice via venous circulation. As a result of this transfer, mutant genes were repaired in a large number of liver cells and consequently the symptoms of the disease were reduced.
This study showed that editing by CRISPR / Cas9 is particularly suitable for diseases such as trizonemia. In such applications, the number of cells initially manipulated although was small (0.4%),It is possible to achieve numerical superiority due to survival advantages of such cells afterwards. In another study on the mouses, with a liver enzyme proprotein convertase subtilisin-kexin type 9 (PCSK9) gene was focused on(Ding et al. 2014). PCSK9 is released into the plasma from hepatocytes and destroys the LDL receptors and prevents LDL nodule uptake. In this study, the PCSK9 gene was screened using the CRISPR / Cas9 vector, resulting in an increase in the number of LDL receptors and this has reduced cholesterol levels.
In this application, the cutting regions have been repaired mostly by NHEJ, and a processing success of about 50% has been achieved. On the contrary, this level is a level that allows for clinical applications.
A study by(Lin et al. 2014)showed that hepatitis B infection could be treated via CRISPR / Cas9. Although different antiviral treatments have been developed so far, they have not completely eliminated the virus in the liver. In this study, researchers targeted the HBV DNA in cells by injecting the CRISPR / Cas9 system by circulating in HBV infected mice. The rate of HBV surface antigens in serum after administration has decreased significantly in serum.
Since mouse cells could not generate circular DNA intermediates (cccDNA) required for in vivo virus replication, researchers performed a similar procedure on human cell lines. As a result of this application, they showed that HBV can be eliminated in
16
human cell lines via CRISPR / Cas9. However, the possibility of recurrent virulence, even in the presence of a small viral residue in cells, lowers the clinical applicability of this procedure for this disease.
When we look at the in vivo studies so far, it seems that these studies are done especially on mice. There are important technical limitations to be overcome in order for these practices to be practiced on a clinically-based basis. These include the effectiveness of the CRISPR / Cas9 system and the difficulties of targeting the vectors to the desired tissue.
Until the last period, such applications have been carried out based on classical gene therapy methods(Mingozzi and High 2011). In this process, a large number of viral and non-viral vectors for gene therapy have also been developed. These improvements have facilitated the adaptation of the vectors to the CRISPR / Cas9 system. Among these, the adeno-associated virus has been shown to allow efficient transduction to different cells and low cytotoxic and immunogenic properties.
Short Cas9 nuclease genes from recent staphylococcus aureus could be transferred to adeno-associated virus particles with sgRNA(Mingozzi and High 2011; Ran et al. 2013). The second major problem encountered in gene editing processes using the in vivo CRISPR / Cas9 system is that HDR is less effective in this system compared to NHEJ. This limits the effective results that are planned to be achieved. To overcome this, methods have been developed based on the formation of single chain fragments by Cas9 nuclease. These methods were able to obtain more effective results(Ran et al. 2013).
The third concern that arises around CRISPR / Cas9 technology is the consequences of non-adverse effects. It is possible that DBS that can occur in undesired regions cause gene mutations or that DBS in different chromosomes lead to chromosomal rearrangements.
Despite the fact that the proportion of non-target changes in these applications is not fully known, Recently developed DBS analysis methods have provided important data in this respect(Ran et al. 2013). As a result of these analyzes It was determined that this ratio varied between 0-150 depending on the sgRNAs used . Even at very low rates it appears if it is thought that important genes (such as protoncogen) may be affected are likely to be available for clinical applications.
Recent studies have shown that sgRNA can be increased in specificity by adding 2 nucleotides to the 5 'end or by removing 17 nucleotides (Cho et al. 2014, Fu et al. 2014).
17
However, it has been determined here that Cas9 nikases that forming single chain fractures rather than DBS, or cut only dimers, and catalytically ineffective Cas9 by using dCas9-Fok fusion proteins reduce the formation of non-target mutations (Tsai et al. 2014, Guilinger et al. 2014).
1.10. Ex vivo genom editing applications
Ex vivo approaches are based on transferring the cells from the organism to the organism after the applications in the culture medium. Recently, Takahashi et al. (Takahashi et al., 2007) demonstrated that pluripotent stem cells (iPSCs) could be obtained by inducing human fibroblasts through some gene transfers. Thus, an ex vivo source that can be differentiated and persisted into different cell types has been provided.
This development provided important expansions in terms of ex vivo gene therapies. New protocols have been increasingly included in the iPSCs produced in the province (Huch et al. 2014- Sato et al 2011). Some of these protocols have focused on making Takahashi and his colleagues' approaches more secure.
The presence of the CRISPR / Cas9 system provided important contributions to these steps. In this respect, the first ex vivo CRISPR / Cas9 treatment was performed on iPSC cells established for the treatment of B-thalassemia disease(Liang et al. 2015).
Xi et al. (2014) generated iPSCs in vitro from fibroblasts obtained from patients homozygous for the b-thalassemia mutation and transfected with CRISPR / Cas9 vectors to correct the respective genes. After transfection, iPSCs could be differentiated to normal erythrocyte precursors. This showed that the combined use of iPSC and CRISPR / Cas9 could provide important opportunities for the treatment of such diseases.
A study conducted by Hans Clevers et al. (2013) showed that it is possible to perform CRISPR / Cas9-mediated genome editing in somatic stem cells. Here, multipotent stem cells found abundantly in the intestines were targeted to correct common alleles causing cystic fibrosis. In this study stem cells isolated from intestinal cells of patients with cystic fibrosis were transfected with CRISPR / Cas9 carrying vectors for the CFTR gene. After the transfection, it was expected to repair the fractures of the targeted locus by HDR using template DNA. The desired transfer has been successfully demonstrated through sequence analysis.
18
The identification of very few non-target mutations in this study has increased hopes for clinical applicability of this method.
In two recent independent studies, mutations in the dystrophin gene leading to DMD could be corrected in vitro using iPSC and CRISPR / Cas9 methods (Lin et al. 2014; Ousterout et al. 2015).
In a first study,(Lin et al. 2014) attempted to correct the function of the dystrophin gene by transferring the CRISPR / Cas9 system to iPSC cells in DMD patients in the presence of templet DNA. Here, successfully transferred iPSC cells were differentiated into muscle cells and functional dystrophin proteins production was found.
The second study related to this disease was performed by Ousterout et al(Ousterout et al. 2015). Here we attempted to establish multiple exon deletions and point mutations in myoblast cells immortalized from DMD patients to obtain functional dystrophin. Dystrophin expression and function improvement of the generated deletions were determined in vitro. These cells were then transplanted into the dystrophin mutant mice and the DMD findings were resolved.
The most important problem in antiviral treatments for HIV is known to be latent infections in T cells. Recently, two approaches based on genome editing technologies have been developed to overcome this problem. The first is by targeting of latent HIV DNA in T cells via nucleases.
Liao et al. (Liao et al. 2014) targeted the highly conserved LTR (long terminal repeats) in the HIV-1 DNA chain, which is latent in CD41T-cells using the CRISPR / Cas9 system. It has been found that the number of viral particles already in contact with the degradation of LTR regions after application has declined significantly.
A second approach developed for the elimination of HIV is the detection of the CCR5 chemokine receptor that HIV-1 uses as a receptor for T cells, via CRISPR / Cas9. In this direction, Mandal et al. (Mandal et al. 2015) attempted to silence the CCR5 gene in CD34HSPC (CD341hematopoietic stem and progenitor Cell) cells using the CRISPR / Cas9 system. As a result, approximately 30% of the cells were silenced in the CCR5 gene.
After transplantation of mice with CCR5 genetically silenced cells, HSPCs maintained progenitor properties and were found to carry a small number of non-target
19
mutations. CCR5-targeted practices against HIV infection increase this success (Liao et al. 2014).
Ex vivo gene editing applications have significant advantages. With this approach, it is possible to select the cells to which the proper transfer is made and to sort out the cells to which the non-target transfer is made. Thus, only the successful transfer of stem cells can be transplanted into the disease.
This suggests that ex vivo approaches are more effective than in vitro approaches. (Jinek et al. 2012).
20 2. MATERIALS AND METHODS
2.1 collecting Blood samples from patients
In our study, peripheral blood samples taken from patients diagnosed with sickle cell anemia at clinical and laboratory level were obtained. Approximately 5 cc peripheral blood was obtained from the patients via the intervenous route.
2.2. Cell Culture
Cell culture procedures were performed in sterile laminar air flow cabins. Peripheral blood was drawn from the patients and transferred to EDTA hemogram tubes. 400 μl of blood samples were taken from patients transferred to tubes containing 15 ml of culture medium. To obtain the maximum amount of cells, the cells were ampilified and then transferred to new tubes, which were applied as approximately 2-3 passages. During culture, the cells were cultured in RPMI medium1640 (Gibco® - Invitrogen, USA) supplemented with 10% FBS (Fetal Bovine Serum) (Biochrome, Invitrogen, USA) and 1% penicillin / streptomycin (100 U/ml penicillin and 100 μg/ml streptomycin) (Biochrome, Germany; Invitrogen, USA). Incubation of the cells was carried out in 5% CO2 and 37 ° C.
2.3. CRISPR gRNA design
The PAM sequences present around the mutated region have been identified. From the presence of these PAM sequences, we designed gRNA with a maximum length of 20 nucleotides to cover the mutated region. The efficiency and specificity of the designed gRNA was checked so that the Cas9 enzyme correctly cut the target site.
The suitability of the constructed gRNA design for the target region was checked via databases such as www.genome-engineering.org, www.zincfingers.org, chopchop.rc.fas.harvard.edu, http://crispr.mit.edu, benchling.com.
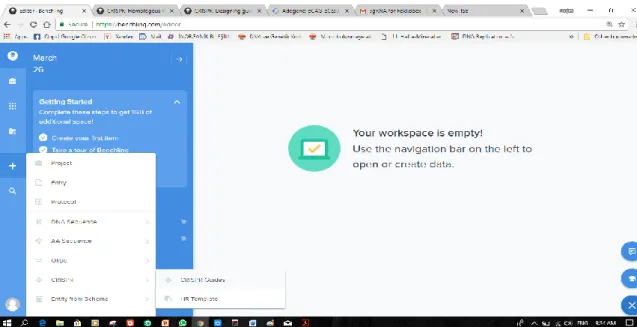
Here we designed guide RNAs for the HBB gene using Benchling‟s CRISPR tool. As the following steps:
21
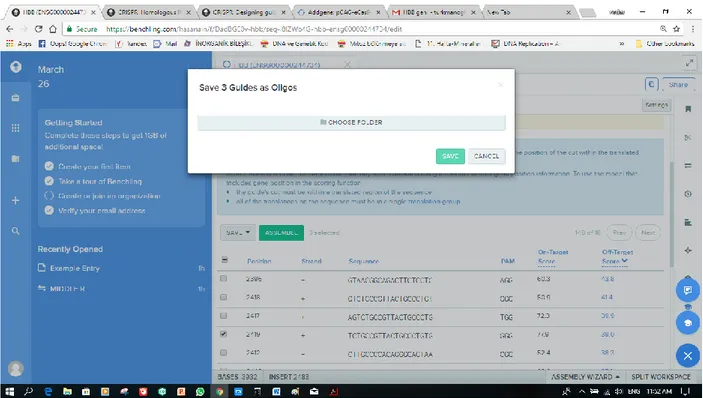
Figure 2.1. Image Show how to beginning CRISPR gRNA design.
Search for the HBB gene. Ensure all settings (including the hg38 genome) are the same as the image below.
Figure 2.2. Image Show page of selecting the gene of interest (HBB gene).
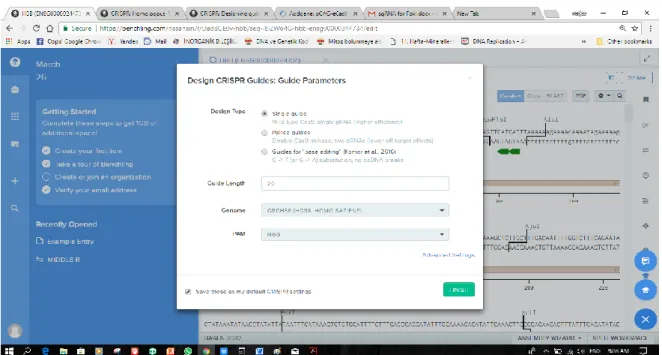
We Selected Single guide, and ensure that the guide length is set to 20, the genome is hg38, and that the PAM is NGG, and click Finish.
22
Figure 2.3. Image Show guide parameters.
Then Use the Annotations tab on the right side panel, and click on Exon 1 to select the first exon‟s amino acids.
23
Figure 2.5. Image Show how to set the target region on selected Exon.
On the Design CRISPR tab, click on the + to set the target region as the selected Exon 1.
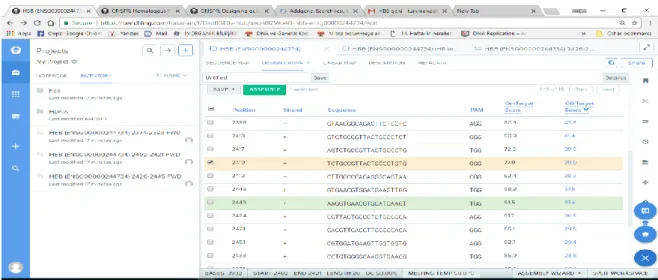
Figure 2.6. Image Show how to set off-target and on-target scores.
By clicking on the target-score column we Sort all sequences using the off-target score. We should look at both the on-off-target and off-off-target scores, When choosing guide RNAs.
24
Figure 2.7. Image Show how selecting gRNAs with high off-target and on target scores. Off-target scores above 50 are considered to be good guides. On-target scores above 60 are considered to be good guides. Use these scores to rank guides relative to each other.
Figure 2.8. Image Show Presence of three selecting gRNAs on sequence map.
Go to Save above your list of guides and select oligos on Benchling. Save the guides in your project. Go to the top of the Design CRISPR tab and save the analysis as “HBB Exon 1 CRISPR Analysis”.
25
Figure 2.9. Image Show how to save the selected gRNAs .
Figure 2.10. Image Show one of the selected gRNAs which saved in a clipboard. Determine the reverse complement (rc) of each guide sequence.
26
Figure 2.11. Image Show how to create the Reverse complement for each guide.
Obtain 24- or 25-mer oligos for each guide and its associated reverse complement including additional nucleotides for cloning and expression purposes.
Add “CACC” before the 20-mer guide sequence and “AAAC” before the guide‟s reverse complement for cloning into the ( pCAG-eCas9-GFP-U6-gRNA )vector using BbsI restriction enzyme.
Add a G nucleotide after the CACC sequence and before the 20-mer if the first position of the 20-mer is not G. sgRNA expression from the U6 promoter of the vector is enhanced by the inclusion of a G nucleotide after the CACC sequence. Add a C at the 3′ end of the reverse complement oligo. The resultant oligos would be 25-mer oligos.
However, if the first position of the 20-mer (protospacer sequence) is G, do not add another G and do not add C to the final position of the reverse complement oligo. In this case, the resultant oligos would be 24-mer oligos.Then ordered as below:
sgRNA for( lentiCRISPR)
HBB g1 F caccgTCTGCCGTTACTGCCCTGTG HBB g1 R aaacCAAACAGACACCATGGTGCAc HBB g2 F caccgCATGGTGCATCTGACTCCTG HBB g2 R aaacCAAACAGACACCATGGTGCAc HBB g3 F caccgAAGGTGAACGTGGATGAAGT HBB g3 R aaacTGGTGCATCTGACTCCTGAGc
27 2.4. HR SSODN templates design for CRISPR-directed HR
Choose your gRNA from your guide RNA design list ( we will choose TCTGCCGTTACTGCCCTGTG on Exon 1)
Figure 2.12. Image Show choosing gRNA from the list of designing SSODN.
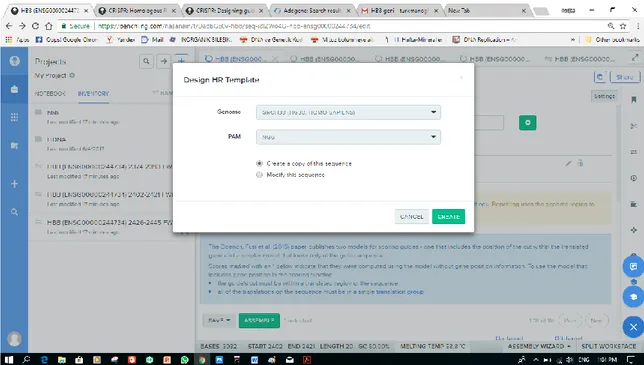
Click the CRISPR icon on the side panel, and then Create HR Template (SSODN)
Figure 2.13. Image Show the beginning for HR Template(SSODN) design.
Ensure that you are using the hg38 genome, and that Create a copy of this sequence is selected.
28
Figure 2.13. Image Show creation a copy of HR template sequence after selecting (Genome and PAM type).
Figure 2.14. Image Show use of annotation tab to select Exon.
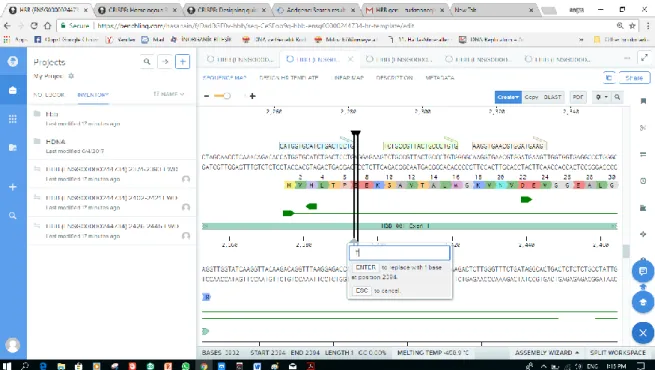
Use the annotation tab to go to Exon 1, and make a point mutation at 2394 from A to T, so that new sixth amino acid will be Valin (V) instead of Glutamic acid (E), and click next. The wizard will record these changes for you as well.
29
Figure 2.15. Image Show how to make a point mutation at 2394 from A to T.
Figure 2.16. Image Show changing of Glutamic Acid amino acid to Valin.
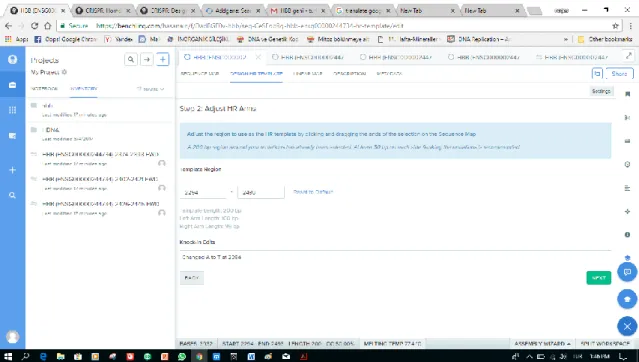
Adjust the length of HR arms on both sides by dragging the highlighted sequence. Click next, and you‟ve now designed your HR template that is ready to be copied into your clipboard for de novo synthesis.
30
Figure 2.17. Image Show how to adjust HR arms.
Copy your guide RNA (CATGGTGCATCTGACTCCTG) into the Guide box. The table will show a list of possible silent mutations to change the target site in your HR template.
Figure 2.18. Image show list of possible silent mutations in HR Template to change the target site.
To avoid the HR template being degraded by Cas9, it is most effective to mutate the PAM sequence. The wizard will automatically select the best mutation for you.
31
Figure 2.19. Image show summary designed HR template.
And then HR ssODN templates for CRISPR-directed HR ordered as below. ssODN
ACATTTGCTTCTGACACAACTGTGTTCACTAGCAACCTCAAACAGACACCATGGTGCATCT GACTCCTGTGGAGAAGTCTGCCGTTACTGCCCTGTGGGGCAAGGTGAACGTGGATGAAGTT GGTGGTGAGGCCCTGGGCAG
The verified gRNA sequence, resistance gene, GFP (green fluorescent protein) and promotor region were placed in the vector system and vector design was performed. The designed vector( pCAG-eCas9-GFP-U6-gRNA)(Addgene#79145) was ordered.
32
Figure 2.20 Full Sequence Map for pCAG-eCas9-GFP-U6-gRNA(addgene).
2.5. Primer Design For The HBB Gene
Primers were designed using the PrimerBank-MGH-PGA online program (https://pga.mgh.harvard.edu/primerbank). Designed primers synthesized by Macrogen.
33 2.6. Culture of Bacteria
plasmid # 79145 that obtained through commercially as stab culture from (Addgene, USA) were cultured on plates. Solid LB (Luria-Bertani) medium was prepared to produce specific bacterial colonies. Therefore, after the LB medium was prepared with 15 g/L of Bacto Tryptone agar, the mixtures were autoclaved. After autoclaving, the agar was cooled to 55 ° C and antibiotics (Ampicillin and/or Kanamycin etc.) were added at appropriate concentrations. After forming a homogeneous mixture, and the mixture was poured into sterile petri plates and allowed to solidify and the plates stored at +4°C until used.
2.7. Bacterial Colonies
5 ml of LB medium with antibiotics were placed in 12-ml culture tubes. A single
bacterial colony was taken from agar plates that were previously cultivated with a sterile stick and each tube was sterile inoculated.
The tubes were then incubated for 8 hours approximately in the shaking incubator at 37°C and 150-250 rpm. Antibiotic-free LB medium without any bacterial inoculation, as a negative control one of the tubes was incubated under equal conditions.
After incubation, the tubes were checked for turbidity. As a result, the lack of bacterial growth in the control tubes and presence of bacterial growth (turbidity) in the culture tubes were evaluated as an indicator of success. At the end of mini-culture, the contents of the 12-ml culture tubes were transferred to 100-250 ml flasks containing medium and antibiotic. then, the flasks containing bacteria culture were incubated for 15 hours in the shaking incubator (with 37°C and 150-250 rpm). After incubation in the incubator, DNA plasmid was extracted.
34 2.8. Lentiviral vector digestion, oligo annealing, and cloning into digested vector
1. Digest and dephosphorylate 5ug of the lentiviral CRISPR plasmid with BsmBI for 30 min at 37C:
5 ug lentiCRISPR
3 ul FastDigest BsmBI (Fermentas) 3 ul FastAP (Fermentas)
6 ul 10X FastDigest Buffer
0.6 ul 100 mM DTT (freshly prepared) Completed with ddH2O to 60ul total volume.
2. Gel purify digested plasmid using Nucleospin Gel Extraction Kit and elute in EB. 3. Phosphorylate and anneal each pair of oligos:
1 ul Oligo 1 (100 μM) 1 ul Oligo 2 (100 μM)
1 ul 10X T4 Ligation Buffer (NEB) 6.5 ul ddH2O
0.5 ul T4 PNK (NEB M0201S) 10 ul total
Put the phosphorylation/annealing reaction in a thermocycler(LightCycler® 480 II (Roche, Germany) using the following parameters:
37oC 30 min
95oC 5 min and then ramp down to 25oC at 5oC/min.
4. Dilute annealed oligos from at a 1:200 dilution into sterile water or EB. 5. Set up ligation reaction and incubate at room temperature for 10 min:
X ul BsmBI digested plasmid from (50ng) 1 ul diluted oligo duplex from
5 ul 2X Quick Ligase Buffer (NEB)
Completed with ddH2O to 10ul subtotal volume. 1 ul Quick Ligase (NEB M2200S)
11 ul total
6. Transformation into Dh5a bacteria. Lentiviral transfer plasmids contain Long-Terminal Repeats (LTRs) and must be transformed into recombination-deficient bacteria.
2.9. Agarose Gel Electrophoresis(1% Agarose Gel)
1. Measure 1 g of agarose.
35
3. Microwave for 1-3 min until the agarose is completely dissolved. 4. Let agarose solution cool down to about 50 °C about 5 mins.
5. Add ethidium bromide (EtBr) to a final concentration of approximately 0.2-0.5 μg/mL (usually about 2-3 μl of lab stock solution per 100 mL gel). EtBr binds to the DNA and allows you to visualize the DNA under ultraviolet (UV) light.
6. Pour the agarose into a gel tray with the well comb in place.
7. Place newly poured gel at 4 °C for 10-15 mins OR let sit at room temperature for 20-30 mins, until it has completely solidified.
2.10. Loading Samples and Running an Agarose Gel
1. Add loading buffer to each of your DNA samples. 2. Once solidified, place the agarose gel into the gel box . 3. Fill gel box with 1xTAE (or TBE) until the gel is covered.
4. Carefully load a molecular weight ladder into the first lane of the gel. 5. Carefully load your samples into the additional wells of the gel.
6. Run the gel at 80-150 V until the dye line is approximately 75-80% of the way down the gel. A typical run time is about 1-1.5 hours, depending on the gel concentration and voltage.
7. Turn OFF power, disconnect the electrodes from the power source, and then carefully remove the gel from the gel box.
8. Optional) If you did not add EtBr to the gel and buffer, place the gel into a container filled with 100 mL of TAE running buffer and 5 μL of EtBr, place on a rocker for 20-30 mins, replace EtBr solution with water and destain for 5 mins. 9. Using any device that has UV light, visualize your DNA fragments. The fragments
of DNA are usually referred to as „bands‟ due to their appearance on the gel.
2.11. Making Calcium Competent Cells
1. Streak out frozen glycerol stock of bacterial cells (DH5α) onto an LB plate (no
antibiotics since these cells do not have a plasmid in them). Work sterile. Grow plate overnight at 37°C.
2. Prepare starter culture of cells Select a single colony of E. coli from fresh LB plate and
inoculate a 10 mL starter culture of LB (or your preferred media – no antibiotics). Grow culture at 37°C in shaker overnight.
3. Inoculate 1 L of LB media with 10 mL starter culture and grow in 37°C shaker.
Measure the OD600 every hour, then every 15-20 minutes when the OD gets above 0.2.