ContentslistsavailableatSciVerseScienceDirect
Pathology
–
Research
and
Practice
j ou rn a l ho m e p a g e :w w w . e l s e v i e r . d e / p r p
Original
article
Evaluation
of
apoptosis
along
with
BCL-2
and
Ki-67
expression
in
patients
with
intestinal
metaplasia
Gulbanu
Erkan
a,∗,
Ipek
Isik
Gonul
b,
Ugur
Kandilci
a,
Ayse
Dursun
b aGaziUniversityHospital,DepartmentofGastroenterology,FacultyofMedicine,Ankara,TurkeybGaziUniversityHospital,DepartmentofPathology,FacultyofMedicine,Ankara,Turkey
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received12August2011
Receivedinrevisedform8December2011 Accepted14December2011 Keywords: Intestinalmetaplasia Bcl-2 Ki-67 Apoptosis
a
b
s
t
r
a
c
t
Theprimaryaimistocompareindividualswithintestinalmetaplasia(IM),chronicactivegastritis(CAG), andnormalgastricmucosa(NGM)intermsofapoptosis,proliferation,andBcl-2expression.The sec-ondaryaimistodeterminewhethertheseparametersaredifferentbetweenpatientswithandwithout gastriccancerinfirst-degreerelatives.Weenrolled106patientswhosehistopathologicalresultswere consistentwithIM(n:42),CAG(n:51),orNGM(n:13).Antralbiopsieswereimmunohistochemically stainedforBcl-2andKi-67expression.ApoptosiswasdetectedusingTUNELassay.Whilenosignificant differencewasdeterminedbetweenthreegroupswithregardtoapoptosisandBcl-2expression(p>0.05), Ki-67expressionwassignificantlyhigherintheIMgroupwhencomparedwiththeCAGandNGMgroups (29.90±22.87vs.18.18±16.22vs.18.54±20,respectively;p=0.012).Helicobacterpyloriwasdetermined toincreaseapoptosis(49.3%vs.25.7%,p<0.05),nevertheless,ithadnosignificanteffectonproliferation andBcl-2expression.Bcl-2andKi-67expressionandapoptosiswerenotdifferentamongpatientswith andwithoutahistoryofgastriccancerinfirstdegreerelatives.Althoughintestinalmetaplasiacases demonstrateanincreaseinproliferation,noelevationisobservedinapoptosis.Thiscanbeanimportant factorintheprogressiontogastriccancer.
© 2011 Elsevier GmbH. All rights reserved.
Introduction
Helicobacterpylori(H.pylori)isagram-negative,microaerophilic bacteriumthat is helicalin shape andis foundin thestomach ofmorethanhalfofthepeopleworldwide.Acausalrelationship between H. pylori infection and gastric cancer has been con-sistentlyreported in many epidemiological and clinicalstudies [3,26,27].
Gastriccancerisgenerallyassumedtodevelopina stepwise fashionfromchronicgastritis,atrophicgastritis,intestinal metapla-sia,anddysplasia.Theseriesofchangesingastriccarcinogenesis, referredtoasCorrea’scascade[5],isoftenaconsequenceofH. pyloriinfection.In this context, atrophicgastritisand intestinal metaplasiaareconsideredtobetheprecursorsofgastriccancer, especiallyfortheintestinaltypeofgastriccancer.However,all indi-vidualswithintestinalmetaplasiadonotprogresstogastriccancer, andthefactorsinfluencingthisprogressionneedtobeelucidated [16].H.pyloriinfectionisalsocausallyrelatedtothedevelopment ofpepticulcer.H.pyloriinfectionresultsintwodifferentclinical
∗ Correspondingauthorat:UfukUniversitesiTıpFakultesi,Dr.RıdvanEge Has-tanesi,MevlanaBulvarı,No:86-88,06520Balgat,Ankara,Turkey.
Tel.:+903122044172;fax:+903122044055. E-mailaddress:gcanbaloglu@gmail.com(G.Erkan).
entities: duodenalulcer or gastric ulcer/cancer.Duodenal ulcer patientsusuallydisplaytheantralpredominant,non-atrophictype gastritis.Incontrast,gastriccancerpatientstendtodevelop mul-tifocalorextensiveatrophicgastritisofthecorpus.Nevertheless, themechanismsleadingtothesetwodifferentphenotypesarenot fullyunderstood[16].
Intestinal metaplasia (IM) is defined as the replacement of thegastricmucosabyanepitheliumresemblingthesmallbowel mucosa.Intestinalmetaplasiaarisesfromgastricstemcells trans-formingintosmallbowelepithelialcellssuchasabsorptivecells, gobletcells,and Panethcells,in placeofproliferatingintocells specifictothestomach[16].
Employingvarioushistologicalandhistochemicaltechniques, intestinal metaplasia can be classified into different subtypes. While severalclassification systems are used, the most widely acceptedsystemhasbeenproposedbyJassandFilipe[12].Inthis classification,intestinalmetaplasiaisclassifiedintocompleteand incompletetypes.
Theclassificationofintestinalmetaplasiahasprognostic signif-icance.CompleteortypeIintestinalmetaplasiacarriesalowrisk forgastriccancer,ontheotherhand,typeIII(colonic)intestinal metaplasiahasthestrongestassociationwithcancer[7,18].
Cellproliferation,whichisessentialfornormalcellturnover, facilitatestheaction of carcinogensthat target DNAwhen it is excessive.Thismayeventuallyresultincarcinogenesis[21].
0344-0338/$–seefrontmatter © 2011 Elsevier GmbH. All rights reserved. doi:10.1016/j.prp.2011.12.002
Apoptosis,whichisprogrammedcelldeath,playsa counter-regulatoryrole to cell proliferation in the maintenance of cell population.Thealterationofthebalancebetweenapoptosisand cellproliferationiscrucialin thedevelopmentofgastriccancer [28].
Bcl-2genewasinitiallyidentifiedduetoitsinvolvementinthe chromosomaltranslocationt(14;18)observedinlowgradeB-cell lymphomas[2].Theexcessiveproductionoftheproteinprolongs celllifespandespiteapoptoticstimuli,andtheproteinisknownto suppressapoptosis[1].
Ki-67isanantigenassociatedwithnuclearproliferation,and it is expressed during thegrowth and synthesis phases of the cellcycle,butnotduringtherestingphase.Thisantigenprovides informationabouttheproportionofactivecellsinthecellcycle [8].
Theaimsofthisstudywere:
(1)To compare individuals with intestinal metaplasia, chronic activegastritis,andnormalgastricmucosawithregardtoBcl-2 expression,apoptosis,andproliferation.
(2)TodeterminewhetherornotBcl-2expression,apoptosis,and proliferationaredifferentbetweenthepatientswithand with-outhistoryofgastriccancerinfirst-degreerelatives.
Materialsandmethods
In this study, we enrolled106patients who had previously undergone upper gastrointestinal endoscopy due to dyspeptic symptoms and had been referred to our outpatient clinic of gastroenterology department with their biopsy results. Their histopathological results were consistent withintestinal meta-plasia (IM), chronic active gastritis (CAG), or normal gastric mucosa (NGM). Informed consents were obtained from each patient, and the study was approved by the university ethical committee.
Fiftyofthecasesweremale,56werefemale;agerangewas 18–81years(meanage:46.1±14.5).
Patients with a history of H. pylori eradication treatment, gastric surgery, nonsteroid anti-inflammatory drug use, proton pumpinhibitororH2receptorantagonistuse,COX-2inhibitoror acetylsalicylicaciduse,antibioticuseduringthepreviousmonth, exposuretocorrosivesubstances,andthosewithmalignancyand vasculitiswereexcludedfromthestudy.
Familyhistoryofgastriccancerwasquestionedinallpatients. Thestudypopulationwasdividedintotwogroups:Patientswith ahistory of gastriccancerin first-degreerelatives andpatients withoutahistoryofgastriccancerinfirst-degreerelatives. Histopathologicalanalysis
Histopathologicalspecimens ofeachpatientwerereassessed andcategorizedintointestinalmetaplasia,chronicactive gastri-tis, and normal gastric histopathology by a second pathologist who was blinded to the previous diagnoses. Histopathological sections of all the patients were evaluated according to the UpdatedSydneySystemScore[6].Moreover, immunohistochem-icalanalysiswasperformedin ordertodetermineBcl-2, Ki-67, andapoptosis.H.pyloripositivitywasevaluatedby Hematoxylin-Eosin.Weperformedsubsequentextrathin-sectionsforthecases in which we did not detect H. pylori by Hematoxylin-Eosin. Thinsectionswere evaluatedby Hematoxylin-Eosin withX100 objective.
Immunohistochemical staining was performed by an indi-rectimmunoperoxidasemethodusingstreptavidin-biotin3.The antibodiesemployed for Ki-67and Bcl-2 were of IgG type and
monoclonalcharacter.For Ki-67 expression,RM-9106-R7rabbit monoclonalantibodyspecifictothehumanKi-67nuclearantigen, whichisexpressedduringtheG1,S,M,andG2phasesofthecell cycleinallproliferatingcells,wasused(LabVision/NeoMarkers, Fremont, CA, USA). For Bcl-2 expression, we used MS-597-R7 specifictothehumanBcl-2 oncoprotein(DiagnosticBiosystem, Pleasanton,CA,USA).Theentireantibodieswerein“ready-to-use” form. We used commercially available kits of biotinized bind-ing(secondary)antibody,streptavidin-biotincomplex,andAECin ready-to-useform.
Thesectionswitha thicknessoffourmicrons were deparaf-finizedbyleavingthemat56◦Cfor12hinasterilizer,beforeputting theminxylenefor30min.Theywerehydratedindescending alco-holsolutions(100%,95%,and90%).Afterwashingthemwithtap water,theywereplacedin 3%hydrogenperoxidefor 10minin ordertoinhibitendogenousperoxidase.Thesectionswerewashed withPBS(phosphatebufferedsaline)solution(pH:7.6)for5min twiceandputinamicrowaveovenfor5minina0.01Msodium cit-ratebuffer(pH:6.0).Theywerewashedwithdistilledwaterthree times.Thenthesectionswereplacedinanon-immuneserumfor 20minforproteinblock.Primaryantibodieswereappliedsoas tocoverthesections, andtheywerekeptatroomtemperature for2h.They werewashedwithPBS for5min twiceand dried. Incubationwithbinding(secondary)antibody(Multi-speciesultra streptavidin detection system-HRP,Signet,Massachusetts, USA) wasperformed atroom temperaturefor 20min.Sectionswere washedwithPBSfor5mintwice.
Streptavidin-biotincomplexwasappliedandallowedtosetfor 30min.ThenpreparationswerewashedwithPBSfor5mintwice. IncubationwithDAB(diaminobenzidinetetrachloride,Novocastra, Newcastle-upon-Tyn,UK)wasperformedfor10min,andthe sec-tionswereagainwashedwithdistilledwaterfor5min.Background stainingwascarriedoutwithhematoxylinbyrapidstaining tech-nique.Thesectionsweredehydratedinascendingalcoholsolutions bytreatingthem5minineach(90%,95%,and100%).Finally,the sec-tionswererenderedmoretransparentbyxyleneandsealedwith Entellan.
TonsillartissuewasusedasthepositivetissuecontrolforKi-67 andBcl-2.NuclearstainingwasrecognizedaspositiveforKi-67and Bcl-2.Apartfromthepositivecontrol,anegativecontrolstaining withoutanyprimaryantibodywasapplied.Degreeofstainingwas determinedwithasemiquantitativemethod.
ApoptosisassociatedwithDNAfragmentationwasdetectedby TUNEL assay.Inthis aim,ApopTag Plusperoxidasein situ apo-ptosis detection kit (Chemicon-S7101, Temecula, CA,USA) was used. The staining wascarried out as per instructions for use. DABwasemployedastheperoxidasesubstrate.Nuclearstainings withdark-brownish colorwererecognizedas positive.Staining index wasdetermined by counting nuclearstaining in at least 1000cellsatrandomlyselected10microscopicfieldsunderhigh magnification.
Statisticalanalysis
ThedataacquiredinthisstudywereanalyzedbySPSS12.0 pack-ageprogram.In normaldistribution,comparison oftwo groups wasperformedbyStudent’st-test,whereascomparisonofthree groupswascarriedoutbyANOVA.Incaseswheretherewasno normaldistribution,comparisonoftwogroupswasperformedby Mann–WhitneyUtest,whereascomparisonofthreegroupswas carriedoutbyKruskal–Wallistest.Incategoricalvalues,Pearson chi-squareandFisher’sexactchi-squaretestswereusedfor depen-dency.p<0.05wasrecognizedaspresenceofstatisticalsignificance anddependence,whereasp>0.05wasrecognizedasabsenceof statisticalsignificanceanddependence.
Table1
Demographicdataofthestudygroup.
IM CAG NGM
Numberofthepatients(n:106) 42(39.6%) 51(48.1%) 13(12.3%)
Age(years) 52.02±13.74 43.04±14.21 39.23±11.94 p=0.002
Gender(male/female) 22/20 22/29 6/7 p>0.05
IM,intestinalmetaplasia;CAG,chronicactivegastritis;NGM,normalgastricmucosa.
Table2
Bcl-2,Ki-67expressionandapoptoticcellratiobasedonhistopathologyresults.
IM(n:42) CAG(n:51) NGM(n:13) p
Bcl-2expressionrate(%) 45.2%(n:19) 43.1%(n:22) 15.4%(n:2) >0.005
Positiveapoptoticcellratio(%) 50%(n:21) 37.2%(n:19) 30.8%(n:4) >0.005
Ki-67expressionrate(%) 29.90±22.87 18.18±16.22 18.54±20 0.03
IM,intestinalmetaplasia;CAG,chronicactivegastritis;NGM,normalgastricmucosa.
Results
Inthisstudy,106patientswereincluded.Theypresentedtoour
outpatientclinicbecauseofdyspepticcomplaintsandwere
endo-scopically diagnosed withintestinalmetaplasia (n: 42),chronic
activegastritis(n:51),andnormalgastricmucosa(n:13)based
onthebiopsiesacquiredfromthecorpusandantrum.While42
(39.6%)of106caseswereintestinalmetaplasia,51(48.1%)were
chronicactivegastritis,and13(12.3%)hadnormalgastricmucosa
(Table1).
Therewasnostatisticaldifferencebetweenthethreegroups interms ofgender(p>0.05).Meanagewasstatistically signifi-cantlyhigherintheIMgroupthanintheCAGandNGMgroups (p<0.05)(Table1).However,nostatisticallysignificantdifference wasfoundbetweentheNGMandCAGgroupswithregardtoage (39.23±11.94vs. 43.04±14.21;p>0.05).Allthepatientsinthe NGMgroupwereH.pylori-negative.Therewasnostatistically sig-nificantdifferencebetweentheCAGandIMgroupswithregardto H.pyloriinfection(%76.5vs.%76.2,respectively;p>0.05).
AlthoughBcl-2expressionwashigherintheIMandCAGgroups thanthatintheNGMgroup,nostatisticallysignificantdifference wasobservedbetweenthethreegroupsrelativetoBcl-2 expres-sion(p>0.05)(Table2).Nostatisticallysignificantdifferencewas determinedbetweentheH.pyloripositiveandnegativegroupsin termsofBcl-2expression(%43.7vs.%34.3,respectively;p>0.05).
AlthoughapoptosistendedtooccurmorefrequentlyintheIM groupthanintheCAGandNGMgroups,nostatisticallysignificant differencewasobservedbetweenthethreegroupswithregardto apoptosisdetectedbyTUNELassay(p>0.05)(Table2).
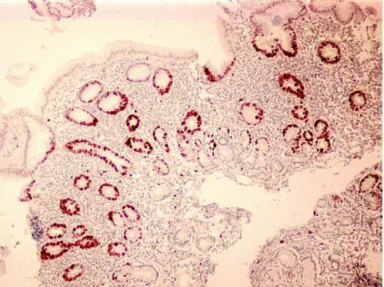
ApoptosisdetectedbyTUNELmethodwasstatistically signifi-cantlyhigherinH.pylori-positivepatientsthaninH.pylori-negative patients(p=0.02)(Table3andFig.1).
TherewasastatisticallysignificantdifferencebetweentheIM, CAG,andNGMgroupswithregardtoKi-67expression(p<0.05). Ki-67expressionwasstatisticallysignificantlyhigherinintestinal metaplasiapatients(Table1andFig.2).
Pairedcomparisonofthehistopathologicalgroupsintermsof Ki-67expressionrevealednosignificantdifferencebetweenthe NGMandCAGgroups(p>0.05),butdemonstratedastatistically significantdifferencebetweentheCAGandIMgroupsinfavorofthe IMgroup(18.18±16.22vs.29.90±22.87,respectively;p=0.012).
Table3
RatioofpositiveapoptoticcellsrelativetoH.pyloripresence.
H.pylori positive(n:71) H.pylori negative(n:35) p Positiveapoptotic cellsratio(%) 49.3%(n:35) 25.7%(n:9) <0.005
Fig.1.DetectionofapoptosisbyTUNELassayinapatientwithH.pyloriinfection.
TherewasnosignificantdifferencebetweentheH.pylori-positive
and-negativepatientsintermsofKi-67expression(21±17.02vs.
23.79±20.85,respectively;p=0.74).
Comparisonofthepatientswithandwithouthistoryofgastric
cancerinfirst-degreerelativesshowednostatisticallysignificant
differencewithregardtopresenceofintestinalmetaplasia(55.6%
vs.36.4%,respectively)(p>0.05).
Asthestudy groupwascategorized into patientswho have
a first-degree relative with a history of gastric cancer (n: 18)
andpatientswhodonothaveafirst-degreerelativewitha
his-toryofgastriccancer(n:88),nostatisticallysignificantdifference
wasobservedbetweenthesetwogroupswithregardtoapoptosis
(%38.9vs.%42,respectively),Bcl-2(%55.6vs.%37.5,respectively)
andKi-67(%94.4vs.%93.2,respectively)expression(p>0.05).
Discussion
Thedevelopmentofgastriccancer,especiallytheintestinaltype,
is a stepwiseprocess, beginning fromchronic gastritisto
atro-phy,intestinalmetaplasia,dyplasia,andeventuallyinvasivecancer
[5].
H.pylori,acommonculpritcausingchronicgastricdisorders, hasbeenclassifiedasaclassIgastriccarcinogen[11].
JassandFilipeproposedaclassificationforintestinal metapla-sia[12],whichhasbeenwidelyaccepted:complete(typeI)and incomplete(typesIIandIII).
Itiswell-knownthattheriskofgastriccancerisrelatedtothe typeofintestinalmetaplasia.InacohortstudyfromSloveniawith afollow-upperiodof10years,patientswhohadintestinal meta-plasiahada10timeshigherriskofdevelopinggastriccanceron averagewhencomparedtothosewhodonothaveintestinal meta-plasia[7].PatientswithtypeIIIintestinalmetaplasiahada4-fold higherriskofgastriccancerwhencomparedtothosewithtypeI [7].
Whilesomestudies[14,20]foundapositivecorrelationbetween H.pyloriinfectionandapoptosisandproliferationofthecellsof nor-malgastricepithelium,Leungetal.[15]foundthatapoptoticindex issignificantlyloweredinH.pylori-associatedintestinal metapla-sia.Ontheotherhand,Hoshietal.performedastudyin which theyevaluatedapoptosisbyTUNELassayandproliferationbyKi-67 immunohistochemicalstainingingastricmucosaspecimenswith andwithoutH.pyloriinfection,andnostatisticallysignificant dif-ferencewasfoundbetweenthetwogroupsintermsofapoptosis andproliferation[10].
Wambura et al. showed that before H. pylori eradication, there was a higher proliferation index in intestinal metapla-sia,whereasapoptosiswassignificantlyhigherinnon-intestinal metaplasia. This was associated with a significantly lower apoptosis/proliferationratioinintestinalmetaplasiathanin non-intestinalmetaplasia.Aftersuccessfuleradication,apoptosisand proliferation decreased in both intestinal metaplasia and non-intestinalmetaplasia[25].
Itisgenerallyagreeduponthatapoptosishasarelativelylow profiledespitean ongoingincrease inproliferationin intestinal metaplasia.Thestudiesshowinganincreaseinproliferation paral-leltodecreaseinapoptosisingastriccancertissuesinfectedwith H.pylorisupportthisview[9,24].
Inourstudy,wedeployedTUNELassayfordetectionof apopto-sisdevelopingwithDNAfragmentation.Inviewofallthreegroups, althoughapoptosiswashighestintheIMgroup,nostatistically sig-nificantdifferencewasfoundbetweenthem.However,apoptosis wasobservedtobestatisticallysignificantlyhigherinpatientswith H.pyloriinfectionthaninthosewithnoH.pyloriinfection.Thiswas aresultconsistentwiththefindingsofsomepreviousstudiesinthe literature[14,20].
Ki-67 is an antigen associated with cell proliferation. As it is expressed during the proliferation and synthesis phases of thecellcycle(G1, S, G2,and M),it isnot expressedduringG0 phase. Therefore, this antigen providesinformation aboutcells undergoing active phases of the cell cycle [8]. In the current study,weevaluatednuclearKi-67proliferationinordertoassess proliferation. The groups were compared withregard to Ki-67 expression,andalthoughnostatisticallysignificantdifferencewas
determinedbetweentheNGMand CAG groups(p>0.05),there was a significant difference between the CAG and IM groups (p=0.012).
ThereareconflictingreportsaboutKi-67expressioninpatients withintestinal metaplasia andin patientswithand withoutH. pyloriinfection.
InastudybyJungetal.[13],20endoscopicallydiagnosedcases ofintestinaltypegastriccarcinoma,20casesofgastricadenoma, and40casesofcontrol(normalorgastritis)wereenrolled.Inthree groups,H.pyloriinfectionrates werenotsignificantly different. Expressionsof apoptosis, Ki-67,and p53 werenot significantly differentinthreegroups.
Inanotherstudy,Ki-67expressionwasfoundtobeelevated duetoH.pyloriinfection,andKi-67expressionwasobservedtobe higherinbothatrophicgastritisandintestinalmetaplasiacasesas comparedwiththepatientswithnormaltissues[28].Cabraletal. fromBrazilfoundthatKi-67expressionwassignificantlyhigherin patientswithH.pyloriinfectioncomparedtothosewithnoH.pylori infection.Inthisstudy,H.pylori-positivepatientswerecompared withregardtoCagApositivity,and Ki-67expressionwas deter-minedtobestatisticallysignificantlyhigherinpatientspositivefor CagA[4].
Hoshietal.performedastudyinwhichtheyevaluatedapoptosis by TUNEL assay and proliferation by Ki-67 immunohistochem-ical stainingin gastric mucosa specimens with and withoutH. pyloriinfection,andnostatisticallysignificantdifferencewasfound betweenthetwogroupsintermsofapoptosisandproliferation [10].
Whilewedidnotobserveastatisticallysignificantdifference intermsofapoptosisinthreegroups,IMgroupdemonstrateda statisticallysignificantlyhigherproliferationascomparedwiththe CAGgroup.Nonetheless,therewasnostatisticallysignificant dif-ferencebetweenthepatientswithandwithoutH.pyloriinfection interms ofKi-67expression.Apoptosisand proliferationareof greatimportancefornormalcellcycle,andtheunchanged apo-ptosisexpressiondespiteelevatedproliferationlevelsinourstudy suggeststhatthereisnodevelopmentofapoptoticresponseagainst uncontrolledcellproliferationinintestinalmetaplasiapatients.In theliterature,manystudiesreportthatH.pyloriinfectionincreases proliferation[4,28].OnlythestudyofHoshietal.foundno sta-tisticallysignificantdifferencebetweenpatientswithandwithout H.pyloriinfectionintermsofKi-67expression[10].Inourstudy, wedeterminednostatisticallysignificantdifferencebetweenthe patientswithandwithoutH.pyloriinfectionwithregardtoKi-67 expression.ThestatisticallysignificantlyhigherKi-67expression inIMpatientsthaninCAGpatientsindicatesthattheremaybe fac-torsinvolvedotherthanH.pyloriinIMpatientswhichmightraise theKi-67expression.
Bcl-2isaproto-oncogeneandsuppressorofapoptosis.Excessive productionofthisproteinprolongsthelifespanofcellsdespite classicapoptoticstimulations[1].
Intheliterature,thereareonlyafewstudiesfocusingonBcl-2 expressioninpatientswithintestinalmetaplasia. Anagnostopou-losetal.evaluatedtheBcl-2andBaxexpressioninchronicgastritis, atrophicgastritis,intestinalmetaplasia,anddysplasiacases[1].Bax belongstotheBcl-2familyandisknownasapro-apoptoticprotein. Inthisstudy,allthechronicgastritiscasesshowedBaxexpression intheirepithelialcells.Twenty-sixpercentoftheatrophicgastritis casesdidnotexhibitBax.Baxisfurthersuppressedinthefaceof intestinalmetaplasiadevelopment.Baxexpressionwasobserved onlyin12%ofthebiopsyspecimenscollectedfromdysplasiacases. WhileBcl-2proteinwasnotfoundinchronicgastritiscases, aber-rantexpressionwasobservedinintestinalmetaplasiaanddysplasia cases.Thus,theyreportedsuppressionofBaxandoverexpression ofBcl-2duringtheearlyphaseofcarcinogenesis,priortothe dys-plasticchanges.
InTurkey,Topaletal.conductedastudyandobservedthatH. pyloriincreasedBcl-2expressionviainducingatrophyand intesti-nalmetaplasiainanindirectfashion.Bcl-2positivitywashigherin intestinalmetaplasiathaninatrophy[23].
Inanotherstudy,overexpressionofBcl-2wasobservedonlyin atrophicgastritiscases,however,itwasnotdeterminedin non-atrophicintestinalmetaplasiaandantralgastritisassociatedwith H.pylori[17].
Inourstudy,nostatisticallysignificantdifferencewas deter-minedbetweenthethreegroupsintermsofBcl-2expressionbased onhistopathologicalanalysis.Moreover,therewasnostatistically significantdifferencebetweenthepatientswithand withoutH. pyloriinfectionwithregardtoBcl-2expression.Inourstudy,the comparisonof patientswithand withouta first-degreerelative havinggastriccancerrevealednostatisticallysignificantdifference withregardtoBcl-2,Ki-67,andapoptosis(p>0.05).Therewasno studyintheliteraturefocusingonBCL-2andapoptosisexpression inpatientswithafirst-degreerelativehavinggastriccancer.There are3studiesevaluatingKi-67expressioninpatientshavinga first-degreerelativewithgastriccancer.Meiningetal.reportedelevated antralproliferationinpatientshavingafirst-degreerelativewith gastriccancer,andnotedincreasedproliferationbothinthe cor-pusandtheantrumwhenpatientswithH.pyloriinfectionwere excluded[19].Intheothertwostudies,whenfirst-degreerelatives ofgastriccancerpatientswerecomparedwiththecontrolgroup,no statisticaldifferencewasobservedintermsofproliferation[22,29]. In conclusion, weobserved nostatisticallysignificant differ-encebetweentheIM,CAG,andNGMgroupswithregardtoBcl-2 expressionandapoptosis,whereaswedeterminedastatistically significantlyhigherKi-67expressionintheIMgroup.H.pyloriwas foundtoelevate apoptosis,however,it had nostatistically sig-nificantinfluenceonproliferationandBcl-2expression.Intestinal metaplasiacasesdisplayednoincreaseinapoptosisbutshowed elevatedproliferation,whichsuggested thatadequateapoptotic responsedidnotoccuragainstuncontrolledcellproliferationand thatitmightbeanimportantfactorintheprogressionfrom intesti-nalmetaplasiatocancer.
In ourstudy, wefound nostatisticallysignificant difference betweenthepatientswithandwithoutfirst-degreerelatives suf-feringfromgastriccancerintermsofBcl-2andKi-67expression, andapoptosis.Intheliterature,thereareinvestigationsstudying Ki-67expressioninfirst-degreerelativesofgastriccancerpatients. However,ourstudywasthefirsttoevaluateapoptosisandBcl-2 expressioninpatientshavingafirst-degreerelativewithgastric cancer. Althoughthis wasthefirst studyfocusing onthat sub-jectintheliterature,thelimitationofourstudywastherelatively smallnumberofpatientshavingafirst-degreerelativewithgastric cancer.Analyzingonly18patientsmaynotreflectthegeneral pop-ulationaccurately.Moreover,therewasnodataonwhetherthose patientshadanygeneticpredisposingfactorornot.Furtherstudies, includinglargerseries,shouldbeconductedtoverifytheseresults. Conflictofinterest
None. References
[1] G.K.Anagnostopoulos,D.Stefanou,E.Arkoumani,G.Sakorafas,G.Pavlakis,D. Arvanitidis,E.Tsianos,N.J.Agnantis,BaxandBcl-2proteinexpressioningastric precancerouslesions:immunohistochemicalstudy,J.Gastroenterol.Hepatol. 20(2005)1674–1678.
[2]B.Antonsson,Baxandotherpro-apoptoticBcl-2familykiller-proteinsandtheir victim,themitochondrion,CellTissueRes.306(2001)347–361.
[3]J.C.Atherton,ThepathogenesisofHelicobacterpylori-inducedgastro-duodenal diseases,Annu.Rev.Pathol.1(2006)63.
[4] M.M.Cabral,C.A.Oliveira,C.M.Mendes,J.Guerra,D.M.Queiroz,G.A.Rocha, A.M.Rocha,A.M.Nogueira,GastricepithelialcellproliferationandCagAstatus
inHelicobacterpylorigastritisatdifferentgastricsites,Scand.J.Gastroenterol. 42(2007)545–554.
[5]P.Correa,Humangastriccarcinogenesis:amultistepandmultifactorial pro-cess.FirstAmericanCancerSocietyAwardLectureonCancerEpidemiology andPrevention,CancerRes.52(1992)6735–6740.
[6]M.F.Dixon,R.M.Genta,J.H. Yardley,P.Correa, Classificationandgrading ofgastritis. TheupdatedSydney System.InternationalWorkshoponthe HistopathologyofGastritis,Houston1994,Am.J.Surg.Pathol.20(10)(1996) 1161–1181.
[7] M.I.Filipe,N.Mu ˜noz,I.Matko,I.Kato,V.Pompe-Kirn,A.Jutersek,S.Teuchmann, M.Benz,T.Prijon,Intestinalmetaplasiatypesandtheriskofgastriccancer:a cohortstudyinSlovenia,Int.J.Cancer57(1994)324–329.
[8]J.Gerdes,L.Li,C.Schlueter,M.Duchrow,C.Wohlenberg,C.Gerlach,I.Stahmer, S.Kloth,E.Brandt,H.D.Flad,Immunobiochemicalandmolecularbiologic char-acterisationofthecellproliferation-associatednuclearantigenthatisdefined bymonoclonalantibodyKi-67,Am.J.Pathol.138(1991)867–873.
[9]T.Hoshi,H.Sasano,K.Kato,N.Yabuki,S.Ohara,R.Konno,S.Asaki,T.Toyota,H. Tateno,H.Nagura,ImmunohistochemistryofCaspase3/CPP32inhuman stom-achanditscorrelationwithcellproliferationandapoptosis,AnticancerRes.18 (6A)(1998)4347–4353.
[10]T.Hoshi,H.Sasano,K.Kato,S.Ohara,T.Shimosegawa,T.Toyota,H.Nagura,Cell damageandproliferationinhumangastricmucosainfectedbyHelicobacter pylori–acomparisonbeforeandafterH.pylorieradicationinnon-atrophic gastritis,Hum.Pathol.30(12)(1999)1412–1417.
[11]InternationalAgencyforResearchonCancer,Schistosomes,liverflukesand Helicobacterpylori,in:IARCMonographsontheEvaluationofCarcinogenic RiskstoHumans,IARCMonograph,vol.61,1994,pp.177–241.
[12] J.R.Jass,M.I.Filipe,Sulphomucinsandprecancerouslesionsofthehuman stom-ach,Histopathology4(1980)271–279.
[13]J.T.Jung,C.H.Lee,S.S.You,H.K.Ha,J.S.Bae,J.G.Kwon,E.Y.Kim,H.G.Kim,C.H. Cho,I.H.Shin,Gradingofhistology,expressionofapoptosisandcell prolifera-tioningastricmucosaadjacenttogastricadenomaoradenocarcinoma,Korean J.Gastroenterol.46(4)(2005)269–275.
[14]W.K.Leung,K.F.To,F.K.Chan,T.L.Lee,S.C.Chung,J.J.Sung,InteractionofH. pyloriandNSAIDongastricepithelialcellapoptosisandproliferation: implica-tionsonulcerogenesis,Aliment.Pharmacol.Ther.14(2004)879–885. [15]W.K.Leung,J.Yu,K.F.To,M.Y.Go,P.K.Ma,F.K.Chan,J.J.Sung,Apoptosisand
proliferationinHelicobacterpylori-associatedgastricintestinalmetaplasia, Ali-ment.Pharmacol.Ther.15(2001)1467–1472.
[16] W.K.Leung,J.J.Sung,Reviewarticle:intestinalmetaplasiaandgastric carcino-genesis,Aliment.Pharmacol.Ther.16(2002)1209–1216.
[17]Y.Maor-Kendler,G.Gabay,J.Bernheim,T.Naftali,I.Lesin,G.Leichtman,I. Pomeranz,B.Novis,ExpressionofBcl-2inautoimmuneandHelicobacter pylori-associatedatrophicgastritis,Dig.Dis.Sci.44(4)(1999)680–685.
[18] N.Matsukura,K.Suzuki,T.Kawachi,M.Aoyagi,T.Sugimura,H.Kitaoka,H. Numajiri,A.Shirota,M.Itabashi,T.Hirota,Distributionofmarkerenzymesand mucininintestinalmetaplasiainhumanstomachandrelationtocompleteand incompletetypesofintestinalmetaplasiatominutegastriccarcinomas,J.Natl. CancerInst.65(1980)231–240.
[19]A.Meining, A. Hackelsberger, C. Daenecke,M. Stolte,E. Bayerdörffer, T. Ochsenkühn,Increasedcellproliferationofthegastricmucosainfirst-degree relativesofgastriccarcinomapatients,Cancer83(5)(1998)876–881. [20] S.F.Moss,J.Calam,B.Agarwal,S.Wang,P.R.Holt,Inductionofgastricepithelial
apoptosisbyHelicobacterpylori,Gut38(4)(1996)498–501.
[21]S.Preston-Martin,M.C.Pike,R.K.Ross,P.A.Jones,B.E.Henderson,Increasedcell divisionasacauseofhumancancer,CancerRes.50(1990)7415–7421. [22]A.Romiti, A. Zullo,S. Tomao,C. Hassan, I. Sarcina,V. De Francesco, E.
Ierardi,F.Tomao,A.Vecchione,S.Morini,Gastricmucosaalterationsin first-degreerelativesofgastriccancerpatients,AnticancerRes.25(3c)(2005) 2567–2572.
[23]D.Topal,V.Göral,F.Yılmaz,I.H.Kara,TherelationofHelicobacterpyloriwith intestinalmetaplasia,gastricatrophyandBcl-2,Turk.J.Gastroenterol.15(3) (2004)149–155.
[24] C.Wambura,N.Aoyama,D.Shirasaka,T.Sakai,T.Ikemura,M.Sakashita,S. Maekawa,K.Kuroda,T.Inoue,S.Ebara,M.Miyamoto,M.Kasuga,Effectof Helicobacterpylori-inducedcyclooxygenase-2ongastricepithelialcellkinetics: implicationsforgastriccarcinogenesis,Helicobacter7(2002)129–138. [25]C.Wambura,N.Aoyama,D.Shirasaka,K.Kuroda,Y.Watanabe,I.Miki,T.
Tamura,M.Kasuga,Cellkineticbalanceingastricmucosawithintestinal meta-plasiaafterHelicobacterpylorieradication:2-yearfollow-upstudy,Dig.Liver Dis.36(2004)178–186.
[26]R.TWang,T.Wang,K.Chen,J.Y.Wang,J.P.Zhang,S.R.Lin,Y.M.Zhu,W.M.Zhang, Y.X.Cao,C.W.Zhu,H.Yu,Y.J.Cong,S.Zheng,B.Q.Wu,Helicobacterpylori infec-tionandgastriccancer:evidencefromaretrospectivecohortstudyandnested case-controlstudyinChina,WorldJ.Gastroenterol.8(6)(2002)1103–1107. [27]M.Welin,N.M.Holmgren,P.Nilsson,H.Enroth,Statisticalmodelofthe
inter-actionsbetweenHelicobacterpyloriinfectionandgastriccancerdevelopment, Helicobacter8(1)(2003)72–78.
[28]H.H.Xia,G.S.Zhang,N.J.Talley,B.C.Wong,Y.Yang,C.Henwood,J.M.Wyatt,S. Adams,K.Cheung,B.Xia,Y.Q.Zhu,S.K.Lam,Topographicassociationofgastric epithelialexpressionofKi-67,Bax,andBcl-2withantralizationinthegastric incisura,bodyandfundus,Am.J.Gastroenterol.97(12)(2002)3023–3031. [29]A.Zullo,C.Hassan,S.Marangi,O.Burattini,A.Romiti,V.DeFrancesco,C.Panella,
S.Morini,E.Ierardi,Gastricepithelialcellproliferationandrasoncogenep21 expressioninfirst-degreerelativesofgastriccancerpatients:acase-control study,Eur.J.Gastroenterol.Hepatol.18(8)(2006)921–926.