Abstract.
Background: The Fas receptor is known to be
widely expressed in various tissues and FasL is highly expressed
on cells of the immune system and also on cells of
immune-privileged areas such as the eyes and brain. Ovarian cells are
known to exhibit marked FasL immunoreactivity throughout
follicular development; there may also be a relationship
between Fas and FasL polymorphisms and the immune
privileges of the epithelial ovarian cells. Patients and Methods:
The study included 47 epithelial ovarian carcinoma patients
and 41 healthy subjects. Polymerase chain reaction (PCR) and
restriction endonucleases were used to determine the
polymorphic Fas and FasL genes. Results: The FasL CC
genotype was found to increase the risk of ovarian carcinoma
and a protective effect of the GGCT genotype was observed.
Conclusion: Because of the expressional aspects of the FasL
-844T→C polymorphism, individuals carrying the FasL-844C
allele would be expected to have higher FasL expression on
tumour cells compared with those carrying the FasL-844T
allele. People with such a genotype show a tendency to develop
various tumours.
One of the two convergent pathways of apoptosis is the
extrinsic type occuring in response to activation of specific
cell membrane receptors termed “death receptors” (1, 2).
Fas is one such death receptor which usually works with its
ligand FasL. It is thought that tumour cells have the
capacity to escape from immune surveillance. Some obtain
immune privileges which prevent them from being
recognised by the immune system while others fight actively
with immune system cells (3-5). It is thought that the
Fas/FasL pathway plays an important role in the
development of those immune privileges (3, 6-9).
Fas (ApoI=CD95) is a type I membrane protein of 48
kDa consisting of 335 amino acids and belongs to one of the
subfamilies of death receptors which is part of the
TNF-receptor superfamily (10, 11). FasL is a type II
transmembrane protein that has been shown to be
expressed by NK cells and activated T-cells and by some
immune-privileged cells such as the eye and brain (8). It is a
~37 kDa member of the TNF-family and can be cleaved by
metalloproteinases into a 26 kDa soluble form (3). Both
forms have the ability to trimerize and then bind to their
receptor, Fas (3), but the membranal form is more efficient
than the soluble form (3). Some in vivo
models
demonstrated that tumors expressing cell surface
membranal FasL are rejected in association with a
neutrophilic inflamatory response (3). This means that the
tumour cells with the ability to express FasL have the
advantage of not being damaged by infiltrating lymphocytes,
which confers an immune privilege on the tumour (12).
Ovarian cancer is one of the most common tumors for
women and it is known that >90% of all ovarian cancers
are of epithelial origin (9). The ovaries are covered by the
ovarian surface epithelium (OSE); this single layer is an
important site of ovarian oncogenesis (13). Fas is known
to be expressed by human ovarian cells and several studies
have indicated a role for the Fas-FasL system in various
gynecological problems, (2, 5, 13-16). Thecal ovarian cells
are known to exhibit marked FasL immunoreactivity
throughout follicular development. The exact physiological
significance of this observation is not clear yet, but there
is a strong possibility that this death factor provides
immune privilige for the follicle as it does in the testes and
eyes (13, 16, 17).
The aim of this study was to determine the relationship
between Fas and FasL polymorphisms and the development
of ovarian cancer.
The most frequent polymorphisms of Fas and FasL that
each cause some expressional sequela of the gene products
991
Correspondence to: Prof. Dr. Turgay Isbir, Institute for Experimental Medical Research, Department of Molecular Medicine, Istanbul University, Capa, Istanbul, Turkey. Tel/Fax: +90 212 6351959, e-mail: tisbir@superonline.com
Key Words: Fas/FasL pathway, immune privilege, polymorphism, ovarian cancer.
A
NTICANCERR
ESEARCH 27: 991-994 (2007)Fas-1377A/G and FasL-844 T/C Gene Polymorphisms
and Epithelial Ovarian Cancer
UZAY GORMUS
1, ARZU ERGEN
1, HULYA YILMAZ
1,
BURAK DALAN
1, SINAN BERKMAN
2and TURGAY ISBIR
11
Istanbul University, Institute for Experimental Medical Research, Department of Molecular Medicine and
2Faculty of Medicine, Department of Obstetrics and Gynecology, Istanbul University, Capa, Istanbul, Turkey
were investigated. It is known that the T
→C transition at
position -844 in the promoter region of FasL is located in
the binding motif for some important transcription factors.
It is thought that considerably higher basal expression of
FasL is associated with the FasL -844C allele compared with
the FasL-844T allele (5). Also G
→A transition at point
Fas-1377 decreases Fas expression (5).
Patients and Methods
Study groups. The study included 47 epithelial ovarian carcinoma patients and 41 healthy subjects. The healthy control subjects were chosen from women attending the Istanbul University Hospital, Obstetrics and Gynecology Clinics for non-specific minor complaints or regular health examinations. Exclusion criteria included a previous history of any kind of carcinoma in the subjects themselves or their first degree relatives. At recruitment, written informed consent was obtained from each subject. The mean age ± standard deviations was 48.62±11.50 years in the control group and 39.66±6.78 years in the patient group and 18.8% of the control group and 30.9% of the patient group were smokers.
Polymorphism analysis. The previously described method of Sun et al. was used (18). The primers used for polymerase chain reaction (PCR) for the -1377 G/A polymorphism were Fas forward, 5’-TGT GTG CAC AAG GCT GGC GC-3’ and Fas reverse, 5’-TGC ATC TGT CAC TGC ACT TAC CAC CA-3’; and for the FasL-844 T/C polymorphism the primer sequences were FasL forward, 5’-CAG CTA CTC GGA GGC CAA G-3’ and FasL reverse, 5’-GCT CTG AGG GGA GAG ACC AT-3’ from IDT (Integrated DNA Technologies Inc, Iowa, USA).
In the Fas procedure, in order to introduce a restriction endonuclease site, the 3’ end of the primer FasIF was changed from CAC to CGC to create a BstU1 site. These fragments were amplified using a 25 ÌL reaction mixture containing approximately 100 ng of template DNA, 1 ÌL of each primer, all four deoxyribonucleoside 5’ triphosphates (each at 0.2 mM), 2 mM MgCl2 and 1 U of Taq polymerase in 1x reaction buffer (MBI Fermentas, Vilnius, Lithuania).
For FasL amplification, a similar reaction mixture was used, except that 2.5 mM MgCl2was included.
For both Fas and FasL gene amplifications, the reactions were carried out with an initial melting step of 2 min at 94ÆC; followed by 35 cycles of 45 sec at 94ÆC, 45 sec at 62ÆC, 60 sec at 72ÆC; and a final elongation step of 7 min at 72ÆC.
PCR and restriction products of Fas were electrophoresed in 3% (w/v) and of FasL in 2% (w/v) agarose gels and stained with ethidium bromide.
The restriction endonuclease BstU1 was used to determine the Fas-1377 G/A polymorphism. The products of this reaction were separated on agarose gels containing ethidium bromide. BstU1 digestion generated the following fragments for the -1377G allele, fragments of 104 bp and 18 bp; Fas-1377A allele, a single fragment of 122 bp. For determinations of the FasL-844 T/C polymorphisms, the BsrDI restriction endonuclease was used which generated for the FasL-844 T allele, a single uncut fragment of 401 bp, and for the -844 C allele, two fragments of 233 and 168 bp.
Statistical analysis. The Student's t-test was used to determine whether or not significant differences in sex and ages existed between patient and control groups. Pearson's Chi-square analyses were used to examine differences in genotype distribution between cancer patients and control subjects.
Results
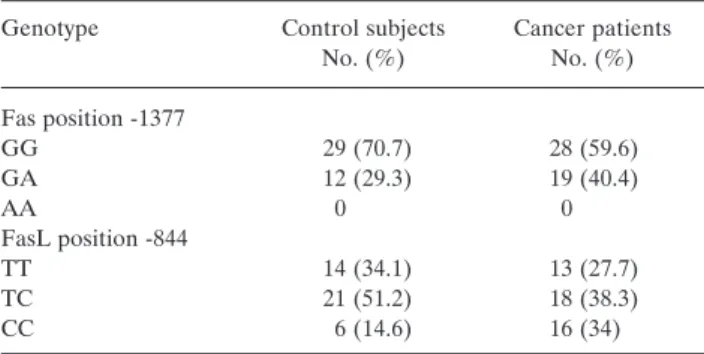
There was no significant difference in the distribution of the
Fas A and G alleles between cancer patients and the control
group (¯
2: 0, 367; p>0.05; df:1) and also no difference of
distribution of the FasL T and C alleles between the study
groups (¯
2: 2, 94; p>0.05; df:1) (Table I).
When the Fas and FasL genes were evaluated
independently of each other, the CC genotype individuals
had a statistically significant tendency to develop epithelial
ovarian cancer (¯
2: 4, 15; p<0.05; df:1) (Table I). No other
statistically significant differences were found for any other
genotype.
Because Fas and FasL form a receptor–ligand system
working together in apoptotic cell death, the possibility of
an interaction between the single nucleotide polymorphism
(SNP) in Fas and FasL that was associated with the risk of
ovarian cancer was examined. No significant differences
were detected between any of the genotypic combinations
of the two genes except the GGCT combination. The
GGCT genotype had a protective effect against epithelial
ovarian cancer (¯
2: 6, 604; p<0.025; df: 1) (Table II).
Discussion
Several studies of various carcinomas evaluated the
relationship between the risk of developing malignancy and
genetic polymorphisms. One focus of such investigation has
been the genes with a role in apoptotic pathways of which
the Fas/FasL system is one of the most important. Two
mechanisms of action of the Fas/FasL system in cancer have
been proposed (18, 19): Firstly, since FasL on
T-lymphocytes can promote apoptosis in Fas-expressing
A
NTICANCERR
ESEARCH 27: 991-994 (2007)992
Table I. Genotype distribution of Fas and FasL.
Genotype Control subjects Cancer patients No. (%) No. (%) Fas position -1377 GG 29 (70.7) 28 (59.6) GA 12 (29.3) 19 (40.4) AA 0 0 FasL position -844 TT 14 (34.1) 13 (27.7) TC 21 (51.2) 18 (38.3) CC 6 (14.6) 16 (34)
cancer cells, this system could play an important role in
cell-mediated cytotoxic reactions against malignant cells and
secondly malignant cells could escape immune surveillance
by down-regulation of FasL and by killing the lymphocytes
which the express FasL.
Sun et al. have observed that subjects carrying the FasL
-844CC genotype had a three-fold increased risk of developing
cervical cancer compared with those carrying the TT genotype
(5), which is in agreement with our results where the FasL CC
genotype was found to increase the risk of ovarian carcinoma.
This could be explained by the considerably higher basal
expression of FasL associated with the FasL-844C allele
compared with the FasL-844T allele (5), which is consistent
with the increase of Fas and related protein expressions in
ovarian carcinoma suggested by some other studies (9, 20-22).
Further studies have indicated that in general CC genotype
women have a higher tendency to develop gynecological
carcinomas. Sun et al. also found that the heterozygous CT
genotype presented a higher risk of cervical cancer (5). No
such relationship between the CT genotype and ovarian
carcinoma was found in the present study.
Van Haaften-Day et al. have found increased expression
of Fas in borderline ovarian carcinoma compared to normal,
benign and malignant cases, but they did not find any
relationship between Fas and FasL expressions in malignant
ovarian tumours (9). The present study was confined to
malignant ovarian carcinoma patients and only a protective
effect of the GGCT genotype was observed.
In conclusion, because of the expressional aspects of
the FasL-844T→C polymorphism, it would be expected
that individuals carrying the FasL-844C allele would also
have higher FasL expression on tumour cells compared
with those carrying the FasL-844T allele. People with such
a genotype for this cell death pathway gene show a
tendency to develop various tumours possibly due to their
immune status.
References
1 Martin TR, Hagimoto N, Nakamura M and Matute-Bello: G. Apoptosis and epithelial injury in the lungs. Proc Am Thorac Soc 2: 214-220, 2005.
2 Dufournet C, Uzan C, Fauvet R, Cortez A, Siffroi JP and Darai E: Expression of apoptosis-related proteins in peritoneal, ovarian and colorectal endometriosis. J Reprod Immunol 70: 151-162, 2006.
3 Abrahams VM, Straszewski SL, Kamsteeg M, Hanczaruk B, Schwartz PE, Rutherford TJ and Mor G: Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res 63: 5573-5581, 2003.
4 Liu K, Caldwell SA and Abrams SI: Cooperative disengagement of Fas and intercellular adhesion molecule-1 function in neoplastic cells confers enhanced colonization efficiency. Cancer Res 65(3): 1045-1054, 2005.
5 Sun T, Zhou Y, Li H, Han X, Shi Y, Wang L et al: FasL-844C polymorphism is associated with increased activation-induced T cell death and risk of cervical cancer. JEM 202(7): 967-974, 2005. 6 Myong NH: Tissue microarray analysis of Fas and FasL expressions in human non-small cell lung carcinomas; with reference to the p53 and bcl-2 overexpressions. J Korean Med Sci 20: 770-776, 2005.
7 Zhang X, Miao X, Sun T, Tan W, Qu S, Xiong P, Zhou Y and Lin D: Functional polymorphisms in cell death pathway genes Fas and FasL contribute to risk of lung cancer. J Med Genet 42: 479-484, 2005.
8 Niederkorn JY: See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol 7(4): 354-359, 2006.
9 Van Haaften-Day C, Russel P, Davies S, King NJC and Tattersall MHN: Expression of Fas and FasL in human serous ovarian epithelial tumors, Hum Pathol 34(1): 74-79, 2003. 10 Lee H and Ferguson TA: Biology of FasL. Cytokine Growth
Factor Rev 14: 325-335, 2003.
11 Poulaki V, Mitsiades CS and Mitsiades N: The role of Fas and FasL as mediators of anticancer chemotherapy, Drug Res Updates 4: 233-242, 2001.
12 Bohana-Kashtan O and Civin CI: Commentary on Kim et al., Profiling tumor counterattack: do Fas Ligand-containing microvesicles reduce anticancer immunity? Clinic Cancer Res 11(3): 968-970, 2005.
13 Quirk SM, Cowan RG and Huber SH: Fas antigen-mediated apoptosis of ovarian surface epithelial cells. Endocrinology 138(11): 4558-4566, 1997.
14 Inoue N, Maeda A, Matsuda-Minehata F, Fukuta K and Manabe N: Expression and localization of Fas Ligand and Fas during atresia in porcine ovarian follicles. J Reprod Devel [Epub ahead of print], 2006.
15 Dharma SJ, Kelkar RL and Nandedkar D: Fas and Fas Ligand protein and mRNA in normal and atretic mouse ovarian follicles. Reproduction 126: 783-789, 2003.
Gormus et al: Gene Polymorphisms and Ovarian Cancer
993 Table II. The genotype combinations of Fas and FasL.
TT CT CC Total
Case Control Case Control Case Control Case Control
AG 3 6 11 5 5 1 19 12
GG 10 8 7 16 11 5 28 29
16 Kim JM, Yoon YD and Tsang BK: Involvement of the Fas/Fas Ligand system in p53-mediated granulosa cell apoptosis during follicular development and atresia. Endocrinology 140(5): 2307-2317, 1999.
17 Quirk SM, Cowan RG, Joshi SG and Henrikson KP: Fas antigen-mediated apoptosis in human granulosa/luteal cells. Biology of Reproduction 52: 279-287, 1995.
18 Sun T, Miao X, Zhang X, Tan W, Xiong P and Lin D: Polymorphisms of death pathway genes Fas and FasL in esophageal squamous cell carcinoma. J Nat Cancer Inst 96(13): 1030-1036, 2004.
19 Liu K, Caldwell SA and Abrams SI: Immune selection and emergence of aggresive tumor variants as negative consequences of Fas-mediated cytotoxicity and altered IFN-Á-regulated gene expression. Cancer Res 65(10): 4376-4388, 2005.
20 Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S and Ramesh R: Activation of the Fas-FasL signaling pathway by MDA-7/IL-24 kills human ovarian cancer cells. Cancer Res 65(8): 3017-3024, 2005.
21 Akhmedkhanov A, Lundin E, Guller S, Lukanova A, Micheli A, Ma Y et al: Circulating soluble Fas levels and risk of ovarian cancer. BMC Cancer 3: 1-7, 2003.
22 Konno R, Takano T, Sato S and Yajima A: Serum soluble Fas level as a prognostic factor in patients with gynecological malignancies. Clin Cancer Res 6: 3576-3580, 2000.