EFFECT OF DIETARY CONJUGATED LINOLEIC ACID
ON PERFORMANCE AND BLOOD PARAMETERS OF
BROILER CHICKENS EXPOSED TO HEAT STRESS
Ladine ÇelikÇukurova University, Agricultural Faculty Department of Animal Science 01330 Adana, Turkey
SUMMARY
The objectives of the present study were to evaluate whether dietary conjugated linoleic acid (CLA) would affect performance and blood glucose, cholesterol and triglyceride levels of broiler chicks exposed to heat stress. One-day-old male broiler chicks (Ross 308) were fed a diet supplemented with 0, 2, 4, or 6% CLA for a period of 42 days.
Inclusion of CLA in broiler diets, especially 2%, in-creased (P<0.05) body weight gain from 4 to 6 weeks of age. Increased dietary CLA linearly improved (P<0.05) feed conversion ratio at 4th week of age. Dietary CLA increased
(P<0.05) cold carcass weight, carcass yield, abdominal fat weight, relative abdominal fat weight, and liver weight. Plasma glucose and cholesterol concentrations were in-creased (P<0.01) by dietary CLA, while triglycerides` con-centration was not statistically affected.
The results showed that 2% CLA inclusion to the diet improved growth performance and fatness in broiler chickens under heat stress conditions.
KEYWORDS: conjugated linoleic acid (CLA), broiler, heat stress, performance, blood parameters.
INTRODUCTION
Conjugated linoleic acid (CLA) refers to a group of positional and geometric octadecadienoic acid isomers of linoleic acid (C18:2), containing conjugated double-bonds at positions 8 and 10, 9 and 11, and 10 and 12 or 11 and 13. CLA has long been discovered as a functional food for human nutrition, playing a crucial role with its anticarcino-genic [1], antiatheroanticarcino-genic [2], antidiabetic [3], and anti-in- flammatory [4] effects. CLA has also been reported to en-hance immune function [5], increase bone formation [6], reduce body fat and increase lean body mass [7] in several species of growing monogastric animals.
Martin et al. [8] indicated that t10,c12-CLA increased activity of hepatic and adipose carnitine palmitoyltrans-ferase, the responsible enzyme for entry of long-chain fatty acids into mitochondria for energy–generating processes via ß-oxidation, in male weanling Wistar rats that consumed a diet containing 1% t10,c12-CLA for 6 weeks. Park et al. [9] also showed that trans-10,12-CLA, but not the cis-9,trans-11-CLA isomer, could inhibit fatty acid synthesis. It is, therefore, speculated that CLA supplementation of diets could be beneficial for energy metabolism; thereby facili-tating fatty acid oxidation and balancing energy deficit by utilization of long-chain fatty acids under stressful condi-tions.
CLA has been shown to reduce body fat accumulation and to improve lean mass in mice [7], rats [10] and pigs [11]. However, the body fat-lowering and performance-improving effects of CLA were contradictory in other ex-perimental animals, such as broiler chicks [12, 13], pigs [14], and rabbits [15]. Szymczyk et al. [12] suggested that the effectiveness of dietary CLA (0.0, 0.5, 1.0 or 1.5% for 34 days) linearly reduced body weight gains. Du and Ahn [16] evaluated the effects of CLA on broiler chicks in two consecutive studies. In the first study, dietary CLA, given at 0.25, 0.5 or 1% from 3 to 6 weeks of age, had no effect on growth performance, abdominal fat weight, total body fat and protein contents of body muscles. In the second study, 2 or 3% dietary CLA, given from 3 to 8 weeks of age, reduced total body fat significantly, and body weight gain numerically. Additionally, Badinga et al. [13] reported that dietary CLA (5%) had no positive effects on growth performance of broiler chicks. However, hepatic lipid and triacylglycerol concentrations were significantly reduced. Similarly, reduced hepatic triglycerides and increased cho-lesterol concentration in plasma have been reported in broiler chicks receiving dietary CLA at 2 or 4%, but with no improvements in growth performance from 3 to 6 weeks of age [17].
The present literature regarding dietary CLA in broil-ers exhibited contradictory results, besides no reports on
the effects of dietary CLA in broilers having stress stimuli, such as heat, which has been well-documented with its de-trimental effects on metabolism and also growth perfor-mance in broilers. It is well-known that many dietary methods have been tried with varying degrees of success over the past 30 years in order to identify the most limiting nutrient(s) or re-establish physiological equilibri-um to correct nutrients` utilization and metabolism in the body during heat stress (e.g. Kutlu and Forbes [18]). CLA with its regulatory effects on energy metabolism could be of value in diets of broilers under heat stress, during which energy metabolism of broilers is disturbed, and fat accumulation is increased (e.g. Baziz et al. [19].
The purpose of the present study was to evaluate whether CLA would affect performance and energy me-tabolism (monitoring by fatness, plasma glucose, choles-terol, and triglycerides` concentrations) of broiler chicks exposed to heat stress.
MATERIALS AND METHODS
Eighty, one-day-old male broiler chicks (Ross 308) ob-tained from a commercial hatchery were assigned to four
dietary treatments with a similar mean weight (45 ± 3.50 g), comprising 20 birds each. Standard broiler starter (230 g/kg CP and 13.4 MJ/kg AME, from 1-3 weeks) and finisher (200 g/kg CP and 13.4 MJ/kg AME, from 4-6 weeks) diets were formulated (Table 1), with a similar nutrient profile of each, but differing in CLA contents (0, 2, 4, or 6% CLA, Luta-CLA 60 (Table 1), obtained from BASF, The Chemi-cal Company, Ludwigshafen, Germany). The birds were housed in an air-conditioned room at a conventional ambi-ent temperature (20-22 C) with a relative humidity of 60-70%, except during the period of heating, when the envi-ronmental temperature fluctuated from 34 to 36 C with a relative humidity of 40-50% for 8 h per day.
During the experiment, which lasted 42 days, each group received feed and water ad libitum. The birds were housed individually in cages of three-tiers battery blocks to allow measurement of individual feed intake and body weight every week. Feed conversion ratio (g feed/g gain) was calculated weekly as the amount of feed consumed per unit of body weight gain. At the end of the experiment, when chickens were 42 days of age, all birds were slaugh-tered, immediately plucked, eviscerated, and then weighed and chilled overnight in a fridge, in order to facilitate the removal of abdominal fat before estimation of cold car-
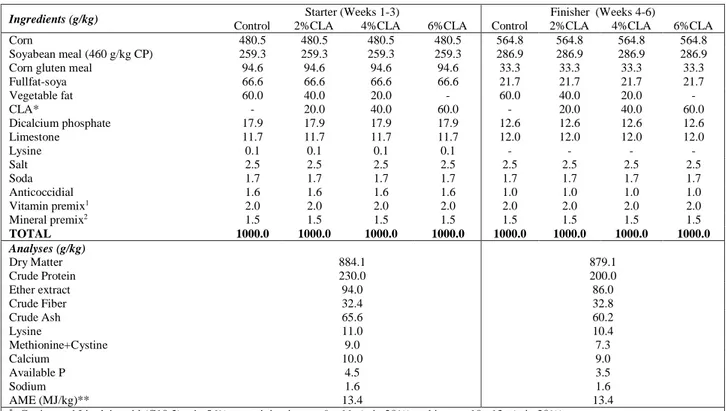
TABLE 1 - Composition and nutrients of experimental diets.
Ingredients (g/kg) Starter (Weeks 1-3) Finisher (Weeks 4-6)
Control 2%CLA 4%CLA 6%CLA Control 2%CLA 4%CLA 6%CLA Corn 480.5 480.5 480.5 480.5 564.8 564.8 564.8 564.8 Soyabean meal (460 g/kg CP) 259.3 259.3 259.3 259.3 286.9 286.9 286.9 286.9 Corn gluten meal 94.6 94.6 94.6 94.6 33.3 33.3 33.3 33.3 Fullfat-soya 66.6 66.6 66.6 66.6 21.7 21.7 21.7 21.7 Vegetable fat 60.0 40.0 20.0 - 60.0 40.0 20.0 - CLA* - 20.0 40.0 60.0 - 20.0 40.0 60.0 Dicalcium phosphate 17.9 17.9 17.9 17.9 12.6 12.6 12.6 12.6 Limestone 11.7 11.7 11.7 11.7 12.0 12.0 12.0 12.0 Lysine 0.1 0.1 0.1 0.1 - - - - Salt 2.5 2.5 2.5 2.5 2.5 2.5 2.5 2.5 Soda 1.7 1.7 1.7 1.7 1.7 1.7 1.7 1.7 Anticoccidial 1.6 1.6 1.6 1.6 1.0 1.0 1.0 1.0 Vitamin premix1 2.0 2.0 2.0 2.0 2.0 2.0 2.0 2.0 Mineral premix2 1.5 1.5 1.5 1.5 1.5 1.5 1.5 1.5 TOTAL 1000.0 1000.0 1000.0 1000.0 1000.0 1000.0 1000.0 1000.0 Analyses (g/kg) Dry Matter 884.1 879.1 Crude Protein 230.0 200.0 Ether extract 94.0 86.0 Crude Fiber 32.4 32.8 Crude Ash 65.6 60.2 Lysine 11.0 10.4 Methionine+Cystine 9.0 7.3 Calcium 10.0 9.0 Available P 4.5 3.5 Sodium 1.6 1.6 AME (MJ/kg)** 13.4 13.4
*: Conjugated Linoleic acid (C18:2) min 56%; containing isomer 9c, 11t (min 28%) and isomer 10t, 12c (min 28%). **: AME (MJ/kg) was calculated using EC equation (Larbier and Leclercq [21], p. 71)
1: each 2 kg aliquot of vitamin premix contains 12 000 000 IU Vitamin A, 3 500 000 IU Vitamin D
3, 100 g Vitamin E, 3 g Vitamin K3, 2.5 g Vitamin B1, 6 g Vitamin B2, 25 g Niacin, 12 g Ca-D-Pantothenate, 4 g Vitamin B6, 15 mg Vitamin B12, 1.5 g Folic Acid, 150 mg D-Biotin, 100 g Vitamin C, and 450 g Choline Chloride,
cass weight. During the slaughtering process, 5 birds from each group were randomly chosen to take blood samples into heparinized tubes. After centrifugation, the plasma was collected and stored at -20 C, pending analysis for glucose, cholesterol and triglycerides. After collecting blood samples, they were analysed with commercial kits for glu-cose (gluglu-cose GOD-PAP; Roche Diagnostics, GmbH,
Ger-many), cholesterol (cholesterol CHOD-PAD; Roche Di-agnostics, GmbH, Germany), or triglyceride (triglycerides GPO-PAP; Roche Diagnostics, GmbH, Germany) levels.
The data obtained in the experiment were analysed us-ing the General Linear Models (GLM), and orthogonal polynomial of SAS [20]. Linear, quadratic and cubic ef-fects (P<0.05) were determined by orthogonal polynomial contrasts.
RESULTS
The effects of CLA on feed intake, body weight gain, feed conversion ratio, carcass characteristics, and some plasma parameters are presented in Tables 2-6, respec-tively.
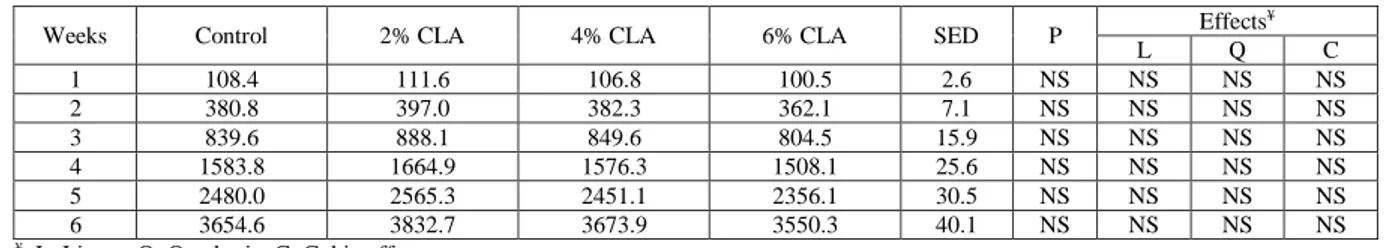
Feed intake of the birds was not affected (P>0.05) by CLA, and all them consumed similar amounts of feed during the experimental period (Table 2). However, body weight gain of broilers showed a significant (P<0.05) increase, when feeding 2% dietary CLA during the last 3 weeks of the experiment. Body weight gain decreased (P<0.05) linearly from 4 to 5 weeks of age, when dietary CLA increased. Body weight gain also had a quadratic res-
TABLE 2 - Effect of dietary CLA on cumulative feed intake (g/bird) of broiler chicks exposed to a high ambient temperature.
Weeks Control 2% CLA 4% CLA 6% CLA SED P Effects ¥ L Q C 1 108.4 111.6 106.8 100.5 2.6 NS NS NS NS 2 380.8 397.0 382.3 362.1 7.1 NS NS NS NS 3 839.6 888.1 849.6 804.5 15.9 NS NS NS NS 4 1583.8 1664.9 1576.3 1508.1 25.6 NS NS NS NS 5 2480.0 2565.3 2451.1 2356.1 30.5 NS NS NS NS 6 3654.6 3832.7 3673.9 3550.3 40.1 NS NS NS NS ¥: L: Linear; Q: Quadratic; C; Cubic effects
SED: Standard error of difference between means NS: Not significant (P>0.05).
TABLE 3 - Effect of dietary CLA on body weight gain (g/bird) of broiler chicks exposed to a high ambient temperature. Weeks Control 2% CLA 4% CLA 6% CLA SED P Effects
¥ L Q C 0 44.9 45.4 44.7 45.0 0.5 NS NS NS NS 1 82.9 83.3 79.7 79.4 2.2 NS NS NS NS 2 291.3 305.0 280.6 273.4 5.6 NS NS NS NS 3 633.1 653.3 610.1 590.6 11.1 NS NS NS NS 4 1125.8 1181.7 1093.1 1045.6 17.2 * * NS NS 5 1654.3 1694.8 1627.1 1550.4 17.8 * * NS NS 6 2306.6 2415.1 2303.7 2212.2 24.3 * NS * NS ¥: L: Linear; Q: Quadratic; C; Cubic effects
SED: Standard error of difference between means NS: Not significant (P>0.05).
*: P<0.05.
TABLE 4 - Effect of dietary CLA on feed conversion ratio (g feed/g gain) of broiler chicks exposed to a high ambient temperature. Weeks Control 2% CLA 4% CLA 6% CLA SED P Effects
¥ L Q C 1 1.33 1.37 1.34 1.29 0.03 NS NS NS NS 2 1.31 1.31 1.36 1.33 0.01 NS NS NS NS 3 1.33 1.36 1.39 1.36 0.01 NS NS NS NS 4 1.41 1.41 1.44 1.44 0.01 NS * NS NS 5 1.50 1.51 1.51 1.52 0.01 NS NS NS NS 6 1.59 1.59 1.60 1.61 0.01 NS NS NS NS ¥: L: Linear; Q: Quadratic; C; Cubic effects
SED: Standard error of difference between means NS: Not significant (P>0.05).
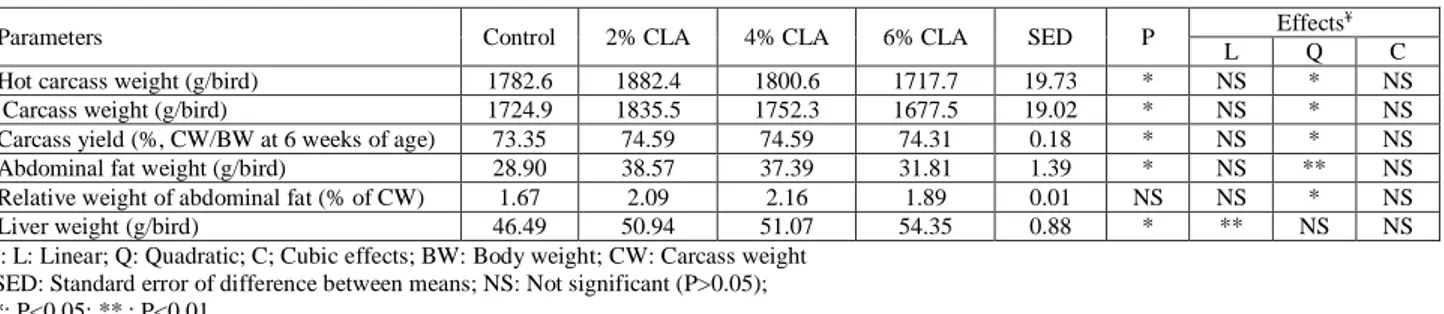
TABLE 5 - Effect of dietary CLA on carcass parameters of broiler chicks exposed to a high ambient temperature. Parameters Control 2% CLA 4% CLA 6% CLA SED P Effects
¥
L Q C Hot carcass weight (g/bird) 1782.6 1882.4 1800.6 1717.7 19.73 * NS * NS Carcass weight (g/bird) 1724.9 1835.5 1752.3 1677.5 19.02 * NS * NS Carcass yield (%, CW/BW at 6 weeks of age) 73.35 74.59 74.59 74.31 0.18 * NS * NS Abdominal fat weight (g/bird) 28.90 38.57 37.39 31.81 1.39 * NS ** NS Relative weight of abdominal fat (% of CW) 1.67 2.09 2.16 1.89 0.01 NS NS * NS Liver weight (g/bird) 46.49 50.94 51.07 54.35 0.88 * ** NS NS ¥: L: Linear; Q: Quadratic; C; Cubic effects; BW: Body weight; CW: Carcass weight
SED: Standard error of difference between means; NS: Not significant (P>0.05); *: P<0.05; ** : P<0.01.
TABLE 6 - Effect of dietary CLA on plasma glucose, cholesterol and triglyceride concentrations (mg/dl) exposed to a high ambient temperature. Parameters Control 2% CLA 4% CLA 6% CLA SED P Effects
¥
L Q C Glucose 174.2 241.0 232.0 251.2 6.28 ** ** * NS Cholesterol 88.8 128.0 122.2 139.2 5.48 ** ** NS NS Triglycerides 32.4 46.4 40.8 40.2 2.20 NS NS NS NS ¥: L: Linear; Q: Quadratic; C; Cubic effects
SED: Standard error of difference between means NS: Not significant (P>0.05).
*: P<0.05 ** : P<0.01
ponse to increasing CLA inclusion during the last week of the experiment (Table 3). Increased CLA content in the diet linearly increased (P<0.05) feed conversion ratio at 4th week of the experiment, without any significant effect
before or after (Table 4).
As indicated in Table 5, cold carcass weight, ab-dominal fat weight, carcass yield, and liver weight signif-icantly increased (P<0.05) by dietary CLA. Cold carcass weight, abdominal fat weight, carcass yield, and relative abdominal fat weight had quadratic responses to increas-ing dietary CLA. The increase in liver weight was dose-dependent.
Plasma glucose and cholesterol concentrations in-creased (P<0.05) linearly, whereas triglycerides` concen-trations were not influenced (P>0.05) by dietary CLA. In-creasing dietary CLA supplementation also caused quadrat-ic change in plasma concentration of glucose (Table 6).
DISCUSSION AND CONCLUSION
In the present study, dietary inclusion of CLA, in par-ticular at 2%, resulted in a maximal benefit in perfor-mance of broiler chicks exposed to a high ambient tem-perature, as the group receiving the diet containing 2% CLA attained higher body weigh gain and feed intake. However, body weight gain, carcass weight, carcass yield, and abdominal fat weight showed significant declines as the dietary CLA level increased over 2%. The improve-ments with 2% CLA under heat stress could be attributed to CLA’s regulatory effects on energy metabolism, which
is greatly disturbed by heat stress. It is well-documented that under heat stress birds undergo a number of complex and imperfectly understood physiological changes, such as increased blood corticosterone and catecholamines and their subsequent catabolic effects, reduced secretion of thyroid hormones, growth hormones and insulin (e.g. Web-ster [22]). As consequences of metabolic changes due to heat stress, birds reduce protein synthesis, and increased fat accumulation, paving to less energy utilization and higher heat increment in the body. Under these circum-stances, increased fat utilization to maintain energy ex-penditure could increase energy efficiency and reduce heat increment in the body, due to specific dynamic effects of fat. As CLA was shown to improve fat utilization and en-ergy expenditure in the body (e.g. Mersmann [23]), diets containing CLA could be expected to lower heat incre-ment and improve animal performance under heat stress conditions. However, 6% dietary CLA induced reductions in weight gain and fat accumulation, in contrast to 2% CLA. The possible explanation is that an excessive CLA inclusion (6% in our study) may stimulate fatty acid oxida-tion and, thus, enhance metabolic rate, leading to less fat accumulation and less weight gain due to reduced fat depo-sition in the body, as the weight gain is the sum of protein, fat and bone as dry matter basis.
However, the results obtained in this experiment were not totally in agreement with previous reports. Szymczyk et al. [12] demonstrated a decreased feed intake and body weight gain, when given CLA particularly at the 1.5% in broiler diets. Du and Ahn [16] also reported that perfor-mance characteristics of broiler chickens were not
influenced by dietary CLA at levels of 2 and 3% diet for 5 weeks. Aletor et al. [17] also found that body growth of broilers, feeding a low protein diet containing 2 or 4% CLA, were not significantly influenced. Badinga et al. [13] showed that, when the dietary CLA level was 5%, broilers exhibited reduced feed intake and weight gain. Takahashi et al. [24] showed that feeding CLA, at a concentration of 1% diet, did not affect growth performance. Former ex-periments have observed that the effectiveness of dietary CLA for decreasing weight gain and abdominal fat weight of broilers could be associated with decreasing fatty acid and tri-glyceride synthesis, but increasing energy ex-penditure, lipolysis, and fatty acid oxidation. Additional studies are needed in broilers to determine how CLA affects body weight gain and/or fat deposition, and their relations to CLA level in the diet.
Our results also showed that CLA significantly in-creased liver weight. The inin-creased liver weight associat-ed with CLA treatment is likely due to lipid accumulation in the liver, as observed by visual examination in the present study. Similarly, Szymczyk et al. [12] and Badinga et al. [13] found that dietary CLA supplementation tended to elevate liver weight, due to increased fat metabolism. In our study, CLA supplementation also increased glucose, cholesterol and triglyceride concentrations. These results may indicate that dietary CLA improves energy metabo-lism via fat utilization. However, our results, with respect to blood glucose, cholesterol and triglyceride concentra-tions, did not support the results of Choi et al. [25] for rats. They reported that dietary CLA at about 1% tended to decrease serum glucose, cholesterol or triglyceride lev-els, compared with high fat diet in rats. They also claimed that beneficial effects of CLA on glucose metabolism may depend on the dose and isomer specificity. Therefore, age, sex, dietary CLA levels, type of isomer of CLA, feeding programme, nutrient composition of the diet, nutritional status of broilers, and/or managerial environmental condi-tions may be factors affecting growth performance and some plasma parameters.
In conclusion, the present study showed that dietary CLA at 2% of diet provides an optimal performance in broiler chicks exposed to a high ambient temperature. Die-tary CLA at that level could be considered as a protective management process in diets, reducing the negative effect of heat stress on broiler chicks. Dietary CLA used in the present study could have the potential to regulate energy metabolism via fat utilization, as observed with the changes in plasma glucose, cholesterol and triglyceride concentra-tions of birds examined.
ACKNOWLEDGEMENTS
The author is grateful to Çukurova University, Agri-cultural Faculty, Research Fund and BASF, The Chemical Company, Ludwigshafen, Germany for their supports.
REFERENCES
[1] Ip, C. (1997) Review of the effects of trans fatty acids, oleic acid, n-3 polyunsaturated fatty acids, and conjugated linoleic acid on mammary carcinogenesis in animals. Am. J. Clin. Nutr., 66, 15235-15295.
[2] Lee, K. N., Kritchevsky, D. and Pariza, M. W. (1994) Conju-gated linoleic acid and atherosclerosis in rabbits. Atheroscle-rosis, 108, 19-25.
[3] Houseknecht, K, Heuvel, K., Moya-Camarena, S., Portocar-rero, C., Peck, L., Nickel, K. and Belury, M. (1998) Dietary conjugated linoleic acid normalizes impaired glucose toler-ance in Zucker diabetic fatty fa/fa rat. Biochem. Biophys, Res. Commun. 244, 678-682.
[4] Takahashi K., Akiba, Y., Iwata, T. and Kasai, M. (2002) Die-tray conjugated linoleic acids alleviate early inflammatory re-sponse caused by lipopolysaccharide injection in male broiler chicks. Animal Science Journal, 73, 47-50.
[5] Cook, M. E., Miller, C. C., Park, Y. and Pariza, M. (1993) Immune modulating by altered nutrient metabolism: Nutri-tional control of immune-induced growth depression. Poult. Sci., 72, 1301-1305.
[6] Li, Y. and Watkins, B.A. (1998) Conjugated linoleic acids al-ter bone fatty acid composition and reduce ex vivo prosta-glandin E-2 biosynthesis in rats fed n–6 or n–3 fatty acids. Lipids, 33, 417–425.
[7] Park, Y., Albright, K.J., Liu, W., Storkson, J. M., Cook, M. E. and Pariza, M. W. (1997) Effect conjugated linoleic acid on body composition in mice. Lipids, 32, 853-858.
[8] Martin, J.C., Gregoire, S., Siess, M.H., Genty, M., Chardigny, J.M., Berdeaux, O., Juaneda, P., Sebedio, J.L. (2000) Effects of conjugated linoleic acid isomers on lipid-metabolizing en-zymes in male rats. Lipids, 35, 91–98.
[9] Park, Y., Storkson, J.M., Ntambi, J.M., Cook, M.E., Sih, C.J. and Pariza, M.W. (2000) Inhibition of hepatic stearoyl-CoA desaturase activity by trans-10, cis-12 conjugated linoleic ac-id and its derivatives. Biochimica et Biophysica Acta 1486, 285-292.
[10] Koba, K., Akahoshi, A., Yamasaki, M., Tanaka, K., Yamada, K., Iwata, T., Kamegai, T., Tsutsumi, K. and Sugano, M. (2002) Dietary conjugated linolenic acid in relation to CLA differently modifies body fat mass and serum and liver lipid levels in rats. Lipids, 37, 343-350.
[11] Tischendorf, F., Schöne, F., Kirchheim, U. and Jahreis, G. (2002) Influence of a conjugated linoleic acid mixture on growth, organ weights, carcass traits and meat quality in growing pigs. J. Anim. Physiol. A. Anim. Nutr. 86, 117-128. [12] Szymczyk, B., Pisulewsky, P.M., Szczurek, W, Hanczakow-sky, P. (2001) Effects of conjugated linoleic acid on growth performance, feed conversion efficiency and subsequent car-cass quality in broiler chickens. Br. J. Nutr., 85, 465-473. [13] Badinga, L., Selberg, K.T., Dinges, A.C., Comer, C.W. and
Miles R.D. (2003) Dietary conjugated linoleic acid alters he-patic lipid content and fatty acid composition in broiler chickens. Poult. Sci. 82, 111-116.
[14] Ostrowska, E., Cross, R.F., Muralitharan, M., Bauman, D.E. and Dunshea, F.R. (2003) Dietary conjugated linoleic acid-differently alters fatty acid composition and increases conju-gated linoleic acid content in porcine adipose tissue. Br. J. Nutr., 90, 915-928.
[15] Corino, C., Mourot, J., Magni, S., Pastorelli, G. and Rosi, F. (2002) Influence of dietary conjugated linoleic acid on growth, meat quality, lipogenesis, plasma leptin and physio-logical variables of lipid metabolism in rabbits. J. Anim. Sci. 80, 1020-1028.
[16] Du, M. and Ahn, D.U. (2002) Effect of dietary conjugated linoleic acid on the growth rate of live birds and on the ab-dominal fat content and quality of broiler meat. Poult. Sci. 81, 428-433.
[17] Aletor, V.A., Eder, K., Becker, K., Paulicks, B.R., Roth, F.X. and Roth-Maier, D.A. (2003) The effects of conjugated lino-leic acids or an α-glucosidase inhibitor on tissue lipid concen-trations and fatty acid composition of broiler chicks fed a low-protein diet. Poult Sci. 82(5), 796-804
[18] Kutlu, H.R. and Forbes, J.M. (1993) Alleviation of the effect of heat stress by dietary methods in broilers: a review. World-Rev-Anim-Prod 28, 15-26.
[19] Baziz, H.A., Geraert, P.A., Padilha, J.C.F. and Guillaumin, S. (1996) Chronic heat exposure enhances fat deposition and modifies muscle and fat deposition in broiler carcasses. Poult. Sci. 75, 505-513.
[20] SAS Institute (1985) SAS User's Guide, Statistics. Version 5th Edition. SAS Institue Inc., Cary, NC.
[21] Larbier, M. and Leclerq, B. (1994) Nutrition and feeding of poultry. Nottingham University Press, ISBN:1-897676-52-2, pp:305.
[22] Webster, A.J.F. (1983) Nutrition and the thermal environ-ment in nutritional physiology of farm animals. Rook, J.A.F. and Thomas, P.C. (eds.) New York. Longman, pp: 639-669 [23] Mersmann, H.J. (2002) Mechanisms for conjugated linoleic
acid-mediated reduction in fat deposition. J. Anim. Sci. 80(E. Suppl. 2), E126-E134.
[24] Takahashi, K., Akiba, Y., Iwata, T. and Kasai, M. (2003) Ef-fect of a mixture of conjugated linoleic acid isomers on growth performance and antibody production in broiler chicks. British Journal of Nutrition, 89, 691-694.
[25] Choi, J.S., Jung, M.H., Park, H.S. and Song, J. (2004) Effect of conjugated linoleic acid isomers on insulin resistance and mRNA levels of genes regulating energy metabolism in high-fat–fed rats. Nutrition, 20 (11-12), 1008-1017.
Received: December 27, 2005 Accepted: February 13, 2006 CORRESPONDING AUTHOR Ladine Çelik Çukurova University Agricultural Faculty
Department of Animal Science 01330 Adana
Turkey
Phone: ++90.322.338 70 27 Fax: ++90.322.338 65 76 e-mail: ladine@cu.edu.tr