Forest Pathology. 2019;49:e12499. wileyonlinelibrary.com/journal/efp
|
1 of 12 https://doi.org/10.1111/efp.12499© 2019 Blackwell Verlag GmbH
1 | INTRODUCTION
Wood‐decay fungi are among the most important biotic factors in damage of timber causing on both wood quality decline and eco‐ nomic losses (Sivrikaya & Can, 2014). In addition, lignicolous fungi decrease the functional life of wood if sufficient measures are not taken to mitigate the threat. Accordingly, to replace the lost wood material, more trees must be cut from the forests, adversely af‐ fecting the important forestry goal of sustainability (Komut, 2011). For this reason, the control of wood‐decay fungi is important for the sustainability of forests and the prevention of economic losses (Bozkurt, Göker, & Erdin, 1993; Glaeser & Lindner, 2011). Effectively
combating these destructive fungi requires an understanding of the species involved and their damage patterns.
Wood‐decay fungi (typically macrofungi) belong to the divisions Basidiomycota or Ascomycota. Under suitable climatic conditions, various fungi can colonize wood. Wood that is stored over a period of time in outdoor weather conditions and in contact with soil is es‐ pecially vulnerable to decay (Pavlidis, Ilieva, Bencheva, & Stancheva, 2005).
Logs cut from forests are stored in log depots until the time of sale. Confiscated stocks of wood are also held in the depots for long periods of time. The prolonged storage of confiscated wood stocks in particular leads to the development and spread of decay fungi. Thus, log depots Received: 26 December 2017
|
Revised: 9 January 2019|
Accepted: 9 January 2019DOI: 10.1111/efp.12499 O R I G I N A L A R T I C L E
Identification of wood‐decay fungi and assessment of damage
in log depots of Western Black Sea Region (Turkey)
Mesut Yalçın
1| Hasan Hüseyin Doğan
2| Çağlar Akçay
11Department of Forest Products Engineering, Faculty of Forestry, Düzce University, Düzce, Turkey
2Department of Biology, Faculty of Science, Selçuk University, Konya, Turkey Correspondence
Hasan Hüseyin Doğan, Department of Biology, Faculty of Science, Selçuk University, Konya, Turkey.
Email: hhuseyindogan@yahoo.com Funding information
the Directorate of Scientific Research Projects of Düzce University, Grant/Award Number: BAP 2015.02.03.389
Editor: S. Woodward
Abstract
The aim of this study was to determine and quantify the wood‐decay fungi found on logs of forest tree species (beech, oak, hornbeam, Scots pine and fir) stored in log depots located in six different provinces in the Western Black Sea Region of Turkey. Additionally, it was aimed to determine the natural durability of some important wood species against the most commonly detected wood‐decay fungi. Eighteen fam‐ ilies, 31 genera and 45 species belonging to the division Basidiomycota were de‐ tected; Antrodia crassa was identified for the first time in Turkey. The abundance of Panus neostrigosus, Polyporus meridionalis, Trametes hirsuta, T. versicolor and Stereum hirsutum increased significantly with the holding time of the logs (r = 0.99, 0.87, 0.53, 0.57 and 0.78, respectively, p < 0.05). The majority of the fungal species were de‐ tected on logs stored in depots for 4–6 years (66%). The percentage of fungal species found on the logs with a holding time of three years or less was 29%, whereas the percentage for those detected on logs stored for seven or more years was 31%. Among the wood species, the greatest number of fungal species (29) and highest amount of fungi (2,539) occurred on beech wood. Natural durability tests showed that T. versicolor caused the greatest loss of wood mass, with an average of 23%. Field studies and natural durability tests performed in the laboratory showed that beech wood lost the most mass among the timber species studied.
K E Y W O R D S
are major habitats of wood‐decay fungal species, and healthy wood stocks freshly cut from the forest can be contaminated by the fungi in the depots (Kantay & Köse, 2009). The present study was carried out specifically on logs and confiscated stocks that were held in log depots.
A number of studies have investigated wood‐decay fungi in Turkey (Abatay, 1988; Doğan, Karadelev, & Işıloğlu, 2011; Selik, 1973; Sümer, 1982). Studies on wood‐decay fungi have also been conducted in the Western Black Sea Region. However, these studies have pri‐ marily examined fungal damage to trees planted in forests. Afyon, Konuk, Yağız, and Helfer (2005) found 80 wood‐decay fungi species in 48 forest areas in the Western Black Sea Region. Sümer (1982) car‐ ried out work in Bolu, one of the provinces included in the present study. However, to date, no study has been conducted to investigate the wood‐decay fungi present in log depots in Turkey. Even though decay is a major problem in many log depots in Turkey, there is a lack of knowledge about wood‐decay fungi and the extent of damage caused. This study was the first work conducted on the wood‐decay fungi on logs held in a variety of log depots. The Western Black Sea Region was selected for the work due to its high decay index (climate index) value resulting from the high relative humidity and tempera‐ tures present throughout the year (Gündüz, 2007).
The aim of the work the reported here was to investigate the impact of the holding time of logs in the depot on wood‐decay fungal
species number and abundance in depots in the Western Black Sea Region of Turkey. The study also examined the damage caused by widespread and concentrated fungal load on wood species with im‐ portant industrial applications in the region. Findings obtained from field studies and laboratory tests were compared to determine the natural durability of these timber species. This study also contrib‐ utes information on the mycobiota of Turkey.
2 | MATERIALS AND METHODS
2.1 | Study areas
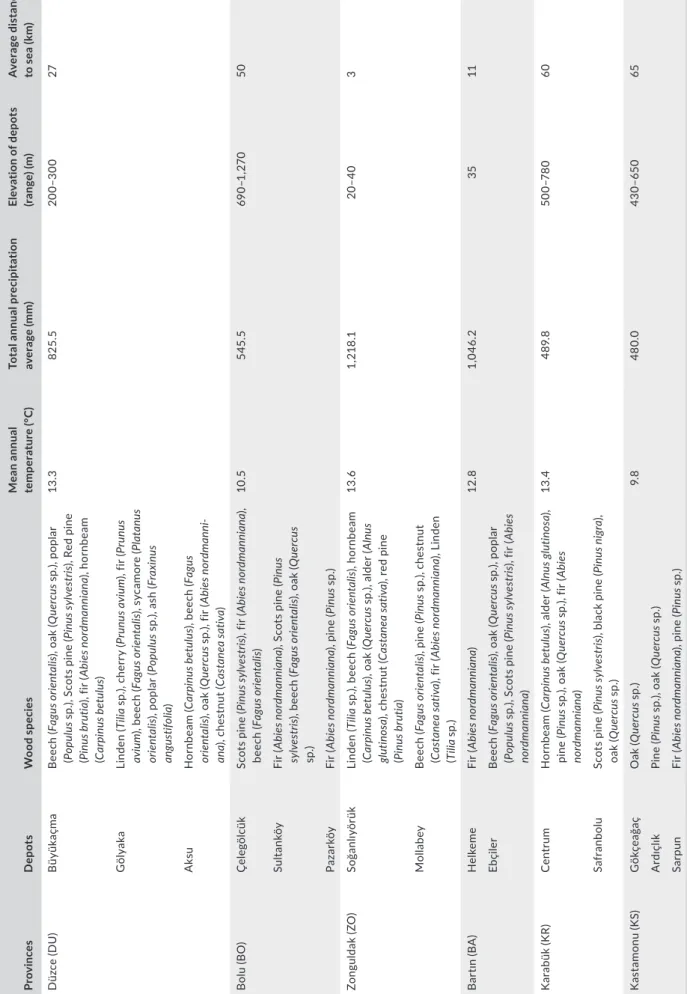
The study was carried out in log depots in the Western Black Sea Region of Turkey between February and November 2016 and in‐ cluded depots in the provinces of Bolu (BO), Düzce (DU), Zonguldak (ZO), Karabük (KR), Bartın (BR) and Kastamonu (KS). A total of 15 different log depots were investigated [Düzce (Büyükaçma, Gölyaka, Aksu), Bolu (Çelegölcük, Sultanköy, Pazarköy), Zonguldak (Ereğli Soğanlıyörük, Mollabey), Karabük (Karabük Merkez, Safranbolu), Bartın (Helkeme, Ebçiler Kadıköy) and Kastamonu (Gökçeağaç, Ardıçlık, Sarpun)] (Figure 1).
Generally, Scots pine (Pinus sylvestris L.), beech (Fagus orienta‐
lis Lipsky), oak (Quercus robur L.), fir (Abies nordmanniana (Steven)
T A B LE 1 So m e d et ai ls a bo ut w oo d s pe ci es a nd e nv iro nm en ta l f ac to rs i n s tu dy p rov in ce s a nd l og d ep ot s e xa m in ed Pr ov in ce s D ep ot s W oo d spec ies Mea n ann ual tem per at ur e (° C ) To ta l a nn ua l p re ci pi ta tio n av er ag e ( m m ) El ev at io n o f d ep ot s (ra ng e) (m) A ve ra ge d is ta nc e to s ea ( km ) D üz ce ( D U ) B üy ük aç ma Bee ch (F ag us o rie nt al is) , o ak ( Q uer cu s s p. ), p op la r (P op ulu s s p. ), S co ts p in e ( Pi nus sy lv es tri s) , R ed p in e (P inu s b ru tia ), f ir ( Ab ie s no rd m an ni ana ), h or nb ea m (C ar pi nus b et ul us ) 13 .3 825 .5 200 –3 00 27 G öl yak a Li nden (T ili a sp .), c he rr y ( Pr un us aviu m ), f ir ( Pr un us aviu m ), b ee ch ( Fag us o rie nt al is) , s yc am or e ( Plat an us or ien ta lis ), p op la r ( Po pu lu s s p. ), a sh ( Fra xi nu s an gus tif ol ia ) A ksu H or nb ea m (C ar pi nus b et ul us ), b ee ch ( Fa gu s or ien ta lis ), o ak ( Q uer cu s s p. ), f ir ( Ab ie s no rd m an ni ‐ ana ), c he st nu t ( Ca st an ea sat iv a) B ol u ( BO ) Ç ele göl cük Sc ot s p in e ( Pi nus sy lv es tri s) , f ir ( Ab ie s no rd m an ni ana ), bee ch (F ag us o rie nt al is) 10 .5 54 5. 5 69 0– 1, 27 0 50 Sult an kö y Fi r ( Ab ie s no rd m an ni ana ), S co ts p in e ( Pinu s sy lv es tri s) , b ee ch ( Fag us o rie nt al is) , o ak ( Q uer cu s sp .) Pa za rkö y Fi r ( Ab ie s no rd m an ni ana ), p in e ( Pinu s s p.) Zo ngu ld ak (Z O ) Soğ anl ıy ör ük Li nden (T ili a sp .), b ee ch ( Fag us o rie nt al is) , h or nb ea m (C ar pi nus b et ul us ), o ak ( Q uer cu s s p. ), a lder (A ln us gl ut in osa ), c he st nu t ( Ca st an ea sat iv a) , r ed p in e (P inu s b ru tia ) 13 .6 1, 21 8.1 20 –4 0 3 M ol la be y Bee ch (F ag us o rie nt al is) , p in e ( Pinu s s p. ), c he st nu t (C as ta ne a s at iv a) , f ir ( Ab ie s no rd m an ni ana ), L in de n (T ili a sp .) B ar tın (B A ) H el keme Fi r ( Ab ie s no rd m an ni ana ) 12 .8 1, 04 6. 2 35 11 Eb çi le r Bee ch (F ag us o rie nt al is) , o ak ( Q uer cu s s p. ), p op la r (P op ulu s s p. ), S co ts p in e ( Pi nus sy lv es tri s) , f ir ( Ab ie s no rd m an ni ana ) K ar ab ük (K R) Cen tr um H or nb ea m (C ar pi nus b et ul us ), a lder (A lnu s g lu tin os a) , pin e ( Pinu s s p. ), o ak ( Q uer cu s s p. ), f ir ( Ab ie s no rd m an ni ana ) 13 .4 48 9. 8 50 0–7 80 60 Sa fr an bo lu Sc ot s p in e ( Pi nus sy lv es tri s) , b la ck pin e ( Pi nu s n ig ra ), oa k ( Q uer cu s s p.) K as ta m on u (K S) G ök çe ağ aç O ak ( Q uer cu s s p.) 9. 8 48 0.0 43 0– 65 0 65 A rdı çl ık Pi ne ( Pinu s s p. ), o ak ( Q uer cu s s p.) Sa rp un Fi r ( Ab ie s no rd m an ni ana ), p in e ( Pinu s s p.)
Spach) and hornbeam (Carpinus betulus L.) wood were stocked in log depots in the study areas. Linden wood (Tilia sp.), cherry (Prunus
avium L.) and black pine (P. nigra J.F.Arnold) were rarely stocked in
the depots. There was no standard log size or method of stacking the logs held in any of the depots. For example, logs of different sizes and different species were stored together. Almost all wood species were present in Büyükaçma (DU), Ereğli Soğanlıyörük (ZO), Epciler Kadköyü (BR), Sultanköy (BO) and Karabük Merkez (KR) log depots. When the log depots were examined, wood species which had been held for two or more years were found in large numbers in the Büyükaçma, Epciler Kadıköyü and Sarpun (KS), Gölyaka (DU) and Karabük Merkez (KR) log depots. Compared with other depots, Büyükaçma, Gölyaka and Epciler Kadıköyü had damp earth floors.
In Table 1, some variations among the provinces in terms of cli‐ mate and location can be seen. When compared with the other prov‐ inces, Düzce has a high mean annual temperature and total annual precipitation and, along with Bartın and Zonguldak, have both a low elevation and proximity to the sea (TSMS, 2018). The depots were located at elevations of approximately 200‒300 m in DU province, 690‒1,270 m in BO, 20‒40 m in ZO, 500‒780 m in KR, 35 m in BR and 430‒650 m in KS.
One month prior to collecting the fungal species, data loggers were placed in each depot in each province. When fungal samples were collected, temperature and humidity values were extracted and the monthly average calculated. The study sites were visited eight different times during the study period. The average tempera‐ ture and relative humidity data for these dates as measured and re‐ corded in the data loggers are given in Table 2.
2.2 | Collection of fungal samples
Before samples of the macrofungi species were collected from the substrates, digital colour photographs were taken of different posi‐ tions. The fruiting bodies along with a piece of the substrate were then carefully cut from the dead wood using a knife or hand saw. Each sample was coded and given a collection number. In addition, the location where the sample was collected, the wood species, the holding time in the depot, the type of decay, the detection date
and the amount of fungal fructification per 1 m3 were recorded for
each study area. A certain area was needed to determine the fungi abundance on the log stacks. Fungal abundance was determined by
selecting an area of 1 m3 in each depot. Fungi samples were placed
in aluminium foil, stored in containers to preserve physical integrity, and transported to the laboratory.
Fungal samples brought to the laboratory were dried to prevent deterioration. Drying was carried out at 40–45°C for 2–3 days on av‐ erage. The dried samples were placed in zip‐lock nylon bags, labelled and stored for identification.
2.3 | Identification of fungal samples
Sections of the fungal fruiting body were taken from the hymenial parts and the basidiocarps. Under the microscope, spores, cystidia,
T A B LE 2 M on th ly av er ag e te m pe ra tu re a nd h um id ity d at a fo r t he s tu dy p rov in ce s St udy P ro vi nc es 1s t v is it 25 .01 .2 01 6 26. 02 .2 01 6 2n d vi si t 27. 02 .2 01 6 28 .0 3. 20 16 3r d v is it 29 .0 3. 20 16 15 .0 4. 20 16 4t h v is it 16 .0 4. 20 16 11 .0 5. 20 16 5t h v is it 12 .0 5. 20 16 13 .0 6. 20 16 6t h v is it 14. 06 .2 01 6 11 .0 7. 20 16 7t h v is it 12 .0 7. 20 16 10. 08 .2 01 6 8t h v is it 11 .0 8. 20 16 08 .0 9. 20 16 T H T H T H T H T H T H T H T H D üz ce 10 .9 78 .7 12 .8 83 .4 15 .4 86 .3 18 .9 86 .1 24 .8 79 .7 25 .2 78 .3 23 .1 82 .7 17. 4 78 .1 B ar tın 16 54 15 .1 79 .5 18 85 23 .4 80. 2 24 74 .4 21 .2 81 .5 15 .3 80 .9 15 .5 84. 6 B olu 7. 3 70 .2 11 .7 70 .8 13 .5 82 .5 16 .9 76 .9 20 .8 66 .9 22 .5 64 .4 19 .1 66 .6 13 .2 73 K as ta m on u 9. 4 66 .2 14 .3 71 19 81 .2 22 .6 70 .3 24 .4 55 .4 22 .3 60 .8 19 .9 68 .1 13 .7 74 .4 K ar ab ük 9. 8 65 .6 13 .7 70 .6 17. 5 70 .2 21 .6 64 .7 22 62 .7 26 .5 58 22 .5 58 .6 17. 4 65 .3 Zo ngu ld ak 12 82 14 80. 5 18 84 20 .4 85 .4 23 .9 83 22 .0 81 18.8 87. 4 17. 9 84 .9 N ote . T he s pe ci fie d te m pe ra tu re a nd h um id ity v al ue s re pr es en t m on th ly a ve ra ge s pr io r t o th e vi si t d at e. H : M on th ly a ve ra ge re la tiv e hu m id ity (% ); T: M on th ly a ve ra ge te m pe ra tu re .
hyphae and mycelia forms were determined, and relevant meas‐ urements were taken. In addition to microscopic measurements, reactions with chemical reagents were noted. Chemical reagents (Melzer's reagent, KOH in 10%, 5%, 3%, and 2% solutions, cotton blue; Congo red) were used for the macroscopic and microscopic studies. The hymenium, pileus, or body sections were prepared and measured under a light microscope (Leica DM 3000, Leica Microsystems, Wetzlar‐Germany). Specimens were identified fol‐ lowing published descriptions (Bernicchia, 2005; Breitenbach & Kränzlin, 2000; Horak, 2005) and checked according to recent list‐ ings (Doğan, Aktaş, Öztürk, & Kaşık, 2012; Doğan et al., 2011; Sesli & Denchev, 2008; Sesli & Helfer, 2013; Solak, Işıloğlu, Kalmış, & Allı, 2015). Taxa, family, and species authorities were recorded according to previous works (Index Fungorum, 2016; Kirk, Cannon, Minter, & Stalpers, 2008; MycoBank, 2018). A description of the fungal spe‐ cies reported for the first time in Turkey was presented.
After the fungal species were identified, the number of fungal species and fungal abundance percentages were calculated using the formulae in Equations (1) and (2), respectively.
2.4 | Natural durability tests of wood species
Natural durability tests were carried out to determine how wood species were affected by the fungi detected in greatest abundance in the region, which included T. hirsuta, T. versicolor, S. hirsutum,
Fomitopsis pinicola, Neolentinus lepideus and P. neostrigosus. In the
natural durability tests, native wood species commonly utilized in the forest industry were selected, including beech (F. orientalis), oak (Quercus spp.), Scots pine (P. sylvestris) and aspen (Populus tremula L.).
First, small pieces cut from the fresh fruiting bodies collected from the study areas were cultured on potato dextrose agar (PDA). Cultures were incubated at 25–28°C and 75%–85% relative humid‐ ity. Wood samples were cut into pieces of 0.5 × 1.5 × 3 cm with a minor modification of the EN 2006 standard and dried at 103°C for
24 hr to determine the full dry weights (W0). All samples were steril‐
ized by autoclaving at 121°C for 20 min before the decay tests. Two wood samples were placed in each fungal culture in Petri dishes after incubation period at 26 ± 2°C and 70 ± 2% relative humidity until the mycelium covered the whole of the agar surface.
Two different experimental periods were applied to determine the differences in fungal damage dependent on holding time. Five replicate Petri dishes were used for each fungus–wood combi‐ nation and for each incubation time. The 1st period was set as 12 weeks as indicated in the EN 113 standard and the 2nd period
as 16 weeks. To minimize the effects of climatic conditions and contamination on the results, one of the two wood samples placed in each Petri dish was taken at the end of the 1st period and the other was taken at the end of the 2nd period. At the end of each test period, the wood samples were cleaned, dried (103°C) and
weighed (W1) and weight losses calculated according to Equation
(3).
The weight loss (%) after 12 weeks and after 16 weeks was cal‐ culated to determine the differences in the average weight losses for the two incubation periods.
2.5 | Statistical analysis
Statistical analyses were conducted using SPSS 19 software. The Pearson correlation test was used to determine the inter‐relation‐ ships between the number of fungal species and their abundance, with the holding times of the wood samples. Mean weight losses for tree and fungal species and their interaction were evaluated using to one‐way analysis of variance (ANOVA). In addition, the Duncan test was used to compare the mean weight loss values between fungi and wood species.
3 | RESULTS
3.1 | Variety and abundance of fungal species
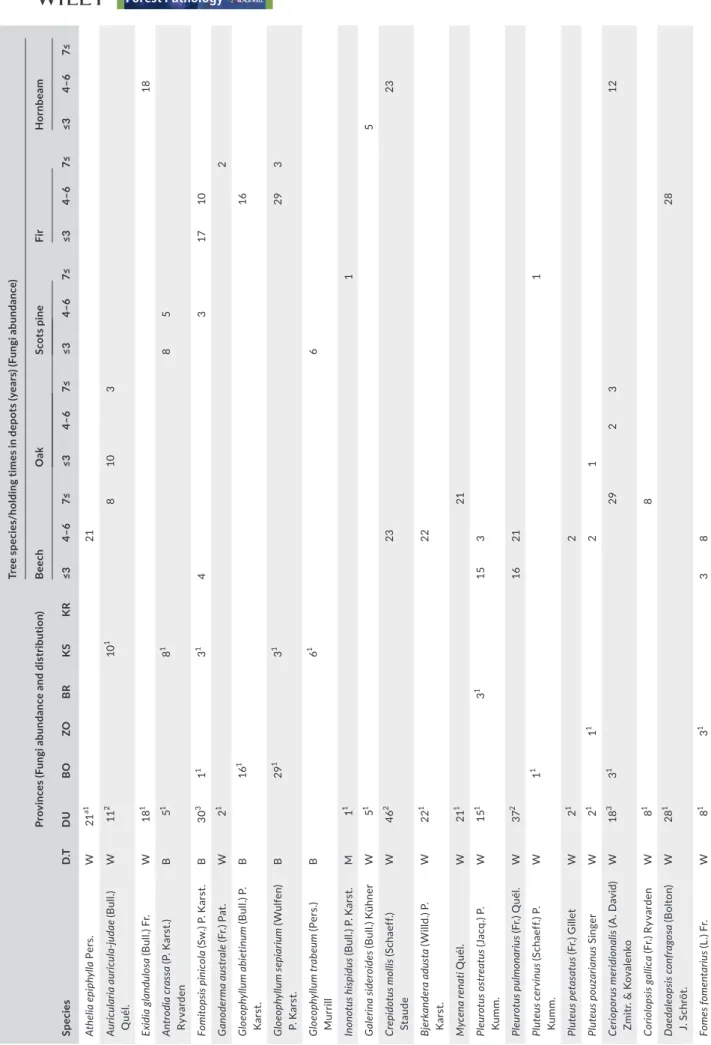
Among the 15 log depots studied, 34 fungal species were identified in DU, 13 in BO, five in ZO, 13 in BR, two in KR and 12 in KS.
Findings related to the number of species and abundance of wood‐decay fungi ranked by province are shown in Table 3. The maximum number of different fungal species and their abundance were detected in the depots in DU Province. Although the lowest distributions were seen in the depots within KR Province, the lowest fungal abundance was found in the depots in ZO Province.
Schizophyllum commune was present in all the studied depots
(Table 3). A high abundance of four species—T. hirsuta, S. hirsutum,
Trichaptum fuscoviolaceum and T. versicolor—was recorded in all
provinces.
Fungal abundance by province and wood species is shown in Table 4. The highest fungal abundance was found in wood in the DU log depots. Similar fungal abundance was found in BR, BO and KS, while the lowest fungal abundance was detected in KR Province and ZO Province.
The greatest number of different fungal species was recorded in DU and the least variety in KR (Figure 2).
The distribution of fungi species according to wood species is shown in Figure 3. A total of 29 fungal species were detected on beech, 10 species on oak, 11 species on fir, 10 species on pine and eight species on hornbeam wood. According to Table 3, 84% (39 spe‐ cies) of all fungal species identified were white rot fungi.
(1) Number of Fungal Species (%) =
Number of different species for each wood or province Total number of species for all woods and study areas ×100
(2)
Fungal Abundance (%) =
Total fungal fructification number for each wood or province Total fungal fructification number for all woods and study areas×100
(3) Weight loss (%) = [(W0−W1)∕W0] × 100
T A B LE 3 Fu ng al a bu nd an ce a nd f un ga l d is tr ib ut io n b as ed o n p rov in ce s a nd t re e s pe ci es /h ol di ng p er io ds Sp ec ies D .T Pr ov in ce s ( Fu ng i a bu nd an ce a nd d is tr ib ut io n) Tr ee s pe ci es /h ol di ng t im es i n d ep ot s ( ye ar s) ( Fu ng i a bu nd an ce ) Beec h Oa k Sc ot s p in e Fir H or nb ea m DU B O ZO B R K S KR ≤3 4– 6 7≤ ≤3 4– 6 7≤ ≤3 4– 6 7≤ ≤3 4– 6 7≤ ≤3 4– 6 7≤ At hel ia e pi ph yl la Pe rs . W 21 a1 21 Au ric ula ria a uri cu la ‐ju da e (B ul l.) Q uél. W 11 2 10 1 8 10 3 Exi di a g la ndu lo sa (B ul l.) F r. W 18 1 18 An tr od ia c ra ss a (P . Ka rst .) Ry va rden B 5 1 8 1 8 5 Fo m ito ps is pi ni cola (S w .) P . Ka rst . B 30 3 1 1 3 1 4 3 17 10 G ano der m a a us tr ale (F r.) P at . W 2 1 2 G lo eo ph yllu m a bi et inu m (B ul l.) P . Ka rst . B 16 1 16 G loe op hy llum se pi ar ium (Wu lfe n) P. Ka rst . B 29 1 3 1 29 3 G loe op hy llum tr ab eum (P er s. ) M ur rill B 6 1 6 In on ot us h ispid us (B ul l.) P . Ka rst . M 1 1 1 G aler ina si der oi de s ( B ul l.) K ühne r W 5 1 5 Cr ep id ot us m ol lis (S ch ae ff. ) St au de W 46 2 23 23 Bj er ka nd er a a du st a (W ill d. ) P . Ka rst . W 22 1 22 M yc ena rena ti Q uél. W 21 1 21 Pl eu rotu s o st re atu s ( Ja cq .) P. Ku m m . W 15 1 3 1 15 3 Pl eu ro tus p ul m on ari us (F r.) Q uél. W 37 2 16 21 Plu te us c er vinu s ( Sc ha ef f.) P . Ku m m . W 1 1 1 Pl ut eus p et as at us (F r.) G ill et W 2 1 2 Pl ut eus p ou za ria nus S in ge r W 2 1 1 1 2 1 Cer iop or us m er id io na lis (A . D av id ) Zm itr . & K ov al en ko W 18 3 3 1 29 2 3 12 Co rio lo ps is g al lic a (F r.) R yv ar den W 8 1 8 D ae da leo ps is c on fr ag os a (B ol to n) J. S ch rö t. W 28 1 28 Fo m es f om en ta riu s ( L. ) F r. W 8 1 3 1 3 8 (C on tinue s)
Sp ec ies D .T Pr ov in ce s ( Fu ng i a bu nd an ce a nd d is tr ib ut io n) Tr ee s pe ci es /h ol di ng t im es i n d ep ot s ( ye ar s) ( Fu ng i a bu nd an ce ) Beec h Oa k Sc ot s p in e Fir H or nb ea m DU B O ZO B R K S KR ≤3 4– 6 7≤ ≤3 4– 6 7≤ ≤3 4– 6 7≤ ≤3 4– 6 7≤ ≤3 4– 6 7≤ Le nt in us a rc ula rius (B at sch ) Z mi tr . W 4 1 4 Len zi te s b et uli na (L .) F r. W 39 1 41 2 17 53 Ne ol en tin us le pid eus (F r.) Red he ad & G in ns B 30 2 1 29 Pa nu s n eo st rig os us (D re ch sl er ) W 28 0 2 10 4 2 4 19 8 18 2 Poly po rus a rc ula rius (B at sc h) Zm itr. W 6 1 6 Poly po rus m erid io na lis (A .D ) Zm itr .& K ov al en ko W 44 4 12 1 29 1 14 12 Tr am et es g ib bo sa (P er s. ) F r. W 11 1 11 Tr am et es h irs ut a (Wu lfe n) L lo yd W 317 4 32 3 13 2 72 3 17 9 3 1 28 0 53 15 17 0 72 2 13 4 Tr am et es oc hr ac ea (P er s. ) G ilb . & Ry va rden W 13 1 13 Tr am et es p ub es ce ns (S ch um ach .) Pil át W 17 9 2 8 17 1 Tr am et es suav eo len s ( L. ) F r. W 1 1 14 1 15 Tr am et es t ro gi i B er k. W 33 3 16 2 14 21 6 8 Tr am et es v er rs ic ol or (L .) L lo yd W 70 6 5 71 3 56 2 14 9 3 25 29 0 129 18 34 15 6 12 36 52 23 0 Tr ic hap tum ab ie tin um (D ick s. ) Ry va rden W 46 1 46 Tr ic ha pt um b ifo rm e (F r.) R yv ar den W 42 1 11 1 53 Tri ch ap tu m fus co vi ola ceu m (E hr enb .) R yv ar de n W 26 1 28 9 1 15 1 26 3 2 41 1 26 0 18 41 26 28 9 Co pr in ellu s m icac eu s ( B ul l.) V ilg al ys W 16 1 16 Ps at hy rel la c an dol lea na (F r.) M aire W 13 1 13 Sc hi zo ph yllu m c om mu ne F r. W 161 3 214 3 14 1 28 1 18 6 1 66 1 66 176 48 37 7 H yp ho don tia ra du la (P er s. ) L an ge r & A nd st erh. W 5 1 5 St er eu m h irs utu m (W ill d. ) P er s. W 382 4 16 6 2 303 1 129 2 42 0 181 32 19 1 15 6 Tr em el la m es en te ric a Re tz . W 2 1 2 N ote . B : B ro w n ro t, D .T : D ec ay ty pe , M : M ou ld ; W : W hi te ro t. aN um be r o f o cc ur re nc es i n t he r eg io n a t d iff er en ce t im e ( 1– 5 t im es ). T he n um be r o f a pp ea ra nc es o f f un ga l s pe ci es i n e ac h p ro vi nc e i s s ho w ; e .g ., Sc hi zo ph yllu m c om mu ne a bu nd an cy w as f ou nd a s a t ot al o f 16 1 o ve r t hr ee t im e p er io ds , a s 2 14 o ve r t w o t im e p er io ds a nd a s 1 4 o ve r o ne t im e p er io d i n D U , B O a nd Z O p ro vi nc es , r es pe ct iv el y. T A B LE 3 (Co nti nue d)
Fungal abundance on the main wood species is shown in Table 4. Abundance on beech wood (55.1%) was considerably higher than on the other wood species.
When the most abundant fungal species found on each wood species were compared, S. hirsutum was the most abun‐ dant fungus on beech (601) and oak (379), S. commune (377) on Scots pine, T. fuscoviolaceum (315) on fir and T. versicolor (230) on hornbeam.
3.2 | Description of first‐time recorded species
A. crassa was recorded for the first time in Turkey (Figure 4). Theperennial resupinate basidiocarp is soft when fresh and brit‐ tle when dry, and has cream‐coloured, roundish surface pores (3–6 per mm) and a yellowish tube layer embedded in a resin‐ ous matrix. The hyphal system was dimitic. Generative hyphae were hyaline, with 2‐ to 4‐µm‐wide, thin‐walled clamp connec‐ tions, while the skeletal hyphae were thick‐walled. Cystidia were absent, the basidia clavate (10–14 × 5–6 µm) with a basal clamp, and the basidiospores thin‐walled, hyaline, smooth and ellipsoi‐ dal to sub‐cylindrical in shape (6.2–7 × 3.2–3.9 μm). In a previous study, the size of A. crassa basidiospores was reported to be 4.9– 8.2 × 3.0–3.8 μm (Spirin, Runnel, Vlasak, Miettinen, & Poldmaa, 2015). Examination of the literature confirmed our microscopic measurements for A. crassa.
3.3 | Effect of wood retention time
The maximum number of fungal species (34) was found on wood stocks held for four to six years, followed by 20 on stocks held for seven years or more. Eighteen fungi species occurred on wood held for three years or less.
There was a weak but significant positive relationship between the wood stock holding time and fungal abundance, irrespective of wood type or province (r = 0.197, p = 0.27, N = 126).
Correlation analysis of the relationship between the wood stock holding time for each wood species and fungal abundance re‐ vealed strong positive correlations for oak (r = 0.487) and hornbeam (r = 0.666, p < 0.05), but no significant correlation for beech, fir or pine wood stocks (p > 0.05).
A positive correlation was found between wood holding time and abundance of P. neostrigosus, P. meridionalis, T. hirsuta, T. versi‐
color and S. hirsutum (r = 0.99, 0.87, 0.53, 0.57 and 0.78, respectively; TA B L E 4 Fungal abundance according to provinces and tree
species Provinces Fungi abundance (%) Tree species Fungi abun‐ dance (%) Düzce 48.1 Beech 55.1 Bartın 16.5 Oak 18.7 Bolu 17.4 Fir 10.2
Kastamonu 11.9 Scots pine 9.8
Karabük 4.7 Hornbeam 6.8
Zonguldak 1.4
F I G U R E 2 Percentage of fungal species variety according to
provinces 0 10 20 30 40 50 60 70 80
Düzce Bolu Zonguldak Bart n Karabük Kastamonu
Percentage
(%
)
Study regions
F I G U R E 3 Percentage of fungal species variety according to
tree species 0 10 20 30 40 50 60 70
Beech Oak Fir Scots pine Hornbeam
Percentage (%)
Wood species
F I G U R E 4 A new record for Turkey— Antrodia crassa: (a) basidiocarp; (b)
basidiospores
p < 0.05). T. hirsuta and T. versicolor in particular were detected in al‐
most all wood species.
3.4 | Natural durability test results
The effects of wood species, fungi and their interaction on wood weight loss were significant (p < 0.005).
Comparisons of the weight loss of the wood species due to vari‐ ous fungal species are given in Table 5. The highest mean weight loss was in beech wood (30.4%), which was significantly higher than that of the other wood species (p < 0.005). A mean weight loss of 15% was found in the oak, Scots pine and fir species, respectively, with no significant differences among these species.
When all wood types were considered, although the highest mean weight loss according to fungal species was detected for
T. versicolor (23.4%), no significant difference was found between P. neostrigosus and N. lepideus. The lowest weight loss was found for F. pinicola (12.7%), with no significant difference between T. hirsuta
and S. hirsutum.
The lowest weight loss occurred in fir tree wood colonized by
S. hirsutum and F. pinicola (Table 5). The highest mean weight loss
was for beech wood (42.1%) damaged by T. versicolor. Mean weight loss differed significantly among fungal species in all variations. Beech wood generally exhibited high weight losses after decay by five of the fungi, but low weight loss after incubation with F. pinicola.
4 | DISCUSSION
The number of fungal species varied between the provinces; how‐ ever, the number of fungal species found on each wood species may not have been representative and may have been influenced by the fact that not all wood species were present in all the depots examined (Table 1). It is thought that the variation in the number of
species among the provinces was related to the humidity and tem‐ perature differences among the study localities. For example, the relative humidity values of DU Province varied during the year, rang‐ ing from 78% to 86%, while in KR Province the range was between 58% and 70% (Table 2).
Most species identified in the study have already been identi‐ fied in many studies conducted in Turkey. However, A. crassa was recorded for the first time in Turkey. This species was recorded on Scots pine wood. It was first detected on 01.06.2016 in the Hanönü district of KS Province. At the time, the average weekly tempera‐ ture and humidity were 18°C and 84%, respectively. The second recording was made on 21.06.2016 in the Sultanköy district of BO Province. At that time, the average weekly temperature and humid‐ ity were 20.1°C and 67%, respectively (Table 2).
In addition to the effect of wood species × fungal species inter‐ action, the wood stock holding period in the log depots proved to be an important criterion the number of decay fungi present, and the abundance of various species. Additional factors such as the number of species in the different storage areas, wood contact with soil, level of humidity on the depot floor, temperature of the area and the wood might also have had an effect on fungal abundance and species number. It has been reported previously that storage conditions, humidity, temperature and wood contact with soil were important for the development of macrofungi species (Bozkurt et al., 1993). Talley, Colley, and Kursar (2002) also noted that increas‐ ing relative humidity had a positive effect on fungal abundance and number of species. The high variety and abundance of fungal species in DU province were due to the damp depot floors and high relative humidity throughout the year compared with other depots. High relative humidity and moisture content provide a favourable habitat for fungal growth (Bills & Foster, 2004). Temperature and humidity varied among the study provinces in the present work (Table 2).
When the most abundant fungi on each wood species were com‐ pared, S. hirsutum was the most abundant species on beech and oak
TA B L E 5 Mean weight losses (%) for tree and fungal species and their interaction Fungi species Tree species Mean WL of fungi species
Oak Beech Scots pine Fir
Mean HG Mean HG Mean H.G Mean HG Mean HG
Tv 19.2 (4.1) fg* 42.1(2.3) ı* 15.2 (3.0) bcde* 16.4 (3.2) cde* 23.43 (11.4) C†
Th 13.8 (1.1) Abcd* 27.0 (2.2) g* 10.4 (0.2) ab* 16.6 (1.2) cde* 16.99 (6.6) ab†
Fp 12.6 (0.8) Abc* 12.3 (3.0) abc* 15.3 (5.9) bcde* 10.2 (3.5) a* 12.65 (3.6) a†
Pn 14.3 (0.9) abcd* 33.0 (7.9) h* 18.3 (4.5) def* 13.4 (1.7) abc* 19.79 (9.1) bc†
Sh 13.9 (0.7) abcd* 33.9 (6.5) h* 12.7 (2.4) abc* 9.9 (1.0) a* 17.64 (10.3) ab†
Nl 22.3 (4.5) f* 33.8 (5.3) h* 15.3 (2.4) bcde* 16.4 (3.4) cde* 21.99 (8.4) bc†
Mean WL of tree species
16.16 (4.3) a‡ 30.4 (10.2) b‡ 14.58 (4.2) a‡ 13.85 (3.6) A‡
Note. HG: Homogeneity group; Tv: Trametes versicolor, Th: Trametes hirsuta, Fp: Fomitopsis pinicola, Pn: Panus neostrigosus, Sh: Stereum hirsutum, Nl: Neolentinus lepideus. Data in parentheses represent standard error.
*Means within each column and line followed by the same letter are not significantly different (p < 0.05)
†Means within each column followed by the same letter are not significantly different (p < 0.05)
wood. S. commune, T. fuscoviolaceum and T. versicolor were the most abundant fungi on Scots pine, fir and hornbeam, respectively. It is possible that the number of fungal species differed according to the wood because of the chemical and physical properties particu‐ lar to each tree species. Some species of decay fungi show prefer‐ ence for certain wood components and, thus, grow only on certain tree species. For this reason, various fungi may prefer coniferous or, broad‐leaved or both coniferous and broad‐leaved trees (Bozkurt et al., 1993; Messner et al., 2003). In addition, there are cases in which wood‐decay fungi prefer different parts of the wood.
Many lignicolous fungi grow on sapwood, while other species tend to develop on the heartwood. The tendency to grow on sap‐ wood is attributed to the higher moisture content in the sapwood than the heartwood (Kazemi, Dickinson, & Murphy, 2001). In par‐ ticular, extractives which display antifungal activity against wood‐ decay fungi are more abundant in heartwood than in sapwood. Therefore, several fungi do not favour heartwood (Taylor, Gartner, & Morrell, 2002). In this case, the ratio of sapwood to heartwood in a tree species can play an important role in the wood preference of fungi (Goodell, Qian, & Jellison, 2008).
In this work, several white rot fungi were identified on the wood from deciduous species (beech, oak, hornbeam), while brown rot fungi were found on the coniferous wood species (Scots pine, fir). Important nutrients for the development of wood‐decay fungi, such as sugar, starch, protein and oils, are present in wood in ad‐ dition to the cellulose, hemicellulose and lignin contained in the main walls of the wood (Bozkurt et al., 1993; Marzuki, Rossiana, & Normanita., 2017). Since the proportion of these substances var‐ ies between tree species, the development of fungi on the wood also varies (Patachia & Croitoru, 2016). It is believed that the num‐ ber of fungal species and their abundance were highest on beech wood in the log depots of the region due to the large proportion of sapwood present in the species. In addition, the amount of al‐ cohol‐soluble extractive substance in beech is low (about 1.9% by weight) (Košikova, Slavikova, & Kacik, 2008; Kurtoğlu, 1984; Sixta, Promberger, Koch, Gradinger, & Messner, 2004). It was also ex‐ pected that the number of fungi would be high on beech and oak wood because the vast majority of the fungal species identified were in the white rot group. In general, white rot fungi are more prevalent on timber of broad‐leaved tree species, while brown rot fungi are more dominant on coniferous tree species (Kirk & Farrell, 1987; Sigoillot et al., 2012). Enzyme systems of the fungi play an important role in this distinction (Tuor, Winterhalter, & Fiechter, 1995). A key–lock fit between the enzyme and the substrate is required for enzymes to degrade wood. Specific enzymes are re‐ quired for the degradation of each polysaccharide. Glucoronoxylan is the main hemicellulose component of hardwoods, whereas ga‐ lactoglucomannan is found in softwoods. Lignin composition also differs between hardwoods and softwood. Guaiacyl lignin in soft‐ wood is higher than found in hardwoods. Additionally, guaiacyl lig‐ nin in softwoods is more extensively cross‐linked than hardwood guaiacyl–syringyl lignin (Timell, 1967). In the degradation of soft‐ wood hemicellulose, fungi‐producing mannanases are favoured,
while fungi‐producing xylanases are required to degrade hardwood (Álvarez, Reyes‐Sosa, & Díez, 2016). Lignin is degraded by lignin peroxidase, manganese‐dependent peroxidase, versatile peroxi‐ dase and laccase (Janusz et al., 2017). White rot fungi degrade the lignin component of wood first, cellulose and hemicellulose in later stages of the degradation while brown rot fungi initially degrade cellulose (Pandey & Pitman, 2003).
When the five different wood species were compared propor‐ tionally for fungal abundance, both the highest abundance and the highest species variety were found on beech wood. Similar findings were reported previously. Deflorio, Johnson, Fink, and Schwarze (2008) and Deflorio, Fink, and Schwarze (2008) reported that Douglas fir and oak are more resistant than beech to decay. In contrast to Douglas fir and oak, the sapwood of beech was highly susceptible to decay. This finding was attributed to the fact that the ratio of chemical extractives in the composition of beech wood is lower than in other species. These extracts include compounds that naturally protect wood from biological degradation (Toshiaki, 2001; Windeisen, Wegener, Lesnino, & Schumacher, 2002). There are many studies on this subject, and high of phenolic contents have been demonstrated to be effective in protection especially against brown rot fungi that destroy wood (Harju et al., 2003; Martínez‐ Inigo, Immerzeel, Gutierrez, Río, & Sierra‐Alvarez, 1999).
Although the amount of extractive content is low in hornbeam and beech (Rowe & Conner, 1979; Vek, Oven, & Poljanšek, 2016), the reason for low abundance of decay fungi on hornbeam timber was an artefact due to absence of the species in the entire several log depots examined. Beech wood, in contrast, was present in all the storage depots examined (Reinprecht, 2016).
The high weight loss in beech wood during, the laboratory tests, can be explained by the fact that white rot fungi have extra‐ cellular enzymes that decompose lignin (Lekounougou, Petrissans, Jacquot, Gelhaye, & Gerardin, 2009). In certain previous stud‐ ies, beech wood also showed very high weight loss. Malakani, Khademieslam, Hosseinihashemi, and Zeinaly (2014) observed a 36% weight loss, and Olfat, Karimi, and Parsapajouh (2007) found a weight loss of up to 42% in their studies with beech wood and
T. versicolor.
In conclusion, 45 species of Basidiomycota in 18 families and 31 genera were identified in the forest log storage depots in the Western Black Sea Region.
The longer the wood stock was held in the log depots, the greater was the abundance of the fungal species. There was a weak but significant and positive relationship between the hold‐ ing period of the wood and fungal abundance, irrespective of wood species or province (p < 0.05). The greatest variety of fungal species and the highest abundance of fungi were found when the wood was stored for 4–6 years. With P. neostrigosus, P. meridiona‐
lis, T. hirsuta, T. versicolor and S. hirsutum, there was a strong pos‐
itive correlation between the holding time of the wood and the abundance of fungi causing decay.
A large number of wood‐decay fungi were detected in the wood depots. Destructive effects on commercially important
wood species were most severe on beech wood. Moreover, of the fungal species tested, T. versicolor caused the greatest weight loss. Differences emerged among the weight losses that occurred during different damage exposure periods. These data suggest that increasing fungal abundance in older timber is a matter of time giving the opportunity for more rare decay fungi to establish in the exposed wood.
ACKNOWLEDGEMENTS
This research was supported financially by the Directorate of Scientific Research Projects of Düzce University (BAP 2015.02.03.389).
ORCID
Mesut Yalçın https://orcid.org/0000‐0002‐5181‐9484 Hasan Hüseyin Doğan https://orcid.org/0000‐0001‐8859‐0188 Çağlar Akçay https://orcid.org/0000‐0003‐1246‐3056
REFERENCES
Abatay, M. (1988). Investigations on edible fungus species developed on
wood in various ecologies, V. Turkish Phytopathology Congress,
Antalya, Turkey, 18–21 October. (pp. 35).
Afyon, A., Konuk, M., Yağız, D., & Helfer, S. (2005). A study of wood decaying macrofungi of the western Black Sea Region, Turkey.
Mycotaxon, 93, 319–322.
Álvarez, C., Reyes‐Sosa, F. M., & Díez, B. (2016). Enzymatic hydrolysis of biomass from wood. Microbial Biotechnology, 9, 149–156.
Bernicchia, A. (2005). European fungi, Polyporaceae, Vol. 10. Alassio, Italy: Edizioni Candusso.
Bills, G. F., & Foster, M. S. (2004). Biodiversity of fungi, inventoring and
monitoring methods. Burlington: Elsevier Academic Press.
Bozkurt, Y., Göker, Y., & Erdin, N. (1993). Impregnation technique. Istanbul: Istanbul University Faculty of Forest Publications. Publication No. 425. (In Turkish).
Breitenbach, J., & Kränzlin, F. (2000). Fungi of Switzerland, Vol. 5. Lucerne: Verlag Mykologia.
Deflorio, G. S., Fink, S., & Schwarze, F. W. M. R. (2008). Detection of incipient decay in tree stems with sonic tomography after wounding and fungal inoculation. Wood Science and Technology, 42, 117–132. Deflorio, G., Johnson, C., Fink, S., & Schwarze, F. M. W. R. (2008). Decay
development in living sapwood of coniferous and deciduous trees in‐ oculated with six wood decay fungi. Forest Ecology and Management,
255, 2373–2383.
Doğan, H. H., Aktaş, S., Öztürk, C., & Kaşık, G. (2012). Macrofungi dis‐ tribution of Cocakdere valley (Arslanköy, Mersin). Turkish Journal of
Botany, 36, 83–94.
Doğan, H. H., Karadelev, M., & Işıloğlu, M. (2011). Macrofungal diver‐ sity associated with the scale‐leaf juniper trees, Juniperus excelsa and
Juniperus foetidissima, distributed in Turkey. Turkish Journal of Botany, 35, 219–237.
EN 113 standards (2006). Wood preservatives ‐ Test method for deter‐ mining the protective effectiveness against wood destroying basidi‐ omycetes ‐ determination of the toxic values.
Glaeser, J. A., & Lindner, D. L. (2011). Use of fungal biosystematics and molecular genetics in detection and identification of wood‐decay fungi for improved forest management. Forest Pathology, 41, 341– 348. https://doi.org/10.1111/j.1439‐0329.2010.00681.x
Goodell, B., Qian, Y., & Jellison, J. (2008). Fungal decay of wood: Soft rot, brown rot, white rot. In T. P. Schultz, H. Militz, M. H. Freeman, B. Goodell, & D. D. Nicholas (Eds.), Development of commercial wood
preservatives: Efficacy, environmental and health issues, Vol. 982 (pp.
9–31). Washington, DC: American Chemical Society.
Gündüz, G. (2007). Mapping of climate index on wood preservation: Case of Turkey. ZKU Journal of Bartin Faculty of Forestry, 11, 26–33. Harju, A. M., Venäläinen, M., Anttonen, S., Viitanen, H., Kainulainen, P.,
Saranpää, P., & Vapaavuori, E. (2003). Chemical factors affecting the brown‐rot decay resistance of Scots pine heartwood. Trees, 17, 263–268.
Horak, E. (2005). Tubed and gilled fungi in Europe. Munich: Elsevier. Index Fungorum (2016). Retrieved from http://www.indexfungorum.
org/Names/Names.asp. [Accessed on 23 April 2017].
Janusz, G., Pawlik, A., Sulej, J., Swiderska‐Burek, U., Jarosz‐Wilkolazka, A., & Paszczynski, A. (2017). Lignin degradation: Microorganisms, en‐ zymes involved, genomes analysis and evolution. FEMS Microbiology
Reviews, 41(6), 941–962.
Kantay, R., & Köse, C. (2009). Forest enterprise depots and storage tech‐ niques. Journal of the Faculty of Forestry Istanbul University, 59, 75–92. Kazemi, S. M., Dickinson, D. J., & Murphy, R. J. (2001). Effects of initial mois‐ ture content on wood decay at different levels of gaseous oxygen con‐ centrations. Journal of Agricultural Science and Technology, 3, 293–304. Kirk, P., Cannon, P. F., Minter, D. W., & Stalpers, J. A. (2008). Ainsworth &
Bisby’s dictionary of the fungi, 10th ed. Wallingford: CAB International.
Kirk, T. K., & Farrell, R. L. (1987). Enzymatic “combustion”: The microbial degradation of lignin. Annual Review of Microbiology, 41, 465–505. https://doi.org/10.1146/annurev.mi.41.100187.002341
Komut, O. (2011). Blue stain damage occurring in Scotch pine logs and its
effect on sale price. Dissertation, Artvin: Artvin Coruh University.
Košikova, B., Slavikova, E., & Kacik, F. (2008). Biodegradability of ex‐ tractives in sound and biologically decayed beech by various yeast species. Wood Research, 53(3), 9–16.
Kurtoğlu, A. (1984). Wood material weight relations. Journal of the Faculty
of Forestry Istanbul University, 34, 150–163.
Lekounougou, S., Petrissans, M., Jacquot, J. P., Gelhaye, E., & Gerardin, P. (2009). Effect of heat treatment on extracellular enzymatic activ‐ ities involved in beech wood degradation by Trametes versicolor.
Wood Science and Technology, 43, 331–341. https://doi.org/10.1007/
s00226‐008‐0236‐z
Malakani, M., Khademieslam, H., Hosseinihashemi, S. K., & Zeinaly, F. (2014). Influence of fungal decay on chemi‐mechanical properties of beech wood (Fagus orientalis). Cellulose Chemistry and Technology, 48, 97–103.
Martínez‐Inigo, M. J., Immerzeel, P., Gutierrez, A., Río, J. C., & Sierra‐ Alvarez, R. (1999). Biodegradability of extractives in sapwood and heartwood from scots pine by sapstain and white‐rot fungi.
Holzforschung, 53, 247–252. https://doi.org/10.1515/HF.1999.042
Marzuki, B. M. (2017). Diversity of macrofungi on wood in forest na‐ ture reserve of Bojonglarang Jayanti Cianjur West Java. Journal
of Bacteriology & Mycology: Open Access, 4(1), 00080. https://doi.
org/10.15406/jbmoa.2017.04.00080
Messner, K., Fackler, K., Lamaipis, P., Gindl, W., Srebotnik, E., & Watanabe, T. (2003). Overview of white‐rot research: Where we are today. In B. Goodell, D. D. Nicholas, & T. P. Schultz (Eds.), Wood deterioration
and preservation (pp. 73–96). Washington DC: ACS Symposium series
845.
MycoBank (2018). Retrieved from http://www.mycobank.org. [Accessed on May 5 2017].
Olfat, A. M., Karimi, A. N., & Parsapajouh, D. (2007). Biological method to quantify progressive stages of decay in five commercial woods by
Coriolus versicolor. Pakistan Journal of Biological Sciences, 10, 1073–
1077. https://doi.org/10.3923/pjbs.2007.1073.1077
Pandey, K., & Pitman, A. (2003). FTIR studies of the changes in wood chemistry following decay by brown‐rot and white‐rot fungi.
International Biodeterioration & Biodegradation, 52(3), 151–160.
https://doi.org/10.1016/S0964‐8305(03)00052‐0
Patachia, S., & Croitoru, C. (2016). Biopolymers for wood preservation. In F. P. Torgal, V. Ivanov, N. Karak & H. Jonkers (Eds.), Biopolymers
and biotech admixtures for eco‐efficient construction materials (pp.
305–332). Waltham, MA: Woodhead Publishing.
Pavlidis, T., Ilieva, M., Bencheva, S., & Stancheva, J. (2005). Researches on wood destroying fungi division Ascomycota, classis Ascomycetes.
Zbornik Matice Srpske Za Prirodne Nauke, 109, 143–148. https://doi.
org/10.2298/ZMSPN0519143P
Reinprecht, L. (2016). Wood deterioration, protection and maintenance. West Sussex: Wiley‐Blackwell.
Rowe, J. W., & Conner, A. H. (1979). Extractives in eastern hardwoods ‐
a review. Madison, WI: Department of Agriculture, Forest Service,
Forest Products Laboratory.
Selik, M. (1973). Eastern Black Sea region fungi especially in the region of Trabzon. Journal of the Faculty of Forestry Istanbul University, Serial
A, 23, 27–38.
Sesli, E., & Denchev, C. M. (2008). Checklists of the myxomycetes, larger ascomycetes, and larger basidiomycetes in Turkey. Mycotaxon, 106, 65–67.
Sesli, E., & Helfer, S. (2013). New fungal records for the Turkish mycota from Trabzon. Turkish Journal of Botany, 37, 414–417.
Sigoillot, J. C., Berrin, J. G., Bey, M., Meessen, L. L., Levasseur, A., Lomascolo, A., … Boukhris, E. U. (2012). Fungal strategies for lignin degradation. In L. Jouanin, & C. Lapierre (Eds.), Lignins: Biosynthesis,
Biodegradation and Bioengineering (pp. 263–308). vol 61. London:
Academic Press, Elsevier.
Sivrikaya, H., & Can, A. (2014). Performance of copper‐azole and water repellents against some wood rot fungi, Turkey II. Forest Entomology and Pathology Symposium, Antalya.
Sixta, H., Promberger, A., Koch, G., Gradinger, C., & Messner, K. (2004). Influence of beech wood quality on bisulfite dissolving pulp manu‐ facture. Part 1: Influence of log storage on pulping and bleaching.
Holzforschung, 58, 14–21.
Solak, M. H., Işıloğlu, M., Kalmış, E., & Allı, H. (2015). Macrofungi of Turkey,
checklist. Vol. 2. Bornova: Universiteliler Ofset (In Turkish).
Spirin, V., Runnel, K., Vlasak, J., Miettinen, O., & Poldmaa, K. (2015). Species diversity in the Antrodia crassa group (Polyporales,
Basidiomycota. Fungal Biology, 119, 1291–1310. https://doi. org/10.1016/j.funbio.2015.09.008
Sümer, S. (1982). Important fungi in the Western Black Sea region of Turkey, especially in and around Bolu province. Istanbul University Faculty of Forest Publications, Publication No: 2907/312. Istanbul. (In Turkish).
Talley, S. M., Colley, P. D., & Kursar, T. A. (2002). The effects of weather on fungal abundance and richness among 25 commu‐ nities in the Intermountain West. BMC Ecology, 2, 7. https://doi. org/10.1186/1472‐6785‐2‐7
Taylor, A. M., Gartner, B. L., & Morrell, J. J. (2002). Heartwood forma‐ tion and natural durability—a review. Wood and Fiber Science, 34(4), 587–611.
Timell, T. E. (1967). Recent progress in the chemistry of wood hemicellu‐ loses. Wood Science and Technology, 1(1), 45–70.
Toshiaki, U. (2001). Chemistry of extractives. In M. Ekker (Ed.), Wood and
cellulosic chemistry (pp. 213–241). New York: Marcel Dekker Inc.
TSMS (2018). Turkish state meteorological service. Turkey: Ankara. Tuor, U., Winterhalter, K., & Fiechter, A. (1995). Enzymes of white‐rot
fungi involved in lignin degradation and ecological determinants for wood decay. Journal of Biotechnology, 41(1), 1–17. https://doi. org/10.1016/0168‐1656(95)00042‐O
Vek, V., Oven, P., & Poljanšek, I. (2016). Review on lipophilic and hydro‐ philic extractives in tissues of common beech. Drvna Industrija, 67(1), 85–96. https://doi.org/10.5552/drind.2016.1511
Windeisen, E., Wegener, G., Lesnino, G., & Schumacher, P. (2002). Investigation of the correlation between extractives content and nat‐ ural durability in 20 cultivated larch trees. Holz Als Roh‐ Und Werkstoff,
60, 373–374. https://doi.org/10.1007/s00107‐002‐0314‐0
How to cite this article: Yalçın M, Doğan HH, Akçay Ç.
Identification of wood‐decay fungi and assessment of damage in log depots of Western Black Sea Region (Turkey).