Contents lists available atScienceDirect
Gene Reports
journal homepage:www.elsevier.com/locate/genrep
Investigation of XRCC1 Arg399Gln, Arg280His, and Arg194Trp
polymorphisms e
ffects on the induction of micronucleus by Aflatoxin B1 in
in vitro
Muhsin Ayd
ın
a,⁎, Mehmet Arslan
b, Eyyüp Rencüzo
ğulları
a, Cengiz Gözayd
ın
c, Ahmet Genç
d,
Süleyman Bayram
eaAdıyaman University, Faculty of Science and Letters, Department of Biology, 02040 Adıyaman, Turkey. bArdahan University, Vocational School of Health Services, 75700 Ardahan, Turkey
cAdıyaman University, Institute of Science, Department of Biology, 02040 Adıyaman, Turkey dAdıyaman University, Vocational School of Health Services, 02040 Adıyaman, Turkey
eAdıyaman University, Adıyaman School of Health, Department of Nursing, 02040 Adıyaman, Turkey
A R T I C L E I N F O
Keywords: Aflatoxin B1 XRCC1 Arg399Gln Arg280His Arg194Trp PolymorphismA B S T R A C T
Aflatoxin B1 (AFB1) is a genotoxic mycotoxin that can contaminate a wide variety of foods. The aim of this study was to examine the associations between XRCC1 Arg399Gln, Arg280His, and Arg184Trp polymorphisms and the frequencies of spontaneous and AFB1-induced DNA damage in peripheral blood lymphocytes from 100 healthy individuals in Turkish population. In vitro cytokinesis-blocked micronucleus (CMBN) assay was used to detect the spontaneous and AFB1-induced DNA damage. Genotyping of XRCC1 Arg399Gln, Arg280His, and Arg194Trp polymorphisms were carried out by using polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) assay. Treatment of the lymphocytes with AFB1 slightly increased (for Arg399Gln 0.73 ± 0.4 vs. 0.40 ± 0.26, Arg280His 0.80 ± 0.4 vs. 0.41 ± 0.28) the overall frequencies of micronucleus (MN) when compared to untreated cultures. No results were obtained for Arg194Trp polymorphism. Obtained overall results do not correspond to any significant (P < 0.05) association between XRCC1 Arg399Gln, Arg280His, and Arg194Trp Polymorphisms and MN frequencies. The results of this study indicate that Arg399Gln, Arg280His, and Arg194Trp polymorphisms do not play an important role in human sensitivity to the genotoxic effects of AFB1.
1. Introduction
Mycotoxins are secondary metabolites produced by fungi. They are toxic chemical products that readily colonize crops (Gross-Steinmeyer and Eaton, 2012; Turner et al., 2009). Additionally, mycotoxins are known among the most effective human dietary carcinogens that con-taminate the crops in the field or after harvest (Aslan et al., 2012; Richard, 2007; Turner et al., 2009; Türkez and Sişman, 2007). A fla-toxins are difuranocoumarin derivatives that are produced by a poly-ketide pathway by many strains of Aspergillusflavus and A. parasiticus, which contaminate a wide variety of food substances (Bennett and Klich, 2003; Gross-Steinmeyer and Eaton, 2012; Aslan et al., 2012; Turner et al., 2009; Türkez and Sişman, 2007). Aflatoxin B1 (AFB1) (Fig. 1) is the most toxic and carcinogenic aflatoxin among all known
aflatoxins (Gross-Steinmeyer and Eaton, 2012; Aslan et al., 2012; Türkez and Sişman, 2007). The International Agency for Research on Cancer (IARC) has been classified AFB1 as a class 1 human carcinogen (IARC, 1987). High-level AFB1 exposure may produce an acute hepatic necrosis resulting in cirrhosis or carcinoma of the liver (Marin et al., 2002). Along with hepatotoxicity, AFB1 is also known to cause im-munotoxicity, teratogenicity, cancer, and even death in mammals (Ağar et al., 2011; Aslan et al., 2012; Gross-Steinmeyer and Eaton, 2012; Türkez and Sişman, 2007). Aflatoxin B1 is metabolized primarily in the liver by cytochrome P450 into two forms of aflatoxin-8,9-epoxide, which are AFB1-8,9-exo-epoxide and 8,9-endo-epoxide (Santella, 2007). The exo-epoxide form is able to intercalate adducts into DNA, this is followed by reaction of C8 position of the epoxide with N7 position of guanine and cause DNA damage (Bbosa et al., 2013; Gross-Steinmeyer
http://dx.doi.org/10.1016/j.genrep.2017.08.002
Received 12 April 2017; Received in revised form 6 June 2017; Accepted 2 August 2017
⁎Corresponding author.
E-mail address:m.aydin@adiyaman.edu.tr(M. Aydın).
Abbreviations: AFB1, Aflatoxin B1; CBMN, Cytokinesis-blocked micronucleus; PCR-RFLP, Polymerase chain reaction restriction fragment length polymorphism; MN, Micronucleus; SCE, Sister chromatid exchange; CA, Chromosome aberrations; PARP, Poly-ADP-ribose polymerase; SNP, Single nucleotide polymorphisms
Available online 04 August 2017
2452-0144/ © 2017 Elsevier Inc. All rights reserved.
and Eaton, 2012). Aflatoxin B1 has been tested extensively in a wide variety test models including in vivo and in vitro test models, and have been consistently found to be positive for genotoxicity ( Gross-Steinmeyer and Eaton, 2012; Aslan et al., 2012; Türkez and Sişman, 2007; IARC, 1987). AFB1 induces micronucleus (MN), sister chromatid exchanges (SCEs), chromosome aberrations (CAs), and intercalate DNA and forms adducts in human cells (Aslan et al., 2012; Gross-Steinmeyer and Eaton, 2012; IARC, 1987; Santella, 2007; Türkez and Sişman, 2007).
Environmental and individual genetic susceptibility/resistance fac-tors are the main reasons of cancer. Genotoxic effects after exposure to carcinogens appears to be affected by genetic determinants, or inherited individual genetic susceptibility/resistance factors which contribute to define the cancer risk (Godderis et al., 2006). An appropriate human data should be used as a basis for risk assessment. In order to determine the effects of a cancer, exposure limits are predicated upon assumptions or information on the relationships between exposure and dose. Also, the frequency of the critical effect in the population exposed to a given hazardous agent corresponds to the frequency of that effect to the general population (Silbergeld, 1998). For example, an increased fre-quency of CAs in a population is considered an indication of an in-creased risk of cancer. Therefore, it is essential to determine which environmental and genetic susceptibility factors are accountable for a particular increase in the frequency of a genotoxic biomarker (Norppa, 1997; Hagmar et al., 1998). Using various biomarker approaches such as CAs, SCEs, and MN tests have led to correctly predict cancer risk in humans exposed to known or potential environmental geno-toxicants (Norppa, 1997). Among these approaches, MN seems to be the most suitable approach for epidemiological studies because it is very simple test with a better precision and can reflect variations due to initial-stage carcinogenesis (Iarmarcovai et al., 2008; Wu et al., 2010). Moreover, it has been suggested that MN plays an important role in genetic in-stability and cancer (Iarmarcovai et al., 2008). The cytokinesis-block micronucleus (CBMN) assay is one of the most commonly used cyto-genetic tests for cyto-genetic toxicology testing in human and mammalian cells due to its reliability and reproducibility (Fenech, 2007). It is the most reliable method for measuring the MN in peripheral blood lym-phocytes
The human X-ray cross-complementing group 1 (XRCC1) is a DNA repair gene (Gene ID 37414; OMIM 21171001 and 21,174,504), and located on chromosome 19q13.2–13.3 (Chi et al., 2015; Gu et al., 2013). It is 33 kb long, consists of 17 exons, and encodes a 2.2 kb transcript, which corresponds to a putative protein of 633 amino acids (Thompson and West, 2000; Trask et al., 1993). Some studies have shown that XRCC1 is involved in single-strand breaks and the base excision repair pathways, and reported to be responsible for the e ffi-cient repair of DNA damage caused by ionization, ionizing radiation, X-rays, oxygen, and alkylating agents (Brem and Hall, 2005; Dianova
et al., 2004; Kubota et al., 1996; Mutamba et al., 2011; Reynolds et al., 2015; Sterpone and Cozzi, 2010). The XRCC1 gene also encodes XRCC1 protein, which is the most well-known DNA repair protein that makes a complex with at least three different enzymes, ADP-ribose poly-merase (PARP), DNA ligase III, and DNA polypoly-meraseβ (Jiang et al., 2010).
There are over 300 validated single nucleotide polymorphisms (SNPs) in the XRCC1 gene reported in the dbSNP database (http:// www.ncbi.nlm.nih.gov/SNP). Three of them are more common than other and are among the most studied ones (Yi et al., 2013; Shen et al., 1998). These three SNPs lead to amino acid substitutions in XRCC1 at codon 399 (exon 10, base G to A, amino acid Arg to Gln, dbSNP ID: rs25487 = Arg399Gln), codon 280 (exon 9, base G to A, amino acid Arg to His, dbSNP ID: rs25489 = Arg280His) (Yang et al., 2014; Yi et al., 2013), and at codon 194 (exon 6, base C to T, amino acid Arg to Trp, dbSNP ID: rs1799782 = Arg194Trp) (Mao et al., 2013). The change of Arg-to-Gln at codon 399 (Arg399Gln) has been associated with several phenotypic alterations including glycophorin A mutations, higher levels of AFB1-DNA adducts, and polyphenol DNA adducts in human tissues (Lunn et al., 1999; Duell et al., 2000; Kelsey et al., 2004). Likewise, it has been determined that the change of Arg-to-His at codon 280 (Arg280His) diminishes genomic stability (Kiuru et al., 2005). Additionally,Hu et al. (2005)reported that XRCC1 Arg280His poly-morphism could be a biomarker of cancer susceptibility.
According to our knowledge, no research has been conducted to evaluate the association between XRCC1 Arg399Gln Arg280His, and Arg194Trp polymorphisms and the frequencies of spontaneous and AFB1-induced DNA damage in Turkish population. The main purpose of this study was to gain further insights about the roles of XRCC1 Arg399Gln, Arg280His, and Arg194Trp polymorphisms in DNA da-mage. In order to explore the association between XRCC1 Arg399Gln, Arg280His, Arg194Trp polymorphisms with the induction of MN by AFB1, in vitro CBMN assay was used to detect the spontaneous and AFB1-inducedchromosomal damage, and polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) was carried out to genotype XRCC1 Arg399Gln, Arg280His, and Arg194Trp poly-morphisms.
2. Materials and methods 2.1. Ethics statement
This study was confirmed by Human Ethics Committee of the Medical Faculty of the Adıyaman University, Adıyaman-Turkey. Date and number of the ethics committee approval is 15.05.2013 and 57831858-24, respectively. All of the volunteers provide their written informed consent to be included in the study regarding the use of their whole blood samples for research studies. The study continued in agreement with the statement on the Declaration of Helsinki approved by the World Medical Association meeting in Edinburgh.
2.2. Samples and chemicals
One hundred non-smokers healthy Turkish volunteers (61 females and 39 males), living in the same region (Adiyaman province) of Turkey, not occupationally exposed to geno-toxicants, not exposed to any known mutagens (e.g. X-rays, medicines), and not regular drinkers were enrolled to this study. Five milliliters of heparinized venous blood were collected by venipuncture from each of volunteers. An additional 5 mL specimen of venous blood was gathered from each subject into a test tube containing EDTA as anticoagulant, stored frozen at−20 °C until processed for genomic DNA (gDNA) isolation.
AFB1 (Cas No: 1162–65-8) and cytochalasin-B (CyteB) (Sigma, C6762) were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). Giemsa and all other chemicals were purchased from Merck (Darmstadt, Germany). All of the test solutions were freshly prepared
Fig. 1. The chemical name of aflatoxin B1 is (6aR,9aS)-4-Methoxy-2,3,6a,9a-tetra-hydrocyclopenta[c]furo[3′,2′:4,5]furo[2,3-h]chromene-1,11-dione (CAS registry number: 1162–65-8). It has a molecular formula of C17H12O6 and a molecular weight of 312.3 g/ mol.
before each experiment. 2.3. gDNA Isolation
gDNA was isolated from the 250μL of whole blood sample of all volunteers by the AxyPrep Blood Genomic DNA Miniprep Kit AP-MN-BL-GDNA-250 (Wujiang,Jiangsu, China) with respect to the manufac-turer's directions. The quantity and quality of DNA was calculated by the Qubit® Fluorometer (Invitrogen, Carlsbad, CA, USA). The gDNA specimens were stored at−20 °C until analysis.
2.4. Genotyping of XRCC1 Arg399Gln, Arg280His, and Arg194Trp polymorphisms
Polymerase chain reaction-restriction fragment length poly-morphism (PCR-RFLP) analysis were performed to determine the gen-otypes of the Arg194Trp, Arg280His and Arg399Gln polymorphisms of XRCC1 gene, as described previously (Yanatatsaneejit et al., 2013; Zhang et al., 2005). The primers, length of amplified fragments, re-striction pattern, and rere-striction enzymes used are listed inTable 1. The 25μL PCR mixture included approximately 125 ng gDNA, with 0.5 μM of both primers, 0.2 mM of each dNTP, 1 X PCR buffer, 2.5 mM MgCI2,
and 0.5 unit (U) Taq polymerase (Sigma-Aldrich, St. Louis, MO, USA). The following PCR cycling conditions were used: an initial denaturation step of 5 min at 95 °C; 30 cycles of 30 s at 94 °C, 30 s at 60 °C (for Arg194Trp and Arg399Gln) or 62 °C (for Arg280His), and 30 s at 72 °C; a final elongation step of 7 min at 72 °C. After confirmation of suc-cessful PCR amplification by 1.5% agarose gel electrophoresis, restric-tion enzymes MspI and RsaI (New England BioLabs, Beverly, MA) were used distinguish the Arg194Trp, Arg280His and Arg399Gln genotypes, respectively. To provide quality control, 15% random sample of in-dividuals was genotyped twice by different researchers; reproducibility of the test was 100%.
2.5. Preparation of S9 mix
3-methylcolanthrene-induced rat liver microsomal fraction was used as the metabolic activator (S9 mix) (Maron and Ames, 1983).The albino male rats (Rattus norvegicus var. albinos) 200 g in weight were pretreated with 80 mg/kg concentration of 3-methylcholanthrene (melted in sunflower oil) for 5 days, and the S9 fraction and S9 mix were prepared as described byMaron and Ames (1983). The freshly prepared S9 fraction was distributed to 1 mL portions into small plastic tubes and frozen immediately in a crushed dry form, and stored at −80 °C. The S9 mix was prepared freshly for each mutagenicity assay. For the preparation of S9 mix, nicotinamide adenine dinucleotide phosphate (4 mM) (Sigma, N5755), Glucose 6-phosphate (5 mM) (Sigma, G7879), MgCl2 (8 mM) (Merck), KCl (33 mM) (Merck) and 6.2 mL of phosphate buffer (0.2 mM) were added to 18 mL of sterile bidistilled water supplemented with 2 mL of microsome fraction. S9 mix (0.5 mL) was used for each of the culture tubes (0.5 mL of S9 mix/ tube).
2.6. CBMN assay
The analysis of MN in binucleated lymphocytes was carried out by following the modified methods described by Fenech (2000) and Kirsch-Volders et al. (2003). Fresh whole blood specimen (0.2 mL) of each donor (100 healthy Turkish volunteers) was added into two dif-ferent culture tubes that contain 2.5 mL of PB-Max chromosome medium (Gibco, Grand Island, NY 12552-013). For each individual, two series of cultures were set up, with two parallel cultures (duplicates). One series was used to determine the spontaneous MN frequencies (control or untreated group); the second series was used to detect the MN frequencies after AFB1 treatment (AFB1/treated group). The test concentration of AFB1 was selected 1.56μg/mL because resulted in roughly 50% (LD50) decrease in mitotic index. In the treated group lymphocytes were co-treated with 1.56μg/mL concentration of AFB1 and 0.5 mL S9 mix for 3 h. Aflatoxin B1 and S9 mix were added 48 h after initiating the lymphocyte culture. The S9 mix was also added to the control tubes without AFB1. Both control groups and treated groups (1.56μg/mL AFB1) were performed for each 100 healthy individuals. The AFB1 and S9 mix were removed from the lymphocyte culture by centrifugation at 2000 rpm for 5 min. The pellet of lymphocytes was washed two times with 2.5 mL RPMI 1640 medium (Sigma, R8758) and re-suspended in freshly prepared PB-Max chromosome medium (Gibco, Grand Island, NY 12552-013). Both control groups and treated groups' lymphocytes cultures were incubated for a total of 68 h at 37 °C. Cyt-B (final concentration of 6 μg/mL) was added after 44 h of incubation in order to block cytokinesis and achieve binucleated (BN) cells. At the end of the 68 h, the lymphocytes were harvested by centrifugation, and the lymphocytes' pellet was re-suspended in a hypotonic solution of 0.4% KCl for 5 min at 37 °C. Then, lymphocytes werefixed in a cold fixative consisting of methanol: glacial acetic acid: 0.9% NaCl (5:1:6 v/ v/v) at room temperature (22 °C ± 1 °C). After centrifugation, the lymphocytes werefixed further two times in a cold solution consisting of methanol-glacial acetic acid (5:1 v/v) at room temperature (22 °C ± 1 °C). Finally, the centrifuged cells were dropped onto clean slides and then air-dried. The air-dried slides were stained with 5% Giemsa stain solution (i.e., with 5% Giemsa in Sorensen Buffer, pH 6.8) for 15 min.
2.7. Microscopic evaluation
For the evaluation of MN frequency, 2000 binucleated (BN) cells with well-preserved cytoplasm were scored both in control culture and treated culture of each individuals with a light microscope at 1000 × magnification (Nikon, Eclipse E200, Japan) according toFenech et al. (2003). The total number of MN present in 2000 BN cells (MN/2000 BN) were used as indices of genotoxicity.
2.8. Statistical analysis
Statistical Package for Social Sciences (SPSS) software version 16.0 (SPSS Inc., Chicago, IL, USA) was used for data handling, dataset
Table 1
Primer sequences, amplicon size, restriction enzyme and restriction pattern for XRCC1 Arg194Trp, Arg280His and Arg399Gln polymorphisms.
Polymorphism Primer sequences Length (bp) Restriction enzyme Restriction pattern (bp)
Arg194Trp (rs1799782) F: 5′GCC CCG TCC CAG GTA 3′ R: 5′AGC CCC AAG ACC CTT TCA CT-3′
491 MspI Arg/Arg: 292, 178, 21 Arg/Trp: 313, 292, 178, 21 Trp/Trp: 313, 178 Arg280His (rs25489) F: 5′ TTG ACC CCC AGT GGT GCT 3′
R: 5′ TGC CTT CTC CTC GGG GTT T 3′
133 RsaI Arg/Arg: 133
Arg/His: 133, 70, 63 His/His: 70, 63 Arg399Gln (rs25487) F: 5′-TTG TGC TTT CTC TGT GTC CA 3′
R: 5′-TCC TCC AGC CTT TTC TGA TA-3′
615 MspI Arg/Arg: 377, 238
Arg/Gln: 615, 377, 238 Gln/Gln: 615
management, and statistical analyses. In this study, descriptive statistics of healthy volunteers were presented as the mean (standard deviation, SD) for continuous variables, while frequencies (%) were used for ca-tegorical variables. Genotype and allele frequencies of XRCC1 Arg399Gln, Arg280His, and Arg194Trp polymorphisms were computed and checked for deviation from the Hardy–Weinberg equilibrium (HWE) in healthy volunteers by using HWE calculator software (http:// www.oege.org/software/hwe-mr-calc.shtml) (Rodriguez et al., 2009). The statistical analyses of differences in micronucleus frequency in in-dividuals with different XRCC1 Arg399Gln Arg280His, and Arg194Trp genotypes were carried out using the Student's t-test, Mann-Whitney U test and Kruskal Wallis H test (whichever is appropriate). All these statistical tests were performed after assessing of assumption of nor-mality by Kolmogorov-Smirnov test and Shapiro- Wilk test. All tests were two sided, and a P value≤ 0.05 was considered to be statistically significant.
3. Results
3.1. General characteristics of the healthy volunteers
A total of 100 Turkish volunteers (61% females and 39%) were enrolled in this study. The average age of the volunteers is 21.91 ± 0.24 (age range 18–32). With respect to XRCC1 Arg399Gln polymorphism, 33% of the individuals revealed wild-type homozygous genotype (Arg/Arg), 47% heterozygous genotype (Arg/Gln), and 20% homozygous genotype (Gln/Gln) (Table 2). When they tested for XRCC1 Arg280His polymorphism, 85% of the volunteers revealed wild-type homozygous genowild-type (Arg/Arg), 13% heterozygous genowild-type (Arg/His), and 2% homozygous genotype (His/His) (Table 3). When they examined for XRCC1 Arg194Trp polymorphism, 89% of the in-dividuals revealed wild-type homozygous genotype (Arg/Arg), 11% heterozygous genotype (Arg/Trp), and none of the volunteers (0%) revealed homozygous genotype (Trp/Trp) (Table 4).
3.2. Induction of micronuclei by AFB1
Since individual variation in the baseline levels of the MN was considerable, the individual baseline frequencies of the MN were sub-tracted from the values of the AFB1 treated cultures and AFB1-control group was formed by this way.Fig. 2 represents data form two in-dependent experiments (control group and AFB1-treated group) as well as the AFB1-control group on the induction of micronucleus. When the micronucleus results were analyzed regardless to genotypes of XRCC1 Arg399Gln, Arg280His, and Arg194Trp polymorphisms, there was no significant increase in all three polymorphisms (Tables 2, 3, and 4).
Although the frequencies of MN produced by AFB1-control group were different from control, this did not mean to any significant difference (Fig. 2). For all XRCC1 Arg399Gln, Arg280His, and Arg194Trp poly-morphisms, there was no significant variation among the percentage of binucleated cells (%BN) for the control and AFB1-treated cells.
4. Discussion
There have been an increasing effort to clarify the effects of cancer risk factors on human populations. These factors could be related to the environment, genetic susceptibility, and lifestyle. Damage in DNA is the most significant initiator event in carcinogenesis. Unrepaired damage can result in the formation of DNA lesions or apoptosis or may even lead to unregulated cell growth and cancer (Yan et al., 2015; Zhang et al., 2012).
A wide variety of endogenous and exogenous factors can damage cellular DNA. The aim of this study was to examine the associations between XRCC1 Arg399Gln, Arg280His, and Arg184Trp polymorph-isms and the frequencies of spontaneous and AFB1-induced DNA da-mage in peripheral blood lymphocytes. Many studies have been done on the XRCC1 Arg399Gln, Arg280His, and Arg184Trp polymorphisms and many types of cancers in different populations. In this study, al-though there was a very small increase in XRCC1 Arg399Gln and Arg280His polymorphisms of AFB1-induced groups and the control, no significant association between XRCC1 Arg399Gln and Arg280His polymorphisms and AFB1-induced DNA damage was found in Turkish population (Tables 2 and 3). Additionally, we could not create domi-nant, recessive, and over-dominant models using the XRCC1 Arg194Trp polymorphism because none of the patients displayed the Trp/Trp ge-netic model (Table 4). As a result, XRCC1 Arg399Gln, Arg280His, and Arg194Trp polymorphisms do not play any significant role in human sensitivity to the genotoxic effects of AFB1 in Turkish population.
Our results could have also faced some possible limitations as fol-lows: (i) the genetic susceptibility to AFB1 exposure is likely to result from the contribution of many genetic variants in multiple pathways that may have a joint or additive effect on DNA damage. As a results, lack of more comprehensive genetic analyses of different genes in-volved in the different mechanisms of the cellular response to AFB1 limits our study. (ii) The small sample size is another crucial study limitation. Because of small number of individuals per category, it is possible that some statistical associations may subject to change. A more comprehensive and larger scale genetic study with different ethnic populations is needed to further explore the effects of genetic susceptibility to AFB1 exposure.
Table 2
Genotoxicity of AFB1 in in vitro human lymphocytes considering different genetic models of XRCC1 Arg399Gln Polymorphism. Summary table presenting mean values and respective ± SD of total number of micronucleus in 2000 binucleated cells (MN/2000BN).
Genetic models of XRCC1 Arg399Gln polymorphism
Control group (n = 100) %MNBN ± SD AFB1 group (n = 100) %MNBN ± SD AFB1-control group (n = 100) %MNBN ± SD
Codominant model Arg/Arg (n = 33) 0.42 ± 0.23 0.79 ± 0.43 0.38 ± 0.45 Arg/Gln (n = 47) 0.39 ± 0.25 0.69 ± 0.35 0.30 ± 0.33 Gln/Gln(n = 20) 0.39 ± 0.30 0.74 ± 0.47 0.35 ± 0.46 Dominant model Arg/Arg (n = 33) 0.42 ± 0.23 0.79 ± 0.43 0.38 ± 0.45 Arg/Gln + Gln/Gln (n = 67) 0.39 ± 0.27 0.71 ± 0.39 0.32 ± 0.38 Recessive model Arg/Arg + Arg/Gln (n = 80) 0.40 ± 0.24 0.74 ± 0.39 0.34 ± 0.39 Gln/Gln (n = 20) 0.39 ± 0.30 0.74 ± 0.47 0.35 ± 0.46 Overdominant model Arg/Arg + Gln/Gln (n = 53) 0.41 ± 0.26 0.77 ± 0.45 0.37 ± 0.45 Arg/Gln (n = 47) 0.39 ± 0.25 0.69 ± 0.35 0.30 ± 0.33
Conflict of interest statement
The authors report no conflict of interest and are responsible for the content and writing of the paper.
Acknowledgments
The author thanks all volunteers who participated in this study. The authors also would like to thank all Adıyaman University Scientific Research Unit staffs (Sel, A.; İlgin, K.; Elbir, F.; Işik, Y.; Abaci, U.).
Funding
This work was supported by Adıyaman University Research Fund (No: FEFYL/2013-0008).
References
Ağar, G., Aslan, A., Kotan Sarıoğlu, E., Alpsoy, L., Ceker, S., 2011. Protective activity of the methanol extract of Usnea longissima against oxidative damage and genotoxicity caused by aflatoxin B1 in vitro. Turk. J. Med. Sci. 41, 1043–1049.
Aslan, A., Agar, G., Alpsoy, L., Kotan, E., Ceker, S., 2012. Protective role of methanol extracts of two lichens on oxidative and genotoxic damage caused by AFB1 in human lymphocytes in vitro. Toxicol. Ind. Health 28, 505–512.
Bbosa, G.S., Kitya, D., Odda, J., Ogwal-Okeng, J., 2013. Aflatoxin metabolism, effects on epigenetic metabolism and their role in carcinogenesis. Health 5, 14–34. Table 3
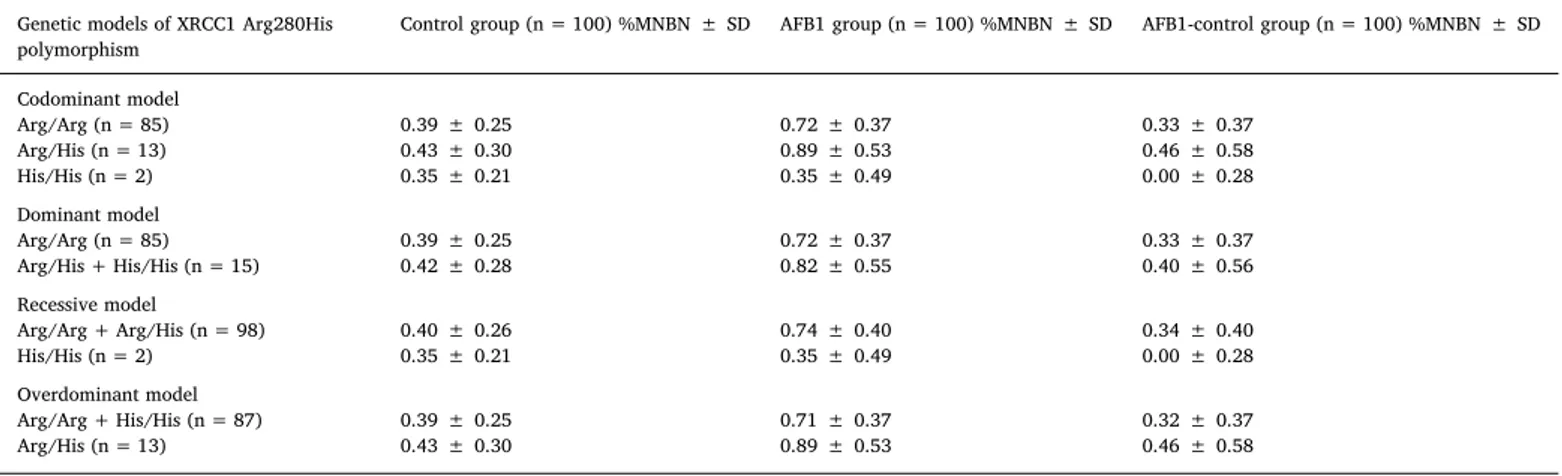
Genotoxicity of AFB1 in in vitro human lymphocytes considering different genetic models of XRCC1 Arg280His Polymorphism. Summary table presenting mean values and respective ± SD of total number of micronucleus in 2000 binucleated cells (MN/2000BN).
Genetic models of XRCC1 Arg280His polymorphism
Control group (n = 100) %MNBN ± SD AFB1 group (n = 100) %MNBN ± SD AFB1-control group (n = 100) %MNBN ± SD
Codominant model Arg/Arg (n = 85) 0.39 ± 0.25 0.72 ± 0.37 0.33 ± 0.37 Arg/His (n = 13) 0.43 ± 0.30 0.89 ± 0.53 0.46 ± 0.58 His/His (n = 2) 0.35 ± 0.21 0.35 ± 0.49 0.00 ± 0.28 Dominant model Arg/Arg (n = 85) 0.39 ± 0.25 0.72 ± 0.37 0.33 ± 0.37 Arg/His + His/His (n = 15) 0.42 ± 0.28 0.82 ± 0.55 0.40 ± 0.56 Recessive model Arg/Arg + Arg/His (n = 98) 0.40 ± 0.26 0.74 ± 0.40 0.34 ± 0.40 His/His (n = 2) 0.35 ± 0.21 0.35 ± 0.49 0.00 ± 0.28 Overdominant model Arg/Arg + His/His (n = 87) 0.39 ± 0.25 0.71 ± 0.37 0.32 ± 0.37 Arg/His (n = 13) 0.43 ± 0.30 0.89 ± 0.53 0.46 ± 0.58 Table 4
Genotoxicity of AFB1 in in vitro human lymphocytes considering different genetic models of XRCC1 Arg194Trp Polymorphism. Summary table presenting mean values and respective ± SD of total number of micronucleus in 2000 binucleated cells (MN/2000BN).
Genetic models of XRCC1 Arg194Trp Polymorphism
Control Group (n = 100) %MNBN ± SD AFB1 Group (n = 100) %MNBN ± SD AFB1-Control Group (n = 100) %MNBN ± SD
Codominant model Arg/Arg (n = 89) 0.41 ± 0.25 0.74 ± 0.41 0.34 ± 0.41 Arg/Trp (n = 11) 0.35 ± 0.29 0.69 ± 0.37 0.35 ± 0.28 Trp/Trp (n = 0) – – –
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
Mean
of
%MNBN
Control
AFB1
Control-AFB1
Fig. 2. Genotoxicity induced by AFB1 in human being lymphocytes. Total number of micronucleus in 2000 binucleated cells (MN/1000BN). Results expressed as mean values ( ± SD) from two independent experiments (control group and AFB1 group) as well as formed AFB1-control group performed with cultured lymphocytes from 100 Turkish healthy volunteers.
Bennett, J.W., Klich, M., 2003. Mycotoxins. Clin. Microbiol. Rev. 16, 497–516. Brem, R., Hall, J., 2005. XRCC1 is required for DNA single-strand break repair in human
cells. Nucleic Acids Res. 33, 2512–2520.
Chi, X.X., Liu, Y.Y., Shi, S.N., Cong, Z., Liang, Y.Q., Zhang, H.J., 2015. XRCC1 and XPD genetic polymorphisms and susceptibility to age-related cataract: a meta-analysis. Mol. Vis. 21, 335–346.
Dianova, I.I., Sleeth, K.M., Allinson, S.L., Parsons, J.L., Breslin, C., Caldecott, K.W., Dianov, G.L., 2004. XRCC1-DNA polymeraseβ interaction is required for efficient base excision repair. Nucleic Acids Res. 32, 2550–2555.
Duell, E.J., Wiencke, J.K., Cheng, T.J., Varkonyi, A., Zuo, Z.F., Ashok, T.D.S., Mark, E.J., Wain, J.C., Christiani, C., Kelsey, K.T., 2000. Polymorphisms in the DNA repair genes XRCC1 and ERCC2 and biomarkers of DNA damage in human blood mononuclear cells. Carcinogenesis 21, 965–971.
Fenech, M., 2000. The in vitro micronucleus technique. Mutat. Res. 455, 81–95. Fenech, M., 2007. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2,
1084–1104.
Fenech, M., Chang, W.P., Kirsch-Volders, M., Holland, N., Bonassi, S., Zeiger, E., 2003. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocytes cultures. Mutat. Res. 534, 65–75.
Godderis, L., Aka, P., Mateuca, R., Kirsch-Volders, M., Lison, D., Veulemans, H., 2006. Dose-dependent influence of genetic polymorphisms on DNA damage induced by styrene oxide, ethylene oxide and gamma-radiation. Toxicology 219, 220–229. Gross-Steinmeyer, K., Eaton, D.L., 2012. Dietary modulation of the biotransformation and
genotoxicity of aflatoxin B(1). Toxicology 299, 69–79.
Gu, X., Sun, H., Chang, L., Sun, R., Yang, H., Zhang, X., Cong, X., 2013. Correlation between X-ray cross-complementing group 1 polymorphisms and the onset risk of glioma: a meta-analysis. Neural Regen. Res. 8, 2468–2477.
Hagmar, L., Bonassi, S., Strömberg, U., Brøgger, A., Knudsen, L.E., Norppa, H., Reuterwall, C., 1998. Chromosomal aberrations in lymphocytes predict human cancer: a report from the European study group on cytogenetic biomarkers and health (ESCH). Cancer Res. 58, 4117–4121.
Hu, Z., Ma, H., Chen, F., Wei, Q., Shen, H., 2005. XRCC1 polymorphisms and cancer risk: a meta-analysis of 38 case-control studies. Cancer Epidemiol. Biomark. Prev. 14, 1810–1818.
IARC, 1987. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. Suppl. 7:440.
Iarmarcovai, G., Ceppi, M., Botta, A., Orsière, T., Bonassi, S., 2008. Micronuclei frequency in peripheral blood lymphocytes of cancer patients: a meta-analysis. Mutat. Res. 659, 274–283.
Jiang, J., Liang, X., Zhou, X., Huang, R., Chu, Z., Zhan, Q., Lin, H., 2010. DNA repair gene X-ray repair cross complementing group 1 Arg194Trp polymorphism on the risk of lung cancer: a meta-analysis on 22 studies. J. Thorac. Oncol. 5, 1741–1747. Kelsey, K.T., Park, S., Nelson, H.H., Karagas, M.R., 2004. A population-based case-control
study of the XRCC1 Arg399Gln polymorphism and susceptibility to bladder cancer. Cancer Epidemiol. Biomark. Prev. 13, 1337–1341.
Kirsch-Volders, M., Sofuni, T., Aardema, M., Albertini, S., Eastmond, D., Fenech, M., Ishidate Jr., M., Kirchner, S., Lorge, E., Morita, T., Norppa, H., Surralle's J, Vanhauwaert A, Wakata A., 2003. Report from the in vitro micronucleus assay working group. Mutat. Res. 540, 153–163.
Kiuru, A., Lindholm, C., Heilimo, I., Ceppi, M., Koivistoinen, A., Ilus, T., Hirvonen, A., Norppa, H., Salomaa, S., 2005. Influence of DNA repair gene polymorphisms on the yield of chromosomal aberrations. Environ. Mol. Mutagen. 46, 198–205. Kubota, Y., Nash, R.A., Klungland, A., Schar, P., Barnes, D.E., Lindahl, T., 1996.
Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J. 15, 6662–6670. Lunn, R.M., Langlois, R.G., Hsieh, L.L., Thompson, C.L., Bell, D.A., 1999. XRCC1
poly-morphisms: effects on aflatoxin B1-DNA adducts and glycophorin a variant fre-quency. Cancer Res. 59, 2557–2561.
Mao, Y., Xu, X., Lin, Y., Chen, H., Wu, J., Hu, Z., Zhu, Y., Xu, X., Xie, L., 2013. Quantitative assessment of the associations between XRCC1 polymorphisms and
bladder cancer risk. World J. Surg. 11, 58.
Marin, D.E., Taranu, I., Bunaciu, R.P., Pascale, F., Tudor, D.S., Avram, N., Sarca, M., Cureu, I., Criste, R.D., Suta, V., Oswald, I.P., 2002. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. J. Anim. Sci. 80, 1250–1257.
Maron, D.M., Ames, B.N., 1983. Revised methods for the salmonella mutagenicity test. Mutat. Res. 113, 173–215.
Mutamba, J.T., Svilar, D., Prasongtanakij, S., Wang, X.H., Lin, Y.C., Dedon, P.C., Sobol, R.W., Engelward, B.P., 2011. XRCC1 and base excision repair balance in response to nitric oxide. DNA Repair (Amst) 10, 1282–1293.
Norppa, H., 1997. Cytogenetic markers of susceptibility: influence of polymorphic car-cinogenmetabolizing enzymes. Environ. Health Perspect. 105, 829–835. Reynolds, P., Cooper, S., Loomax, M., O'neill, P., 2015. Disruption of PARP1 function
inhibits base excision repair of a sub-set of DNA lesions. Nucleic Acids Res. 43, 4028–4038.
Richard, J.L., 2007. Some major mycotoxins and their mycotoxicoses–an overview. Int. J. Food Microbiol. 119, 3–10.
Rodriguez, S., Gaunt, T.R., Day, I.N., 2009. Hardy-Weinberg equilibrium testing of bio-logical ascertainment for Mendelian randomization studies. Am. J. Epidemiol. 169, 505–514.
Santella, R.M., 2007. Aflatoxin B1: mechanism of mutagenesis. Iatreia 20, 38–39. Shen, M.R., Jones, I.M., Mohrenweiser, H., 1998. Nonconservative amino acid
substitu-tion variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 58, 604–608.
Silbergeld, E.K., 1998. Toxicology. In: Stellman, J.M. (Ed.), Encyclopaedia of Occupational Health and Safety, 4th ed. ILO Publications (Chapter 33). Sterpone, S., Cozzi, R., 2010. Influence of XRCC1 genetic polymorphisms on ionizing
radiation-induced DNA damage and repair. J. Nucleic Acids 2010 (Article ID 780369).http://dx.doi.org/10.4061/2010/780369.
Thompson, L.H., West, M.G., 2000. XRCC1 keeps DNA from getting stranded. Mutat. Res. 459, 1–18.
Trask, B., Fertitta, A., Christensen, M., Youngblom, J., Bergmann, A., Copeland, A., de Jong, P., Mohrenweiser, H., Olsen, A., Carrano, A., Tynan, K., 1993. Fluorescence in situ hybridization mapping of human chromosome 19: cytogenetic band location of 540 cosmids and 70 genes or DNA markers. Genomics 15, 133–145.
Türkez, H., Sişman, T., 2007. Anti-genotoxic effect of hydrated sodium calcium alumi-nosilicate on genotoxicity to human lymphocytes induced by aflatoxin B1. Toxicol. Ind. Health 23, 83–89.
Turner, N.W., Subrahmanyam, S., Piletsky, S.A., 2009. Analytical methods for determi-nation of mycotoxins: a review. Anal. Chim. Acta 632, 168–180.
Wu, C., Lu, Y., Morimoto, K., 2010. Effect of gene polymorphisms and ethanol con-sumption on micronucleus frequency in human reticulocytes: a preliminary study. Environ. Health Prev. Med. 15, 188–193.
Yan, J., Wang, X., Tao, H., Deng, Z., Yang, W., Lin, F., 2015. Meta-analysis of the re-lationship between XRCC1-Arg399Gln and Arg280His polymorphisms and the risk of prostate cancer. Sci Rep 5, 9905.
Yanatatsaneejit, P., Boonsuwan, T., Mutirangura, A., Kitkumthorn, N., 2013. XRCC1 gene polymorphisms and risk of ameloblastoma. Arch. Oral Biol. 58, 583–589. Yang, D., Liu, C., Shi, J., Wang, N., Du, X., Yin, Q., Wang, Y., 2014. Association of XRCC1
Arg399Gln polymorphism with bladder cancer susceptibility: a meta-analysis. Gene 534, 17–23.
Yi, L., Xiao-feng, H., Yun-tao, L., Hao, L., Ye, S., Song-tao, Q., 2013. Association between the XRCC1 Arg399Gln polymorphism and risk of cancer: evidence from 297 case-control studies. PLoS One 8 (10), e78071.
Zhang, Z., Wan, J., Jin, X., Jin, T., Shen, H., Lu, D., Xia, Z., 2005. Genetic polymorphisms in XRCC1, APE1, ADPRT, XRCC2, and XRCC3 and risk of chronic benzene poisoning in a Chinese occupational population. Cancer Epidemiol. Biomark. Prev. 14, 2614–2619.
Zhang, K., Zhou, B., Wang, Y., Rao, L., Zhang, L., 2012. The XRCC1 Arg280His poly-morphism contributes to cancer susceptibility: an update by meta-analysis of 53 in-dividual studies. Gene 510, 83–101.
![Fig. 1. The chemical name of aflatoxin B1 is (6aR,9aS)-4-Methoxy-2,3,6a,9a-tetra- (6aR,9aS)-4-Methoxy-2,3,6a,9a-tetra-hydrocyclopenta[c]furo[3′,2′:4,5]furo[2,3-h]chromene-1,11-dione (CAS registry number: 1162–65-8)](https://thumb-eu.123doks.com/thumbv2/9libnet/3910298.45074/2.892.109.387.83.275/chemical-aflatoxin-methoxy-methoxy-hydrocyclopenta-chromene-registry-number.webp)