Contents lists available atScienceDirect
Food Chemistry
journal homepage:www.elsevier.com/locate/foodchem
The anti-in
flammatory potential of Portulaca oleracea L. (purslane) extract
by partial suppression on NF-
κB and MAPK activation
Lingchao Miao
a,1, Hongxun Tao
a,1, Yu Peng
a, Shengpeng Wang
a, Zhangfeng Zhong
a,
Hesham El-Seedi
b, Simona Dragan
c, Gokhan Zengin
d, Wai San Cheang
a, Yitao Wang
a,⁎,
Jianbo Xiao
a,⁎aState Key Laboratory of Quality Control in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau bDepartment of Medicinal Chemistry, Uppsala University, Biomedical Centre, Box 574, SE-75 123 Uppsala, Sweden
cUniversity of Medicine and Pharmacy Victor Babes, 2 Eftimie Murgu Sq, 300041 Timisoara, Romania dDepartment of Biology, Science Faculty, Selcuk University, Konya, Turkey
A R T I C L E I N F O Keywords: Portulaca oleracea L. Flavonoids Lipopolysaccharide Anti-inflammatory effects NF-κB MAPK A B S T R A C T
Portulaca oleracea L. (Purslane) has great potential as food and traditional drugs in several countries. The purpose of this study was to evaluate the anti-inflammatory effects of purslane extract on lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. Purslane extracts significantly reduced LPS-induced synthesis of NO in a dose-dependent manner, as well as the expression levels of iNOS and COX-2. The productions of TNF-α and IL-6 were also significantly reduced at the higher dose of 400 μg/ml. Meanwhile, the expression levels of P65, P65, p-MEK and p-IκB-α were inhibited dose-dependently. The nuclear translocation of P65 was partially prevented by the extract, which explained the inhibition of NF-κB pathway. In addition, three reported flavonoids, named luteolin, kaempferol and quercitrin, were identified in the extract, which might be responsible for its anti-inflammatory effects. Above all, our research has partially proved that purslane could be considered as a natural anti-inflammatory agent in further applications.
1. Introduction
Portulaca oleracea L., commonly known as purslane in English and Ma-Chi-Xian (马齿苋) in Chinese, belongs to the Portulacaceae family (Zhou et al., 2015). This plant is used diffusely as a potherb, being
added in soups or salads; and has also been used in traditional medicine in many countries (Pinela, Carvalho, & Ferreira, 2017). Purslane pos-sesses a number of nutritional benefits due to the presence of its con-stituents: omega-3 fatty acids,flavonoids, terpenoids, alkaloids, sterols, vitamins, proteins and minerals (Delfan-Hosseini, Nayebzadeh, Mirmoghtadaie, Kavosi, & Hosseini, 2017; Iranshahy et al., 2017; Petropoulos, Karkanis, Martins, & Ferreira, 2016). Previous studies supported that it owns many pharmacological activities, including an-tidiabetic activity (El-Sayed, 2011; Gong et al., 2009), anti-cancer ac-tivity (Zheng et al., 2014), hepatoprotective activity (Eidi, Mortazavi, Moghadam, & Mardani, 2015), anti-microbial effect (Oh, Chang,
Hwang, & Mar, 2000), and antioxidant activity (Karimi, Aghasizadeh, Razavi, & Taghiabadi, 2011). Therefore, the World Health Organization (WHO) listed this plant as one of the most used medicinal plants and named it as“Global Panacea”.
Inflammation is a recognized hallmark of many diseases, such as cancer (Chen et al., 2018; Shalapour & Karin, 2015), diabetes (Calle & Fernandez, 2012) and cardiac diseases (Fioranelli et al., 2018). A recent study has shown that purslane modulates lung inflammation and im-mune markers (Kaveh, Eidi, Nemati, & Boskabady, 2017). Several al-kaloids obtained from purslane have been reported to present anti-in-flammatory activity (Meng et al., 2016). Furthermore, tumor necrosis factor-α (TNF-α) activity is reduced by purslane extract in vascular endothelial cells (Lee, Kim, Lee, Kang, & Lee, 2012). Although these studies have indicated the anti-inflammatory potential of purslane, the underlying molecular mechanism remains unclear and needs further exploration. The aim of the present work was to evaluate the
anti-https://doi.org/10.1016/j.foodchem.2019.04.005
Received 18 November 2018; Received in revised form 2 March 2019; Accepted 1 April 2019
⁎Corresponding authors at: Room 1050, Building N22, University of Macau, Avenida da Universidade, Taipa, Macau (Y. Wang). Room 8005, Building N22,
University of Macau, Avenida da Universidade, Taipa, Macau (J.B. Xiao).
E-mail addresses:swang@um.edu.mo(S. Wang),annacheang@umac.mo(Z. Zhong),hesham.el-seedi@ilk.uu.se(H. El-Seedi),
simona.dragan@umft.ro(S. Dragan),gokhanzengin@selcuk.edu.tr(G. Zengin),annacheang@umac.mo(W.S. Cheang),ytwang@umac.mo(Y. Wang), jianboxiao@umac.mo(J. Xiao).
1Lingchao MIAO and Hongxun TAO contributed equally to this work.
Available online 02 April 2019
0308-8146/ © 2019 Elsevier Ltd. All rights reserved.
inflammatory properties of the purslane extract and explore its poten-tial mechanisms. During an inflammatory process, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) play a key role in magnifying the inflammatory response (Choi et al., 2014). The iNOS enzymatically catalyzes the conversion of L-arginine to nitric oxide (NO) (Jaffrey & Snyder, 1995). iNOS expression is induced in response to TNF-α, interleukin-6 (IL-6) and lipopolysaccharide (LPS). In the present study, LPS-stimulated macrophage RAW 264.7 cells were se-lected as an in vitro model to assess the anti-inflammatory activity of the extract, the release of NO, IL-6 and TNF-α in LPS-induced cells. It has been well-accepted that the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, and further translocation into the nuclei of cytoplasmic complexes, play a central role in inflammation (Tak & Firestein, 2001). The increased activities of mitogen-activated protein kinases (MAPKs) during inflammation, as well as their participation in the regulation of cytokines, make these pathways potential targets for anti-inflammatory therapeutics (Kaminska, 2005). Therefore, the effects of purslane on the cascades of
NF-κB and MAPKs signaling pathways were also investigated. To un-derstand the material basis of purslane, chemical compositions in the extract were analyzed using UPLC/Q-TOF-MS system. We aimed to investigate whether purslane extract presented anti-inflammatory effect and whether the effect was mediated through NF-κB and MAPK sig-naling pathways using LPS-induced RAW 264.7 cells.
2. Materials and methods 2.1. Chemicals and reagents
Thiazolyl blue tetrazolium bromide (MTT) reagent, Griess reagent and 4′,6-diamidino-2-phenylindole (DAPI) dye were purchased from Sigma-Aldrich. All the primary antibodies and secondary antibodies of Western Blotting assay were obtained from Cell Signaling Technology. Interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) ELISA kits were acquired from Neobioscience Technology Co., Ltd (Shenzhen, China). Cedar oil was purchased from Leica and Alexa Fluor 568 goat anti-rabbit IgG (H+L) was purchased from Life Technologies. 2.2. Preparation of purslane ethanol extract
The whole plants were collected and manufactured during October 2016 in Huangshan Mountain (Anhui, China), and identified as P. oleracea L. by Prof. Jianbo Xiao from the Institute of Chinese Medical Sciences, University of Macau (Macau, China). The voucher specimen (1.0 kg) was kept at above institution. Ten grams of the shade dried plant materials were smashed and soaked in 100 ml water-ethanol (1:1, v/v) overnight. Then the soaked solution was collected and the powder was extracted with another fresh 100 ml water-ethanol (1:1, v/v) by ultrasonic treatment. Then, the extracts were mixed, filtered and en-riched to 10.0 g under the negative pressure. The concentrated extract was stored at −80 °C overnight immediately followed by the freeze-drying process for 2 days. Thefinal freeze-dried powder (4.81 g) was attained. The freeze-dried powder was then dissolved in culture medium before its application. Then the extract media wasfiltered by 0.22μm sterile filters to keep the media sterilized prior to use in the experiments.
2.3. Analysis of major bioactive compounds of purslane extract
The freeze-dried powder was weighed and dissolved in DMSO so-lution, then the solution was diluted in the ethanol-water (1:1, v/v) to achieve the concentration of 2, 5 and 10 mg/ml. Afterwards, the solu-tion was centrifuged at 14,800 rpm for 15 min at 4 °C. The supernatant was all transferred to new tubes and stored at−20 °C. All the standard samples were dissolved in DMSO and all the samples were performed on a Waters ACQUITY UPLC system with a Waters SYNAPT G2-Si QTOF
HDMS (Waters Technology, USA). During the detection, all samples were placed in cooled autosamplers (4 °C) to maintain sample stability throughout the analysis. Chromatographic separation on the system was achieved on a Waters ACQUITY UPLC HSS T3 2.1 × 150 mm, 1.8μm column (Waters Technology, USA). The following phase was a gradient of water (A) and Acetonitrile (B) at aflow velocity of 0.3 ml/ min. Gradient starting composition was 5% B increasing to 95% B over 1 min followed by a 28 min post-run. For the instrument, detection was carried out by using electrospray ionization (ESI) with nebulizer pres-sure of 5.5 Bar, cone gasflow of 70 L/h, and desolvation gas flow of 600 L/h at 20 °C. The temperature of source was set as 120 °C. Capillary voltage was set to 3.0 kV and cone voltage was set to 40 V. Acquisition type for Q-TOF was in Resolution mode at a scan rate of 0.3 s/spectra in positive ion mode. The acquisition time was set as 40 min and the ac-quire TOF MS was over the range from 50 Da to 1200 Da. A differential analysis of the Q-TOF data was performed using MassLynx V4.1 soft-ware (Waters Technology, USA). Compounds were identified based on their accurate mass according to the standard samples run under the same UPLC/Q-TOF-MS conditions.
2.4. Cell culture and treatments
Mouse RAW 264.7 macrophage cells were purchased from the ATCC (TCM 13, American Type Culture Collection, USA). The RAW 264.7 cells were cultured in DMEM/HIGH GLUCOSE medium (GE Healthcare Life Sciences HyClone Laboratories, Utah, America) with 10% FBS (Gibco by Life Technologies, South America), 50 U/ml penicillin and 100 mg/l streptomycin (Gibco, America). Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. Inflammatory RAW
264.7 cells were induced by treatment with LPS (1μg/ml). Purslane extract was added to the medium 4 h before LPS.
2.5. Cell viability
The cell viability was measured by the MTT assay according to the method of Mosmann (1983) with a few transformations. The RAW 264.7 cells (5 × 103/well) were seeded in 96-well plates and incubated at 37 °C with 5% CO2 overnight. Then, the cells were treated with
DMEM medium alone or different concentrations (0, 6.25, 12.5, 25, 50, 100, 200 and 400μg/ml) of the purslane extract medium for 24 h. After the removal of the medium, 10μl MTT (10 mg/ml) was added into the 100μl flesh DMEM medium in each well, followed by incubation at 37 °C for another 3 h. Finally, all of the MTT media were thrown away and the formazan crystals were dissolved in 100μl DMSO in every well. Afterwards, the 96-well plates were shaken for 30 min. The absorbance was detected at 570 nm using a microplate spectrophotometer reader. The optical density of the control group was regarded as 100% viability. The results were presented as a percentage of surviving cells over control cells. The experiments were performed in triplicate.
2.6. Determination of nitric oxide (NO) production
The production of NO was estimated by Griess assay (Sreejayan & Rao, 1997). The RAW 264.7 cells were seeded in 24-well plates (8 × 103cells/well) and incubated at 37 °C with 5% CO
2 overnight.
After pre-treatment with different concentrations (0, 6.25, 12.5, 25, 50, 100, 200 and 400μg/ml) of the purslane extract for 4 h, RAW 264.7 cells were subsequently co-treated with LPS (1μg/ml) for another 12 h. The control group was treated with DMEM medium without the pur-slane extract and LPS, while the model group was treated with DMEM medium containing LPS (1μg/ml). Then, 100 μl supernatant of each well was drawn from the 24-well plates into a new 96-well plate and 50μl Griess reagent was added to each well. The 96-well plates were shaken for 30 min under dark conditions. The absorbance was de-termined at 540 nm on a microplate spectrophotometer reader. The inhibitory rate was expressed as a percentage of released NO from the
purslane extract and LPS co–treated groups over LPS treated RAW 264.7 cells. All assays were performed in triplicate.
2.7. Western blotting analysis
The RAW 264.7 cells were seeded in 6-well plates (8 × 103/well)
and incubated at 37 °C with 5% CO2overnight. Two time points were
chosen to do the western blotting assay. On the following day, the RAW 264.7 cells were pre-treated with different concentrations (0, 100, 200 and 400μg/ml) of the purslane extract medium for 4 h. Then the cells were harvested after induced by LPS (1μg/ml) for another 1 or 12 h respectively, followed by centrifugation at 2000 rpm for 6 min at room temperature, and removal of supernatant. Then, 100μl of the RIPA solution containing 1% phenylmethanesulfonylfluoride (PMSF) and 1% Protease Inhibitor Cocktail (Beyotime, China) was added into each tube for another 40 min incubation on the ice. Afterwards, the lysates were centrifuged at 15,000 rpm for 15 min at 4 °C. The supernatant was collected, and the total protein concentration was determined with a Thermo Pierce BCA protein assay kit. Proteins were dissolved in SDS/ PAGE loading buffer and boiled for 10 min at 99 °C. The protein sam-ples were then resolved and separated by 8–12% SDS/PAGE gels. Afterwards, the blots were electrophoretically transferred to PVDF membranes, and blocked by 5% de-fatted milk in TBST buffer for 2 h at room temperature. Then, the PVDF membrane was incubated with corresponding primary antibodies overnight at 4 °C (iNOS, COX-2, phosphor-JNK, phosphor-P65, P65, phosphor-MEK, phosphor-IκB-α and GADPH). After washing with TBST for 3 times, the PVDF membrane was incubated with the corresponding secondary antibodies (1:5000) for another 2 h at room temperature, and followed by washing for 3 times with TBST. Finally, protein bands on PVDF membranes were vi-sualized with an enhanced chemiluminescence (ECL) system (BIO-RAD, America) and scanned by ChemiDoc MP Imaging System (BIO-RAD, America). The strips were quantified relative to GAPDH as standard. All the experiments were run in triplicate.
2.8. Enzyme-linked immunosorbent assay (ELISA)
The supernatant samples of the cytokines were gained while the RAW 264.7 cells were treated the same as the procedure of Western Blotting. The identical time points were chosen to obtain the super-natant samples for the analysis of IL-6 and TNF-α cytokine levels. The cytokine levels were measured with the corresponding ELISA kits ac-cording to the manufacturer’s instructions. The absorbance of each sample was read at 450 nm with a microplate spectrophotometer. And the concentrations of IL-6 and TNF-α cytokines were calculated ac-cording to the standard curves by Origin 8 (America).
2.9. Confocal microscopy
The RAW 264.7 cells were seeded (5 × 103cells/well) in the con-focal dishes and incubated overnight. Then the cells were pretreated with the purslane extract medium (400μg/ml) for 4 h and immune-stimulated by LPS (1μg/ml) for another 12 h. After that, the super-natant was removed and the dishes were washed by ice cold PBS for three times, 3 min every time, and cells were fixed with 4% paraf-ormaldehyde solution for 15 min. Then the supernatant was removed and the dish was washed for three times with cold PBS. Cell permea-bilization was performed by adding 0.5% Triton X-100 solution in PBS for 20 min and washed again. The cells were blocked with the corre-sponding solution which contained 3% BSA for another 30 min at 37 °C in the cell incubator. Next, the cells were incubated with primary an-tibody rabbit anti-P65 (1:800, CST, America) at 4 °C overnight. After the primary antibody was recovered, the cells were washed by ice cold PBS for three times. Then the secondary antibody, Alexa Fluor 568 goat anti-rabbit IgG (H+L), was used at a concentration of 1:1000 for in-cubation at 37 °C for 1 h in the dark. Following that, the cells were
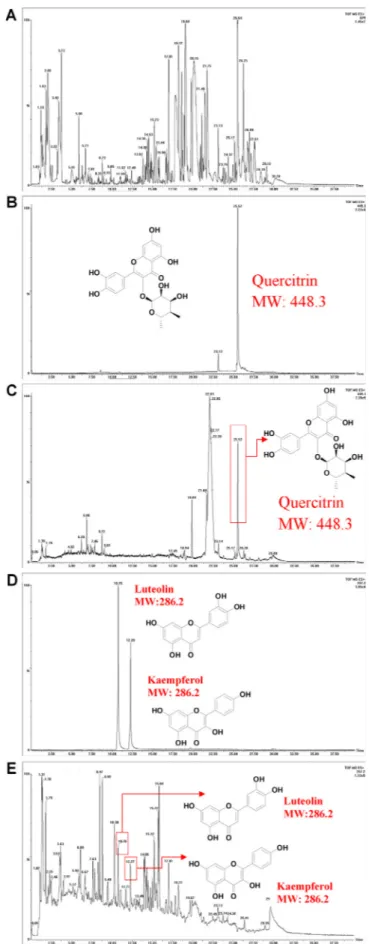
Fig. 1. UPLC-MS chromatograms of the purslane extract. (A) TIC spectrogram of the purslane extract. (B) The chromatogram of the standard sample querci-trin. (C) The chromatogram of quercitrin in the purslane extract sample. (D) The chromatogram of the standard sample luteolin and kaempferol. (E) The chromatogram of luteolin and kaempferol in the purslane extract sample.
stained by Hoechst 33342 dye (1μg/ml) for 5 min at room temperature in dark conditions to label cell nucleus (Sigma). Finally, the image acquisition was performed using the Leica TCS SP8 Confocal Laser Scanning Microscope System (Leica Microsystems, Germany). 2.10. Statistical analysis
The results are shown as mean ± SD for the all of representative experiments. Multiple comparisons between the experimental groups were distinguished by one-way ANOVA. The level of significance was set at p < 0.05. For statistical analysis, GraphPad Prism version 7 (America) and Origin version 8 (America) were used.
3. Results
3.1. UPLC-MS analysis of the purslane extract
According to the TIC spectrogram of the purslane extract, many constituents in the extract were detected (Fig. 1A). Compared with the standards, three natural products (luteolin, kaempferol and quercitrin) were identified in the purslane extract sample. The retention time (RT) of theseflavonoids in the purslane extract was the same as their cor-responding standards. The RT of quercitrin was 25.52 min (Fig. 1B and C) both in the standard and the extract sample. The RT of luteolin was 10.75 min (Fig. 1D) and 10.76 min (Fig. 1E) in the standard and the extract sample respectively. The RT of kaempferol was 12.28 min (Fig. 1D) and 12.27 min (Fig. 1E) in the standard and the extract sample respectively.
3.2. Effects of purslane extract on the cell viability and LPS-induced NO release in RAW 264.7 cells
The cell viability assay evidenced the absence of toxic effects of the purslane extract when added to the medium for 24 h with 12.5, 25, 50, 100, 200 and 400μg/ml (Fig. 2A). Interestingly, at the concentration of 25–400 μg/ml, the cell viability of the RAW 264.7 cells was even above 100%, indicating the proliferation of RAW 264.7 cells might be pro-moted by the purslane extract. Therefore, the concentration range of 12.5–400 μg/ml was thought to be the safe window. In the following experiments, the concentration of the purslane extract was chosen based on this safe window. Purslane extract inhibited NO release in a
dose-dependent manner (Fig. 2B). In the Dunnett’s multiple
compar-isons test, the inhibitory rates of NO release by the group of 50, 100, 200 and 400μg/ml were 17.52%, 20.71%, 32.62% and 46.16% re-spectively, which all showed significant differences to the LPS group. 3.3. Modulation of cellular production of IL-6 and TNF-α by purslane extract
To confirm the anti-inflammatory potential of the purslane extract, the release of the pro-inflammatory cytokines IL-6 and TNF-α in the cultured medium was investigated by ELISA kits. In RAW 264.7 cells, the synthesis of IL-6 and TNF-α was up-regulated by LPS (1.0 μg/ml) sharply by 53.45 times and 33.07 times. However, this LPS-stimulated production of IL-6 and TNF-α was significantly down-regulated by the pre-treatment of the purslane extract (400μg/ml,Fig. 3A and B). The concentration of the purslane extract (400μg/ml) was of great effi-ciency to inhibit the production of IL-6 by 48.97% and the production of TNF-α by 34.11%, which means the inflammation of RAW 264.7 cells activated by LPS (1μg/ml) can be suppressed by the effective concentration of the purslane extract (400μg/ml).
3.4. Effects of purslane extract on protein expression of iNOS, COX-2, proteins of NF-κB and MAPKs pathways in LPS-induced RAW 264.7 cells
In RAW 264.7 cells, the expression of iNOS and COX-2 was sharply up-regulated by LPS (1.0μg/ml). However, the up-regulation of both proteins was significantly ameliorated by the purslane extracts in a dose-dependent way. As shown inFig. 4A, no significant decrease of the expression of iNOS was found at the concentration of 100 or 200μg/ml of the purslane extract. Nevertheless, the purslane extract was found to obviously inhibit the expression of iNOS at 400μg/ml. Meanwhile, the LPS-induced expression of COX-2 was decreased even from 100μg/ml to 400μg/ml of the purslane extracts. Taken together, the anti-in-flammatory potential of purslane extract was confirmed at protein level.
To further clarify the anti-inflammatory mechanism of the purslane extract, both the MAPKs and NF-κB pathways were investigated. The expression levels of phosphor-JNK, phosphor-P65, MEK, phosphor-MEK, IκB-α, IκB-α, P38, P38, IKK-α, IKK-β and phosphor-IKK-α/β proteins were analyzed by western blotting assays, while GADPH andβ-actin were used as internal standards. When RAW 264.7
Fig. 2. Effects of purslane extract on cell viability and NO release on RAW 264.7 cells. (A) Cell viability of RAW 264.7 cells treated with different concentrations of the purslane extract medium for 24 h. Values are the means ± SD (n = 3).*P < 0.05,**P < 0.01 purslane extract vs control. (B) Inhibitory rate of NO production in
RAW 264.7 cells pretreated with the purslane extract (12.5, 25, 50, 100, 200 and 400μg/ml) for 4 h, followed by co-treatment with LPS (1 μg/ml) for another 12 h.
cells were pre-treated with the purslane extracts for 4 h and stimulated by LPS (1.0μg/ml) for another 12 h, the phosphorylation of P65 was up-regulated by LPS but suppressed by the purslane extracts especially at the concentration of 400μg/ml (Fig. 4B). Beyond that, the
expressions of IκB-α and phosphor-IκB-α were also suppressed appar-ently by the purslane extract at the concentration of 400μg/ml while they were not affected by LPS regularly (Fig. 4B). Besides, the pro-ductions of phosphor-IKK-α/β, IKK-β, P38 and phosphor-P38 were found Fig. 3. Effects of the purslane extract on LPS-stimulated pro-inflammatory cytokines. (A) IL-6 and (B) TNF-α production in RAW 264.7 cells pre-treated with 400 μg/ ml purslane extract for 4 h followed by LPS stimulation (1.0μg/ml) for 12 h. Values are the means ± SD.***P < 0.001 LPS vs control or purslane extract vs LPS.
Fig. 4. Effects of the purslane extract on the expression of iNOS, COX-2, NF-κB and MAPKs pathway related proteins. RAW 264.7 cells were treated with different concentrations (0, 100, 200 and 400μg/ml) of the purslane extract medium for 4 h ahead of treatment with LPS (1.0 μg/ml) for another (A–C) 12 h or (D, E) 1 h.
to maintain unchanged when the RAW 264.7 cells were treated by LPS or the purslane extract (Fig. 4B and C). The expression levels of MEK and phosphor-MEK were increased by LPS, but they were not overturned by the purslane extract, yet were suppressed slightly at the concentra-tion of 400μg/ml (Fig. 4C). Apart from this, when the RAW 264.7 cells were incubated with the purslane extract for 4 h and activated with LPS (1.0μg/ml) for another 1 h, the productions of MEK, phosphor-P38, phosphor-P65 and phosphor-IκB-α were found to be up-regulated by LPS, but down-regulated by the purslane extracts in a dose-dependent response (100–400 μg/ml) (Fig. 4D and E), which was different from when the RAW 264.7 cells were activated with LPS (1μg/ml) for 12 h. The expression of IKK-α and IKK-β were inhibited when the cells were stimulated by LPS but were overturnedfirstly and then down-regulated when co-treated by the purslane extract dose-dependently (Fig. 4E). All the results indicated that MAPKs and NF-κB pathways were partially activated. Pre-treatment with the purslane extracts effectively inhibit the expression of phosphor-MEK and phosphor-IκB-α, phosphor-P65 and phosphor-P38 in a slight dose-dependent manner (100–400 μg/ml). Particularly, the purslane extracts showed potent inhibition at the concentration of 400μg/ml. Taken together, the purslane extracts might down-regulate the phosphorylation of both IκB and P65 by at-tenuating the phosphorylation of MEK and P38.
3.5. Effects of purslane extracts on the expression and nuclear translocation of P65 in LPS-induced RAW 264.7 cells
At the same microscope magnification, the cell size of RAW 264.7 cells stimulated by LPS (1.0μg/ml) was larger than the control group, and the cell shape was also changed, which reflected the inflammatory changes. Interestingly, the purslane extracts pre-treatment reversed the changes of cell size and shape resulted from LPS induction (Fig. 5A). Compared with control group, the redfluorescence indicating P65 level was stronger in LPS group, which was inhibited by the purslane extract (400μg/ml). The results of western blotting analysis of P65 confirmed the down-regulating effect of the purslane extracts, which occurred in a dose-dependent manner (100–400 μg/ml) (Fig. 5B). In addition, the
P65 subunit, which is required for TNF-α-dependent genes’ induction, was introduced into cell nucleus by LPS activation. This translocation of P65 was significantly blocked by the purslane extract (seeFig. 6). 4. Discussion
In the present study, we found that the mechanism of anti-in-flammatory effects of the purslane extracts rich in flavonoids on LPS-stimulated RAW 264.7 cells may be attributed to the inhibition to the protein expression of iNOS, COX-2 and partial suppression on NF-κB and MAPK activation. Significant evidence illustrated that dietary fla-vonoids showed anti-inflammatory activity in vitro and in vivo (Chen et al., 2018; Xiao, 2017).
Excessive release of NO associated to the iNOS induction is involved in the production of the highly reactive peroxynitrite, and is also in-volved in the inflammatory response. Thus, NO release is regarded as an inflammatory marker (Guzik, Korbut, & Adamek-Guzik, 2003). As
TNF-Fig. 5. Effect of the purslane extract on the translocation and expression of P65 in LPS-induced RAW 264.7 cells. (A) Confocal immunofluorescence photo-micrography for RAW 264.7 cells pre-treated with the purslane extract (400μg/ml) for 4 h and induced by LPS (1 μg/ml) for another 12 h. P65 protein was indicated by redfluorescence, while the nuclear was indicated by blue fluorescence. (B) Western blotting analysis of P65 for RAW 264.7 cells pre-treated with different concentrations of the purslane extract (0, 100, 200 and 400 µg/ml) for 4 h followed by LPS treatment (1μg/ml) for another 12 h. (For interpretation of the references to colour in thisfigure legend, the reader is referred to the web version of this article.)
α is considered not only a pro-inflammatory but also an im-munoregulatory cytokine, it has differential effects depending on the target immune cell and also on the apoptotic process (Sabry et al., 2006). IL-6 is a pleiotropic cytokine, with a central role in inflammation
and as an activator of signal transducer and activator of transcription 3, leading to the blockage of apoptosis in cells during the inflammatory process, maintaining them alive in toxic environments (Hodge, Hurt, & Farrar, 2005). The induction of iNOS in macrophages is a pre-requisite for high NO release, mediating numerous bactericidal and tumoricidal responses in immune cells (Connelly, Palacios-Callender, Ameixa, Moncada, & Hobbs, 2001). The COX-2 protein is an inducible pro-in-flammatory molecule (Hooshmand et al., 2015).Li et al. (2017)isolated three novel alkaloids from P. oleracea L. and demonstrated that one of the alkaloids oleracimine decreases the secretions of NO, IL-6, TNF-α and prostaglandin E2as well as mRNA levels of iNOS and COX-2 in
LPS-induced RAW 264.7 cells. In line with the earlier work, we here showed that purslane extract remarkably inhibited NO release, as well as sup-pressed the expressions of iNOS and COX-2 dose-dependently; and significantly decreased LPS-induced inflammatory factors (IL-6 and TNF-α) at 400 μg/ml in LPS-stimulated RAW 264.7 cells. These results strongly supported the anti-inflammatory role of the purslane extract. It has already been reported that MAPK regulates the LPS-induced NF-κB activation pathway, which contributes to the phosphorylation of IκB-α or P65 (Chen et al., 2003). Studies performed in mice with de-ficient NF-κB subunits reported that this transcription factor is neces-sary for the lymphocyte response to antigens and cytokine-inducible gene expression. Specifically, the P65 subunit is essential for the in-duction of TNF-α-dependent genes (Beg & Baltimore, 1996) and binding site for P65 is also present in the promoter of the IL-6 genes (Matsusaka et al., 1993). After dissociation from IκB, the nuclear
translocation of P65 is an important step in the NF-κB signaling cascade (Oeckinghaus & Ghosh, 2009). In consistence, we found that purslane extract inhibited the phosphorylation of P65, MEK, P38 and IκBα in LPS-treated RAW 264.7 cells. Furthermore, the extract diminished the expression and nucleus translocation of P65 subunit in cells.
Purslane consists of many natural compounds, includingflavonoids, terpenoids, alkaloids, sterols, vitamins, proteins and minerals (Iranshahy et al., 2017; Petropoulos et al., 2016). Although we con-firmed the presence of major flavonoids luteolin, kaempferol and quercitrin in the purslane extract, whether the anti-inflammatory effect is mediated by these bioactive compounds, or whether and how these compounds act on NF-κB and MAPK pathways to modulate its function is not fully understood, which is yet to be explored in the future studies. Moreover, the metabolism of the purslane extract and characterization for the metabolites of the bioactive compounds during the incubation in RAW 264.7 cells can be studied in the future.
Acknowledgements
This study was supported by Multi-Year Research Grant of University of Macau (MYRG2018-00169-ICMS). The authors also thank Ms. Conghui Liu and Mr. Jason Kuang for their valuable assistance. Conflict of interest
None. References
Beg, A. A., & Baltimore, D. (1996). An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science, 274(5288), 782–784.
Calle, M. C., & Fernandez, M. L. (2012). Inflammation and type 2 diabetes. Diabetes & Metabolism, 38(3), 183–191.
Chen, Y. H., Layne, M. D., Chung, S. W., Ejima, K., Baron, R. M., Yet, S. F., & Perrella, M. A. (2003). Elk-3 is a transcriptional repressor of nitric oxide synthase 2. Journal of Biological Chemistry.
Chen, L., Teng, H., Jia, Z., Battino, M., Miron, A., Yu, Z. L., ... Xiao, J. B. (2018). Intracellular signaling pathways of inflammation modulated by dietary flavonoids: The most recent evidence. Critical Reviews in Food Science and Nutrition, 58(17), 2908–2924.
Choi, W. S., Jeong, J. W., Kim, S. O., Kim, G. Y., Kim, B. W., Kim, C. M., ... Kim, G. D. (2014). Anti-inflammatory potential of peat moss extracts in lipopolysaccharide-stimulated RAW 264.7 macrophages. International Journal of Molecular Medicine, 34(4), 1101–1109.
Connelly, L., Palacios-Callender, M., Ameixa, C., Moncada, S., & Hobbs, A. J. (2001). Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. Journal of Immunology, 166(6), 3873–3881.
Delfan-Hosseini, S., Nayebzadeh, K., Mirmoghtadaie, L., Kavosi, M., & Hosseini, S. M. (2017). Effect of extraction process on composition, oxidative stability and rheological properties of purslane seed oil. Food Chemistry, 222, 61–66.
Eidi, A., Mortazavi, P., Moghadam, J. Z., & Mardani, P. M. (2015). Hepatoprotective effects of Portulaca oleracea extract against CCl4-induced damage in rats. Pharmaceutical Biology, 53(7), 1042–1051.
El-Sayed, M. I. K. (2011). Effects of Portulaca oleracea L. seeds in treatment of type-2 diabetes mellitus patients as adjunctive and alternative therapy. Journal of Ethnopharmacology, 137(1), 643–651.
Fioranelli, M., Bottaccioli, A. G., Bottaccioli, F., Bianchi, M., Rovesti, M., & Roccia, M. G. (2018). Stress and inflammation in coronary artery disease: a review psychoneur-oendocrineimmunology-based. Frontiers in Immunology, 9, 2031.
Gong, F., Li, F., Zhang, L., Li, J., Zhang, Z., & Wang, G. (2009). Hypoglycemic effects of crude polysaccharide from purslane. International Journal of Molecular Sciences, 10(3), 880–888.
Guzik, T. J., Korbut, R., & Adamek-Guzik, T. (2003). Nitric oxide and superoxide in in-flammation and immune regulation. Journal of Physiology and Pharmacology, 54(4), 469–487.
Hodge, D. R., Hurt, E. M., & Farrar, W. L. (2005). The role of IL-6 and STAT3 in inflammation and cancer. European Journal of Cancer, 41(16), 2502–2512.
Hooshmand, S., Kumar, A., Zhang, J. Y., Johnson, S. A., Chai, S. C., & Arjmandi, B. H. (2015). Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food & Function, 6(5), 1719–1725.
Iranshahy, M., Javadi, B., Iranshahi, M., Jahanbakhsh, S. P., Mahyari, S., Hassani, F. V., & Karimi, G. (2017). A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. Journal of Ethnopharmacology, 205, 158–172.
Jaffrey, S. R., & Snyder, S. H. (1995). Nitric oxide: A neural messenger. Annual Review of Cell and Developmental Biology, 11, 417–440.
Kaminska, B. (2005). MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochimica et Biophysica Acta (BBA)-Proteins and. Proteomics, 1754(1–2), 253–262.
Karimi, G., Aghasizadeh, M., Razavi, M., & Taghiabadi, E. (2011). Protective effects of aqueous and ethanolic extracts of Nigella sativa L. and Portulaca oleracea L. on free radical induced hemolysis of RBCs. Daru, 19(4), 295–300.
Kaveh, M., Eidi, A., Nemati, A., & Boskabady, M. H. (2017). Modulation of lung inflammation and immune markers in asthmatic rats treated by Portulaca oleracea. Avicenna Journal of Phytomedicine, 7(5), 409.
Lee, A. S., Kim, J. S., Lee, Y. J., Kang, D. G., & Lee, H. S. (2012). Anti-TNF-α activity of Portulaca oleracea in vascular endothelial cells. International Journal of Molecular Sciences, 13(5), 5628–5644.
Li, C. Y., Meng, Y. H., Ying, Z. M., Xu, N., Hao, D., Gao, M. Z., ... Ying, X. X. (2017). Three novel alkaloids from Portulaca oleracea L. and their anti-inflammatory effects (vol 64, pg 5836, 2016). Journal of Agricultural and Food Chemistry, 65(4), 993–994.
Matsusaka, T., Fujikawa, K., Nishio, Y., Mukaida, N., Matsushima, K., Kishimoto, T., & Akira, S. (1993). Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proceedings of the National Academy of Sciences, 90(21), 10193–10197.
Meng, Y., Ying, Z., Xiang, Z., Hao, D., Zhang, W., Zheng, Y., ... Ying, X. (2016). The anti-inflammation and pharmacokinetics of a novel alkaloid from Portulaca oleracea L. Journal of Pharmacy and Pharmacology, 68(3), 397–405.
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods, 65(1–2), 55–63.
Oeckinghaus, A., & Ghosh, S. (2009). The NF-κB family of transcription factors and its reg-ulation. Cold Spring Harbor Perspectives in Biologya000034.
Oh, K. B., Chang, I. M., Hwang, K. J., & Mar, W. (2000). Detection of antifungal activity in Portulaca oleracea by a single-cell bioassay system. Phytotherapy Research, 14(5), 329–332.
Petropoulos, S., Karkanis, A., Martins, N., & Ferreira, I. C. F. R. (2016). Phytochemical com-position and bioactive compounds of common Purslane (Portulaca oleracea L.) as affected by crop management practices. Trends in Food Science & Technology, 55, 1–10.
Pinela, J., Carvalho, A. M., & Ferreira, I. C. F. R. (2017). Wild edible plants: Nutritional and toxicological characteristics, retrieval strategies and importance for today's society. Food and Chemical Toxicology, 110, 165–188.
Sabry, A., Sheashaa, H., El-Husseini, A., Mahmoud, K., Eldahshan, K. F., George, S. K., ... Abo-Zenah, H. (2006). Proinflammatory cytokines (TNF-alpha and IL-6) in Egyptian patients with SLE: Its correlation with disease activity. Cytokine, 35(3–4), 148–153.
Shalapour, S., & Karin, M. (2015). Immunity, inflammation, and cancer: An eternal fight be-tween good and evil. Journal of Clinical Investigation, 125(9), 3347–3355.
Sreejayan, & Rao, M. N. (1997). Nitric oxide scavenging by curcuminoids. Journal of Pharmacy and Pharmacology, 49(1), 105–107.
Tak, P. P., & Firestein, G. S. (2001). NF-κB: A key role in inflammatory diseases. The Journal of Clinical Investigation, 107(1), 7–11.
Xiao, J. B. (2017). Dietaryflavonoid aglycones and their glycosides: Which show better bio-logical significance? Critical Reviews in Food Science and Nutrition, 57, 1874–1905.
Zheng, G. Y., Qu, L. P., Yue, X. Q., Gu, W., Zhang, H., & Xin, H. L. (2014). Portulacerebroside A induces apoptosis via activation of the mitochondrial death pathway in human liver cancer HCCLM3 cells. Phytochemistry Letters, 7, 77–84.
Zhou, Y. X., Xin, H. L., Rahman, K., Wang, S. J., Peng, C., & Zhang, H. (2015). Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. BioMed Research