/ Published online: 15 August 2019
Role of Melatonin Receptors in Hyperthermia-Induced Acute Seizure
Model of Rats
Rasim Mogulkoc1 &Abdülkerim Kasim Baltaci1&Leyla Aydin2
# Abstract
Melatonin is a neurohormone that has anticonvulsant activity in different experimental seizure models including hyperthermic febrile seizure. However, the mechanisms of this effect are not clear at the receptor level. The aim of the study was to determine which melatonin receptors involve in the hyperthermic febrile seizure model. 22–30 days Wistar male rats were used, and in children, it corresponds to 1.5–2 years. Groups were performed as (1) control, (2) ethanol/saline, (3) DMSO, (4) melatonin (MT), (5) MT + luzindole (LUZ), (6) MT + K-185, (7) MT + prazosin (PRZ), (8) MT + LUZ + K-185, (9) MT + LUZ + PRZ, (10) MT + K-185 + PRZ, and (11) MT + LUZ + PRZ + K-185. The hyperthermic febrile seizure pattern was established by keeping the rats in 45 °C hot water, and the latency, duration, and severity of seizures were determined in all groups. MT, LUZ, K-185, and PRZ were given 15, 45, 15, and 30 min before the induction of seizure, respectively. It was observed that melatonin shortened the duration of seizure, reduced the severity, and did not affect latency and that these effects were not completely blocked by receptor antagonists when compared with control, ethanol/saline, and DMSO groups. In conclusion, the fact that the anticonvulsant effect of melatonin is not completely blocked by all melatonin receptor antagonists. We can conclude that a multimodal mechanism may be responsible for the effect of melatonin receptors alone on the anticonvulsant effect of melatonin. It will be useful to design new pharmacological studies to make the subject clear.
Keywords Febrile seizure . Melatonin . Luzindole . K-185 . Prazosin . Rat
Introduction
Febrile seizures (FS) are the most common convulsive disease of childhood. Recent studies have reported that prolonged febrile seizures cause severe damage in temporal structures of the brain, and increase the risk for development of temporal lobe epilepsy which is a form of epilepsy refractory to medi-cation, in advanced periods (Natsume et al.2007). For this reason, studies conducted about this investigate the role of different compounds in the treatment of febrile seizures (Dubé et al.2010).
Melatonin (MT) regulates the neuronal activity of the cen-tral nervous system. Melatonin levels have been found to in-crease during seizures (Dabak et al. 2016). Similarly,
considering that melatonin regulates cerebral activity via GABA and glutamate receptors, and thus melatonin levels may be changed during seizures, Molina-Carballo et al. (2007) investigated melatonin levels during and after febrile seizures. Similar to the above-mentioned findings, it has been reported that melatonin levels increased in children both with seizures and epilepsy (Molina-Carballo et al.1994).
Manev et al. (1996) drew attention to that MT suppression occurring in pinealectomized rat’s increases cerebral damage induced by kainic acid. Thereafter, they speculated that endog-enous MT has neuroprotective properties (Acuña-Castroviejo et al.1995). In another experimental study, the effect of mela-tonin on febrile convulsion has been examined. Administration of 80 mg/kg melatonin before febrile seizures in Sprague-Dawley offspring produced an important anticonvulsant effect (Aydin et al.2015). Peled et al. (2001) administered 3 mg oral melatonin half hour before going to bed for 3 months in patients with resistant epilepsy in addition to antiepileptic therapy they have used and reported the decreased frequency of seizures. MT can be used as an antiepileptic/anticonvulsant agent, a study has been conducted with MT receptor agonist Ramelteon (Fenoglio-Simeone et al.2009).
* Rasim Mogulkoc
rasimmogulkoc@yahoo.com
1
Medical Faculty, Department of Physiology, Selcuk University, Konya, Turkey
2 Department of Physiology, Meram Medical School, Necmettin
Erbakan University, Konya, Turkey
Springer Science+Business Media, LLC, part of Springer Nature 2019 Received: 14 January 2019 / Accepted: 29 July 2019
In the previous studies, the anticonvulsant effect of MT has been observed in different experimental seizure types (Srivastava et al.2002). Anticonvulsant effects of MT has been studied in maximal electroshock model in mice (Borowicz et al.1999), pilocarpine-induced epilepsy model in rats (Costa-Lotufo et al.2002), pentylenetetrazole (PTZ)-induced seizure model in mice and guinea pigs (Solmaz et al.
2009; Yahyavi-Firouz-Abadi et al. 2007), and penicillin-induced epilepsy model in rats (Yildirim and Marangoz
2006), but no study was found in the literature screening about MT receptors in FS model.
Evaluating the current data from the literature, it is obvious that melatonin is somehow associated with seizures. It is thought that MT will affect FS latency, duration, and severity of seizures in FS model created with hyperthermia in rats.
The objective of this study was to determine which recep-tors of MT exert its effect in the FS model created by hyper-thermia in rats.
Material and Methods
This study was conducted in the Selcuk University, Experimental Medicine Research & Application Center. The ethics committee approved the study (2016-19). The study was performed on 22–30-day Sprague-Dawley male rats. The rats were allowed to undergo laboratory adaptation for 3 days. The rats were kept in standard laboratory conditions (23 ± 1 °C, humidity rate 55% ± 5) at 12-h light/dark cycle and fed with pellet bait, and water was given ad libitum. Rectal body temperatures were measured as soon as possible before the experiment and during seizures.
Febrile Seizure
Hyperthermia-Induced Febrile Seizure Model
HIFS was achieved by placing the rats in 45 °C water that did not reach the rat’s neck. The rats were removed from the water and placed on a dry towel when the seizure began (myoclonic spasm at the edge of the lips or the rat stayed still and sank to the bottom). Seizure latency, the total duration of clinical sei-zure, and seizure stage were evaluated. Seizure latency was defined as the time interval between the first contact with the water and the beginning of the seizure of animal. Clinical seizure duration was the time period from the beginning of the seizure until the rat was conscious and regained move-ment. Seizure severity was evaluated according to Racine’s scale, which assigns scores as follows: 0, no convulsive be-havior; 1, facial clonus; 2, nodding; 3, anterior limb clonus; 4, rearing up movement; and 5, rearing up and falling on one side due to imbalance (Łotowska et al.2011).
Rectal Temperature Measurement Rectal temperatures were measured prior to hyperthermia and just after seizure induc-tion using the medical purpose “Medical Grade YSI 400” temperature probe.
Experiment Groups
A total of 88 male Wistar albino male rats were used to ex-periments and groups were performed as (1) control (n = 8), (2) ethanol/saline (n = 8), (3) DMSO (n = 8), (4) melatonin (MT) (n = 8), (5) melatonin + luzindole (LUZ) (n = 8), (6) melatonin + K-185 (n = 8), (7) melatonin + prazosin (PRZ) (n = 8), (8) melatonin + LUZ + K-185 (n = 8), (9) melatonin + LUZ + PRZ (n = 8), (10) melatonin + K-185 + PRZ (n = 8), (11) melatonin + LUZ + PRZ + K-185 (n = 8).
Preparation and Administrations of Drugs
Ethanol/saline It was subcutaneously given to the animals as 0.5 mL at 2.5% concentration.
DMSO It was subcutaneously given to the animals as 0.5 mL at 20%.
Melatonin (Sigma 5250) Immediately after the use, it was dis-solved in 2.5% v/v ethanol/saline and was administered as 80 mg/kg IP 15 min before the FS model (Aydin et al.2015). Luzindole Non-selective MT1 receptor antagonist was dis-solved in 2.5% v/v ethanol/saline and was administered as 5 mg/kg IP, 45 min before the FS induction(L2407 Sigma), (Moezi et al.2011; Wellman et al.2002).
K-185 Selective MT2 receptor antagonist was dissolved in 20% dimethyl sulfoxide (DMSO) and administered subcuta-neously as 2 mg/kg 15 min before the FS induction (K1888 -Sigma), (Arreola-Espino et al.2007; Srinivasan et al.2012). Prazosin Selective MT3 receptor antagonist was dissolved in isotonic saline and administered as 1 mg/kg IP, 30 min before the FS induction (Sigma Prod. No. P7791), (Moezi et al.2011; Wellman et al.2002).
Vehicles were administered at appropriate doses (intraperitoneal) immediately before the FS protocol (Wellman et al.2002). Considering the harm doses, applica-tion time and the drug doses to be used were determined from the various studies on rats (Srinivasan et al.2012; Wellman et al.2002).
Statistical Analysis
The normality of the distribution of the variables was analyzed with the Shapiro–Wilk test and the homogeneity of the
variances was analyzed with the Levene’s test. The Kruskal– Wallis test and the Bonferroni–Dunn test (to account for mul-tiple comparisons) were used to compare the group medians because it was determined that the assumptions of the para-metric tests could not be provided. The results of all statistical analyses are presented as the mean ± standard deviation (SD) andp value < 0.05 was considered to be statistically signifi-cant. The SPSS 17.0 statistical software package program (SPSS Ver. 17.0, SSPS Inc.; Chicago, IL, USA) was used to analyze all data.
Results
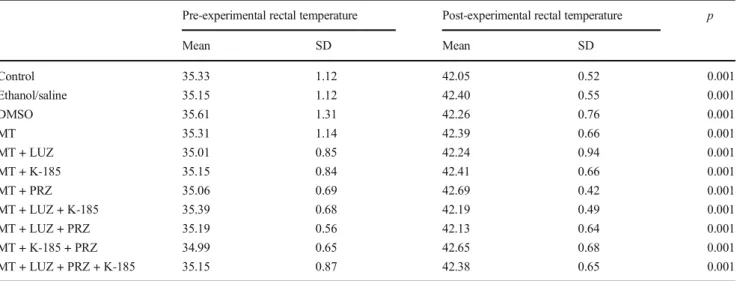
Pre-experimental and post-experimental rectal temperatures and weights of experimental animals were similar and no dif-ference in pre- and post-experimental rectal temperatures was observed within groups. There were no differences among the groups for the body weights of animals (Table1). However, the difference between pre- and post-experimental rectal tem-peratures within groups was significantly different (Table1).
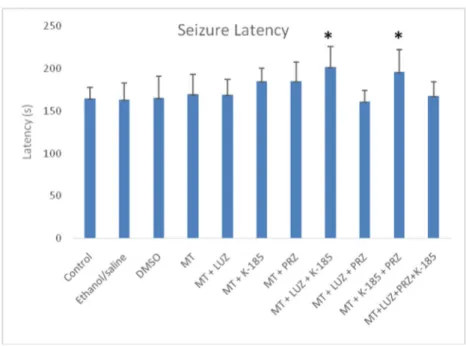
When seizure latencies were evaluated between the groups, no significant differences were found among seizures laten-cies of the group given melatonin and melatonin receptor an-tagonist (except for the group administered melatonin + LUZ + K-185), control group, ethanol/saline group, and DMSO groups (Fig.1). Latency values were increased only in group 8, which received melatonin + LUZ + K-185, and group 10, which administered melatonin + K-185 + PRZ, compared with the other groups (p < 0.05).
Whereas, melatonin application significantly decreased seizure duration compared with the control values. However, K-185 (group 6) and PRZ (group 7) combined with melatonin
significantly increased seizure durations which decreased due to MT supplementation (p < 0.001).
Seizure severity of the groups was examined according to the scale. Melatonin administration significantly suppressed seizure severity compared with the DMSO group (p < 0.001). Melatonin administration inhibited the seizure time (group 4) compared with the control, ethanol/saline, and DMSO groups. However, MT + K-185 and MT + PRZ administration increased the seizure time again compared with the only-melatonin-administered group (group 4) (p < 0.001) (Figs.2and3).
Discussion
As a result of this study, a statistically significant difference was found between pre- and post-experimental rectal temper-atures in all groups administered with melatonin and melato-nin + melatomelato-nin receptor blockers, compared with the control, ethanol/saline, and DMSO groups of Wistar albino rats. Previous studies have reported different results of the effects of melatonin on body temperature. In a study conducted to investigate anticonvulsant activity in Sprague-Dawley rats, it was found that melatonin at low doses (80 and 100 mg/kg, IP) significantly decreased rectal temperature compared with the control group but did not create a significant change in rectal temperature compared with the control group at high doses (150 mg/kg) (Aydin et al.2015). On the other hand, in a study investigating the effects of melatonin to body temperature on healthy cynomolgus monkeys, melatonin was administered to the monkeys at 4 different doses (0.2; 2; 20; 200 mg/kg, oral gavage). During the application, melatonin was given at 9:00 a.m. and the body temperature was measured after 2 h in some groups, and at 4:00 p.m. and the body temperature was Table 1 Pre- and post-experimental rectal temperatures in groups (mean ± SD)
Pre-experimental rectal temperature Post-experimental rectal temperature p
Mean SD Mean SD Control 35.33 1.12 42.05 0.52 0.001 Ethanol/saline 35.15 1.12 42.40 0.55 0.001 DMSO 35.61 1.31 42.26 0.76 0.001 MT 35.31 1.14 42.39 0.66 0.001 MT + LUZ 35.01 0.85 42.24 0.94 0.001 MT + K-185 35.15 0.84 42.41 0.66 0.001 MT + PRZ 35.06 0.69 42.69 0.42 0.001 MT + LUZ + K-185 35.39 0.68 42.19 0.49 0.001 MT + LUZ + PRZ 35.19 0.56 42.13 0.64 0.001 MT + K-185 + PRZ 34.99 0.65 42.65 0.68 0.001 MT + LUZ + PRZ + K-185 35.15 0.87 42.38 0.65 0.001
measured after 11 h in some groups. When body temperatures were evaluated at the end of the study, the body temperature was not changed in animals administered with a low dose of melatonin (0.2–2-20 mg/kg). However, body temperature was found to significantly decrease in the group given 200 mg/kg melatonin compared with the controls (Inui and Hazeki2010). The difference between the studies might result from the dif-ferences in the experimental animals and study methods used. When study results were examined in terms of seizure latency, there were no differences in seizure latency among melatonin group, melatonin + melatonin receptor antagonist group (except for melatonin + LUZ + K-185 groups), control group, ethanol/ saline group, and DMSO group. There are controversial research results in the literature on this topic. Solmaz et al. (2009) reported that 10 mg/kg melatonin significantly prolonged the latency in the seizure in pigs induced by pentylenetetrazole. In their study,
Aydın et al. (2015) demonstrated that 150 mg/kg (IP) adminis-tration of melatonin did not affect FS latency, while 80 and 100 mg/kg (IP) melatonin significantly increased the latency. In the mentioned research, the Sprague-Dawley rats were used. However, in the present study, the use of melatonin in 22–30-day-old Wistar albino rats did not affect seizure latency (although there was a partial numerical increase). In a previous study, the use of 80 mg intraperitoneal melatonin was reported to signifi-cantly increase the latency, and the difference could be said to be completely caused by the rat species because, despite the use of the same experimental methods and the same dose of melatonin, the only difference was the use of a different rat species. However, latency values were increased only in group 8, which received melatonin + LUZ + K-185, and group 10, which re-ceived melatonin + K-185 + PRZ, compared with the other groups. It was thought that the latency increase seen in Wistar Fig. 1 Seizure latency of groups.
*MT + LUZ + K-185– and *MT + K-185 + PRZ–administered groups have the highest latency levels compared with all other groups (groups 8 and 10) (p < 0.05)
Fig. 2 Seizure time of groups. Melatonin administration inhibited seizure time (group 4) compared with the control, ethanol/saline, and DMSO groups. However, MT + K-185 and MT + PRZ administration increased the seizure time again compared with the melatonin (MT) group (group 4) (p < 0.001)
albino rats were due to melatonin receptor 3 (PRZ) in group 8 and the melatonin receptor 1 (LUZ) in group 10 remained active (because the other receptors were suppressed). In a study inves-tigating the anticonvulsant activity of melatonin in seizure model induced with penicillin in female Wistar rats, 20, 40, and 80μg doses of melatonin were administered as intracerebroventricular, and the rats were monitored for 1 h. Whereas no significant change was observed in the rats that received 20μg melatonin compared with the controls, epileptiform activity latency was significantly increased in the groups that received 40 and 80μg melatonin (Yildirim and Marangoz2006). In their study investi-gating the anticonvulsant activity of melatonin alone and the combined use of melatonin with phenobarbital in seizure model induced with pentylenetetrazole, Forcelli et al. (2013) used 7-day female Sprague-Dawley rats. At the end of the study, they found that melatonin alone at 20, 40, and 80 mg/kg did not affect seizure latency in all three doses, while the combined use of melatonin with 20 mg/kg phenobarbital increased the latency only in the group which received 20 mg melatonin.
When the groups were examined in terms of seizure dura-tion, seizure durations were shortened in all the groups that received melatonin and melatonin + melatonin receptor antag-onists compared with the control, ethanol/saline, and DMSO groups, although seizure durations were increased in the mel-atonin + LUZ group and melmel-atonin + K-185 group compared with group 4 which received only melatonin. This suggests that the shortening in seizure duration occurred through mel-atonin receptors 2 and 3. However, no statistically significant difference was found in this parameter between the group administered with melatonin receptor antagonist 1 (melatonin + LUZ) and the melatonin group, suggesting that this might be caused by different mechanisms rather than the receptors. It is known for long years that melatonin modulated GABA recep-tor activity, increasing the effect of GABAergic system and decreasing neuronal excitation, without binding to gamma-aminobutyric acid (GABA) receptor (Acuña Castroviejo
et al.1986; Cheng et al.2012; Golombek et al.1992; Niles et al.1997). Ray et al. (2004) postulated that GABAergic mechanisms may play an important role in the seizure model induced by maximal electroshock. In their study, Bikjdaouene et al. (2003) found that melatonin IP administered at 60 and 160 mg/kg 30 min after in the PTZ-induced seizure model in rats significantly increased GABA in the brain tissue samples collected 3 h after the seizure. In light of these studies, it can be said that a GABA-mediated mechanism may be responsi-ble for the effect of melatonin on seizure duration. On the other hand, the results of the studies on melatonin receptor agonists or antagonists are controversial. Mosinska et al. (2016) compared the anticonvulsant activity in a single-dose acute use of melatonin with a half-life of 30–45 min and 2 new melatonin receptor agonist agents (Neu-P11 and Neu-P97) with a relatively long half-life. For this purpose, comparisons have been made in 3 different mouse models: 6 Hz psycho-motor seizure model, maximal electroshock model, and pentylenetetrazole seizure model. Although melatonin de-creased the duration and severity of seizures in all three models, interestingly, melatonin receptor agonists did not pro-duce an anticonvulsant effect, and it has been argued that further new pharmacological studies should be conducted to understand the anticonvulsive action mechanism of melato-nin. In contrary, in the study of Aygun et al. (2015), 1-week applications of melatonin and agomelatine, which is a differ-ent melatonin receptor agonist and used as an antidepressant (also is a 5HT2c receptor antagonist), were compared in an absence epilepsy model in WAG/Rij rats, and similar anticon-vulsant effects between agomelatine and MT in the absence epilepsy model were found. Fenoglio-Simeone et al. (2009) found that the use of melatonin ½ receptor agonist ramelteon (30 or 100 mg/kg intraperitoneal, 2 × 1, for 5 days) showed an anticonvulsant effect in a rapid kindling model in rats.
In their study, Moezi et al. (2011) found that although the combined use of luzindole and melatonin blocked the Fig. 3 Seizure severity in groups
(scale). MT administration reduced seizure severity (group 4) compared with the control, ethanol/saline, and DMSO groups. However, MT + LUZ (group 5) and MT + K-185 (group 6) have increased Racine scale values compared with the only-MT-administered group (group 4) (p < 0.001)
anticonvulsant effect of melatonin in seizure model induced with PTZ (which blocks GABA-mediated inhibition), prazosin did not produce any change in the anticonvulsant activity of melatonin. However, prazosin reinforced the effect of melatonin in maximal electroshock model in mice (Ray et al.2004).
In the light of the above-mentioned studies, we can say that different activities of melatonin receptor agonists or antago-nists in different experimental animals or different seizure models suggest that the basic mechanisms in the anticonvul-sant activity of melatonin differ depending on the experimen-tal animals and seizure models used.
In our study, melatonin was found to decrease seizure severity, consistently with the other studies (Aydin et al.2015; Aydin et al.
2017; Solmaz et al.2009). Even in their retrospective study conducted in 2018, Yamaguchi et al. (2018) indicate that al-though the severity of seizures was stable over a 24-h period, the occurrence of seizures in our cohort of pediatric patients with complex febrile seizures requiring hospitalization was highest in the evening and lowest at night. On the other hand, in our study, MT administration inhibited seizure severity (group 4). However, melatonin + LUZ (group 5) and melatonin + K-185 (group 6) administration increased again the seizure severity in a febrile seizure. Thereafter, we postulated that the effect of melatonin is related to MT1 and MT2 receptors because MT3 receptor antag-onist PRZ alone or combined with melatonin or other MT recep-tor antagonists did not make any impact on febrile seizure severity.
As a result in the present experimental model, the anticonvul-sant effect of melatonin was not completely blocked by melato-nin receptor antagonists, suggesting that melatomelato-nin receptors alone are not responsible, and a multimodal mechanism may also be responsible for the anticonvulsant effect of melatonin. Further new studies would be beneficial to clarify this issue. Studies in the literature were utilized to not increase the use of experimental animals, which is a limitation of the present study.
Funding This study was supported by a grant from the Selcuk University, Scientific Research Council (grant number is 16401130). Authors would like to thank Begüm Aydin Gazi University Faculty of Medicine helps during experiments.
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
References
Acuña Castroviejo D, Rosenstein RE, Romeo HE, Cardinali DP (1986) Changes in gamma-aminobutyric acid high affinity binding to cere-bral cortex membranes after pinealectomy or melatonin administra-tion to rats. Neuroendocrinology 43:24–31
Acuña-Castroviejo D, Escames G, Macías M, Muñóz Hoyos A, Molina Carballo A, Arauzo M, Montes R (1995) Cell protective role of melatonin in the brain. J Pineal Res 19:57–63
Arreola-Espino R, Urquiza-Marín H, Ambriz-Tututi M, Araiza-Saldaña CI, Caram-Salas NL, Rocha-González HI, Mixcoatl-Zecuatl T, Granados-Soto V (2007) Melatonin reduces formalin-induced nociception and tactile allodynia in diabeticrats. Eur J Pharmacol 577:203–210
Aydin L, Gundogan NU, Yazici C (2015) Anticonvulsant efficacy of melatonin in an experimental model of hyperthermic febrile sei-zures. Epilepsy Res 118:49–54
Aydin L, Yurtcu E, Korkmaz Y, Sezer T, Ogus E (2017) Effect of mela-tonin on cytokine levels in a hyperthermia-induced febrile seizure model. Cell Mol Biol (Noisy-le-grand) 63:11–16
Aygun H, Aydın D, Inanır S, Ekici F, Ayyıldız M, Agar A (2015) The effects of agomelatine and melatonin on ECoG activity of absence epilepsy model in WAG/Rij rats. Turk J Biol 39:904–910 Bikjdaouene L, Escames G, León J, Ferrer JM, Khaldy H, Vives F,
Acuña-Castroviejo D (2003) Changes in brain amino acids and ni-tric oxide after melatonin administ ration in rat s with pentylenetetrazole-induced seizures. J Pineal Res 35:54–60 Borowicz KK, Kamiñski R, G1sior M, Kleinrok Z, Czuczwar SJ (1999)
Influence of melatonin upon the protective action of conventional anti-epileptic drugs against maximal electroshock in mice. Eur Neuropsychopharmacol 9: 185–190
Cheng XP, Sun H, Ye ZY, Zhou JN (2012) Melatonin modulates the GABAergic response in cultured rat hippocampal neurons. J Pharmacol Sci 119:177–185
Costa-Lotufo LV, de Fonteles MM, Lim ISP, de Oliveira AA, Nascimento VS, de Bruin VM, Viana GS (2002) Attenuating effects of melato-nin on pilocarpine-induced seizures in rats. Comp Biochem Physiol C 131:521–529
Dabak O, Altun D, Arslan M, Yaman H, Vurucu S, Yesilkaya E, Unay B (2016) Evaluation of plasma melatonin levels in children with afe-brile and feafe-brile Seizures. Pediatr Neurol 57:51–55
Dubé CM, Ravizza T, Hamamura M, Zha Q, Keebaugh A, Fok K, Andres AL, Nalcioglu O, Obenaus A, Vezzani A, Baram TZ (2010) Epileptogenesis provoked by prolonged experimental febrile sei-zures: mechanisms and biomarkers. J Neurosci 30:7484–7494 Fenoglio-Simeone K, Mazarati A, Sefidvash-Hockley S, Shin D, Wilke J,
Milligan H, Sankar R, Rho JM, Maganti R (2009) Anticonvulsant effects of the selective melatonin receptor agonist ramelteon. Epilepsy Behav 16:52–57
Forcelli PA, Soper C, Duckles A, Gale K, Kondratyev A (2013) Melatonin potentiates the anticonvulsant action of phenobarbital in neonatal rats. Epilepsy Res 107:217–223
Golombek DA, Fernández Duque D, De Brito Sánchez MG, Burin L, Cardinali DP (1992) Time-dependent anticonvulsant activity of mel-atonin in hamsters. Eur J Pharmacol 210:253–258
Inui Y, Hazeki O (2010) Acute effects of melatonin and its time of ad-ministration on core body temperature and heart rate in cynomolgus monkeys. J Toxicol Sci 35:383–391
Łotowska MES, Joanna M, Łotowska JM (2011) The neuroprotective effect of topiramate on the ultrastructure of pyramidal neurons of the hippocampal CA1 and CA3 sectors in an experimental model of febrile seizures in rats. Folia Neuropathol 49:230–236
Manev H, Uz T, Kharlamov A, Joo JY (1996) Increased brain damage after stroke or excitotoxic seizures in melatonin-deficient rats. FASEB J 10:1546–1551
Moezi L, Shafaroodi H, Hojati A, Dehpour AR (2011) The interaction of melatonin and agmatine on pentylenetetrazole-induced seizure threshold in mice. Epilepsy Behav 22:200–206
Molina-Carballo A, Acuña-Castroviejo D, Rodriguez-Cabezas T, Muñoz-Hoyos A (1994) Effects of febrile and epileptic convulsions on daily variations in plasma melatonin concentration in children. J Pineal Res 16:1–9
Molina-Carballo A, Muñoz-Hoyos A, Sánchez-Forte M, Uberos-Fernández J, Moreno-Madrid F, Acuña-Castroviejo D (2007) Melatonin increases following convulsive seizures may be related to its anticonvulsant properties at physiological concentrations. Neuropediatrics 38:122–125
Mosińska P, Socała K, Nieoczym D, Laudon M, Storr M, Fichna J, Wlaź P (2016) Anticonvulsant activity of melatonin, but not melatonin receptor agonists Neu-P11 and Neu-P67, in mice. Behav Brain Res 307:199–207
Natsume J, Bernasconi N, Miyauchi M, Naiki M, Yokotsuka T, Sofue A, Bernasconi A (2007) Hippocampal volumes and diffusion-weighted image findings in children with prolonged febrile seizures. Acta Neurol Scand 115(Suppl 186):25–28
Niles LP, Smith LJ, Tenn CC (1997) Modulation of c-fos expression in the rat striatum by diazepam. Neurosci Lett 236:5–8
Peled N, Shorer Z, Peled E, Pillar G (2001) Melatonin effect on seizures in children with severe neurologic deficit disorders. Epilepsia 42: 1208–1210
Ray M, Mediratta PK, Reeta K, Mahajan P, Sharma KK (2004) Receptor mechanisms involved in the anticonvulsant effect of melatonin in maximal electroshock seizures. Methods Find Exp Clin Pharmacol 26:177–181
Solmaz I, Gurkanlar D, Gokcil Z, Cuneyt G, Ozkan M, Erdogan E (2009) Antiepileptic activity of melatonin in Guinea pigs with pentylenetetrazol-induced seizures. Neurol Res 2009(31):989–985
Srinivasan V, Zakaria R, Jeet Singh H, Acuna-Castroviejo D (2012) Melatonin and its agonists in pain modulation and its clinical appli-cation. Arch Ital Biol 150:274–289
Srivastava AK, Gupta SK, Jain S, Gupta YK (2002) Effect of melatonin and phenytoin on an intracortical ferric chloride model of posttrau-matic seizures in rats. Methods Find Exp Clin Pharmacol 24:145– 149
Wellman P, Ho D, Cepeda-Benito A, Bellinger L, Nation J (2002) Cocaine-induced hypophagia and hyperlocomotion in rats are atten-uated by prazosin. Eur J Pharmacol 455:117–126
Yahyavi-Firouz-Abadi N, Tahsili-Fahadan P, Riazi K, Ghahremani MH, Dehpour AR (2007) Melatonin enhances the anticonvulsant and proconvulsant effects of morphine in mice: role for nitric oxide signaling pathway. Epilepsy Res 75:138–144
Yamaguchi H, Nagase H, Ishida Y, Toyoshima D, Maruyama A, Tomioka K, Tanaka T, Nishiyama M, Fujita K, Mariko TI, Nozu K, Morioka I, Nishimura N, Kurosawa H, Takada S, Uetani Y, Iijima K (2018) Diurnal occurrence of complex febrile seizure and their severity in pe-diatric patients needing hospitalization. Epilepsy Behav 80:280–284 Yildirim M, Marangoz C (2006) Anticonvulsant effects of melatonin on
penicillin-induced epileptiform activity in rats. Brain Res 1099:183–188 Publisher’s Note Springer Nature remains neutral with regard to jurisdic-tional claims in published maps and institujurisdic-tional affiliations.