* Corresponding Author DOI: 10.37094/adyujsci.628399
Preparation and Characterization of Polyvinyl Alcohol/Activated Carbon
(PVA/AC) Composite and Its Use in the Adsorption of 4-Nitrophenol (4-NP)
Hatice KARAER YAĞMUR1,*
1Dicle University, Faculty of Science, Department of Chemistry, Diyarbakır, Turkey
profhatice23@hotmail.com, ORCID: 0000-0002-3728-1825
Received: 02.10.2019 Accepted: 26.03.2020 Published: 25.06.2020
Abstract
In this study, polyvinyl alcohol-activated carbon (PVA/AC) composite was synthesized and characterized by SEM, FTIR, DLS and TGA-DSC measurement. The zero point charge for the PVA/AC was determined as approximately 3 pH. The efficiency of PVA/AC as a cheap adsorbent for removal of 4-nitrophenol (4-NP) from aqueous solution was researched at 298, 318 and 328 K. For this purpose, Freundlich and Langmuir isotherm models were used. According to the results, adsorption of 4-NP onto composite best fitted to Freundlich model. The pseudo-first-order, pseudo-second-order and intraparticle diffusion models were utilized to study the adsorption kinetics. The experimental consequences fitted with the pseudo-first-order model. According to Arrhenius equation, Ea was calculated as 14.13 kJmol-1. Thermodynamic
parameters were also calculated, which revealed that the adsorption process is endothermic, not spontaneous.
Keywords: Adsorption; Activated carbon; Polyvinyl alcohol; 4-nitrophenol (4-NP); Zero point charge.
Polivinil Alkol/Aktif Karbon (PVA/AC) Kompozitinin Hazırlanması ve Karakterizasyonu ve 4-Nitrofenol (4-NP) Adsorpsiyonunda Kullanımı
Öz
Bu çalışmada polivinil alkol/aktif karbon (PVA/AC) kompoziti sentezlendi ve SEM, FTIR, DLS ve TGA-DSC ölçümleriyle karakterize edildi. PVA/AC için sıfır noktası yükü yaklaşık 3 pH olarak belirlenmiştir. Sulu çözeltiden 4-nitrofenolün (4-NP) uzaklaştırılması için ucuz bir adsorban olarak PVA / AC'nin etkinliliği 298, 318 ve 328 K'de araştırıldı. Bu amaçla Freundlich ve Langmuir izoterm modelleri kullanıldı. Sonuçlara göre, 4-NP'nin kompozit üzerine adsorpsiyonunda Freundlich model en uygundu. Adsorpsiyon kinetiklerini incelemek için psödo birinci derece, psödo ikinci derece ve partikül içi difüzyon modelleri kullanıldı.Deneysel sonuçlar psödo birinci derece modeline uydu. Arrhenius denklemine göre Ea, 14.13 kJmol-1 olarak
hesaplandı. Termodinamik parametreler hesaplandı, adsorpsiyon sürecinin endotermik olduğu kendiliğinden olmadığı belirlendi.
Anahtar Kelimeler: Adsorpsiyon; Aktif karbon; Polivinil alkol; 4-nitrofenol (4-NP); Sıfır nokta yükü.
1. Introduction
Activated charcoals (ACs) are known homogeneous materials having high surface area and microporous structure. Moreover, they are widely used in industrial applications as adsorbent and catalyst. The features of AC adsorption depend primarily upon size of particle, porosity, ash contents, carbonization degree and activation process [1]. Moreover, they are efficiently used both in purification of drinking water and different industrial applications and in hemoperfusion and based on hemofiltration therapies [2]. Polyvinyl alcohol (PVA) is a water-soluble synthetic polymer and is known as a cheap polymer and not poisonous [3]. Between artificial polymers, PVA has received amazing interest owing to its convenient physicochemical and viscoelastic features and is widely utilized for several biomedical functions for wound dressing and tissue engineering since it is a biodegradable as well as biocompatible artificial polymer [4]. Phenolic compounds have been widely utilized in the production of chemicals such as explosives, dyes, pesticides, solvents and can stay in aqueous media for a long time. Phenols have been encountered in both wastewater and in whole natural aquatic systems, and have toxic features even at low concentrations. 4-nitrophenol (4-NP) is one of the phenol derivatives which is toxic. 4-NP has a hydroxyl group at the opposite side of a nitro group on the benzene ring and is generally found in wastewater especially with pesticides, herbicides and petrochemicals [5]. Elimination of the
such as separation of membrane, adsorption and exchange of ion have been utilized for the elimination of phenolic compounds from industrial effluents. Adsorption method is one of the efficient and preferred processes due to its simplicity and high effectiveness [6]. There are many studies in the literature on activated carbon. However, the studies on polyvinyl alcohol/activated carbon and 4-nitrophenol (4-NP) are limited.
In this study, a new polymer-based composite was prepared from PVA and AC. The composite was characterized by FTIR, DLS, SEM-EDX, TGA-DSC and Zeta sizer. Furthermore, the point of zero charge of PVA/AC composite was determined. Then, PVA/AC composite was used as an adsorbent for the removal of 4-nitrophenol (4-NP) from aqueous solution. Finally, the adsorption of 4-NP by the PVA/AC composite from aqueous solution was studied both thermodynamically and kinetically.
2. Materials and Methods 2.1. Materials
PVA (Poly(vinyl alcohol) was supplied from Sigma–Aldrich (average molecular weight (MW): 30,000-70,000 gmol−1 and 90% hydrolyzed). AC (Activated charcoal, black powder,
100-400 mesh) and 4-Nitrophenol (4-NP) were bought from Sigma and Sigma–Aldrich (MW: 139.11
gmol−1) respectively.
2.2. Preparation of PVA/AC composite
The composite was synthesized according to a reported procedure [2, 7]. A solution of PVA was synthesized from PVA (5 g) and distilled water (100 mL) at 90 °C with vigorous stirring. Then, an aqueous solution of PVA and10 mL sulfuric acid was mechanically stirred in a 500 mL beaker, and temperature of the mixture was diminished to 60 oC. Subsequently, AC (2.5 g) was
added and stirred. The mixture was cooled to room temperature and kept in an ice bath for 30 min. 5 mL of 25% glutaraldehyde solution was added to the cooled solution and the mixture was stirred by a magnetic stirrer for about 3h. PVA/AC composite was acquired after washing with distilled water. It was dried in an oven at about 80 oC. Scheme 2 shows the PVA/AC preparation
Scheme 1: The chemical structure of materials
Scheme 2: Schematic representation of PVA/AC composite preparation
2.3. Characterization
The chemical structure of the composite was elucidated by using FTIR spectroscopy (A Perkin Elmer spectrometer-ATR sampling accessory between the wavelengths of 4000–450 cm−1). TGA–DTA analysis was carried out between 20 and 1000 °C (Perkin Elmer, in N
2, rate
10°C min−1). DSC analysis of the composite was performed between 25 and 450 °C (Perkin Elmer
Pyris sapphire, in N2, rate 10 °C min−1). Surface structure of the composite was identified by
using of SEM (scanning electron microscope, JEOL, JSM-7100 model). The conductivity measurements were performed by Keithley Electrometer (Keithley 2400, Ohio, USA). The pellet was pressed on hydraulic press at 1687 kg/cm2. Iodine doping was performed by exposing the
pellet to iodine steam at 25 oC in a desiccator. Particle dimension of the PVA/AC was detected
by using the DLS (dynamic light scattering) method. Zeta potential measurement of the PVA/AC was performed using a ZetaSizer Nano-ZS (Malvern Nano ZS90).
2.4. Determination of the point of zero charge (pHzpc)
The point of zero charge was determined by a reported procedure [8, 9]. The procedure can be described as follows: to a series of 50 mL conical flasks, 30 mL of 0.1M NaCl solution was added. pH values of the initial NaCl solutions (initial pH) were adjusted in the range of 2 to 12 of using 0.1 M HCl / NaOH solutions. After a constant value of initial pH had been reached, 0.015 g of PVA/AC composite was added into each conical flask and caped them immediately. These samples were shaken for 48 h, after which pH was measured for each sample (pHf). The pH
variation (ΔpH = pHf − pHi) was plotted against the pHi, and the point of intersection of the
resulting curve at which ΔpH = 0 was the pHzpc value. 2.5. Adsorption kinetics and isotherms
The sorption studies were performed on the composite by use of 4-NP (Scheme 1) and can be classified as three parts: (a) effect of temperature (b) defining of a kinetic model and isotherm model (c) thermodynamic studies. Kinetic studies were performed agitating 100 ml of solution of a constant 4-NP concentration with 0.1 g of the composite at the shaking rate 120 rpm, 298, 313 and 328 K, natural pH. Adsorption equilibrium studies were performed by 0.05 g of composite with 100 mL of 4-NP solution of in the range of 50 to 500 mgL-1 concentrations at 298, 313 and
328 K (S.R:120 rpm for 4h). The equilibrium phenol concentration was measured via a UV-visible spectroscopy (λmax:348 nm for 4-NP, Cary 100 Bio UV-visible spectrophotometer). The
capacity of adsorption (qe, mg g-1) may be computed by the following equations:
qe = (Co-Ce) V/m (1) V (L), Co (mgL-1), m (g) and Ce (mg L-1) are the volume of the solution, initial
concentration, the adsorbent mass and equilibrium concentration of phenol in the solution, respectively.
3. Results and Discussion 3.1. FTIR
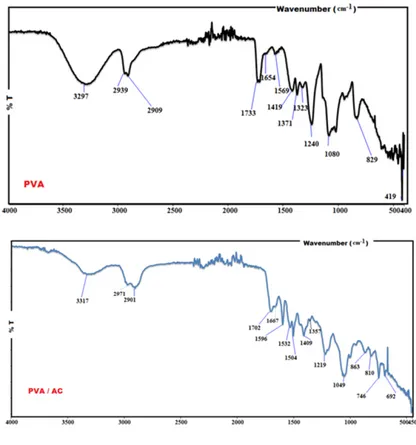
FTIR spectra of PVA and PVA/AC were shown in Fig. 1. The PVA has –CH2, –CH and –
OH groups with C-C backbone and is known as a polar polymer. The formation of intermolecular and free bonded hydroxyl groups of AC and PVA were observed at 3317 cm−1. The aliphatic
stretching peak of C-H was observed at 2971 cm−1 and 2901 cm−1. The peaks at 1702 cm−1 and
1667 cm−1 are due to C=O and C-O stretching, respectively. The peaks at around 1596 cm−1 and
peak at 1080 cm−1 (PVA) was defined C-O stretching peak in alcohols. The broadening of C-O
peak (1080 cm−1) occurred with the existence of a new peak at 1049 cm−1 in the composite is
attributed to the interfacial covalent reaction between AC and PVA. The peak at 829 cm−1 (PVA)
for C-H bending in the PVA/AC sample shifted to 809 cm−1 with AC addition [4].
Figure 1: FTIR spectra of the PVA and PVA/AC
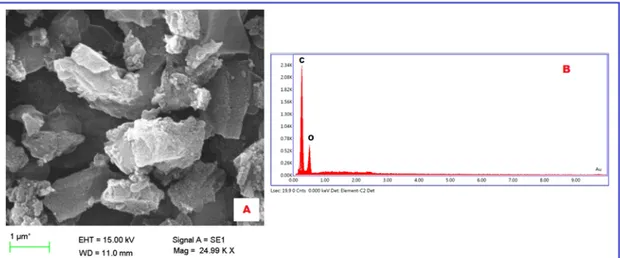
3.2. SEM-EDX analysis
The surface morphology of PVA/AC composite was determined by using SEM (Fig. 2). EDX has been defined as the elements on the composite surface. Fig. 2, shows the SEM images of the composite. One can easily see in the Fig. 2 that the surface of the composite is amorphous and not regular, which shows a relatively high surface area. EDX analysis shows that the composite is composed commonly of carbon (Fig. 2(B)). The peaks related to oxygen are low and there were no other different atoms (N, P, S.) present in the composite.
Figure 2: SEM image of composite (A) and EDX spectra of composite (B)
3.3. Dynamic light scattering (DLS)
633 nm diode laser was used in the instrument. The PVA/AC composite was diluted by using of pure water before measurement. The D-values (D10, D50 and D90) were usually used for describing particle size distributions. D10, D50 and D90 are the intercepts for 10%, 50% and 90% of the cumulative mass [10]. Size distribution of the PVA/AC particles was found as < 14.3 µm < 299 µm < 1170 µm for D10, D50 and D90% cumulative mass, respectively. For example, if the D50 is 299 µm, for PVA/AC particles, this means that 50% of the PVA/AC particles has a size of 299 µm or smaller. An additional parameter to show the width of the size distribution is the span. The span of a volume-based size distribution is determined as Span = (D90–D10)/D50 and gives an indication of how far the 10 percent and 90 percent points are apart, normalized with the midpoint [10]. The value of span PVA/AC was calculated as 3.86.
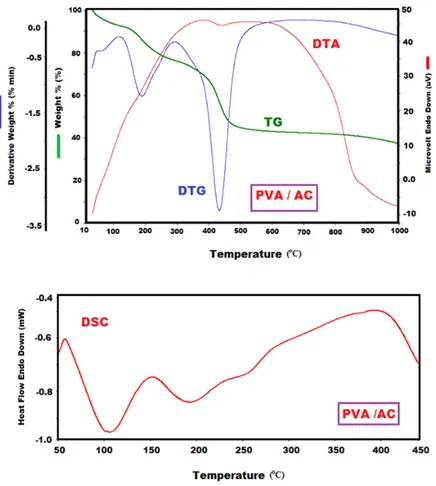
3.4. Thermal studies
TGA, DTA, DTG and DSC are defined as Thermogravimetric analysis, Differential Thermal Analysis, Differential Thermogravimetry and Differential Scanning Calorimetry respectively. TGA, DTG, DTA and DSC curves of the composite were given in Fig. 3. Based on TGA analysis, Tonset (the initial degradation temperatures) of the PVA/AC composite was defined as 170 oC. The values of temperature of T
20 (20%, weight losses) and T50 (50%, weight
losses) were defined as 233 and 446 oC, respectively. Tmax was defined from the DTG curves as
194 and 433oC. The endothermic and exothermic peaks were not observed in DTA curve of the
composite. The glass transition temperature (Tg) and the change in the specific heat (∆Cp) during
the glass transition of the composite were computed from its DSC curve as 147 oC and 0.106 J/g oC.
Figure 3: TGA and DSC analysis curves of the PVA/AC
3.5. Conductivity of the composite
The composite pellet was doped with iodine vapors at 25 oC in desiccators for 24 h. It was
observed that the conductivity of the PVA/AC increased slightly but then tended to level-off. The conductivities of doped and undoped pressed PVA/AC pellets were determined as 2.44x10-5 S
cm-1 and 2.09x10-4 S cm-1, respectively.
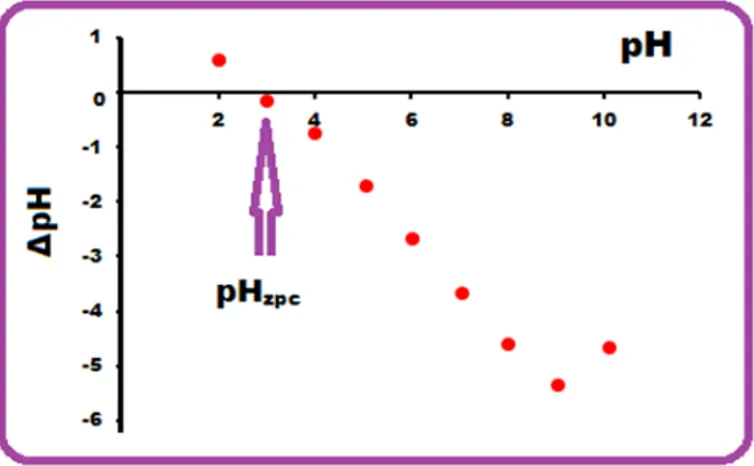
3.6. Point of zero charge
The sorbent surface to charge positively or negatively depends on concentration of protons in the solution. The pHpzc results of the experiments were determined with the PVA/AC composite, where the pH ranged from 2 to 12. Fig. 4 shows that the pHpzc of the PVA/AC was approximately 3. In other words, at this pH, the PVA/AC surface has zero charge [9]. Therefore, when solution pH increases above pHpzc, the surface of PVA/AC is negatively charged, when it
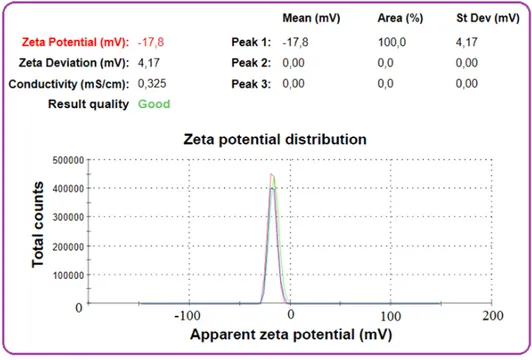
4-NP is a weak acid (pKa value of 7.15). If initial pH of the solution is in acidic range, particularly less than 3, the most of 4-NP molecules remain in molecular form and at the same time the PVA/AC surface possesses positive charge due to pHpzc (i.e. 3). If initial pH of the solution is in alkaline range, the dissociation of 4-NP into 4-NP anions takes place and at the same time the PVA/AC surface possesses negative charge (because pH > pHpzc). In this state electrostatic repulsion dominates between the adsorbate and adsorbent surface, resulting in a considerable decrease in percentage removal of 4-NP in alkaline range. At pH of 3.0 the net charge on the PVA/AC surface was zero, hence, there was no electrostatic repulsion and hence the removal of 4-NP is more than that in alkaline range [13]. Fig. 5 shows the zeta potential curves of PVA/AC in three replicates. Zeta potential value of PVA/AC was -17.8 mV. Therefore, the PVA/AC composite has a negative surface charge and may be moved towards a positively polarized electrode under an electric field [14]. Nanoparticles with zeta potential greater than+30mV or less than ‒30mV are considered strongly cationic and strongly anionic respectively [15]. Paralikar [16] reported that negative zeta potential value of the particles might be owing to adsorption of OH- ions on adsorbent. OH- ion helps in preventing the aggregation.
Figure 5: Zeta potential curves obtained for water suspensions of PVA/AC
3.7. Kinetic study
3.7.1. Effect of temperature
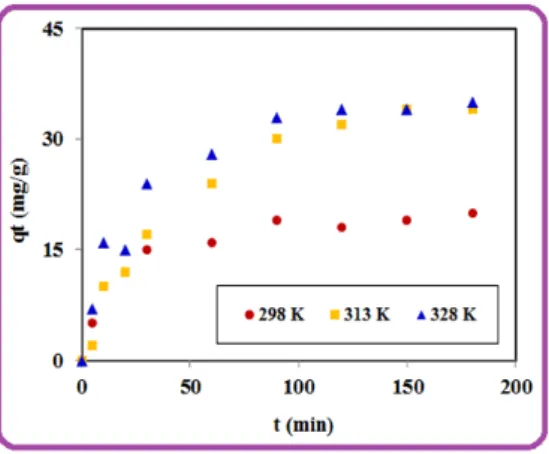
The effect of contact time on the sorption of 4-NP by the PVA/AC was researched for 200 min by using initial 4-NP concentration of 100 mg/L. Fig. 6 shows the quantity of adsorbed 4-NP (qe, mg g-1) at 298, 313 and 328 K. The values of qe (mg g-1) increased from 298 to 313 K,
indicating that the adsorption of 4-NP on the PVA/AC composite is endothermic. Three different kinetic models (Lagergren(PFO), Ho-Mckay(PSO) and Weber-Morris) were used to understand the adsorption process:
Pseudo-first order (PFO)
log (qe−qt ) = log qe –k1 t / 2.303 (2)
Pseudo-second order (PSO)
t / qt = 1 / k2 qe2 + t / qe (3)
Figure 6: Adsorption kinetic of 4-NP on the composite at 298, 313, 328 K
qe (mg g-1) and qt (mg g-1) are the quantity of adsorbed at equilibrium and at any contact
time of adsorption t (min), respectively; PFO, PSO and intraparticle diffusion rate constants were defined as k1 (min-1), k2 (g mg-1 min-1), and kid (mg g-1 min-1/2) respectively. Based on Eqn.(2), the
plot of log (qe–qt) versus t, and based on Eqn.(3) the plot of t/qt versus t and based on Eqn. (4) the plot of qt versus t½ should each give a straight line for the respective model to be applicable
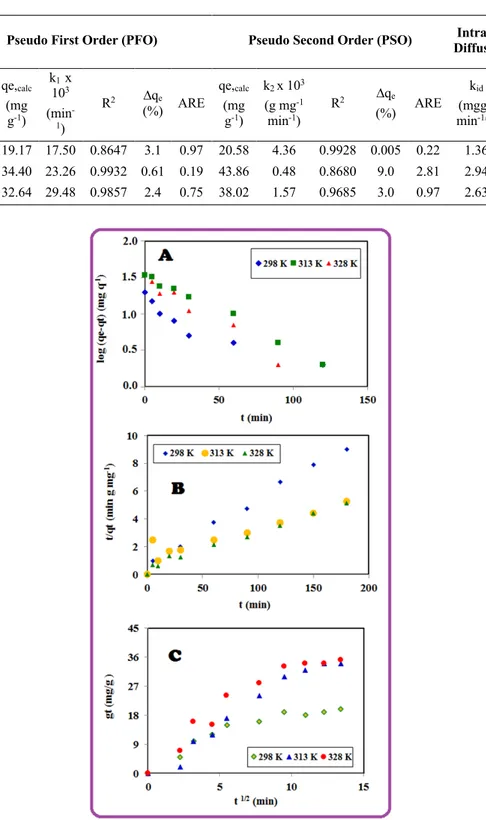
[17]. The rate constants, qe and correlation coefficient values (R2) were shown in Table 1. Table
1 and Fig. 7 show the kinetic models of sorption for 4-NP. Based on the results (Table 1), PFO model has higher values of correlation coefficients (R2) compared to PSO model. Moreover, the
computed qe values agreed with the experimental qe data in the case of the PFO model [18]. Feyzi et al. used raw and modified activated carbon from walnut and pistachio shell in order to remove Dimethyl sulfide from model fuel. Results showed that adsorption kinetic could be determined by pseudo-first order kinetic model [19]. Nam et al. synthesized active carbon from peanut shell with KOH activation for the adsorption of H2S. According to their results, adsorption kinetic was
determined as the pseudo-first order model owing to its higher R2 and smaller difference between
qe,exp and qe,calc as compared to the pseudo-second order model [20]. Moreover, the best-fitted model can be determined using the normalized standard deviation (Δqe (%)) and average relative
error (ARE) [21, 22]. The equations of the functions are given as follows:
∆𝑞!(%) = 100 × +#$"" ∑ -%!,#$%&% $%!,'%( !,#$%& . & # '(" (5) 𝐴𝑅𝐸 = "))# ∑ -%!,#$%&$%!,'%( %!,#$%& . # '(" (6)
qt.meas(mg/g), qt.cal(mg/g) and n are the experimental and calculated equilibrium adsorption
shown in Table 1. Low ARE and Δqe (%) values indicate the accurate fitting of the model [21,
23]. According to our experimental results (Table 1), it may be seen that the pseudo-first order (PFO) model seems to be suitable for modelling the adsorption of 4-NP on PVA/AC.
Table 1: Kinetic data
Pseudo First Order (PFO) Pseudo Second Order (PSO) Intra-Particle Diffusion Model
T (K) qe,exp (mg g-1) qe,calc (mg g-1) k1 x 103 (min -1) R2 ∆qe (%) ARE qe,calc (mg g-1) k2 x 103 (g mg-1 min-1) R2 ∆qe (%) ARE kid (mgg-1 min-1/2) R2 298 21 19.17 17.50 0.8647 3.1 0.97 20.58 4.36 0.9928 0.005 0.22 1.36 0.8639 313 35 34.40 23.26 0.9932 0.61 0.19 43.86 0.48 0.8680 9.0 2.81 2.94 0.8003 328 35 32.64 29.48 0.9857 2.4 0.75 38.02 1.57 0.9685 3.0 0.97 2.63 0.9131
3.7.2. Activation parameters
The adsorption type was defined by using the PFO rate constants(k1) at 298, 313, 328 K
and the Arrhenius eqn. (7),
logk1 = logA − Ea/2.303RT (7)
where Ea, k1, A, R and T are the activation energy (Jmol-1), the PFO rate constant(min-1),
the Arrhenius factor (gmol-1min-1), the gas constant (8.314 J K-1 mol-1) and the temperature (K)
respectively.
The slope of the plot of ln k1 versus 1/T was used to evaluate Ea (Figure not given). Physical
adsorption has low activation energies (between 5–40 kJ mol-1), while chemical chemisorption
has higher ones (40–800 kJ mol-1) [24, 25]. Based on the results, the adsorption of 4-NP on the
composite is physisorption because of Ea=14.13 kJmol-1.
3.8. Adsorption isotherm parameters
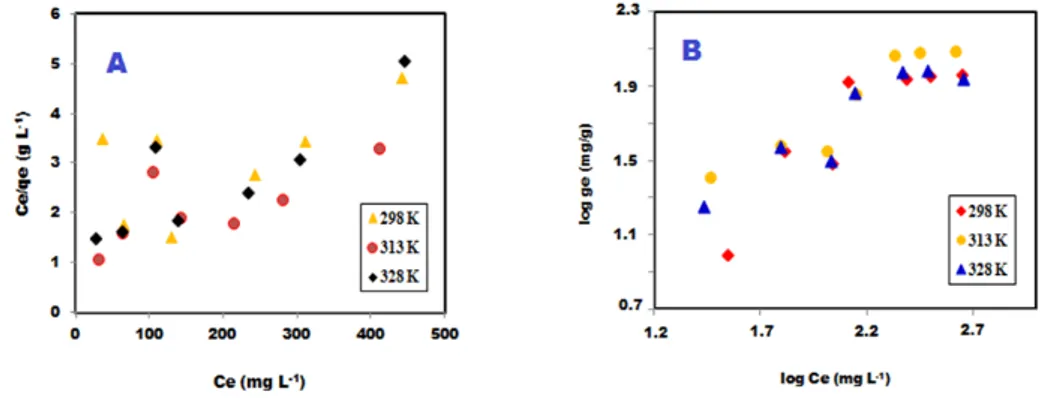
The adsorption isotherms studies for evaluating adsorption process are essential to attain some information about the nature of interaction of 4-NP with adsorbent and to evaluate the applicability and efficiency of any adsorbent [26]. Fig. 8 shows adsorption isotherms at different temperature. The surface property can be determined as a multilayer or monolayer by using of adsorption isotherms. The Langmuir (8) and Freundlich (9) isotherm models are usually used to detect the adsorption isotherm.
Ce /qe = 1 /b Qmax + Ce/Qmax (8)
qe (mgg-1), Ce (mgL-1), Q
max (mgg-1) and b (Lmg-1) are the quantity of phenol adsorbed at
equilibrium, the remaining concentration of phenol in solution at equilibrium, maximum adsorption capacity and Langmuir constant respectively which are relative to adsorption energy [27, 28]. The Langmuir model is monolayer adsorption isotherm. According to Langmuir isotherm, surface of adsorbent is homogeneous, energetically uniform [28]. Linear representation of Langmuir isotherm may be described by Eqn. (8). The Qmaxand b values were computed from
the slopes and intercept of the linear plot of Ce/qe versus Ce in Fig. 9 (A).
log qe = log k + (1/n) log Ce (9) k(mg/g) and n are the adsorbent capacity, the heterogeneous factor respectively and connected to intensity of Freundlich isotherm. According to Freundlich isotherm, adsorbent surface is heterogenous [29] and adsorption capacity is relative to the concentration of adsorbed
phenol at equilibrium. Freundlich isotherm is convenient adsorption at high concentrations and not convenient for low concentration range [30]. The values of k and n may be acquired from the slope and intercept of linear plot of log qe versus log Ce [31]. In Fig. 9 (B) for based on Eqn. (9). The adsorption isotherm parameters, correlation coefficients (R2) and values error RMSE (root
mean squared error) were given in Table 2. The values of RMSE (Eqn.10) can be calculated by the following equations [21]:
𝑅𝑀𝑆𝐸 = 4 "
#$"∑##("(𝑞!,+!,- − 𝑞!,/,0 )& (10)
qe.meas (mg g−1), qe.cal (mg g−1) and n are the experimental, calculated equilibrium
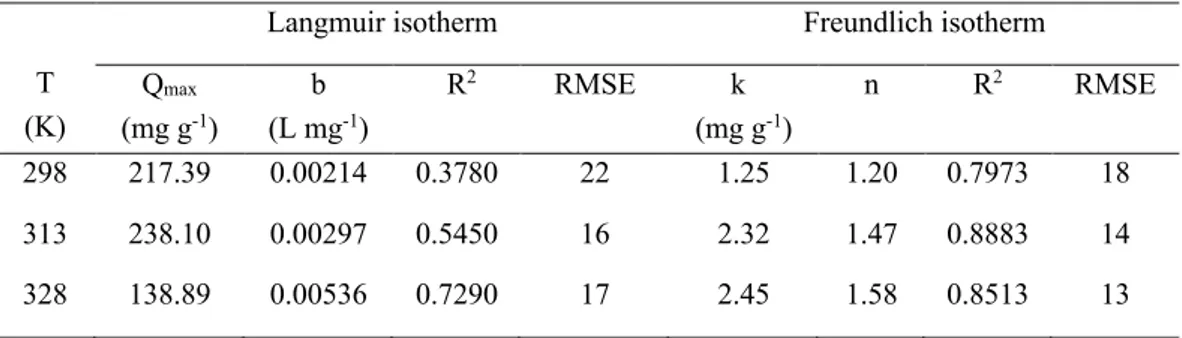
adsorption capacity and the value the number of data points respectively. According to Table 2, the Freundlich model provided a higher correlation coefficient (R2 = 0.7973, 0.8883 and 0.8513)
as compared to Langmuir isotherm model (R2 = 0.3780, 0.5450 and 0.7290). It can be seen that
the values of RMSE obtained from Freundlich were lower than those from the Langmuir. Low RMSE values indicate the accurate fitting of the model [21, 23]. According to the Freundlich isotherm, the significance of n value is as follows: adsorption process is chemical (n < 1), linear (n = 1), or physical (n > 1) [18]. From Table 2, the values of n are 1.2, 1.47 and 1.58 at 298, 313 and 328 K respectively. So, the adsorption of 4-NP on PVA/AC was a physical process due to n > 1.
Based on these results, the Freundlich isotherm model was the best-fitted adsorption isotherm model for the adsorption of 4-NP on PVA/AC. So, the adsorption of 4-NP on the PVA/AC occurred on nonhomogeneous adsorptive sites [31].
Table 2: Equilibrium parameters
T (K)
Langmuir isotherm Freundlich isotherm
Qmax (mg g-1) b (L mg-1) R2 RMSE k (mg g-1) n R2 RMSE 298 217.39 0.00214 0.3780 22 1.25 1.20 0.7973 18 313 238.10 0.00297 0.5450 16 2.32 1.47 0.8883 14 328 138.89 0.00536 0.7290 17 2.45 1.58 0.8513 13
Figure 8: Adsorption isotherm of 4-NP on PVA/AC at different temperature
Figure 9: Langmuir (A) and Freundlich isotherm (B) plots for 4-NP removal by PVA/AC
3.9. Adsorption thermodynamics
The standard Gibbs energy change (ΔGo), standard enthalpy change (ΔHo) and standard
entropy change (ΔSo) which are thermodynamic parameters. They are essential to predict the
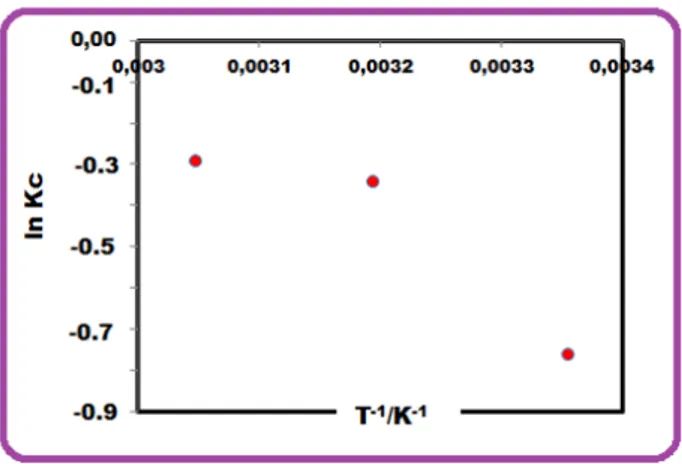
feasibility and mechanism of adsorption process. ΔGo was computed by using Eqn. (11). Kc (L/g)
was calculated by using the Langmuir constants Qmax and b (Kc =Qmaxx b) (Fig. 10) and known
as equilibrium constant [26]. R and T had been explained before.
Kc = Qmax x b (11)
The thermodynamic parameters ΔHo and ΔSo were computed by using the slope and
intercept of the plot of ln Kc versus 1/T, Fig. 10. The ΔG0 was computed as 1.91, 0.91 and 0.82
kJ mol-1 at 298, 313 and 328 K respectively. Physical adsorption was reconfirmed because of ΔGo
being >-20 kJ mol-1 regardless of the temperature, because these ΔGo values are positive.
Therefore, this reaction is nonspontaneous. As the adsorption temperatures increased, accordingly the absolute values increased, which means the process becomes more non spontaneous at higher
temperatures [32]. According to Fig. 10, ΔS0 and ΔHo were calculated as 37.32 J mol-1 K-1 and
12.88 kJ mol-1 respectively. Owing to the positive ΔHo values, adsorption process is endothermic
for 4-NP. Consequently, increasing the temperature from 298 to 328 K causes an increase in phenol adsorption on the PVA/AC. In addition, if the magnitude of ΔH if lies between 80–200 kJ/mol process of the adsorption may be considered as chemical whereas physical adsorption usually is seen in a range of 2.1–20.9 kJ/mol or ≤40 kJ/mol [33]. In this way, adsorption of 4-NP on PVA/AC composite is physical in the nature (ΔHo were calculated as 12.88 kJ mol-1). Positive
ΔSo values show the increasing randomness in phenol solution-sorbent interface during the
sorption process [27].
Figure 10: Van’t Hoff plot of ln Kc versus 1/T
4. Conclusion
In this study, PVA/AC composite was synthesized and characterized. The adsorption of 4-NP from aqueous solution was investigated by utilizing as an adsorbent PVA/AC. This adsorbent can be used for the elimination of 4-NP from aqueous solution. There were two kinds of the adsorption process parameters. They were kinetics and thermodynamic parameters and both of them were calculated. According to results, adsorption process was determined as endothermic. The adsorption isotherms were determined by Langmuir and Freundlich models. The adsorption data was better fitted by the Freundlich isotherm model due to RMSE and R2 values. Adsorption
kinetic model was determined as a pseudo-first-order kinetic model. All the results on the sorption of 4-NP on PVA/AC indicate the applicability of the adsorption study.
References
[1] Aljeboree, A., Alshirifi, A., Alkaim, A., Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon, Arabian Journal of Chemistry, 10, 3381-3393, 2017.
[2] Wang, H., Preparation and properties of porous poly (vinyl alcohol)/powdered activated carbon composite, Material Science and Advanced Technologies in Manufacturing, 852, 117-120, 2014.
[3] Jia, Z., Li, Z., Li, S., Li, Y., Zhu, R., Adsorption performance and mechanism of methylene blue on chemically activated carbon spheres derived from hydrothermally-prepared poly (vinyl alcohol) microspheres, Journal of Molecular Liquids, 220, 56–62, 2016.
[4] Kaur, T., Thirugnanam, A., Effect of Porous Activated Charcoal Reinforcement on Mechanical and In-Vitro Biological Properties of Polyvinyl Alcohol Composite Scaffolds, Journal of Materials Science & Technology, 33(7), 734–743, 2017.
[5] Kumar, A., Kumar, S., Kumar, S.,Gupta, D., Adsorption of phenol and 4-nitrophenol on granular activated carbon in basal salt medium: equilibrium and kinetics, J. Hazard. Mater., 147, 155–166, 2007.
[6] Liu, S., Wang, J., Huang, W., Tan, X., Dong, H., Goodman, B., Du, H., Lei, F., Diao, K., Adsorption of phenolic compounds from water by a novel ethylenediamine rosin-based resin: Interaction models and adsorption mechanisms, Chemosphere, 214, 821-829, 2019.
[7] Ivanov, A., Kozynchenko, O., Mikhalovska, L., Tennison, S., Jungvid, H., Gunko, V., Mikhalovsky, S., Activated carbons and carbon-containing poly(vinyl alcohol) cryogels: characterization, protein adsorption and possibility of myoglobin clearance, Phys. Chem. Chem. Phys., 14, 16267–16278, 2012.
[8] Swamy, M., Nagabhushana, B., Krishna, R., Kottam, N., Raveendra, R., Prashanth, P., Fast adsorptive removal of methylene blue dye from aqueous solution onto a wild carrot flower activated carbon: isotherms and kinetics studies, Desalination and Water Treatment, 71, 399– 405, 2017.
[9] Silva, M., Santos, M., Júnior, M., Fonseca, M., Filho, E., Natural Palygorskite as an Industrial Dye Remover in Single and Binary Systems, Materials Research,19, 1232-1240, 2016. [10] Trivedi, M., Sethi, K., Panda, P., and Jana, S., A comprehensive physicochemical, thermal, and spectroscopic characterization of zinc (II) chloride using X‑ray diffraction, particle size distribution, differential scanning calorimetry, thermogravimetric analysis/differential thermogravimetric analysis, ultraviolet‑visible, and Fourier transform‑infrared spectroscopy, International Journal of Pharmaceutical Investigation, 7, 33-40, 2017.
[11] Cuevas, L., Villacura, E., Toloza, C., Magellan Peat (Sphagnum Magallanicum) As Natural Adsorbent OfRecalcitran Synthetic Dyes, J. Soil Sc. Plant Nutr., 8,31-43, 2008.
[12] Haddad, M., Mamouni, R., Saffaj, N., Lazar, S., Removal of a cationic dye – Basic Red 12– from aqueous solution by adsorption onto animal bone meal, Journal of the Association of Arab Universities for Basic and Applied Sciences, 12, 48–54, 2012.
[13] Dhorabe, P., Lataye, D., Ingole, R., Removal of 4-nitrophenol from aqueous solution by adsorption onto activated carbon prepared from Acacia glauca sawdust, Water Science & Technology, 73, 2016, doi: 10.2166/wst.2015.575.
[14] Chartarrayawadee, W., Moulton, S., Too, C., Wallace, G., Fabrication of Graphene Electrodes by Electrophoretic Deposition and Their Synergistic Effects with PEDOT and Platinum, Chiang Mai J. Sci., 40, 750-762, 2013.
[15] El-Naggar, N., Hussein, M., El-Sawah, A., Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities, Scientific Reports, 8, 2018, doi:10.1038/s41598-018-27276-61.
[16] Paralikar, P., Biogenic Synthesis of Silver Nanoparticles Using Leaves Extract of EpiphyllumOxypetalum and its Antibacterial Activity, Austin Journal of Biotechnology & Bioengineering, 1, 2014.
[17] Bulut, Y., Karaer, H., Adsorption of Methylene Blue from Aqueous Solution by Crosslinked Chitosan/Bentonite Composite, Journal of Dispersion Science and Technology, 36,61-67, 2015.
[18] Salehi, E., Farahani, A., Macroporous chitosan/polyvinyl alcohol composite adsorbents based on activated carbon substrate, J Porous Mater., 24,1197–1207, 2017.
[19] Feyzi, F., Jalilvand, H., Dehghani, M., Adsorption of dimethyl sulfide from model fuel on raw and modified activated carbon from walnut and pistachio shell origins:kinetic and thermodynamic study, doi.org/10.1016/j.colsurfa.2020.124620.
[20] Nam, H., Nam, H., Wang, S., Preparation of activated carbon from peanut shell with
KOH activation and its application for H2S adsorption in confined space, Journal of
Environmental Chemical Engineering, 8, 103683, 2020.
[21] Fajri, L., Nawi, M., Sabar, S., Nawawi, N., Preparation of immobilized activated carbon-polyvinyl alcohol composite for the adsorptive removal of 2,4-dichlorophenoxyacetic acid, Journal of Water Process Engineering, 25, 269–277, 2018.
[22] Almeida, V., Vargas, A., Nogami, E., NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption, Chemical Engineering Journal 174, 117– 125, 2011.
[23] Tseng, T., Wu, F., Juang, R., Characteristics and applications of the Lagergren’s first-order equation for adsorption kinetics, Journal of the Taiwan Institute of Chemical Engineers 41, 661–669, 2010.
[24] Wang, L., Zhang, J., Wang, A., Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly(acrylic acid)=attapulgite composite. Desalination, 266: 33– 39, 2011.
[25] Gürses, A., Hassani, A., Kırans, M.¸ Açıslı, O., Karaca, S., Removal of methylene blue from aqueous solution using by untreated lignite as potential low-cost adsorbent: kinetic, thermodynamic and equilibrium approach, J. Water Process Eng., 2,10-21, 2014.
[26] Daga, K., Gehlot, P., Use of Polyvınylalcohol Coated Carbon Black for Removal of Lınear AlkylbenzeneSulphonate (Las) From Wastewater, Nature Environment and Pollution Technology © Technoscience Publications, 6,725-730, 2007.
[27] Karaer, H., Kaya, I., Synthesis, characterization of magnetic chitosan/active charcoal composite and using at the adsorption of methylene blue and reactive blue4, Microporous and Mesoporous Materials, 232,26-38, 2016.
[28] Iqbal, M., Ashiq, M., Adsorption of dyes from aqueous solutions on activated charcoa, Journal of Hazardous Materials B, 139,57–66, 2007.
[31] Kyzas, G., Deliyanni, E., Lazaridis, N., Magnetic modification of microporous carbon for dye adsorption, Journal of Colloid and Interface Science, 430,166–173, 2014.
[32] Kim, Y., Kim, J., Isotherm, kinetic and thermodynamic studies on the adsorption of paclitaxel onto Sylopute, J. Chem. Thermodynamics, 130,104–113, 2019.
[33] Mahida, V., Patel, M., Removal of some most hazardous cationic dyes using novel poly(NIPAAm/AA/N-allylisatin) nanohydrogel, Arabian J. Chem., 9, 430-442, 2016.