Contents lists available atScienceDirect
Biomedicine & Pharmacotherapy
journal homepage:www.elsevier.com/locate/biophaProtective e
ffects of L-theanine against doxorubicin-induced nephrotoxicity
in rats
Yahya Alt
ınkaynak
a,⁎, Birgül Kural
b, Buket A. Akcan
c, Ak
ın Bodur
b, Serap Özer
b, Esin Yulu
ğ
d,
Sevdegül Mun
ğan
e, Cansu Kaya
d, As
ım Örem
baProgram of Laborant and Veterinary Health, N.D. Göle Vocational High school, Ardahan University, Ardahan, Turkey bDepartment of Medical Biochemistry, Faculty of Medicine, Karadeniz Technical University, Trabzon, Turkey cDepartment of Nursing, Faculty of Health, Ardahan University, Ardahan, Turkey
dDepartment of Histology and Embryology, Faculty of Medicine, Karadeniz Technical University, Trabzon, Turkey eDepartment of Pathology, Faculty of Medicine, Karadeniz Technical University, Trabzon, Turkey
A R T I C L E I N F O Keywords: Antioxidant Apoptosis Glutathione Inflammation L-Theanine A B S T R A C T
Background/aim: L-theanine is the unique amino acid found in tea plants, has antioxidant and antiinflammatory activities, and functions in mental concentration and sleep quality. In this study, it is aimed to investigate the effects of L-theanine on doxorubicin (DOX, a chemotherapeutic agent) induced nephrotoxicity in rats, especially via GSH related enzymes.
Materials and methods: 32 male Sprague Dawley rats weighing 300–400 g were randomly assigned into 4 groups (n = 8) and the substances were given intraperitoneally to them: Control group (saline for 5 days); Theanine group (200 mg/kg/day theanine for 5 days); DOX group (single dose of 20 mg/kg DOX); DOX + Theanine group (20 mg/kg DOX atfirst day and 200 mg/kg/day theanine for 5 days). Kidney tissues were evaluated by histo-pathological analysis. Serum levels of blood urea nitrogen (BUN) and creatinine by spectrophotometrically; percentage of apoptosis indexes (AI%) in the tissues by TUNEL method; caspase-3 levels, reduced and oxidized glutathione (GSH and GSSG), gamma-glutamyltransferase 1 (GGT1), glutathione reductase (GR), glutathione peroxidase (GPx), and glutathione S-transferase (GST), nuclear factor kappa B p65 (NF-kB p65) by commercial kits; malondialdehyde (MDA) by spectrophotometrically were determined in plasma and kidney tissues. Results: According to DOX group, the DOX + Theanine group has much lower tissue and plasma GSSG, GGT1, NF-κB p65 levels and tissue AI%, whereas significantly higher GSH levels and GPx, GR, GST activities (P < 0.05).
Conclusion: It is suggested that L-theanine may have protective effects by enhancing effects on the antioxidant system of GSH and GSH-related enzymes against DOX-induced nephrotoxicity in rats. But thisfinding needs to be supported with further studies.
1. Introduction
Acute kidney injury is a condition that can cause morbidity and mortality. The kidneys are vital organs due to their vital functions in-cluding the maintenance of homeostasis, regulation of the extracellular environment, detoxification and excretion of toxic metabolites and drugs, urinary elimination of metabolic wastes such as blood urea ni-trogen (BUN) and creatinine which are breakdown products of amino acids [1–3].
Toxic reactive intermediates can cause nephrotoxicity via genera-tion of reactive oxygen species, which may lead to damage of renal cellular structures. Nephrotoxicity is defined as nephrotoxic renal in-sufficiency, acute glomerular nephritis, interstitial nephritis, nephrotic syndrome and nephron nephrosis. Kidneys are organs that can be ex-posed to the effects of drugs and toxins due to high blood perfusion, metabolic pathway activity and expression functions [4].
A member of the anthracycline family, doxorubicin (DOX, also called Adriamycin) has an efficient anticancer activity extensively used
https://doi.org/10.1016/j.biopha.2018.09.171
Received 21 August 2018; Received in revised form 28 September 2018; Accepted 28 September 2018
Abbreviations: BUN, blood urea nitrogen; DOX, doxorubicin; GGT, gamma-glutamyl transferase; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; MDA, malondialdehyde; NF-κB, nuclear factor kappa B; H&E, hematoxylin and eosin; TCA, three carboxylic acid cycle; CCL4, carbon
tetra-chloride
⁎Corresponding author.
E-mail address:yahyaaltinkaynak@ardahan.edu.tr(Y. Altınkaynak).
Biomedicine & Pharmacotherapy 108 (2018) 1524–1534
0753-3322/ © 2018 Elsevier Masson SAS. All rights reserved.
as a chemotherapeutic agent in clinics [5]. Its full potential as an an-ticancer agent has not been fully utilized because of dose-limiting toxicities such as cardiotoxicity, hepatotoxicity, nephrotoxicity etc. [6,7]. It was reported that DOX shows its cytotoxic effects via forming reactive oxygen species by complexing with Fe and Cu (Fe+2- DOX + O2→ Fe+3- DOX + O2−), binding to DNA and related enzymes [3]. In
our study, DOX has been applied to produce experimental ne-phrotoxicity.
Antioxidants are able to alleviate the side effects of reactive oxygen species. Glutathione (γ-glutamylcysteiniglycine) (GSH) is a commonly found tripeptide in plants and animals and is a vital compound for the antioxidant systems [8]. There are two forms of glutathione: reduced (GSH) and oxidized (GSSG). Low GSSG/GSH ratio is essential for pro-tection against oxidative stress [9]. Glutathione reductase (GR), glu-tathione peroxidase (GPx), gluglu-tathione S-transferase (GST), and γ-glu-tamyltransferase (GGT) are enzymes that have important roles in glutathione functions [10,11].
GR is an essential enzyme for GSH redox cycle in terms of main-taining appropriate cellular GSH levels [12]. GPx catalyzes the reduc-tion reacreduc-tion of peroxides by using GSH [13]. GSTs conjugate toxins by using GSH, thus neutralizing their electrophilic sites and increasing their solubility in water. GST-α form can be used as an early biomarker of nephrotoxicity in acute renal damage [14,15]. GGT, which hydro-lyzesγ-glutamylethylamide bonds in cell membranes, provides an in-termediate product for intracellular GSH synthesis [16,17].
The beverage of tea plant, which is a member of the Theaceae fa-mily, has been a worldwide popular beverage for centuries. L-theanine is a unique nonprotein derivative amino acid, comprising 50% of the free amino acids present in Camellia sinensis tea species, firstly dis-covered in green tea by Sakato in 1949 [18,19]. It is synthesized from glutamic acid and ethylamine, and its chemical structure is similar to glutamate and gamma-aminobutyric acid (GABA) [20].
Since 1964, L-theanine has been used as a nutritional supplement because of its protective effects such as relaxing, mental concentration, sleep quality, antiapoptotic, antioxidant and anti-inflammatory etc. [21,22]. In addition to these protective features, in a recent study, Nagai et al. suggested that L-theanine prevents against DOX-induced renal toxicity via alterations in MDA, GSH and Creatinine clearance (Ccr) levels in rats [23].
In this study, the effects of theanine on inflammatory, apoptotic, lipid peroxidation and antioxidant parameters (mainly on GSH and GSH metabolism-related enzymes due to structural similarity of theanine to glutathione) in DOX-induced nephrotoxicity in rats were studied in more details for thefirst time.
2. Material and methods 2.1. Chemicals
L-Theanine was purchased from Chem-Impex Intl, Inc. (Kn:14293, Chicago, ABD). DOX hydrochloride was purchased from Cayman Chemical (Item No:15007, Michigan, ABD). L-theanine and DOX hy-drochloride were dissolved in physiological saline, just before i.p. in-jections into rats.
2.2. Animals and experimental design
The experimental study commenced following approval from the Republic of Turkey (TR), Karadeniz Technical University Animal Care and Ethics Committee for Animal Experiments (meeting no: 2016/08, date: 21/06/2016, file no: 2016/26). All experimental and surgical procedures were performed in the TR Karadeniz Technical University Surgical Application and Research Center. Thirty-two Sprague Dawley rats, aged 3–4 months and weighing 300–400 g, were divided into 4 groups. 1 st group (control, n = 8) which was intraperitoneally given saline in parallel with the applications; 2nd group (Theanine, n = 8)
200 mg/kg/day theanine for 5 days; 3rd group (DOX, n = 8) single dose 20 mg/kg DOX; 4th group (DOX + Theanine, n = 8) which was given 20 mg/kg DOX atfirst day and 200 mg/kg/day theanine for 5 days. All the animals were fed a standard rat chow and water ad libitum and kept in a temperature-controlled environment (20–22 °C) with an alter-nating cycle of 12-h light and dark. On day 6th of applications, in ac-cordance with ethical guidelines, the rats were decapitated under an-esthesia [with applying of ketamine (75 mg / kg) + xylazine (10 mg / kg) i.p.] and serum/plasma and tissue samples of rats were collected and stored at−80 °C (Thermo Electron Corp. Farma -86C ULT Freezer, Waltham, MA, USA) for measurement of biochemical parameters. 2.3. Measurement of serum BUN and creatinine levels
The levels of BUN and creatinine in serum were estimated spec-trophotometrically using the Beckman Coulter Autoanalyzer AU5800 and Beckman Coulter commercial kits OSR6134- OSR6678 (California, USA). BUN and creatinine levels were expressed as mg/dL serum. 2.4. Histopathological examination
Renal tissue wasfixed in 10% neutral formaldehyde solution for 48 h, embedded in paraffin, sectioned by a microtome (Leica RM2255, Japan) at 5μm thickness and stained with hematoxylin and eosin for histopathological assessment. Olympus BX51 microscope (Olympus, Tokyo, Japan) was used for examination of kidney tissue preparations. Thefindings were photographed by camera attachment Olympus DP71 (Olympus, Tokyo, Japan) with different magnification areas. All spe-cimens were evaluated by a pathologist and histologist unaware of the study groups in two different ways.
Kidney damage levels were scored by the pathologist according to focal glomerular necrosis, Bowman capsule dilatation, tubular epithe-lial degeneration, tubular epitheepithe-lial necrosis, tubular dilatation, inter-stitial inflammatory, infiltration, and congestion in all sections [24].
As a result of the examinations, it was scored according to the fol-lowing scale;
0 = no damage
1 = (< 25% damage) focal, slight changes
2 = (25–50% damage) multifocal, significant changes 3 = (> 50% damage) common widespread changes.
In the second examination, in which different parameters was scored by the histologist according to dilation in Bowman's cavity, va-cuolization in tubule epithelial cells, degeneration, accumulation of intratubular hyaline and vasocongestion in all sections [25].
As a result of the examinations, it was scored according to the fol-lowing scale;
0= normal kidney (no damage) 1= minimal damage (< 25% damage) 2= slight damage (25–50% damage) 3= medium damage (50–75% damage) 4= severe damage (> 75% damage)
2.5. Immunohistochemical procedures (TUNEL assay and AI%)
TUNEL (Terminal deoxynucleotidyl transferase deoxyuridine tri-phosphate nick end labeling) assay was used to evaluate apoptosis of kidney tissue specimens and DNA fragments of cells in each tissue were identified. Insitu cell death detection POD kit was used in the evalua-tion of apoptosis (Roche, 11684817910, Berlin, Germany). A com-mercial kit containing 3,3′-diaminobenzidine was used on the colora-tion of epithelial cells (Sigma, 11718096001, MO, USA). The cells with homogeneously stained brown nucleus without necrosis areas were identified as TUNEL (+) cells (apoptotic cell). A total of 100 cells (glomerular and tubular cells) in 5 different areas were counted using the Analysis 5 Research program at 400X magnification (Olympus Soft Imaging Solutions, Münster, Germany) on each tissue. Apoptotic and
normal cells were recorded and apoptotic cell percentage was calcu-lated as the apoptotic index (AI) [25]. For immunohistochemical pro-cedures, a light microscope (Olympus, BX51, Tokyo, Japan) with an Olympus DP 71 camera (Tokyo, Japan) was used.
2.6. Determination of NF-κB p65, GGT1, and Caspase-3 levels
Plasma and tissue NF-κB p65, GGT1 and caspase-3 levels were de-termined using commercial ELISA kits (NF-κB; Catalog No: E-EL-R0674, Lot No: AK0017JUL26062, GGT1; Catalog No: E-EL-R0404, Lot No: AK0017JUL26064, Caspase-3; Catalog No: E-EL-R0160, Lot No: AK0017JUL26063, Elabscience Biotechnology Inc., Texas, USA) in line with the manufacturer’s instructions.
Renal tissues were placed in ice-cold PBS (0.01 M, pH = 7.4) and homogenized in PBS (100 mg tissue in 1 mL PBS) with a glass homo-genizer (IKA- ultra turrax T 18 Staufen, Germany) on ice for 30 s. Suspensions were sonicated 3 times for 45 s by using an ultrasonic cell disruptor (Sonics & Materials, Inc., Newtown, USA) The resulting homogenates were centrifuged at 50,000 rpm for 5 min, and the su-pernatants were used for NF-κB, GGT1 and caspase-3 levels measure-ment.
NF-κB, GGT1, and caspase-3 levels were estimated in 96-well plates according to the manufacturer's protocol by measured at 450 nm using VERSA max tunable microplate reader (Molecular Devices in California, USA). Experiments were performed in duplicate. The results were ex-pressed for NF-κB p65; as pg/mL plasma and ng/mg protein tissue, for GGT1 and Caspase-3 as ng/mL plasma and ng/mg protein tissue.
2.7. Measurement of plasma and tissue MDA levels
Tissue MDA levels were determined by Uchiyama and Mihara (1978). Plasma MDA levels were determined following the method described by Yagi (1984). Plasma lipids and proteins were precipitated with a phosphotungstic acid and sulfuric acid mixture. Tetramethoxypropane was used as a standard. Kidney tissue was cut on ice molds and weighed at 100 mg on a precision scale (Mettler Toledo AB 204-S Greifensee, Switzerland). 500μL of cold homogenization buffer was placed and homogenized at 5000 rpm in a homogenizer for 30 s in a cold environment. The Homogenizats were centrifugated at 4 °C, 3000 rpm, 10 min (IKA- ultra turrax T 18, Staufen, Germany),. The supernatant fractions were analyzed at 512 nm by microplate reader (Molecular devices Versa Max, California, United States). MDA levels were expressed as TBARS/mL in serum and nmol TBARS/mg protein in the tissues.
2.8. Measurement of GSH, GSSG and GSSG/GSH levels
Plasma and tissue GSH and GSSG levels were determined using commercial assay kits (Cayman, item no: 703002, Batch: 0512562, Michigan, USA) by the Ellman's reagent based on the enzymatic method. Sample preparation and application in plasma and tissue homogenates were performed as described in the commercial kit.
Plasma and tissue homogenates were deproteinated on the same day that they were taken from the rats. 100 mg of tissue was transferred to 1 ml of homogenization buffer (50 mM MES containing 1 mM EDTA, pH: 7.0). The tissues were homogenized in the ice bath at 5000 rpm for 30 s using a homogenizer. Homogenates were centrifuged at 4 °C, 10,000 g, 15 min so supernatants were taken. After the procedure was performed according to the kit protocol, measurements were made spectrophotometrically at 410 nm at 0 and 30th min. Plasma and tissue total GSSG and GSH levels were determined. GSSG values were calcu-lated by subtracting the GSH values from the total GSSG values in tissue and plasma samples.
2.9. Measurement of GPx, GR and GST activities
Plasma and tissue GPx, GR and GST activities were determined using commercial assay kits (Cayman GPx, item no: 703102, Batch: 0,508,232; GR, item no: 703202, Batch: 0,508,233; GST, item no: 703302, Batch: 0508663, Michigan, USA) in line with the manu-facturer’s instructions. The samples which prepared according to the kit protocol were measured absorbance per minute for 5 min at 340 nm on the microplate reader (VERSA max tunable microplate reader, Molecular Devices, California, USA). Tissue homogenization were pre-pared in the same manner as in GSH measurement. Results were given mU/mL units for plasma and mU/mg protein for the renal tissue. 2.10. Measurement of protein content
Total protein concentration of the kidney tissue homogenates were estimated according to the method of Bradford (1976) using bovine albumin (Sigma, Batch: 017K0724, MO, ABD) as standard.
2.11. Statistical analysis
Data are expressed as the median-inter quarter range (IQR) (25th and 75th percentiles) and %95 confidence interval (CI). The Kolmogorov-Smirnov test was used to test normality, and the groups were compared using the Mann-Whitney U test. Statistical significance was set at p < 0.05 (IBM SPSS Statistics 24.0). In our study, data have not follow a normal distribution.
3. Results
3.1. Effect of L-Theanine on the levels of serum renal function test parameters
There was no difference in serum renal function tests (BUN, crea-tinine and BUN/creacrea-tinine values) between L-theanine and control groups. In DOX group, the levels of these renal function parameters were significantly high when compared to control group. DOX + Theanine group had lower levels of them than those of DOX group (p < 0.05,Fig. 1,Table 1).
3.2. Effect of L-Theanine on renal histopathology
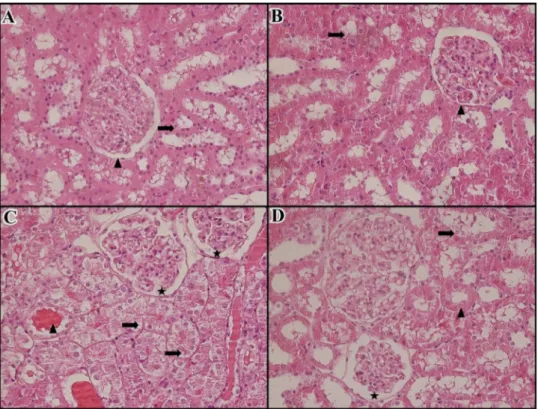
According to the histopathological examinations by pathologist and histologist, no pathologicalfindings were found in renal samples from both theanine and control groups (Fig. 2A, B;Fig.3A, B). Renal sections from DOX-treated rats showed intensive desquamation in tubular epi-thelial cells, single cell necrosis, tubular atrophy, tubular necrosis, and glomerular necrosis (score = 3 > % 50, Fig. 2C). In addition, renal cortical microcysts were noted in two rat kidneys in the DOX group. On the other hand, according to the examinations by the histologist, in all kidney samples in the DOX group, dilation in Bowman's cavity, va-cuolization in tubular epithelial cells, degeneration in tubule epithelial cells and intertubular vasocongestionfindings were medium and severe (Fig. 3C). The prevalence of nephrotoxicityfindings was minimal and light, ranging from 0% to 50% in the DOX + Theanine group. Lighter damagefindings was observed in the DOX + Theanine group compared to the DOX group (score 1–2, % 0 < > % 50) (Figs. 2, 3D).
The Photomicrographs of kidney tissues of groups are shown in Fig. 2(by the pathologist) andFig. 3(by the histologist). Histopatho-logical scores of the groups are shown inTables 2 and 3.
3.3. Effect of L-Theanine on apoptosis levels
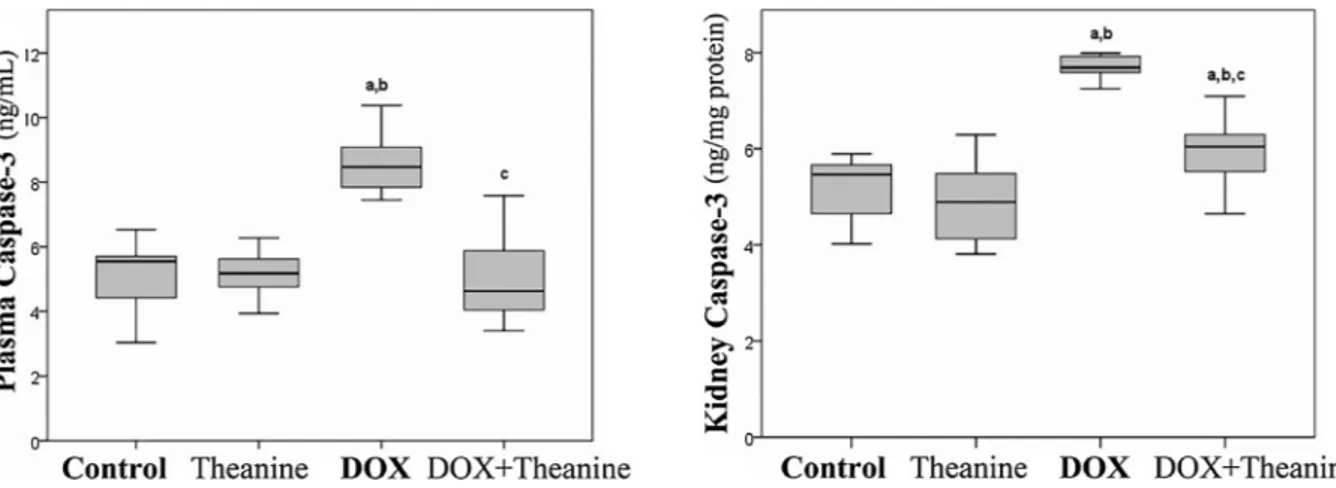
The results of apoptosis evaluation, tissue apoptotic index identified by the TUNEL method (Table 4) and caspase-3 levels in tissues and plasma (Fig. 4) showed that no significant change was observed
between control and theanine group but DOX + Theanine group had significantly lower values than DOX group. The mean values of AI% in control, theanine, DOX and DOX + Theanine groups were 5.75%, 6.62%, 42%, 29% respectively. The DOX group with a high apoptotic index showed a statistically significant difference from all other groups (p < 0.05).
Photomicrographs of cells in kidney tissues of groups were taken.
Apoptotic cells are indicated as TUNEL (+) whereas healthy cells are expressed as TUNEL (−) [25].
In the control and theanine groups, apoptosis was observed in a small number of tubular epithelial cells. The kidney tissues in the DOX group showed very commonly apoptotic cells in the tubular epithelial cells. The number of apoptotic cells in tubular epithelial cells of DOX + Theanine group was less than that of DOX group (Fig. 5). 3.4. Effect of L-Theanine on NF-κB levels
In terms of NF-κB p65 concentrations, there were no differences between theanine and control groups in renal tissue and plasma. The DOX group with the highest plasma and tissue NFKB levels showed significant differences according to the DOX + Theanine group. On the other hand, plasma NF-κB p65 concentrations were significantly higher in the DOX + Theanine group than in the control and theanine groups (P < 0.05,Fig. 6).
3.5. Effect of L-Theanine on plasma and renal oxidant-antioxidant system parameters
3.5.1. Effect of L-Theanine on MDA concentrations
Similar to BUN and Creatinine levels, no significant difference was observed in MDA levels between the control, theanine and DOX + Theanine groups. Relatively higher MDA levels in the DOX Fig. 1. Effect of L-Theanine on serum BUN and creatinine levels in the rats (n = 8).
a: significantly different from control; b: significantly different from Theanine; c: significantly different from the DOX group statistically (p < 0.05). Significance is according to the Mann-Whitney U test.
Table 1
BUN / Creatinine ratios in the each group (n = 8).
Control Theanine DOX DOX + Theanine BUN (mg/dL) [18.5 (4)] [17.5 (3)] [44.1 (39)a,b] [22.5 (19)c] (16 - 20) (15 - 20) (35 - 68) (15 - 36) Creatinine (mg/dL) [0.24 (0.05)] [0.25 (0.05)] [0.60 (0.34)a,b] [0.30 (0.19)c] (0.23– 0.27) (0.22– 0.28) (0.41– 0.75) (0.22– 0.42) BUN / Creatinine [72 (17)] [74 (20)] [126 (25)a,b] [87 (19)a,b,c]
Values are expressed as [Median (IQR)]; (% 95 CI).
a significantly different from control. b significantly different from Theanine.
c significantly different from DOX group statistically (p < 0.05).
Significance is according to the Mann-Whitney U test.
Fig. 2. The Photomicrographs and patholo-gical examinations of kidney tissues stained with hematoxylin and eosin (H&E, 400X). Arrow indicates a typical structural change. A: Control group (saline) (score = 0) (200X). No pathologicfindings in renal cortical area. B: Theanine group (20 mg/kg L-Theanine) (score = 1, 0%–25%). Δ= Light desquamation on the right side of the interstitium. C: DOX group (20 mg/kg DOX (score = 3, >
50%) Findings of common acute tubular da-mage in the cortical area, O = Desquamation in tubule epithelium, single cell necrosis, tub-ular atrophies, ↔ = Glomerular necrosis findings.
D: DOX + Theanine group (20 mg/kg L-Theanine + 20 mg/kg DOX) (score = 2, 25% < > 50%)Δ= desquamation in tubular epithelium in the interstitium, ┴ = Tubuler atrophy,→= Normalproximal tubule, ⟵ = Normalistal tubule.
group were not statistically significant. Plasma and tissue MDA con-centrations were lower in the DOX + Theanine group than DOX group (P < 0.05,Fig.7).
3.5.2. Effect of L-Theanine on GSSG/GSH and GSH related enzymes Theanine group did not show any significant change in plasma and tissue GSH and GSSG levels and therefore GSSG/GSH ratios when compared to control group. But in DOX + Theanine group, significantly higher GSH levels and therefore lower GSSG/GSH ratios in plasma and kidneys were found when compared to the DOX group (P < 0.05, Fig.8, Table 5). Although, DOX + Theanin group had significantly higher tissue GSSG levels than control and theanine group (P < 0.05, Table 6).
Plasma GPx activity levels in theanine group were statistically higher than the control group. Both tissue and plasma GPx activities were higher in the DOX + Theanine group than in all other groups including DOX group who has higher plasma GPx activity than control group (P < 0.05,Tables 5, 6).
Plasma and tissue GR and GST activities and GGT1 levels did not differ between control and theanine groups. On the other hand, these activities were lower and GGT1 levels higher in the DOX group than all other groups include DOX + Theanine group. DOX + Theanine group had significantly higher tissue GR activities than all other groups (P < 0.05, Tables 5, 6); had lower plasma GST activities than the control and theanine group, but not renal tissue activities; had sig-nificantly higher plasma GGT1 levels than the control and theanine group (P < 0.05,Tables 5, 6).
Fig. 3. The morphological changes in kidney tissue photomicrographs of the group (H&E). A: Control group (saline) (no damage) (400×). Normal tubule epithelial cell (↑), normal glo-merulus structure (Δ)
B: Theanine group (20 mg/kg L-Theanine) (no damage). Normal tubule epithelial cells (↑), normal glomerular structure (Δ)
C: DOX group (20 mg/kg DOX) (score = 3, > 50%) Degenerative tubular epithelial cells (↑), dilatation (star) in Bowman space, accumula-tion of intratubular hyaline (Δ)
D: DOX + Theanine group (20 mg/kg L-Theanine + 20 mg/kg DOX) (score = 1–2, 0% < > 50%). Normal tubule epithelial cells (Δ), degenerated tubular epithelial cells (↑), dilatation (star)
Table 2
The histopathological score of the each rat in the each group (n = 8).
Rat No Control Theanine DOX DOX + Theanine
1 0 1 3 2 2 0 0 3a 2 3 1 0 3 1 4 0 0 3 2 5 0 0 3 2 6 0 0 3 1 7 0 0 3 2 8 0 0 3a 2
score 0= no damage; score 1= focal, slight changes (less than 25%); score 2= multifocal, significant changes (25%–50%); score 3= common, widespread changes (more than 50%).
a Cortical microcystfindings.
Table 3
Morphological change scores in kidney tissues of each group (n = 8).
Morphological change Control Theanine DOX DOX + Theanine Dilatation of Bowman's space 1 1 2 2
Vacuolization in tubule epithelial cells
1 1 3 2
Degeneration in tubule epithelial cells 1 1 3 2 Intratubular hyaline accumulation 0 0 3 1 Vasocongestion 0 0 3 1
Score = 0: no damage, 1: minimal (< % 25), 2: slight (% 25 - % 50), 3: medium (% 50-% 75), 4: severe (> % 75).
The scores of each pathological change were calculated separately and the averages were taken, the total score was calculated by adding these scores.
Table 4
Levels of apoptotic index in the each group (n = 8).
Control Theanine DOX DOX + Theanine AI % [6.1 (2.8)] [5.5 (4.7)] [40.5 (5.8)a,b] [30 (5.8)a,b,c]
(3.9 - 7.6) (4.4– 8.8) (40– 45) (25– 33)
Values are expressed as [Median (IQR)]; (% 95 CI), AI%: the percentage of apoptotic index.
a significantly different from control. b significantly different from Theanine.
c significantly different from DOX group statistically (P < 0.05).
4. Discussion
The L-theanine synthesized in tea species such as Camellia sinensis is described as "a unique amino acid that is exclusive to the person" and there are about 500 research articles and about 300 reviews on L-theanine. Surprisingly, theanine has been suggested to increase the bioavailability of anti-neoplastic agents such as DOX in tumor cells whereas inhibits DOX-induced side toxicity in healthy cells [20,26]. In this study, DOX, which is frequently used in cancer chemotherapy, has been applied to produce experimental nephrotoxicity. In accordance with the literature, experimental nephrotoxicity was performed with DOX application of i.p. 20 mg/kg dose (Tables 1, 4,Fig. 4) [27,28]. It is indicated that DOX leads to these present toxic effects by free radical generation, lipid peroxidation production and oxidative damage on
tissues and organs [3,29]. According to our findings, a heavy dose 20 mg/kg DOX administration has serious toxic effects on kidney tissue. In our study, it has been decided that a dose of 200 mg/kg theanine applied considering the different studies about theanine because there is no previous study about the effects of L-theanine on the renal tissue [30,31]. According to ourfindings, the administration of 200 mg/kg theanine intraperitoneally may have protective effects on ne-phrotoxicity and no evidence was found that this causes any toxic ef-fects.
In our study, there was no significant difference in BUN, creatinine and BUN/creatinine levels between control and theanine groups (p < 0.05,Fig.1). It was also found out that theanine did not affect renal function tests in healthy subjects. The fact that the levels of these parameters were lower in the DOX + Theanine group than in the DOX Fig. 4. Effect of L-theanine on Caspase-3 levels in plasma and renal tissue (n = 8).
a: significantly different from control; b: significantly different from Theanine; c: significantly different from the DOX group statistically (P < 0.05). Significance is according to the Mann-Whitney U test.
Fig. 5. TUNEL-stained photomicrographs on kidney tissues of groups (400X). TUNEL (-) cell (↑), TUNEL (+) cell (Δ)
A: Control group (AI% = 5.75), B: Theanine group (AI% = 6.62), C: DOX group (AI% = 42) D: DOX + Theanine group (AI% = 29).
group suggests that Theanine may protect the renal structure and functions by inhibiting DOX-induced oxidative stress (Table 1). Nearly all of the urea synthesis occurs in the liver and is distributed to body fluids. Reabsorption and excretion of BUN and creatinine occur with glomerular filtration by the kidneys. Urine and plasma BUN and
creatinine levels are increased by high protein diet, various drug treatments, damage to various tissues such as muscle, liver and kidney and excessivefluid loss [32,33]. Unlike creatinine, about 40% of the BUN is reabsorbed with water and inorganic substances from the kidney proximal tubules. However, in the case of dehydration and inorganic Fig. 6. Effect of L-theanine on NF-κB p65 levels in plasma and renal tissue (n = 8).
a: significantly different from control; b: significantly different from Theanine; c: significantly different from the DOX group statistically (P < 0.05). Significance is according to the Mann-Whitney U test.
Fig. 7. Effect of L-theanine on MDA levels in plasma and renal tissue (n = 8).
a: significantly different from control; b: significantly different from Theanine; c: significantly different from the DOX group statistically (P < 0.05). Significance is according to the Mann-Whitney U test.
Fig. 8. Effect of L-theanine on GSSG/GSH ratios in plasma and renal tissue (n = 8).
a: significantly different from control; b: significantly different from Theanine; c: significantly different from the DOX group statistically (P < 0.05). Significance is according to the Mann-Whitney U test.
substance losses, BUN reabsorption can be increased due to reducing the urine excretion; and consequently, the ratio of plasma BUN/Crea-tinine may increase [33,34].
The increased level of these parameters in the current study, by a single dose of 20 mg/kg DOX, indicates deterioration of renal function in rats. DOX administration leads to dehydration and thereby may cause the BUN/Creatinine ratio to increase [34]. 200 mg/kg/day theanine supplementation for 5 days can protect the renal functions by
acting on the inhibitory effect on DOX-induced toxicity. There are no studies investigating the effect of L-theanine on serum BUN and crea-tinine levels. But Nagai et al. reported that 10 mg/kg L-theanine for 4 days normalizes clearance of creatinine in DOX-stimulated ne-phrotoxicity [23]. That research supported ourfinding that theanine is effective in decreasing the toxic effect of DOX in terms of renal function parameters. So, the current study suggests that theanine may act in a protective way by preventing dehydration on DOX-stimulated ne-phrotoxicity.
In our study, the number of apoptotic cells in the tubular epithelial cells of the DOX + Theanine group was lower than in the DOX group (Fig. 5,Table 4). Moreover, very common apoptosisfindings were ob-served in the tubular epithelial cells of the DOX group. The mean of AI % was 5.8% in the control group, 6.6% in theanine group, 42% in the DOX group and 29% in the DOX + Theanine group. Several studies have shown that DOX application induces apoptosis through increase in expression and activities of caspase-3 in tissues such as kidney and liver [29,35]. Theanine may have inhibitory effects on the DOX-induced apoptotic cell formation in kidney tissue. In our study, plasma and tissue Caspase-3 levels increased in DOX group, too. Caspase-3 and related pathways are considered to be important in studies on the ef-fects and development of anticancer drugs, and it is emphasized that high caspase-3 levels are an important marker of cell damage [36]. Theanine caused decreases in caspase-3 levels and AI% when admini-strated with DOX but did not show any significant effect change when given to healthy rats (p < 0.05,Fig. 4). There is no study on the effects of theanine on the renal AI% or any tissue in the literature. However, it was reported that theanine inhibits mitochondrial apoptosis by reg-ulation of cytochrome c release and mitochondrial membrane potential in ethanol-induced toxicity in hepatocytes [36]. In addition, protective effect of the theanine was emphasized by reporting that caspase-3 levels were lower in the theanine treated group [36,37]. Our study showed that theanine administration may inhibit apoptosis by inhibiting the increase of tissue and plasma caspase-3 levels and %AI rates on DOX-induced nephrotoxicity.
According to our results, plasma and tissue NF-kBp65 levels were recorded at lower levels in DOX + Theanine group compared to the DOX group (p < 0.05,Fig. 6), while no difference was found between theanine and control groups. DOX application promoted inflammation, and therefore increased NF-κBp65 (a marker of inflammation) levels. As DOX administration increases reactive oxygen species, it can lead to metabolic stress by inhibiting three carboxylic acid cycle (TCA) and oxidative respiratory pathways. Hypoxia and oxidative stress induced by DOX cause increased inflammation and then the apoptosis pathways become active [26,38]. Decreased effects of theanine on NF-kB p65 levels of DOX-induced inflammation suggested that theanine treatment can be effective in suppressing inflammation in kidney and plasma. There is no study on the relationship between theanine and NF-κB levels in DOX-induced nephrotoxicity. Perez et al. suggested that L-theanine inhibits liver fibrosis by inhibiting the activation of NF-κB and in-creasing the active matrix metalloproteinase-13 activities on CCL4-in-duced hepatotoxicity in mice models [30]. Also, according to a recent study, L-theanine has shown a regulatory effect of cytokines release by increasing the expression of the Ras-related protein A1 (Rap1A) and hydroxymethylglutaryl coenzyme reductase (HMGCR), which are in-volved in mevalonate biosynthetic pathways in rat splenic lymphocytes [39]. Thefindings we have obtained in our study suggest that theanine has anti-inflammatory effects in the prophylactic manner against DOX-induced nephrotoxicity. Due to the molecular similarity of theanine with glutamate, the concentration of the intracellular alpha-ketogluta-rate (α-KG) may increase after theanine administration. It has been suggested thatα-KG may inhibit hypoxia, inflammation, NF-κB release and apoptosis by activating prolyl hydroxylases. However, the effects of theanine on these pathways and molecules are still unclear [40–42].
In this study, the effects of L-theanine were determined on plasma and renal tissue MDA levels as the oxidative stress parameter. While Table 5
Levels of plasma GSH and GSH related enzymes in the study groups (n = 8).
Control Theanine DOX DOX + Theanine GSH (μM) [6.08 (0.33)] [6.71 (1.59)] [3.89 (0.93)a,b] [5.25 (1.02)a,b,c] (5.87– 6.39) (6.06– (7.40) (3.34– 4.46) (4.73– 5.69) GSSG (μM) [3.88 (0.22)] [3.02 (1.97)] [3.46 (0.92)] [2.89 (1.17)a,b,c] (3.24 -4.18) (2.37– 4.03) (2.82– 4.21) (1.94– 3.24) GPx (mU/mL) [92.8 (20.6)] [115 (27.8)a] [137 (52.9)a] [182.6 (59.4)a,b,c] (80– 101) (95– 135) (116– 164) (158– 225) GR (mU/mL) [29.4 (18.8)] [36.7 (22.7)] [23.9 (15.8)a,b] [32.9 (6.6)c] (20– 42) (26– 51) (11– 32) (30 - 36) GST (mU/mL) [47.5 (15.5)] [41.9 (8.4)] [25.2 (3.5)a,b] [29.9 (12.2)a,b,c] (45– 59) (37– 48) (18– 29) (25– 40) GGT1 (ng/mL) [15.1 (3.5)] [14 (6.1)] [30.5 (7.1)a,b] [22.3 (3.23)a,b,c] (13– 16) (11– 18) (26– 33) (20– 25)
Values are expressed as [Median (IQR)]; (% 95 CI).
Reduced (GSH) and oxidized (GSSG) glutathione, Glutathione reductase (GR), glutathione peroxidase (GPx), glutathione S-transferase (GST), γ-glutamyl-transferase (GGT).
a significantly different from control. b significantly different from Theanine.
c significantly different from the DOX group statistically (P < 0.05).
Significance is according to the Mann-Whitney U test. Table 6
Levels of renal tissue GSH and GSH related enzymes in the study groups (n = 8).
Control Theanine DOX DOX + Theanine GSH (μM) [58.3 (5.6)] [61.1 (4.2)] [35.9 (8.5)a,b] [42.3 (9.1)a,b,c] (56- 61) (59– 64) (29– 38) (40– 48) GSSG (μM) [44.7 (5.73)] [44.9 (4.88)] [64.1 (12.2)a,b] [54.8 (11.1)a,b,c] (41 - 48) (41– 47) (62– 73) (51– 61) GPx (mU/mg) [64.5 (34.4)] [59 (30.6)] [66.2 (38.3)] [110.5 (20.7)a,b,c] (41– 77) (55– 86) (37– 76) (99– 127) GR (mU/mg) [682 (114)] [684 (150)] [547 (107)a,b] [836 (91)a,b,c] (601– 712) (647– 777) (483– 609) (774– 875) GST (mU/mg) [239 (40)] [236 (27)] [219 (13)a,b] [268 (48)c] (228– 275) (223– 267) (211– 227) (247– 290) GGT1(ng/mg) [4.10 (0.47)] [4.53 (0.73)] [5.66 (2.65)a,b] [4.44 (0.43)a,c] (3.75– 4.43) (3.83– 5.01) (4.90– 7.32) (4.23– 4.62)
Values are expressed as [Median (IQR)]; (% 95 CI).
Reduced (GSH) and oxidized (GSSG) glutathione, Glutathione reductase (GR), glutathione peroxidase (GPx), glutathione S-transferase (GST), γ-glutamyl-transferase (GGT).
a significantly different from control. b significantly different from Theanine.
c significantly different from the DOX group statistically (P < 0.05).
there was no difference between theanine and control groups, MDA levels were lower in DOX + Theanine group than DOX group (p < 0.05,Fig. 3). MDA levels increase in nephrotoxicity generated by DOX or similar toxic agents [43,44]. Nagai et al. suggested that L-theanine prevents MDA formation by antioxidant effects in DOX-in-duced nephrotoxicity [23]. Jiang et al. reported that theanine has shown the protective effect by inhibiting the increase in MDA levels caused by increased oxidative stress [45]. In this context, no other studies on kidney tissue have been found. According to our data, theanine can be regarded as a molecule that shows protective activity against DOX-induced nephrotoxicity by reducing lipid peroxidation.
L-theanine is closely related to glutathione metabolism, but its ef-fects on nephrotoxicity have been investigated for thefirst time in our current study [46,47]. In the present study, both in plasma and tissue samples, there was no difference in GGT1, GSH, GSSG, GSSG/GSH le-vels or GR and GST activities between theanine and control groups, whereas GPx activities were higher in theanine group than control group. Furthermore, the DOX group had statistically significantly lower GSH levels, GR, GPx and GST activities, and higher GGT1and GSSG levels and GSSG/GSH ratios compared with the DOX + Theanine group (Fig. 8,Tables 5, 6). Also, the elevated levels of GSSG and GSSG/GSH in the DOX group is an indicator of oxidative stress (Fig. 8) [48]. It is stated that oxidative stresses are caused by oxygen radicals as a result of complex formation between DOX and Fe+3. DOX inhibits the in-tracellular transport system, GSH levels in the cell, and glutathione-related enzymes such as GST and GR, that is, vital antioxidant defense systems [49–51]. GSH is one of the most important antioxidant com-ponents in cells and plasma. Elevation of GSSG and GSSG/GSH levels is considered as an important indicator of impairment of redox balance and natural functions of intracellular systems [48].
In a study on the effect of L-theanine on nephrotoxicity, it was re-ported that administration of L-theanine increased GSH levels and showed protective activity against DOX-induced renal toxicity [23]. No other study has been found in this regard. Li et al. suggested that 200 mg/kg theanine administration can act as a protective by in-creasing GSH levels in ethanol-induced hepatotoxicity [37]. Deng et al. reported that theanine showed protective activity by increasing GSH levels in hepatotoxicity [52]. Vargas et al. suggested that theanine ex-hibits protective activity by raising GSH and GSH/GSSG levels [30]. It was noted in the literature that theanine can protect cells from harmful effects of toxic agents by raising GSH levels in the healthy cells whereas increasing the activity of anti-tumor agents in tumor cells [20,30].
As a response to injury under oxidative stress, some mechanisms for increasing antioxidant enzymes activities or levels (e.g., GCL, GR, GPx) can be activated therefore GSH precursors such as theanine and glu-tamine are important [53]. In cisplatin-induced nephrotoxicity, GSH levels of glutamine-treated groups were reported to be higher than the control group. It has also been suggested that glutamine has protective effects by raising intracellular GSH levels [53,54]. In DOX-induced cardiotoxicity, glutamine has been reported to increase GSH levels and inhibits lipid peroxidation on plasma and heart tissue [54]. Glutamine is essential in the biosynthesis of nucleotide, asparagine, and hex-osamine. Glutamine-like compounds such as theanine are necessary for the synthesis of GSH; nonessential amino acids such as proline, serine, glycine; the biosynthesis of polyamines, and the anaplerotic fuel sup-port for the citric acid cycle (TCA). On the other hand, it is stated that DOX application oxidizes enzymes and proteins, which play a key role in TCA and electron transport chain (ETS) [55]. It is also suggested that DOX inhibits the mitochondrial complex I [56]. Under increased oxi-dative stress and hypoxia conditions of cells, ATP synthesis is forced to occur by glycolysis. For this reason, the continuity of the TCA cycle is important [57]. The effects of L-Theanine on all these toxic effects and metabolic pathways may not be related to only elevating GSH levels. L-Theanine may have protective effects on enzymes and proteins in me-tabolic pathways such as TCA and ETS via GSH and GSH related en-zymes in DOX-induced nephrotoxicity. Furthermore, L-theanine may
increase intracellular glutamate or glutamine levels and thus may lead to an increase inα-KG levels and this situation may provide a healthy process for the TCA cycle. Through these effects, L-theanine may pre-vent the damage of DOX to the balance of energy metabolism [37,40,58]. In order to support such a thesis, it has been suggested that theanine regulates mitochondrial membrane potential against toxic effects and prevents mitochondrial damage and apoptosis. The basis of these effects is the increase in GSH levels and various metabolic con-tributions [36,40]. Ethylamine, one of the theanine's metabolites, may also have caused GPx increases. Ethylamine inhibits the mevalonate pathway leading to isopentenyl pyrophosphate accumulation. Iso-pentenyl pyrophosphate increases the synthesis of selenoproteins such as GPx [39]. Therefore, it has been thought that theanine may have contributed to increases in GPx enzyme activities via ethylamine. It has been reported that theanine administration has an important protective effect on the liver, leading to an increase in GPx activity levels [22]. In a study examining the activity of the strawberry extract, high GPx levels were obtained on DOX-induced toxicity [59]. Li et al. in their studies on the protective effect of theanine in alcohol-induced liver toxicity, in parallel with ourfindings, no significant difference was found in GPx activity levels between the control group and the toxic group [37]. It is suggested that L-theanine has enhancing effects on catalase, superoxide dismutase, GSH and GR levels in the alcohol-induced hepatocyte da-mage [37]. Similar to ourfindings, Deng et al. evaluated Escherichia coli-induced oxidative damage in the liver of rats, and they suggested that 100–300 mg/kg of theanine administration increased GPx1 ex-pression and activities [52]. Sumathi et al. reported that theanine ex-hibited protective activity by increasing GPx, GR activities and GSH levels in brain cells exposed to toxic effects [60,61]. Similarly, in our study, plasma and tissue levels of GR and GPx were higher in the L-theanine treated groups including DOX + Theanine group. It has been thought that theanine might have similar effects as GSH, and/or also may have supportive effects on glutathione-associated enzymes, which have not yet been elucidated.
As a biomarker of kidney damage, GGT functions in hydrolyzing of extracellular GSHs to cysteinyl glycine andγ-glutamyl products and is important in GSH homeostasis [15]. Amino acids can be taken up into the cell by the specific amino acid transporters and can also be taken up asγ-glutamyl and γ-glutamylcystine via GGT and used in the synthesis of intracellular GSH. GCLC, the catalytic subunit of the γ-glutamylcys-teine ligase (GCL), which is involved in thefirst step of GSH synthesis, can be inhibited by DOX application, and thus result in a decrease in intracellular GSH levels and an increase in GGT expression [16,62]. For this reason, even though GGT levels are increased, GSH synthesis can be inhibited via GCL with DOX administration. Yamamoto et al. have found evidence of increased tissue GGT levels in ischemia-induced renal injury [56]. According to another research, high GGT levels have been reported in cisplatin-induced nephrotoxicity in experimental animals [63]. Parallel to thefindings in literature, DOX group had higher GGT levels than DOX + Theanine group in our study. At this point, it is possible to say that theanine has a protective effect by inhibiting the increase of GGT1 levels in DOX-induced nephrotoxicity.
GST, a member of the phase II detoxification enzyme family, acti-vates the NF-κB signaling pathway by providing phosphorylation of the inhibitor NF-κB and has protective effects against oxidative damage in vitro [64]. The GST-α form can be used as an early biomarker to detect acute renal damage in nephrotoxicity [15]. Studies have shown that DOX administration reduces GST activity on kidney tissue [65,66]. It is emphasized that DOX application inhibits GSTs, thus leading to in-creased toxic metabolites and oxidative stress, which in turn leads to tissue damage [67]. L-theanine administration against DOX-induced nephrotoxicity may protect tissue and plasma GST activities (Tables 5, 6). Our study is thefirst in this regard.
In conclusion, according to our biochemical and histological find-ings, L-theanine, with its anti-oxidant, anti-inflammatory, and anti-ne-crotic properties, may have protective effects against DOX-induced
nephrotoxicity in rats. Due to these properties, L-theanine can be de-fined as a compound that can inhibit the increase of serum BUN and Creatinine levels in DOX-induced toxicities. It is thought that L-thea-nine may also have protective effects against dehydration stimulated by a commonly used chemotherapeutic agent like DOX. It is possible to say that via especially GSH and GSH-related enzymes, L-theanine shifts the oxidant-antioxidant balance in favor of antioxidants by reducing the oxidative stress and/or activating the antioxidant mechanisms (Fig. 9). In addition, detailed studies are needed to establish exactly by which metabolic pathways DOX displays these protective effects.
Study limitation
In our study, levels of the gene expression of glutathione-related enzymes have not been studied due to budget constraints. Since we do not have metabolic cages, the urine levels of BUN and creatinine have not been studied.
Acknowledgments
This research was supported by The Scientific and Technological Research Council of Turkey (TUBİTAK) (3001- 216z025), Republic of Turkey. In addition, thanks to Veterinarian Sait Al and Asst. Prof. Fulya Balaban Yücesan for their assistance in the follow-up and operation of the experimental animals, and Hanife Kara (PhD) and Ertuğrul Yiğit (MSc) for their assistance in biochemical examinations.
References
[1] V. Vaidya, M. Ferguson, J. Bonventre, Biomarkers of acute kidney injury, Annu. Rev. Pharmacol. Toxicol. 48 (2008) 463–493,https://doi.org/10.1146/annurev. pharmtox.48.113006.094615.Biomarkers.
[2] D.L. Gustafson, A.L. Merz, M.E. Long, Pharmacokinetics of combined doxorubicin and paclitaxel in mice, Cancer Lett. 220 (2) (2005) 161–169,https://doi.org/10. 1016/j.canlet.2004.09.007.
[3] C. Carvalho, R. Santos, S. Cardoso, S. Correia, P. Oliveira, M. Santos, P. Moreira, Doxorubicin: the good, the bad and the ugly effect, Curr. Med. Chem. 16 (25) (2009) 3267–3285,https://doi.org/10.2174/092986709788803312. [4] W. Pfaller, G. Gstraunthaler, Nephrotoxicity testing in vitro–what we know and
what we need to know, Environ. Health Perspect. 106 (2) (1998) 559,https://doi. org/10.1289/ehp.98106559.
[5] S.M. Cutts, A. Nudelman, A. Rephaeli, D.R. Phillips, The power and potential of doxorubicin-DNA adducts, IUBMB Life 57 (2005) 73–81,https://doi.org/10.1080/ 15216540500079093.
[6] P. Ben, Z. Zhang, Y. Zhu, A. Xiong, Y. Gao, J. Mu, Z. Yin, L. Luo, L-Theanine
attenuates cadmium-induced neurotoxicity through the inhibition of oxidative da-mage and tau hyperphosphorylation, Neurotoxicology 57 (2016) 95–103,https:// doi.org/10.1016/j.neuro.2016.09.010.
[7] Z. Zhang, X. Yu, Z. Wang, P. Wu, J. Huang, Anthracyclines potentiate anti-tumor immunity: A new opportunity for chemoimmunotherapy, Cancer Lett. 369 (2) (2015) 331–335,https://doi.org/10.1016/j.canlet.2015.10.002.
[8] A. Galant, M.L. Preuss, J.C. Cameron, J.M. Jez, Plant glutathione biosynthesis: di-versity in biochemical regulation and reaction products, Front. Plant Sci. 2 (2011) 45,https://doi.org/10.3389/fpls.2011.00045.
[9] L. Flohé, The fairytale of the GSSG/GSH redox potential, Biochim, Biophys. Acta -Gen. Subj. 1830 (2013) 3139–3142,https://doi.org/10.1016/j.bbagen.2012.10. 020.
[10] V.L. Kinnula, P. Pääkkö, Y. Soini, Antioxidant enzymes and redox regulating thiol proteins in malignancies of human lung, FEBS Lett. 569 (2004) 1–6,https://doi. org/10.1016/j.febslet.2004.05.045.
[11] D.A. Bender, No Title, 3 rd, Wiley-Blackwell, Indianapolis, 2012 doi:doi.org/10. 1002/9781118357514.ch5.
[12] I. Carlberg, B. Mannervik, Glutathione reductase, Methods Enzymol. 113 (1985) 484–490,https://doi.org/10.1002/0471684228.egp05186.
[13] F. Ursini, M. Maiorino, C. Gregolin, The selenoenzyme phospholipid hydroperoxide glutathione peroxidase, BBA - Gen. Subj. 839 (1985) 62–70,https://doi.org/10. 1016/0304-4165(85)90182-5.
[14] L.S. Engel, E. Taioli, R. Pfeiffer, P.M. Marcus, Q. Lan, P. Boffetta, P. Vineis, H. Autrup, D.A. Bell, R.A. Branch, J. Brockmöller, A.K. Daly, S.R. Heckbert, I. Kalina, D. Kang, A. Lafuente, H.J. Lin, M. Romkes, J.A. Taylor, N. Rothman, Am. J. 156 (2002) 95–109.
[15] W.S. Waring, A. Moonie, Earlier recognition of nephrotoxicity using novel bio-markers of acute kidney injury, Clin. Toxicol. 49 (2011) 720–728,https://doi.org/ 10.3109/15563650.2011.615319.
[16] S.J. Chinta, J.M. Kumar, H. Zhang, H.J. Forman, J.K. Andersen, Up-regulation of γ-glutamyl transpeptidase activity following glutathione depletion has a compensa-tory rather than an inhibicompensa-tory effect on mitochondrial complex I activity: implica-tions for Parkinson’s disease, Free Radic. Biol. Med. 40 (2006) 1557–1563,https:// doi.org/10.1016/j.freeradbiomed.2005.12.023.
[17] H.J. Forman, H. Zhang, A. Rinna, Glutathione: overview of its protective roles, measurement, and biosynthesis, Mol. Aspects Med. 30 (2009) 1–12,https://doi. org/10.1016/j.mam.2008.08.006.
[18] H. Kawagishi, K. Sugiyama, Facile and Large-Scale Synthesis of L-Theanine, Biosci. Biotechnol. Biochem. 56 (1992),https://doi.org/10.1271/bbb.56.689689-689. [19] Q.V. Vuong, M.C. Bowyer, P.D. Roach, L-Theanine: Properties, synthesis and
iso-lation from tea, J. Sci. Food Agric. 91 (2011) 1931–1939,https://doi.org/10.1002/ jsfa.4373.
[20] T. Sugiyama, Y. Sadzuka, Theanine and glutamate transporter inhibitors enhance the antitumor efficacy of chemotherapeutic agents, Biochim. Biophys. Acta - Rev. Cancer 1653 (2003) 47–59,https://doi.org/10.1016/S0304-419X(03)00031-3. [21] D. Türközü, N.Şanlier, L-theanine, unique amino acid of tea, and its metabolism,
health effects, and safety, Crit. Rev. Food Sci. Nutr. 57 (2017) 1681–1687,https:// doi.org/10.1080/10408398.2015.1016141.
[22] D. Wang, Q. Gao, T. Wang, F. Qian, Y. Wang, Theanine: The unique amino acid in the tea plant as an oral hepatoprotective agent, Asia Pac. J. Clin. Nutr. 26 (2017) 384–391,https://doi.org/10.6133/apjcn.032017.11.
[23] K. Nagai, S. Fukuno, K. Otani, Y. Nagamine, S. Omotani, Y. Hatsuda, M. Myotoku, H. Konishi, Prevention of doxorubicin-induced renal toxicity by theanine in rats, Pharmacology 101 (2018) 219–224,https://doi.org/10.1159/000486625.
Fig. 9. L-theanine may have protective effects by enhancing effects on the antioxidant system of GSH and GSH-related enzymes against DOX-induced nephrotoxicity in rats.
[24] M. Colbay, S. Yuksel, I. Uslan, G. Acarturk, O. Karaman, O. Bas, H. Mollaoglu, M. Yagmurca, O.A. Ozen, Novel approach for the prevention of contrast nephro-pathy, Exp. Toxicol. Pathol. 62 (2010) 81–89,https://doi.org/10.1016/j.etp.2009. 02.119.
[25] E.B. Sancak, A. Akbas, C. Silan, D.U. Cakir, H. Turkon, S.S. Ozkanli, Protective effect of syringic acid on kidney ischemia-reperfusion injury, Ren. Fail. 38 (2016) 629–635,https://doi.org/10.3109/0886022X.2016.1149868.
[26] L.J. Frederiksen, D.R. Siemens, J.P. Heaton, L.R. Maxwell, M.A. Adams, C.H. Graham, Hypoxia induced resistance to doxorubicin in prostate cancer cells is inhibited by low concentrations of glyceryl trinitrate, J. Urol. 170 (2003) 1003–1007,https://doi.org/10.1097/01.ju.0000081126.71235.e0.
[27] Q. Wang, C. Min, F. Zhu, Y. Xin, S. Zhang, L. Luo, Z. Yin, Production of bioactive γ-glutamyl transpeptidase in Escherichia coli using SUMO fusion partner and appli-cation of the recombinant enzyme to l-theanine synthesis, Curr. Microbiol. 62 (2011) 1535–1541,https://doi.org/10.1007/s00284-011-9891-7.
[28] K. Nagai, A. Oda, H. Konishi, Theanine prevents doxorubicin-induced acute hepa-totoxicity by reducing intrinsic apoptotic response, Food Chem. Toxicol. 78 (2015) 147–152,https://doi.org/10.1016/j.fct.2015.02.009.
[29] Z. Su, J. Ye, Z. Qin, X. Ding, Protective effects of madecassoside against Doxorubicin induced nephrotoxicity in vivo and in vitro, Sci. Rep. 5 (2015) 1–14,https://doi. org/10.1038/srep18314.
[30] J.E. Pérez-Vargas, N. Zarco, P. Vergara, M. Shibayama, J. Segovia, V. Tsutsumi, P. Muriel, l-Theanine prevents carbon tetrachloride-induced liverfibrosis via in-hibition of nuclear factorκB and down-regulation of transforming growth factor β and connective tissue growth factor, Hum. Exp. Toxicol. 65 (2015) 550–553,
https://doi.org/10.1177/0960327115578864.
[31] C. Li, H. Tong, Q. Yan, S. Tang, X. Han, W. Xiao, Z. Tan, L-Theanine improves immunity by altering TH2/TH1 cytokine balance, brain neurotransmitters, and expression of phospholipase C in rat hearts, Med. Sci. Monit. 22 (2016) 662–669,
https://doi.org/10.12659/MSM.897077.
[32] L.D. Mora, L.M.G. Antunes, H.D. Francescato, M.D.P. Bianchi, The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats, Pharmacol. Res. 47 (2003) 517–522,https://doi.org/10.1016/S1043-6618(03)00040-9.
[33] A.O. Hosten, BUN and creatinine, Clin. Methods Hist. Phys. Lab. Exam. (1990),
https://doi.org/10.1159/000254075.
[34] B.E. Robinson, H. Weber, Dehydration despite drinking: beyond the BUN/creatinine ratio, J. Am. Med. Dir. Assoc. 3 (2002) 386–389, https://doi.org/10.1016/S1525-8610(04)70532-0.
[35] F. Tulubas, A. Gurel, M. Oran, B. Topcu, V. Caglar, E. Uygur, The protective effects ofω-3 fatty acids on doxorubicin-induced hepatotoxicity and nephrotoxicity in rats, Toxicol. Ind. Health 31 (2015) 638–644,https://doi.org/10.1177/
0748233713483203.
[36] S. Shalini, L. Dorstyn, S. Dawar, S. Kumar, Old, new and emerging functions of caspases, Cell Death Differ. 22 (2015) 526–539,https://doi.org/10.1038/cdd.2014. 216.
[37] G. Li, Y. Ye, J. Kang, X. Yao, Y. Zhang, W. Jiang, M. Gao, Y. Dai, Y. Xin, Q. Wang, Z. Yin, L. Luo, L-Theanine prevents alcoholic liver injury through enhancing the antioxidant capability of hepatocytes, Food Chem. Toxicol. 50 (2012) 363–372,
https://doi.org/10.1016/j.fct.2011.10.036.
[38] D. Verdegem, S. Moens, P. Stapor, P. Carmeliet, Endothelial cell metabolism: par-allels and divergences with cancer cell metabolism, Cancer Metab. 2 (2014) 19,
https://doi.org/10.1186/2049-3002-2-19.
[39] C. Li, Q. Yan, S. Tang, W. Xiao, Z. Tan, Alteration of mevalonate pathway in rat splenic lymphocytes: possible role in cytokines secretion regulated by L-theanine, Biomed Res. Int. 2018 (2018),https://doi.org/10.1155/2018/1497097. [40] Q. Zhang, M. Liu, J. Ruan, Integrated transcriptome and metabolic analyses reveals
novel insights into free amino acid metabolism in huangjinya tea cultivar, Front. Plant Sci. 8 (2017) 1–11,https://doi.org/10.3389/fpls.2017.00291.
[41] P. Sonveaux, F. Vegran, T. Schroeder, M.C. Wergin, J. Verrax, Z.N. Rabbani, C.J. De Saedeleer, K.M. Kennedy, C. Diepart, B.F. Jordan, M.J. Kelley, B. Gallez, M.L. Wahl, O. Feron, M.W. Dewhirst, Targeting lactate fueled respiration selectively kills hy-poxic tumor cells in mice, J. Clin. Invest. 118 (2008) 3930–3942.
[42] P. Sonveaux, T. Copetti, C.J. de Saedeleer, F. Végran, J. Verrax, K.M. Kennedy, E.J. Moon, S. Dhup, P. Danhier, F. Frérart, B. Gallez, A. Ribeiro, C. Michiels, M.W. Dewhirst, O. Feron, Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis, PLoS One 7 (2012) e33418,https://doi.org/10.1371/journal.pone.0033418.
[43] T. Safari, M. Nematbakhsh, S. Miri, O. Ghofran, F. Fereidooni, A.A. Niazi, H. Bagheri, Gender differences in response to vitamin E and C in gentamicin in-duced nephrotoxicity in Wistar rats, J. Nephropathol. 6 (2017) 338–345,https:// doi.org/10.15171/jnp.2017.54.
[44] S. Ugur, R. Ulu, A. Dogukan, A. Gurel, I.P. Yigit, N. Gozel, B. Aygen, N. Ilhan, The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity, Ren. Fail. 37 (2015) 332–336,https://doi.org/10.3109/0886022x.2014.986005.
[45] W. Jiang, M. Gao, S. Sun, A. Bi, Y. Xin, X. Han, L. Wang, Z. Yin, L. Luo, Protective effect of l-theanine on carbon tetrachloride-induced acute liver injury in mice, Biochem, Biophys. Res. Commun. 422 (2012) 344–350,https://doi.org/10.1016/j. bbrc.2012.05.022.
[46] T. Unno, Y. Suzuki, T. Kakuda, T. Hayakawa, H. Tsuge, Metabolism of theanine, gamma-glutamylethylamide, in rats, J. Agric. Food Chem. 47 (1999) 1593–1596
http://www.ncbi.nlm.nih.gov/pubmed/10564022.
[47] T. Kakuda, E. Hinoi, A. Abe, A. Nozawa, M. Ogura, Y. Yoneda, Theanine, an
ingredient of green tea, inhibits [3H] glutamine transport in neurons and astroglia in rat brain, J. Neurosci. Res. 86 (2008) 1846–1856,https://doi.org/10.1002/jnr. 21637.
[48] D.P. Jones, Protein sensors and reactive oxygen species - part B: thiol enzymes and proteins, Methods Enzymol. 348 (2002) 93–112, https://doi.org/10.1016/S0076-6879(02)48630-2.
[49] K.B. Wallace, Doxorubicin-induced cardiac mitochondrionopathy, Pharmacol. Toxicol. 93 (2003) 105–115http://www.ncbi.nlm.nih.gov/pubmed/12969434. [50] D. Cardinale, A. Colombo, G. Bacchiani, I. Tedeschi, C.A. Meroni, F. Veglia,
M. Civelli, G. Lamantia, N. Colombo, G. Curigliano, C. Fiorentini, C.M. Cipolla, Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy, Circulation 13 (1) (2015) 1981–1988,https://doi.org/10.1161/ CIRCULATIONAHA.114.013777.
[51] E. Ricevuto, V. Cocciolone, M. Mancini, K. Cannita, S. Romano, G. Bruera, M. Pelliccione, M.I. Adinolfi, A. Ciccozzi, A. Bafile, M. Penco, C. Ficorella, Dose-dense nonpegylated liposomal doxorubicin and docetaxel combination in breast cancer: dose-finding study, Oncologist 20 (2015) 109–110,https://doi.org/10. 1634/theoncologist.2014-0129.
[52] Y. Deng, W. Xiao, L. Chen, Q. Liu, Z. Liu, Z. Gong, In vivo antioxidative effects of l-theanine in the presence or absence of Escherichia coli-induced oxidative stress, J. Funct. Foods 24 (2016) 527–536,https://doi.org/10.1016/j.jff.2016.04.029. [53] Y. Cao, R. Kennedy, V.S. Klimberg, Glutamine protects against doxorubicin-induced
cardiotoxicity, J. Surg. Res. 85 (1999) 178–182,https://doi.org/10.1006/jsre. 1999.5677.
[54] H.J. Kim, D.J. Park, J.H. Kim, E.Y. Jeong, M.H. Jung, T.H. Kim, J.I. Yang, G.W. Lee, H.J. Chung, S.H. Chang, Glutamine protects against cisplatin-induced ne-phrotoxicity by decreasing cisplatin accumulation, J. Pharmacol. Sci. 127 (2015) 117–126,https://doi.org/10.1016/j.jphs.2014.11.009.
[55] Y. Zhao, S. Miriyala, L. Miao, M. Mitov, D. Schnell, S.K. Dhar, J. Cai, J.B. Klein, R. Sultana, D.A. Butterfield, M. Vore, I. Batinic-Haberle, S. Bondada, D.K.St. Clair, Redox proteomic identification of HNE-bound mitochondrial proteins in cardiac tissues reveals a systemic effect on energy metabolism after doxorubicin treatment, Free Radic. Biol. Med. 72 (2014) 55–65,https://doi.org/10.1016/j.freeradbiomed. 2014.03.001.
[56] S. Yamamoto, B. Watanabe, J. Hiratake, R. Tanaka, M. Ohkita, Preventive effect of ggstop, a novel and selective gamma -glutamyl transpeptidase inhibitor, on ischemia / reperfusion-induced renal injury in rats, J. Pharmacol. Exp. Ther. 339 (2011) 945–951,https://doi.org/10.1124/jpet.111.183004.ids.
[57] S. Ramos, Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways, Mol. Nutr. Food Res. 52 (2008) 507–526,https://doi.org/10. 1002/mnfr.200700326.
[58] G. Eelen, P. De Zeeuw, M. Simons, P. Carmeliet, Endothelial cell metabolism in normal and diseased vasculature, Circ. Res. 116 (2015) 1231–1244,https://doi. org/10.1161/CIRCRESAHA.116.302855.
[59] F. Giampieri, J.M. Alvarez-Suarez, M. Gasparrini, T.Y. Forbes-Hernandez, S. Afrin, S. Bompadre, C. Rubini, A. Zizzi, P. Astolfi, C. Santos-Buelga, A.M. González-Paramás, J.L. Quiles, B. Mezzetti, M. Battino, Strawberry consumption alleviates doxorubicin-induced toxicity by suppressing oxidative stress, Food Chem. Toxicol. 94 (2016) 128–137,https://doi.org/10.1016/j.fct.2016.06.003.
[60] T. Sumathi, D. Asha, G. Nagarajan, A. Sreenivas, R. Nivedha, L-Theanine alleviates the neuropathological changes induced by PCB (Aroclor 1254) via inhibiting up-regulation of inflammatory cytokines and oxidative stress in rat brain, Environ. Toxicol. Pharmacol. 42 (2016) 99–117,https://doi.org/10.1016/j.etap.2016.01. 008.
[61] T. Sumathi, C. Shobana, M. Thangarajeswari, R. Usha, Protective effect of L-thea-nine against aluminium induced neurotoxicity in cerebral cortex, hippocampus and cerebellum of rat brain - histopathological, and biochemical approach, Drug Chem. Toxicol. 38 (2015) 22–31,https://doi.org/10.3109/01480545.2014.900068. [62] M.H. Hanigan, W.A. Ricketts, Extracellular glutathione is a source of cysteine for
cells that expressγ-glutamyl transpeptidase, Biochemistry 32 (24) (1993) 6302–6306,https://doi.org/10.1021/bi00075a026.
[63] T. Ozaki, S. Ishiguro, H. Itoh, K. Furuhama, M. Nakazawa, T. Yamashita, Cisplatin binding and inactivation of mitochondrial glutamate oxaloacetate transaminase in cisplatin-induced rat nephrotoxicity, Biosci. Biotechnol. Biochem. 77 (8) (2013) 1645–1649,https://doi.org/10.1271/bbb.130172.
[64] T. Nakajima, T. Takayama, K. Miyanishi, A. Nobuoka, T. Hayashi, T. Abe, J. Kato, K. Sakon, Y. Naniwa, H. Tanabe, Y. Niitsu, Reversal of multiple drug resistance in cholangiocarcinoma by the glutathione S-transferase-pi-specific inhibitor O1-hex-adecyl-gamma-glutamyl-S-benzylcysteinyl-D-phenylglycine ethylester, J. Pharmacol. Exp. Ther. 306 (3) (2003) 861–869,https://doi.org/10.1124/jpet.103. 052696.
[65] S. Mahajan, W.M. Atkins, The chemistry and biology of inhibitors and pro-drugs targeted to glutathione S-transferases, Cell. Mol. Life Sci. (2005),https://doi.org/ 10.1007/s00018-005-4524-6.
[66] H.A. Hassan, G.M. Edrees, E.M. El-Gamel, E.A. El-Sayed, Amelioration of cisplatin-induced nephrotoxicity by grape seed extract andfish oil is mediated by lowering oxidative stress and DNA damage, Cytotechnology 66 (2014) 419–429,https://doi. org/10.1007/s10616-013-9589-8.
[67] T.O. Omobowale, A.A. Oyagbemi, U.E. Ajufo, O.A. Adejumobi, O.E. Ola-Davies, A.A. Adedapo, M.A. Yakubu, Ameliorative effect of gallic acid in doxorubicin-in-duced hepatotoxicity in Wistar rats through antioxidant defense system, J. Diet. Suppl. 15 (2) (2018) 183–196,https://doi.org/10.1080/19390211.2017.1335822.