Environmental Nanotechnology, Monitoring & Management 14 (2020) 100380
Available online 15 October 2020
2215-1532/© 2020 Elsevier B.V. All rights reserved.
Azo dye-functionalized magnetic Fe
3
O
4
/polyacrylic acid nanoadsorbent for
removal of lead (II) ions
Omer Sadak
a,b, Robert Hackney
b, Ashok K. Sundramoorthy
c, Galip Yilmaz
d,
Sundaram Gunasekaran
b,e,*
aDepartment of Electrical and Electronics Engineering, Ardahan University, Turkey bMaterials Science & Engineering, University of Wisconsin, Madison, WI, USA cDepartment of Chemistry, SRM Institute of Science and Technology, Chennai, India dTechnical Sciences Vocational School, Bayburt University, Bayburt, Turkey
eDepartment of Biological Systems Engineering, University of Wisconsin, Madison, WI, USA
A R T I C L E I N F O Keywords:
Nanoadsorbent Congo red Heavy metal removal Wastewater treatment
A B S T R A C T
We report the fabrication of a nanoadsorbent for the removal of heavy metals. The nanoadsorbent is comprised of ferric oxide (Fe3O4) magnetic nanoparticles (MNPs) covalently conjugated with polyacrylic acid (PAA) and further functionalized with an azo dye (Congo red, CR) using carbodiimide. This Fe3O4 core and PAA-CR shell system (MNP/PAA-CR) exhibited binding affinity towards various cations (Pb2+, Fe2+, Fe3+, Cd2+, and Cu2+) at room temperature. Using MNP/PAA-CR we studied its heavy metal removal effectiveness and kinetics targeting Pb2+under various reactions conditions including time and pH. The Pb2+removal efficiency and adsorption capacity were maximal at pH 6.5 and 45 min of reaction time, and the Pb2+adsorption kinetics better fit a pseudo second-order model than a first-order model. The adsorption of Pb2+by MNP/PAA-CR was also inves-tigated by the so-called Langmuir and Freundlich isotherms, and the Langmuir isotherm predicted a maximum absorption of 195.3 mg.g-1. Our results further indicated that MNP/PAA-CR is potentially reusable after desorption of the adsorbed metal with only small decline in adsorption ability over five consecutive cycles of regeneration.
1. Introduction
Heavy metals such as lead, cadmium, mercury, nickel, and copper are commonly found in wastewater emanating from mining, metal- plating, power generation, and tanning operations (Liu, Hu et al. 2009). The release of these heavy metals has led to their accumulation in the living cells and the attendant adverse health issues have become a major problem worldwide (Nriagu and Pacyna 1988, Liu, Zhao et al. 2008, Zargoosh, Zilouei et al. 2014). For instance, heavy metals in living cells act as non-competitive enzyme inhibitors and impact several physiological functions (Inbaraj and Chen 2012). To alleviate these harmful effects, stringent policies are being enforced towards the release of heavy metals (Liu, Zhao et al. 2008). Therefore, the industries are forced to address reducing and or removing the heavy metals in the waste streams. The conventional methods of heavy metal removal include chemical precipitation, ion exchange, liquid-liquid extraction, resins, cementation, and with hydrogels (Liu, Hu et al. 2009, El-Hag Ali
2012, Zargoosh, Zilouei et al. 2014). These methods are rather ineffec-tive and suffer from high cost, high energy-requirements, and toxic waste generation (Barakat 2011). In recent years, use of nanoparticles (NPs)-based systems have attracted much attention for their potential use in heavy metal removal (Yantasee, Warner et al. 2007, Shabani, Ardejani et al. 2017, Khaligh and Johan 2018, Yang, Hou et al. 2019).
Core/shell NP structure can comprise a center core, a shell, and a surface layer (Khan et al., 2019), and the NP is referred to by its core composition. The shell is chemically different from the core, which is either synthesized or formed through natural processes. The outer sur-face is usually comprised of a functional entity, such as metal ions, small molecules, surfactants, or polymers, which confers a unique property to the core/shell NP system (Christian, Von der Kammer et al. 2008). An example of core/shell iron NP is where iron core is surrounded by an iron oxide shell (Herman, Ferguson et al. 2011).
Ferric oxide (Fe3O4) NPs, which are magnetic NPs (MNPs), are
widely used biotechnology, biomedical, material science, engineering,
* Corresponding author at: Biological Systems Engineering, University of Wisconsin, Madison, WI, USA E-mail address: guna@wisc.edu (S. Gunasekaran).
Contents lists available at ScienceDirect
Environmental Nanotechnology, Monitoring & Management
journal homepage: www.elsevier.com/locate/enmm
https://doi.org/10.1016/j.enmm.2020.100380
become demagnetized (Estelrich, Escribano et al. 2015, Predescu, Matei et al. 2018). One way to avoid this is by enveloping MNPs with organic compounds such as surfactants or polymers (Laurent, Forge et al. 2008). We synthesized MNPs and modified them via covalent reactions of polyacrylic acid (PAA) and Congo red (CR). The size and structure of this core-shell system (MNP/PAA-CR) were characterized by transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FT-IR), dynamic light scattering (DLS), UV-vis, and zeta potential measurements. The MNP/PAA-CR was used to remove lead (Pb2+) from
water, by optimizing the parameters such as pH, initial Pb2+
concen-tration, and reaction time. Several kinetic models were investigated to determine the adsorption mechanism. The Langmuir and Freundlich isotherms were used to fit the equilibrium data. The reusability of MNP/ PAA-CR was also investigated.
2. Experimental
2.1. Materials
Iron (III) chloride (FeCl3⋅6H2O) and iron (II) chloride (FeCl2⋅4H2O)
were purchased from Fisher (NJ, USA); PAA (average Mw ~100,000, 35 wt. % in H2O), carbodiimide (CDI), cupric chloride (CuCl2⋅5H2O), hy-drochloric acid (HCl), and ammonium hydroxide (NH4OH) were from
Sigma Aldrich (St. Louis, MO, USA); lead(II) chloride (PbCl2) was from
Acros Chemicals (NJ, USA); and CR was from MP Biomedicals (OH, USA). All chemicals were used as received.
2.2. Synthesis of Fe3O4 MNPs
The MNPs were synthesized by a co-precipitation method using ferrous (Fe2+) and ferric (Fe3+) ions (at 1:2 molar ratio) in ammonia
solution (Zhou, Zhang et al. 2013). Briefly, 25 mL of 2 M HCl was added into a 100-mL flask containing 2.70 g of FeCl3⋅6H2O and 0.99 g of FeCl2⋅4H2O. The solution was sonicated for 15 min, followed by
degassing using nitrogen gas at room temperature. Then, 40-mL of aqueous ammonia (28%) was added into the mixture slowly over one hour while stirring under nitrogen at room temperature. The resulting black precipitate was collected with a strong permanent magnet and washed with deionized (DI) water several times. The final product of Fe3O4 MNPs was obtained by drying the precipitate in a vacuum oven at
60 ◦C for 12 h.
2.3. Coating MNPs with PAA
The MNPs were coated with PAA to obtain MNP/PAA. A published method was adapted with some modifications (Liao and Chen 2002). A sample 100 mg of MNPs were placed into a 50-mL flask and mixed with 2 mL of buffer A (3 mM phosphate, pH 6, 0.1 M NaCl), and after adding 0.5 mL of CDI (25 mg.mL-1 in buffer A) the reaction flask was sonicated
for 20 min in a bath sonicator. Then, 3.0 mL of PAA solution (50 mg.mL-1
in buffer A) was added into the mixture. The mixture was homogenized by vortexing for 2 min and sonicating for 20 min. The formed MNP-PAA was recovered from the reaction mixture using a strong permanent magnet and washed twice with DI water.
2.4. Functionalizing MNP-PAA with CR
3,3′-([1,1′-biphenyl]-4,4′-diyl)bis(4-aminonaphthalene-1-sulfonic
2.5. Adsorption and desorption measurements
The ability of MNP/PAA-CR to adsorb metal cations was investigated using Pb2+as the target. Briefly, 30 mg of MNP/PAA-CR was added into
30 mL of Pb2+(150 mg.L-1) under stirring at 350 rpm, and the pH was adjusted by adding NaOH or HCl. After certain contact time, the MNP/ PAA-CR were removed from the mixture using a strong permanent magnet. The removal process is illustrated in Figure S1. The residual Pb2+in the solution was determined with inductively coupled plasma-
mass spectrometry (ICP-MS). Similar experiments were conducted with MNPs and MNP-PAA for comparison. The Pb2+removal efficiency
(Re, %) and adsorption capacity (Q, mg. g-1) were calculated as follows: Re= (C0− Ct) C0 × 100 (1) Q =(C0− Ct) m × V (2)
Where, C0 and Ct are Pb2+ concentrations (mg.L-1) before and after
adsorption studies, respectively, and m is the mass of the adsorbent (g) and V is the volume of Pb2+solution (L).
The desorption of Pb2+from the Pb2+-adsorbed MNP/PAA-CR was performed using 200 mg sample in 10 mL of 0.01 M HNO3 solution.
After 3 h under constant stirring, the MNP/PAA-CR were collected using a strong permanent magnet and washed with diluted NaOH and DI water. This absorption-desorption experiment was performed for five consecutive cycles to determine the reusability of MNP/PAA-CR.
2.6. Characterizations
All the synthesized materials were characterized using UV-vis (Lambda 25, Perkin Elmer) and FT-IR (Spectrum 100, Perkin Elmer) spectroscopies. Dynamic light scattering (DLS) measurements were made by using Zeta Plus (Brookhaven Instruments Corp., Holtsville, NY, USA) to measure the hydrodynamic size and zeta potential of the MNPs at 25 ◦C. The size of MNPs was measured from micrographs obtained
with TEM (Tecnai TF12, FEI Co., Hillsboro, OR) at an acceleration voltage of 80 kV. The concentrations of Pb2+ in test solutions were measured with ICP-MS (Agilent 7800, Santa Clara, CA).
3. Results
3.1. Characterization of the magnetic nanoparticles
The appearance of 1 mM CR solutions in the presence of following metal ions at room temperature is shown in Fig. 1: zinc (Zn2+), nickel (Ni2+), cadmium (Cd2+), iron (Fe2+, Fe3+), chromium (Cr2+), cobalt
(Co2+), copper (Cu2+), potassium (K1+) and Pb2+. The visual color
change of the native CR solution was instantly observed in the presence of only Pb2+, Fe2+, Fe3+, Cd2+, and Cu2+. This is due to the formation of
CR complexes with these cations, which served as the basis for targeting and removing heavy metals from wastewater. To facilitate this process, a CR-based MNP core/shell (Fe3O4 core and covalently linked PAA-CR
shell) system was synthesized, as shown in Scheme 1.
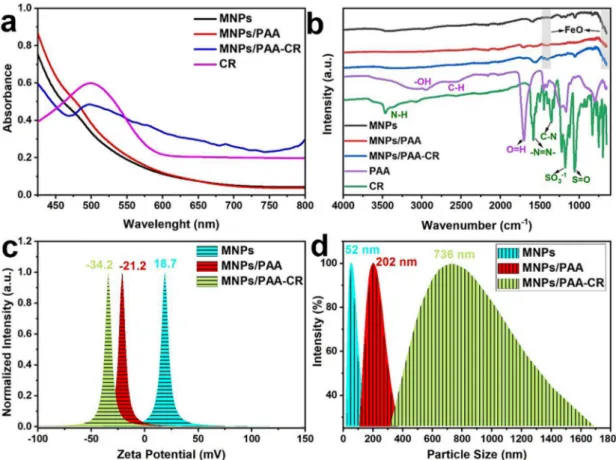
The UV-vis and FT-IR spectra for MNP, MNP/PAA, MNP/PAA-CR and CR are shown in Fig. 2a and b, respectively. The UV-vis peak at
498 nm corresponding to a strong absorption band is clearly seen for CR. This peak blue-shifted to 495 nm for MNP/PAA-CR due to the interac-tion of CR with MNP/PAA. While linking CR with MNP/PAA, CDI was used to activate the carboxylic acid groups on PAA (McCarthy and Weissleder 2008), and the observed blue shift is the evidence for this occurring.
In the FT-IR spectrum of CR, characteristics peaks at 3461, 1595, 1346, 1216, and 1053 cm-1 were assigned to N–H bonds, stretching
vi-bration of –N = N–bond, stretching vivi-bration of C–N, SO3-1 and S = O
stretching vibrations, respectively (Sundramoorthy, Wang et al. 2015). The spectrum of PAA showed a broad peak at ~3440 cm-1, which was
assigned to –OH bonds and peaks at 2937, 1708, 1238 and 1051 cm-1 are
due to stretching vibrations of C-H, C = O, C-C-O, and O-C-C bonds, respectively (Tai, Yang et al. 2013). The spectrum for MNPs showed absorption bands around 600 and 1360 cm-1 due to Fe–O stretching
modes. The MNP/PAA spectrum consists bands of PAA and MNP, which confirm chemical attachment of PAA on MNPs. Likewise, the spectrum of MNP/PAA-CR consists of bands of MNPs, PAA and CR, which sug-gested the successful formation of MNP/PAA-CR nanocomposite.
Zeta potential of NPs is an indicator of their surface charge. The surface charge of MNP changed from positive (18.7 ± 0.5 mV) to negative (‒21.2 ± 0.2 mV) after reacting with negatively charged PAA (Fig. 2c). The surface charge of MNP/PAA-CR is even more negative (‒ 34.2 ± 0.6 mV) than that of MNP-PAA due to CR, which contains functional groups that have a negative charge.
The particle size distribution of the MNP, MNP/PAA and MNP/PAA- CR obtained with DLS indicated their average diameters of 52, 202, 736
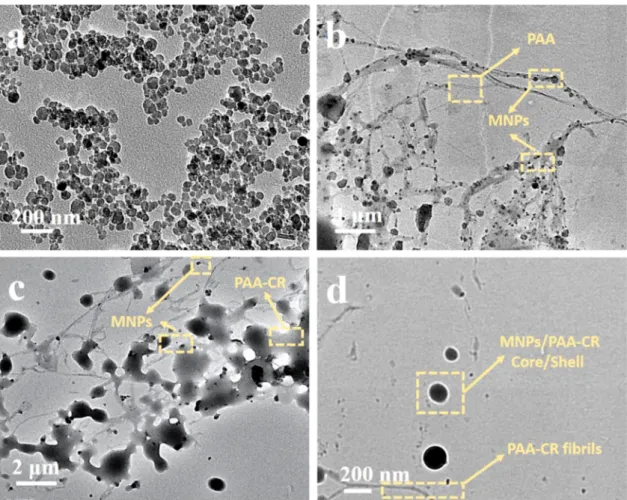
nm, respectively (Fig. 2d). The TEM images of these samples are shown in Fig. 3. The diameter of the MNPs measured from the TEM ranged from 15 nm to 80 nm (Fig. 3a), which is in good agreement with the DLS data. As shown in Fig. 3b and c after the synthesis of MNP/PAA and MNP/
PAA-CR, the composite materials showed a network of randomly interconnected fibril-like morphologies. Higher magnification TEM image (Fig. 3d) exhibits evidence of core/shell structure in MNP/PAA- CR along with fibril-like morphology.
3.2. Adsorption studies
Lead ions are toxic, which contaminate the environment including soils, surface water, and groundwater, even at very low levels (Kaur, Kumari et al. 2020). According to the World Health Organization (WHO), when the amount of Pb2+in blood is 10 μg.dL-1 or above, it
causes serious health effect on human (Rossi 2008). Since the presence of Pb2+is hazardous, we targeted its removal using our MNP/PAA-CR, taking advantage of the fact that Pb2+readily complexes with CR. 3.2.1. Effect of pH
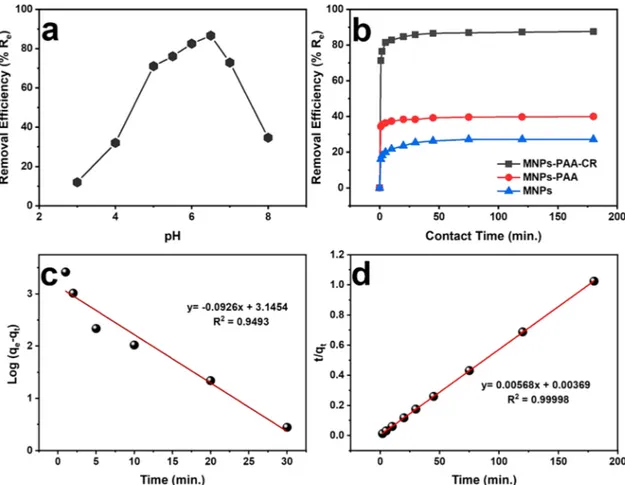
The effect of various parameters on heavy metals removals including contact time, pH, heavy metal concentrations and temperature plays an essential role in the adsorption process. Therefore, we first examined the effect of pH at room temperature using 30 mg of MNP/PAA-CR for the removal of 30 mL of Pb2+(200 mg/L) at various pHs (3, 4, 4.5, 5, 5.5, 6,
6.5, 7, and 8) under shaking condition of 350 rpm for three hours. Samples were collected by placing a strong permanent magnet on the
Fig. 1. Appearance of aqueous 1:1 mixtures of 1 mM CR and 1 mM of different metal cations.
side of the container and collecting 100 μL of samples from another side
via a micropipette. The Re of each sample was calculated based on
equation (1). As shown in Fig. 4a, the Re value was the highest at pH 6.5.
At lower pHs, H+ion concentration is higher, functional groups on the
reaction site such as -COOH, and -NH2 become inactive due to
proton-ation. Electron donors are not available for Pb2+ for chelation or
complexation on the reaction site, because it competes with H+ion and
creates Coulombic repulsion between Pb2+ions and protonated
func-tional groups on the reactions that significantly slow the adsorption process (Ali, Peng et al. 2019). At pHs greater than 6.5, high concen-tration of OH- ions triggers the precipitation of Pb2+ions (Ali, Peng et al.
2019). As a result, fewer of Pb2+ions would chelate or make complex
with MNP/PAA-CR on the reaction site. Thus, the maximal Pb2+
adsorption pH was determined as 6.5 and was adopted for the rest of experiments.
3.2.2. Effect of reaction time and adsorption kinetics
The absorption capacity of MNP/PAA-CR (30 mg) for Pb2+(30 mL,
200 mg/L) was tested at pH of 6.5 and at room temperature over different reaction times (1, 2, 5, 10, 20, 30, 45, 75, 120 and 180 min). As shown in Fig. 4b (and Figure S2, Supplemental Information), the Re
reached 81.4 % within five minutes and a steady state value of 86.6% was attained in 45 min, which according to equation (2) gives Q=173.2 mg.g-1. The as-synthesized MNPs are amine coated and, as shown in
Fig. 4b have low chelating affinity toward Pb2+ions. It may be due to
positive surface charge of MNPs. After MNPs are functionalized it with PAA, their chelating capability improves because of multiple polar carboxylic groups, which have the ability to form complexes with metal cations. Also, as has been reported (Huang and Chen 2009, Mahdavian and Mirrahimi 2010), PAA-coated MNPs can remove heavy metals including Pb2+by ion exchange. Moreover, due to binding affinity of CR
towards Pb2+, covalently attached CR onto MNP/PAA exhibits greater
adsorption capability for Pb2+. However, for Pb2+removal the Re of
MNP/PAA-CR (>85 %) is more than twice as that of MNP/PAA (~40 %). This result suggests that the covalently linked CR substantially improves
Re for MNP/PAA-CR.
3.2.3. Effect of initial Pb2+concentration
The effect of initial Pb2+concentration (30 mL of 50, 100, 150, 200,
250, 300 and 400 mg/L solutions) on Re was studied using 30 mg of
MNP/PAA-CR at pH of 6.5 under shaking condition of 350 rpm for three hours. As listed in Table 1, with increasing Pb2+concentration from 50 to
400 mg/L, Q increased from 49.9 to 223.1 mg.g-1and Re decreased from
99.9 to 55.8%. The decrease in Re is attributed to saturation of limited
number of available binding sites on the nanoadsorbent. Moreover, at higher initial Pb2+concentration, functional groups on MNP/PAA-CR
would be surrounded with more ions that enhance the adsorption ca-pacity (Ghorbani, Younesi et al. 2008). This result indicates that initial ion concentration has a strong effect on the adsorption process.
The heavy metal removal capacity of our MNP/PAA-CR is perhaps the best among the previously reported studies that employed MNPs, especially when considering a very short reaction time (Table 2). While
Alqadami et al. (Alqadami, Naushad et al. 2020) reported a Q value somewhat close to that of our system (205 vs 223 mg.g-1), the reaction
time of theirs is more than three times that of ours (150 vs. 45 min). Thus, our nanoabsorbent shows much promise for heavy metal removal from wastewater, owing to its impressive ability towards the removal of Pb2+and exhibited potential to adsorb other heavy metals such as Fe2+,
Fe3+, Cd2+, and Cu2+. 3.2.4. Adsorption kinetics
The Pb2+ adsorption rate and mechanism of MNP/PAA-CR were
analyzed via pseudo first-order, and pseudo second-order kinetic models. The linearized forms of these models are given in Eqs. (3) and
(4), respectively (Lagergren 1898, Liao, Zheng et al. 2010):
log(Qe− Q) = logQe− k1t 2.303 (3) t Q= 1 k2Qe2 + t Qe (4)
Where, Qe is the amounts of Pb2+adsorbed (mg/g) at equilibrium, Qt is
the amounts of Pb2+adsorbed (mg/g) at time t (min), k
1 is the first-order
rate constant (min-1), and k2 is the second-order rate constant (g. mg-1.
min-1).
Linear plots for Eq. (3) (log (Qe‒Qt) vs. t) and Eq. (4) (t/Qt vs. t) were used to determine k1 and k2, respectively (Fig. 4c and d). The model
parameters determined are listed in Table S1 (Supplemental Informa-tion). While both models statistically fit the experimental data (high R2 value), the Qe determined from the pseudo second-order model (173.2
mg.g-1) was in close agreement with the experimental value (176.1 mg.
g-1). Hence, we believe that the pseudo second-order kinetic model is better suited for describing Pb2+adsorption onto MNP/PAA-CR. 3.2.5. Adsorption isotherms
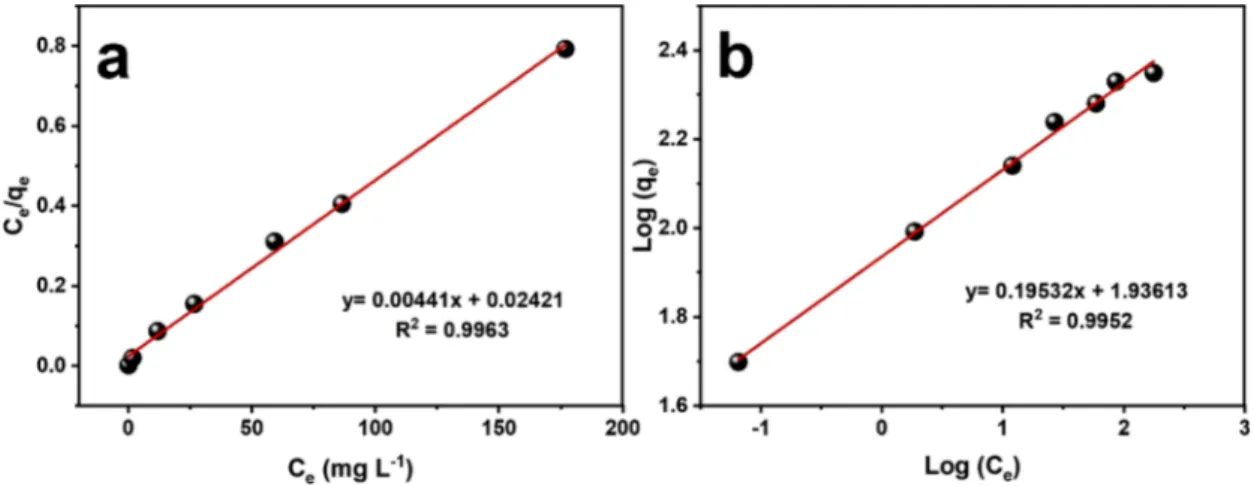
The experimental results from Table 1 were fitted using Langmuir and Freundlich isotherms. Langmuir isotherm describes the system based on monolayer adsorption of the adsorbate on homogenous adsorbent surface (Allen, McKay et al. 2004). The linearized form of Langmuir isotherm is given as (Huang and Chen 2009):
Ce Qe =Ce Qm + 1 QmKL (5)
Where, Ce is the equilibrium concentration of the adsorbate (mg.L-1), Qm
is the maximum amounts of Pb2+ adsorbed (mg. g-1), and K L is the
Langmuir equilibrium constant (L. mg-1).
Freundlich isotherm describes the system based on multilayer adsorption of the adsorbate and considers the surface heterogeneity for the absorbent (Almomani, Bhosale et al. 2020). Unlike Langmuir isotherm, it does not predict the Qm value. The linearized form of
Freundlich isotherm is given as (Ji, Miao et al. 2018):
log qe=logKF+
1
nlog Ce (6)
Where, n is the adsorption intensity (dimensionless) and KF is the
adsorption capacity (mg. g-1) (also known as Freundlich constant). The
numerical value of n is indication of the favorability of adsorption. If 1<n<10, it represents favorable adsorption and If n<1, it represents poor adsorption characteristics (Ali, Hamad et al. 2016). The Langmuir and Freundlich model constants were determined (see Table S2) from the plots of respectively, Ce/Qe vs Ce (Fig. 5a) and log qe vs log Ce
(Fig. 5b). As can be observed, both models fit the data well with R2 >
0.99, indicating their suitability for predicting experimental data. The
Qm value predicted (226.8 mg.g-1) according to Langmuir isotherm is in
close agreement with the experimental value (223.1 mg.g-1, see
Table 1), and n value per Freundlich isotherm is 5.1, which is in the 1 to 10 range, thus confirming the good adsorption capability of MNP/PAA-CR for Pb2+cations.
3.2.6. Reusability of adsorbent
The processes of removing heavy metals from wastewater will be economical if the adsorbents can be regenerated and reused with an acceptable Re. The desorption of Pb2+were carried out with 0.01 M
nitric acid (HNO3) solution and after which MNP/PAA-CR were washed
several times with DI water. In acidic conditions, interaction between metal ions and active sites on magnetic nanoadsorbent is weak due to
protonation of adsorbent surface, which leads to desorption of metal ions (Abdolmaleki, Mallakpour et al. 2015). The reusability of nano-adsorbent was tested for five consecutive adsorption-desorption cycles. The MNP/PAA-CR retained a fairly high Re of >82 % after five cycles
(Fig. 6). When considering this high reusability, along with fairly facile
and inexpensive synthesis process, our nanoadsorbent system has the potential for highly effective and economical heavy metal removal.
4. Conclusions
We prepared core-shell magnetic nanoparticles (MNP) with Fe3O4
core and covalently bound PAA-CR as shell. This nanoadsorbent exhibited the ability to adsorb various heavy metals. When targeting the removal of Pb2+, we achieved a maximum removal capacity of 223.12
mg.g-1 from aqueous solutions at pH 6.5 in 45 min. The MNPs were also
reusable, with over 82 % of its Pb2+removal potential still available
after five consecutive adsorption-desorption cycles. The adsorption ki-netics of the MNPs were best fit with a pseudo second-order kinetic
Fig. 4. Removal efficiency at room temperature (a) of MNP/PAA-CR at various pHs for 180 min reaction time and (b) of MNP/PAA-CR and MNP-PAA at various reaction times at pH 6.5. (c) Pseudo first-order and (d) Pseudo second-order kinetic models at pH 6.5 with initial Pb2+concentration of 200 mg.mL-1.
Table 1
Effect of initial Pb2+concentration on removal efficiency (Re) and adsorption
capacity (Q). Initial Pb2+concentration (mg.L-1) Re (%) Q(mg. g -1) 50 99.87 49.94 100 98.13 98.13 150 92.02 138.03 200 86.58 173.16 250 76.32 190.81 300 71.18 213.54 400 55.78 223.12 Table 2
Comparison of the maximum Pb2+adsorption capacity (Q) of our MNP/PAA-CR
with other MNP-based systems reported in the literature.
Adsorbents* Q(mg. g -1) Reaction time(min) Reference Fe3O4 nanoadsorbents 36.0 <30 (Nassar 2010) Iron-coated sand 1.2 180 (Lai and Chen 2001) Magnetic–cotyledon
biomass 63.6 180 (et al. 2019Mohubedu, Diagboya ) Magnetic–pericarp biochar 58.2 180 (Mohubedu, Diagboya
et al. 2019) Xanthate-modified
magnetic chitosan 76.9 N/A (Zhu, Hu et al. 2012)
EYMC 119.1 60 (Li, Liu et al. 2013)
MNPs@PEI 123.3 30 (Jiang, Zhang et al. 2017) Fe2O3 93.9 45 (Chen et al., 2019) Co - Fe2O3 136.0 30 (Chen et al., 2019) Ni - Fe2O3 97.5 30 (Chen et al., 2019) T-b-CD–MNPs 105.4 120 (Abdolmaleki, Mallakpour et al. 2015) CDpoly-MNPs 64.5 45 (Badruddoza, Shawon
et al. 2013) Fe3O4@TATS@ATA 205.2 150 (Alqadami, Naushad
et al. 2020)
MNP/PAA-CR 223.12 45 This work
*EYMC: Ethylenediamine-modified yeast biomass coated with magnetic chito-san microparticles, MNPs@PEI: polyethyleneimine functionalized magnetic Fe3O4, T-b-CD–MNPs: triazinyl-b-cyclodextrin modified magnetic nanoparticles,
CDpoly-MNPs: carboxymethyl-β-cyclodextrin (CM-β-CD) polymer modified Fe3O4 nanoparticles, Fe3O4@TATS@ATA: 2-aminoterephtalic acid-modified
model. Further, Langmuir and Freundlich isotherm models were inves-tigated for the equilibrium experimental adsorption data. Based on Langmuir isotherm, the maximum adsorption capacity of MNPs is 226.8 mg g-1, and the Freundlich model confirmed our system possesses good
absorption ability. The results suggest an excellent potential of our system for very effective and economical heavy metal removal.
CRediT authorship contribution statement
Omer Sadak: Conceptualization, Methodology, Writing - original
draft. Robert Hackney: . Ashok K. Sundramoorthy: Conceptualiza-tion, Methodology. Galip Yilmaz: . Sundaram Gunasekaran: Super-vision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the
online version, at doi:https://doi.org/10.1016/j.enmm.2020.100380.
References
Abdolmaleki, A., Mallakpour, S., Borandeh, S., 2015. Efficient heavy metal ion removal by triazinyl-β-cyclodextrin functionalized iron nanoparticles. RSC Adv. 5 (110), 90602–90608.
Akbarzadeh, A., Samiei, M., Davaran, S., 2012. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 7 (1), 144. Ali, I., Peng, C., Naz, I., 2019. Removal of lead and cadmium ions by single and binary systems using phytogenic magnetic nanoparticles functionalized by 3-marcaptopro-panic acid. Chinese J. Chem. Eng. 27 (4), 949–964.
Ali, R.M., Hamad, H.A., Hussein, M.M., Malash, G.F., 2016. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 91, 317–332.
Allen, S.J., McKay, G., Porter, J.F., 2004. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 280 (2), 322–333.
Almomani, F., Bhosale, R., Khraisheh, M., kumar, A., Almomani, T., 2020. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 506, 144924.
Alqadami, A.A., Naushad, M., Alothman, Z.A., Alsuhybani, M., Algamdi, M., 2020. Excellent adsorptive performance of a new nanocomposite for removal of toxic Pb(II) from aqueous environment: Adsorption mechanism and modeling analysis. J. Hazard. Mater. 389, 121896.
Badruddoza, A.Z.M., Shawon, Z.B.Z., Tay, W.J.D., Hidajat, K., Uddin, M.S., 2013. Fe3O4/ cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydrate Polym. 91 (1), 322–332.
Barakat, M.A., 2011. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 4 (4), 361–377.
Chen, W., Lu, Z., Xiao, B., Gu, P., Yao, W., Xing, J., Asiri, A.M., Alamry, K.A., Wang, X., Wang, S., 2019. Enhanced removal of lead ions from aqueous solution by iron oxide nanomaterials with cobalt and nickel doping. J. Clean. Prod. 211, 1250–1258. Christian, P., Von der Kammer, F., Baalousha, M., Hofmann, T., 2008. Nanoparticles:
structure, properties, preparation and behaviour in environmental media. Ecotoxicology 17 (5), 326–343.
El-Hag Ali, A., 2012. Removal of heavy metals from model wastewater by using carboxymehyl cellulose/2-acrylamido-2-methyl propane sulfonic acid hydrogels. J. Appl. Polym. Sci. 123 (2), 763–769.
Estelrich, J., Escribano, E., Queralt, J., Busquets, M.A., 2015. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 16 (4), 8070–8101.
Ghorbani, F., Younesi, H., Ghasempouri, S.M., Zinatizadeh, A.A., Amini, M., Daneshi, A., 2008. Application of response surface methodology for optimization of cadmium biosorption in an aqueous solution by Saccharomyces cerevisiae. Chem. Eng. J. 145 (2), 267–275.
Herman, D.A.J., Ferguson, P., Cheong, S., Hermans, I.F., Ruck, B.J., Allan, K.M., Prabakar, S., Spencer, J.L., Lendrum, C.D., Tilley, R.D., 2011. Hot-injection synthesis of iron/iron oxide core/shell nanoparticles for T-2 contrast enhancement in magnetic resonance imaging. Chem. Commun. 47 (32), 9221–9223.
Huang, S.H., Chen, D.H., 2009. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 163 (1), 174–179.
Inbaraj, B.S., Chen, B.H., 2012. In vitro removal of toxic heavy metals by poly(gamma- glutamic acid)-coated superparamagnetic nanoparticles. Int. J. Nanomed. 7, 4419–4432.
Ji, S., Miao, C., Liu, H., Feng, L., Yang, X., Guo, H., 2018. A Hydrothermal Synthesis of Fe (3)O(4)@C Hybrid Nanoparticle and Magnetic Adsorptive Performance to Remove Heavy Metal Ions in Aqueous Solution. Nanoscale Res. Let. 13 (1), 178-178.
Fig. 5. (a) Langmuir and (b) Freundlich isotherms models for MNP/PAA-CR for the removal of Pb2+.
Fig. 6. Reusability of MNP/PAA-CR over five consecutive Pb2+adsorption-
Khan, I., Saeed, K., Khan, I., 2019. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 12 (7), 908–931.
Lagergren, S., 1898. Zur theorie der sogenannten adsorption geloster stoffe." Kungliga Svenska Vetenskapsakademiens. Handlingar 24, 1–39.
Lai, C.H., Chen, C.Y., 2001. Removal of metal ions and humic acid from water by iron- coated filter media. Chemosphere 44 (5), 1177–1184.
Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Elst, L.V., Muller, R.N., 2008. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 108 (6), 2064–2110.
Li, T.-t., Liu, Y.-g., Peng, Q.-q., Hu, X.-j., Liao, T., Wang, H., Lu, M., 2013. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Chem. Eng. J. 214, 189–197.
Liao, D., Zheng, W., Li, X., Yang, Q., Yue, X., Guo, L., Zeng, G., 2010. Removal of lead(II) from aqueous solutions using carbonate hydroxyapatite extracted from eggshell waste. J. Hazard. Mater. 177 (1), 126–130.
Liao, M.-H., Chen, D.-H., 2002. Preparation and characterization of a novel magnetic nano-adsorbent. J. Mater. Chem. 12 (12), 3654–3659.
Liu, J.F., Zhao, Z.S., Jiang, G.B., 2008. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 42 (18), 6949–6954.
Liu, X., Hu, Q., Fang, Z., Zhang, X., Zhang, B., 2009. Magnetic chitosan nanocomposites: a useful recyclable tool for heavy metal ion removal. Langmuir 25 (1), 3–8. Mahdavian, A.R., Mirrahimi, M.A.-S., 2010. Efficient separation of heavy metal cations
by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem. Eng. J. 159 (1), 264–271.
McCarthy, J.R., Weissleder, R., 2008. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 60 (11), 1241–1251.
Biochem. Rev. 29 (2), 63–70.
Sadegh, H., Ali, G.A.M., Gupta, V.K., Makhlouf, A.S.H., Shahryari-ghoshekandi, R., Nadagouda, M.N., Sillanp¨a¨a, M., Megiel, E., 2017. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 7 (1), 1–14.
Shabani, K.S., Ardejani, F.D., Badii, K., Olya, M.E., 2017. Preparation and
characterization of novel nano-mineral for the removal of several heavy metals from aqueous solution: Batch and continuous systems. Arab. J. Chem. 10, S3108–S3127. Sundramoorthy, A.K., Wang, Y.-C., Gunasekaran, S., 2015. Low-temperature solution
process for preparing flexible transparent carbon nanotube film for use in flexible supercapacitors. Nano Res. 8 (10), 3430–3445.
Tai, Z., Yang, J., Qi, Y., Yan, X., Xue, Q., 2013. Synthesis of a graphene oxide–polyacrylic acid nanocomposite hydrogel and its swelling and electroresponsive properties. RSC Adv. 3 (31), 12751–12757.
Yang, J.Y., Hou, B.H., Wang, J.K., Tian, B.Q., Bi, J.T., Wang, N., Li, X., Huang, X., 2019. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 9 (3).
Yantasee, W., Warner, C.L., Sangvanich, T., Addleman, R.S., Carter, T.G., Wiacek, R.J., Fryxell, G.E., Timchalk, C., Warner, M.G., 2007. Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles. Environ. Sci. Technol. 41 (14), 5114–5119.
Zargoosh, K., Zilouei, H., Mohammadi, M.R., Abedini, H., 2014. 4-Phenyl-3- thiosemicarbazide Modified Magnetic Nanoparticles: Synthesis, Characterization and Application for Heavy Metal Removal. CLEAN Soil Air Water 42 (9), 1208–1215. Zhou, C., Zhang, W., Xia, M., Zhou, W., Wan, Q., Peng, K., Zou, B., 2013. Synthesis of
poly(acrylic acid) coated-Fe3O4 superparamagnetic nano-composites and their fast removal of dye from aqueous solution. J. Nanosci. Nanotechnol. 13 (7), 4627–4633. Zhu, Y., Hu, J., Wang, J., 2012. Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto