See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/287245386
Effects of some technological parameters on chemical and sensory qualities
and free fatty and amino acids of various probiotic cultures in Beyaz cheese
during ripening process
Article in Journal of Food Agriculture and Environment · January 2014
CITATIONS
0
READS
59 2 authors, including:
Some of the authors of this publication are also working on these related projects:
Kavılca Lifi Kullanılarak Üretilen Dondurmaların Kalite ve Besinsel Özelliklerinin TespitiView project
Possibilities for the use of whey in TEL KADAYIF (a Turkish dessert) productionView project Filiz Yangılar
Erzincan University
29PUBLICATIONS 178CITATIONS
www.world-food.net
Journal of Food, Agriculture & Environment Vol.12 (3&4): 32-39. 2014
WFL Publisher
Science and Technology
Meri-Rastilantie 3 B, FI-00980 Helsinki, Finland
e-mail: info@world-food.net
Effects of some technological parameters on chemical and sensory qualities and free
fatty and amino acids of various probiotic cultures in Beyaz cheese during
ripening process
Filiz Yangılar 1* and Salih Ozdemir 2
1 Department of Food, Engineering Faculty, Ardahan University, 75000, Ardahan, Turkey. 2 Department of Agricultural, Food Engineering, Atatürk University, 25000, Erzurum, Turkey. *e-mail: f_yangilar@hotmail.com, filizyangilar@ardahan.edu.tr
Received 16 May 2014, accepted 2 October 2014.
Abstract
It was aimed in the present study to assess the effects of four probiotic strains on the chemical, physical and organoleptic properties of Beyaz cheese. For all experimental samples taken into consideration in the study, total chemical parameters were found to increase during ripening (p<0.05) process in the study. The FFAs concentration in control cheeses was significantly higher than that of made with probiotic dahi cheese samples (p<0.05). In addition, probiotic B. bifidum BB-12 appeared to increase the production of FFAs (caproic, 1.67; caprilic, 0.16 and capric, 2.57%). Bifidum BB–
12+L. acidophilus LA–5 increased significantly the rate of nitrogen compounds with low molecular weight and individual free amino acids
(p<0.05). Length of ripening period contributed to a significant increase in the content of free amino acids. For the sensorial characteristics, cheese sample E was found to the least preferred by the panellists.
Key words: Beyaz cheese, probiotics, free fatty acids, free amino acids.
Introduction
Today consumers are concerned in great majority with not only food security and its nutritional values, but also its health benefits 1, 2. Such kind of demands helped shape new concepts in
food industry, e.g. functional food, where probiotic ingredients take important parts 3. Probiotics are defined in an overview to be
living non-pathogenic microorganisms used in food industry to be dietary supplements due to their health benefits 4-9, such as
increasing the intake rate of low-fat dairy products and the reduction of the incidence of cardiovascular diseases (CVD), preclinical atherosclerosis, and cardiovascular risk factors in middle-age and older age persons 3, 10-16. Although the number of
probiotic bacteria that provides health benefits has not been
firmly established, levels between 106 and 109 cfu/g have been
suggested 17, 18. Various parameters must be considered when
adding probiotic bacteria to foods: type of culture to use, addition level required to obtain a physiological effect, survival during process parameters, stability during storage, and effect on the sensory properties 8, 19, 20.
Dairy products have been used as carrier foods for probiotic bacteria, as many of them had already been optimized for survival of lactic cultures 21, 22 , while new products including milk, yoghurt,
fermented milk, desserts, fruit juice and some cheese types have also taken their places among probiotic products 23. Cheese owns
a deserved fame to be a good carrier for probiotic bacteria by allowing them to survive throughout gastrointestinal tract 15, 24-32.
Beyaz cheese (Turkish acronym of white cheese) is a soft or semi-hard cheese type produced from sheep or cow milk or mixture of them 26. Cheese may offer several advantages over fermented milk
products such as yoghurt by serving as a delivery system for
viable probiotic in gastrointestinal tract; tending to increase fat content; and offering protection to probiotic bacteria during storage and passage through the gastrointestinal tract. Cheese can also exhibit larger buffering capacity than yoghurt 33. Various
chemical and biochemical reactions can be seen during the ripening process of Beyaz cheese including glycolysis, lipolysis and especially proteolysis, which is an important process and plays a direct role in the development of cheese flavour and texture 34.
Being among the most widely used probiotic bacteria, lactobacilli and bifidobacteria can contain proteolytic and peptidolytic enzyme types and therefore affect proteolysis 35-40. Addition of lactic acid
bacteria to dairy products was stated to contribute to the production of free fatty acids (FFAs) causing the lipolysis of milk fat 41-43.
The present study was conducted to evaluate the implications of different probiotics [B. bifidum BB-12 (B), B. bifidum BB-12+L.
acidophilus LA-5 (C), B. bifidum (D) and B. longum (E)] in addition
to commercial lactic culture [L. lactis and L. cremoris (A)] in the chemical and physical composition and sensory performance; and determine the fatty acid composition and related properties of Beyaz cheese during storage at 4°C.
Materials and Methods
Cultures: Research and Application Farm of Atatürk University provided cow milk and Lactococcus lactis subsp. lactis and
Lactococcus lactis subsp. cremoris frozen to dry were obtained
from DSM Food Specialties Pty. Ltd. (Moorebank, NSW, Australia) and used in the preparation of starter culture. Strains of various probiotic bacteria were used as adjunct cultures, among which B.
bifidum BB-12 and B. bifidum BB-12+L. acidophilus LA-5 were
obtained from Peyma Hansen (Gayrettepe, Istanbul, Turkey), while
B. bifidum and B. longum were obtained from Christian Hansen
(Christian Hansen, Valinnhos, Brazil). The organisms were activated using the method by Martensson et al. 44 and
experimental cheese material was produced in the pilot dairy plant of the Agricultural Faculty of Atatürk University.
Cheese manufacture: In the study, probiotic Beyaz cheese material manufactured by taking the rules of Demirci and Şimşek 45
into consideration was divided into five experimental groups (one control and four probiotic containing groups). Cheese manufacturing process was initiated by taking 500 L raw milk and adjusting its fat concentration to 3%. Milk sample was pasteurized at 65°C for 30 min, cooled to the incubation temperature of 35°C and divided into five equal parts (batch). A solution of 20 g/100 L CaCl2 was added to each batch and a control batch was prepared using 1 mL/100 mL commercial culture mix consisting of L. lactis and L. cremoris and a four – batch mix [B (Bifidobacterium bifidum BB–12), C (B. bifidum BB–12+L. acidophilus LA–5), D (B.bifidum) and E (B. longum)] was produced using equal concentrations of the probiotic and commercial mixes. Experiments on cheese production were conducted at the beginning with 100 L of raw milk, which was pasteurized at 65°C for 30 min, cooled to 35°C, CaCl2 (20 g/100 L) and a commercial culture mix inoculum (1 mL/ 100 mL;1% v/v) of cheese starter (Lactococcus lactis and
Lactococcus cremoris) was added. Probiotic strains were added
into samples B, C, D and E to inoculate at a level up to 107 cfu/mL.
Then 12 mL chymosin (Peyma Hansen, Turkey) dispersed in 100
mL water was added in each cheese vat in a sufficient rate to coagulate them in 90 min. At the end of curdling process about 1 cm3 small curd blocks were left for storage and compressed, after which formed cheese material was cut into 8 cm3 cubes salted in pasteurized brine (12% w/v, NaCl) for 6 h. After brine-salting, cheese samples in the form of blocks were taken to store at room temperature for 12 h and then transferred to plastic bags containing brine water by leaving ripening at 4±1°C. Such manufacturing process of cheese was triplicated and the samples were left to ripen for 2 months by analysing at the 2nd, 15th, 30th and 60th days of
ripening.
Chemical and physical analysis: Moisture, fat, dry matter fat, salt, dry matter salt (%), ash (%), protein (%), water-soluble protein (WSN; %), ripening degree (%) and titratable acidity (SH) of probiotic Beyaz cheeses were measured three times according to Kurt et al. 46. The pH was measured by adding and mixing 20 mL of
distilled water in grated cheese (10 g) using a digital pH meter
(WTW 340–1 47).
Nitrogen fractions: Probiotic Beyaz cheese fractions soluble in 12% trichloroacetic acid-soluble nitrogen (TCA-SN) were determined according to Polychroniadou et al. 48 and the
micro-Kjeldahl method by IDF 49. In this process, homogenisation of
grated cheese samples (20 g) was performed by adding and blending 40 mL of H2O in the samples an Ultraturrax blender (IKA, Wilmington, NC) for 2 min after which the mixture (homogenate) was stored at 40°C for 1 h and centrifuged at 3000 g for 30 min at 4°C. The fatty slick was removed and the supernatant was filtrated through filter paper (Scleicher & Schuell 589/2). Twenty five mL
extract prepared for WSN was taken in an equal volume of 24% (w/v) and TCA was added in the mixture to fragment nitrogenous compounds. The mixture samples were left for incubation for 2 h at ambient temperature. Precipitates were filtered through white ribbon filter paper (Schleicher & Schuell, 589/2).
Analysis of free fatty acids (FFAs): Direct transesterification gas chromatography was used to define FFA profile of milk and dahi samples as in Akalın et al. 50. One mL of methanol : benzene (3:2
ratio) was added to an aliquot of 100 mL of milk or 100 mg of dahi samples and freshly prepared 1 mL acetyl chloride : methanol (5:1000) was also added in the mixture and the tubes were capped tightly. The mixture tubes were taken in methanolysis process at 100°C for 1 h, cooled to room temperature and 1 mL methylated penta decanoic acid-hexane solution (500 mg dissolved in 1 mL of hexane) and 1 mL water were added. After that, the tubes were shaken and stored at 4°C until the gas chromatography. GC-Agilent 6890 N (USA) equipment had a glass column of 60 m × 0.25 mm ID packed with 10% DC–200 on chromosorb, and a flame ionisation detector. Nitrogen was the carrier gas with the flow rate of 28 mL min-1. The injector port temperature was fixed at 100°C for 2 min by
reaching gradually up to 250°C. Peak rates of each FFA were identified according to the retention times of the reference standards (Sigma Chemical Co., St. Louis, MO, USA).
Free amino acid (FAA) analysis: Free amino acids were extracted from probiotic Beyaz cheese curd slurry samples conveniently to the method in Standara et al. 51. Cheese slurry (10 g) was added in 90 mL trichloroacetic acid (TCA) by mixing to homogenize the mixture. Fatty top layer of the mixture was removed and the remaining sample was stored at 3°C to separate cream from the mixture at more advanced level and then the mixture was centrifuged at 3°C for 10 min at 8000 × g to remove fully the cream remnant and vacuum filtered (No. 1 filter paper, Whatman International Ltd, Maidstone, UK). The isolated free amino acids were derivatized according to protocol of EZ: FaastTM kit (Phenomenex, Torrance,
CA, USA) and subjected to gas chromatography (GC-Agilent 6890 N (USA) with a split injection port and flame ionization detector (FID)). Amino acid samples were injected and separated on a Zebron ZB-PAAC column (10 m × 0.25 mm, Phenomenex) and helium was carrier gas at 60 kPa and injection port and detector were set to 250°C and 320°C, respectively, by increasing oven temperature 35°C in one minute from 110°C to 320°C and leaving at 320°C for one minute. Amino acid standards included in EZ: FaastTM kits were used for the identification of amino acids in the
samples.
Sensorial analysis: Experimental samples stored at 4±1°C for 60 days were evaluated by trained and experienced panellists considering sensorial properties and the principles in Lyne 52.
Each panellist gave scores, ranging from 1 (poor) to 9 (excellent) to the cheese samples taking five sensory properties into account including colour, texture, taste and aroma, foreign flavour and aroma, saltiness and general acceptability. Panellists were permitted to have water and bread to screen the tastes of each sample.
Statistical analysis: The randomized complete block design was adopted for the experiments in the study. Data obtained was
transferred and evaluated in SPSS Statistical Software (version 15.0). Standard deviations of mean chemical and biochemical values were also calculated and statistically significant differences in mean values were compared using Duncan’s multiple range tests. Each sample was subjected to triplicate analysis 53.
Results and Discussion
Probiotic Beyaz cheese samples were produced from the skimmed milk material containing dry matter, fat, protein, ash, % acidity and pH in the rates of 11.68±0.45%; 3.21±0.38%; 3.31±0.17%; 0.59±0.20%; 8.88±0.71°SH and 6.40±0.03, respectively. Mean yields of cheese samples A, B, C, D and E were 15.94ab, 16.85b, 18.15b,
15.48a and 17.60b, respectively. Cheese sample D yielded
significantly lower than B, C and E (p<0.05) possibly caused by the different acidification processes employed in sample D. It was stated by Kindstedt et al. 54and Buriti et al. 55 that when the direct milk acidification process is used in common cheese production, higher yield rate, higher moisture content, as well as improved durability can be obtained due to delayed acidification.
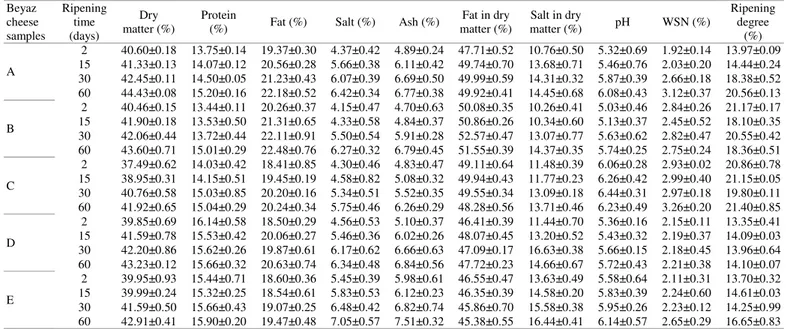
Physical and chemical composition: Table 1 gives the changes observed in the physico-chemical parameters obtained from probiotic Beyaz cheese samples A, B, C, D and E throughout during 60-day storage period. Length of the ripening period was found to have statistically significant effects on the chemical composition of experimental samples. The highest rate of dry matter (44.43%) was determined in control sample (Lactococcus
lactis+Lactococcus cremoris) even though the dry matter content
of all cheese samples increased during the ripening period. Such a situation may be expressed by the positive correlation between dry matter and salt 56, 57. Differences in the rates of dry matter
content between the samples were found to be statistically significant (p<0.01) in the present study, while Atasever et al. 58,
Topcu and Saldamlı 59 and Öksüz et al. 60 found mean dry matter
rates of 35.41 to 39.48%, 39.80 to 41.75% and 30 to 61%, respectively, which are in a similar range with those found in the present study. The highest fat (22.48%) and dry-matter fat
2 15 30 60 18 19 20 21 22 23 24 25
Ripening time (day)
Titratable acidity (SH)
Figure 1. Changes in titratable acidity (SH) of probiotic Beyaz
cheese samples.
Table 1. Some chemical and physical properties of probiotic Beyaz cheese.
** The values presented are the average of three recurrences. A(Control): Only lactic culture. B: B. bifidum BB-12+lactic culture. C: B. bifidum BB-12+L. acidophilus LA-5+lactic culture. D: B. bifidum+lactic culture. E: B. longum+lactic culture. Beyaz cheese samples Ripening time (days) Dry matter (%) Protein
(%) Fat (%) Salt (%) Ash (%)
Fat in dry matter (%) Salt in dry matter (%) pH WSN (%) Ripening degree (%) A 2 40.60±0.18 13.75±0.14 19.37±0.30 4.37±0.42 4.89±0.24 47.71±0.52 10.76±0.50 5.32±0.69 1.92±0.14 13.97±0.09 15 41.33±0.13 14.07±0.12 20.56±0.28 5.66±0.38 6.11±0.42 49.74±0.70 13.68±0.71 5.46±0.76 2.03±0.20 14.44±0.24 30 42.45±0.11 14.50±0.05 21.23±0.43 6.07±0.39 6.69±0.50 49.99±0.59 14.31±0.32 5.87±0.39 2.66±0.18 18.38±0.52 60 44.43±0.08 15.20±0.16 22.18±0.52 6.42±0.34 6.77±0.38 49.92±0.41 14.45±0.68 6.08±0.43 3.12±0.37 20.56±0.13 B 2 40.46±0.15 13.44±0.11 20.26±0.37 4.15±0.47 4.70±0.63 50.08±0.35 10.26±0.41 5.03±0.46 2.84±0.26 21.17±0.17 15 41.90±0.18 13.53±0.50 21.31±0.65 4.33±0.58 4.84±0.37 50.86±0.26 10.34±0.60 5.13±0.37 2.45±0.52 18.10±0.35 30 42.06±0.44 13.72±0.44 22.11±0.91 5.50±0.54 5.91±0.28 52.57±0.47 13.07±0.77 5.63±0.62 2.82±0.47 20.55±0.42 60 43.60±0.71 15.01±0.29 22.48±0.76 6.27±0.32 6.79±0.45 51.55±0.39 14.37±0.35 5.74±0.25 2.75±0.24 18.36±0.51 C 2 37.49±0.62 14.03±0.42 18.41±0.85 4.30±0.46 4.83±0.47 49.11±0.64 11.48±0.39 6.06±0.28 2.93±0.02 20.86±0.78 15 38.95±0.31 14.15±0.51 19.45±0.19 4.58±0.82 5.08±0.32 49.94±0.43 11.77±0.23 6.26±0.42 2.99±0.40 21.15±0.05 30 40.76±0.58 15.03±0.85 20.20±0.16 5.34±0.51 5.52±0.35 49.55±0.34 13.09±0.18 6.44±0.31 2.97±0.18 19.80±0.11 60 41.92±0.65 15.04±0.29 20.24±0.34 5.75±0.46 6.26±0.29 48.28±0.56 13.71±0.46 6.23±0.49 3.26±0.20 21.40±0.85 D 2 39.85±0.69 16.14±0.58 18.50±0.29 4.56±0.53 5.10±0.37 46.41±0.39 11.44±0.70 5.36±0.16 2.15±0.11 13.35±0.41 15 41.59±0.78 15.53±0.42 20.06±0.27 5.46±0.36 6.02±0.26 48.07±0.45 13.20±0.52 5.43±0.32 2.19±0.37 14.09±0.03 30 42.20±0.86 15.62±0.26 19.87±0.61 6.17±0.62 6.66±0.63 47.09±0.17 16.63±0.38 5.66±0.15 2.18±0.45 13.96±0.64 60 43.23±0.12 15.66±0.32 20.63±0.74 6.34±0.48 6.84±0.56 47.72±0.23 14.66±0.67 5.72±0.43 2.21±0.38 14.10±0.07 E 2 39.95±0.93 15.44±0.71 18.60±0.36 5.45±0.39 5.98±0.61 46.55±0.47 13.63±0.49 5.58±0.64 2.11±0.31 13.70±0.32 15 39.99±0.24 15.32±0.25 18.54±0.61 5.83±0.53 6.12±0.23 46.35±0.39 14.58±0.20 5.83±0.39 2.24±0.60 14.61±0.03 30 41.59±0.50 15.66±0.43 19.07±0.25 6.48±0.42 6.82±0.74 45.86±0.70 15.58±0.38 5.95±0.26 2.23±0.12 14.25±0.99 60 42.91±0.41 15.90±0.20 19.47±0.48 7.05±0.57 7.51±0.32 45.38±0.55 16.44±0.41 6.14±0.57 2.65±0.29 16.65±0.83
(52.57%) contents were in B. bifidum BB-12 sample.
In the present study, differences in fat rates between samples were statistically significant (p<0.01) during ripening period in which fat contents of all cheese samples increased. A general decrease in the titratable acidity rates of all probiotic cheese samples was observed throughout ripening period (Fig. 1). The acidity rate of B. bifidum BB–12+L. acidophilus LA-5 (19.12 SH) sample was lower than those in others in the present study, which may be related to the salt contents of C cheese sample. Higher salt and moisture contents might have reduced the activities of lactic acid bacteria found in cheese 61, 62. During the ripening period in
the present study, pH rates of the cheese samples showed a slight increase and the highest increase in pH value was seen in C probiotic cheese sample (6.44), which is convenient with literature, e.g. Atasever et al. 58 reporting a mean pH rate ranging from 4.98
to 5.68. The protein content of cheese samples ranged from 13.44 to 16.14% resulting from the hydrolysis of proteins to water-soluble nitrogenous compounds and to brine 62, 63. Mean protein content
of Beyaz cheese ranged from 12.78 to 17.27% by Hayaloğlu 64,
being in convenience with the present study.
Nitrogen fractions: Table 1 and Fig. 2 summarizes the results of the assessment of proteolysis in the control and probiotic Beyaz cheese samples through the determination of water-soluble
nitrogen (WSN) and trichloroacetic acid-soluble nitrogen (TCA-SN) over 60-day ripening period at 4°C. The ratio of WSN to total nitrogen (TN) in all samples increased consistently. Cheese produced from B. bifidum BB-12+L. acidophilus LA-5 (3.26%) revealed the highest water-soluble protein rate. WSN rates reported by Ong and Shah 65 in the samples of probiotic Cheddar
cheese are similar to those found in the present study.
The rate of TCA-SN also increased continuously and intensified more until 60th day (Fig. 2). Ong and Shah 65 found that the levels
of SN-TCA in probiotic Cedar cheeses increased during ripening period similarly with those in the present study. Reason for such a situation may be the responsibility of the starter and probiotic bacteria proteinases for the formation of TCA-SN. Cheese samples produced from B. longum (2.27%) in general showed the highest rate of TCA-SN. Such a condition may show that upon the formation of soluble peptides by rennet and starter culture, the peptidases and proteinases of probiotic adjuncts hydrolysed these peptides and released more intermediate- and smaller-size peptides. This situation was easily seen at the end of 12 weeks when the primary proteolysis gave products as substrates for the subsequent proteolysis by the probiotic organisms.
Chemical analysis of all types of cheese samples in the present study showed that the addition of tested probiotic microorganisms in Beyaz cheese has no adverse effect on cheese composition and similar results were reported in previous studies, e.g. Gardiner
et al.29, Ong et al.66 and Kilic et al.26.
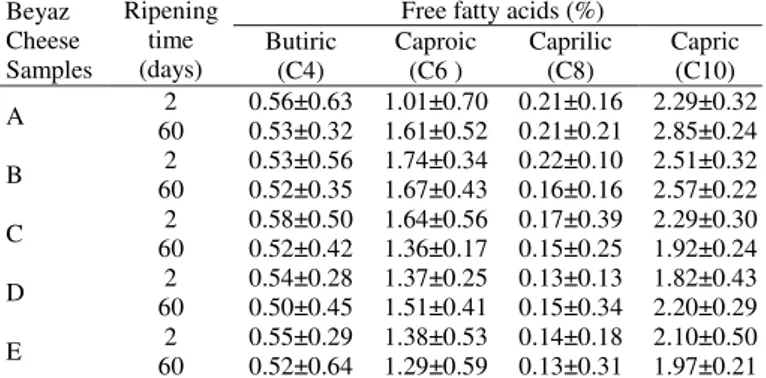
Free fatty acid (FFA) composition: Results of free fatty acid analysis of probiotic Beyaz cheese during ripening period are given in Table 2. The rate of caproic acid in the present study ranged from 1.01 to 1.74% (Fig. 3), which is convenient with the results found in Prandini et al. 67 where this rate was stated to be
1.14% in Alpine cheese samples. Total rate of FFA, capric acid (1.82–2.85%), was higher in probiotic dahi in the present study (Fig. 4). Corbo et al. 19 reported that the rate of capric acid in
Bifidobacterium sp. added Italian cheese was higher than that
found in control samples, which is in agreement with the findings in the present study. It can be seen in literature that butyric acid is produced largely through lipolytic activity of LAB 43, 68 and the
butyric acid content of food may contribute to medicinal properties of the dahi product 43, 69.
Free amino acid (FAA) composition: Proteolysis in ripening process can contribute to the formation of flavour thanks to peptides and free amino acids. Presence of free amino acid in
2 15 30 60 0 0.5 1 1.5 2 2.5
Ripening time (day)
TCA-SN (%)
Figure 2. Changes in trichloroacetic acid-soluble nitrogen
(TCA-SN) of probiotic Beyaz cheese samples.
2 60 0.8 0.9 1 1.1 1.2 1.3 1.4 1.5
Ripening time (day)
Caproic acid (%)
1.6 1.7 1.8
Figure 3. Changes in caproic acid of probiotic Beyaz cheese samples.
2 60 1.4 1.6 1.8 2 2.2 2.4 2.6 2.8
Ripening time (day)
Capric acid (%)
3
Figure 4. Changes in capric acid of probiotic Beyaz cheese samples.
probiotic Beyaz cheese can be attributed to the proteolytic activity of the bacteria during ripening. Proline was found to increase until the 30th day and then decreased until the 60th day while glycine,
serine and isoleucine were stable following the 60th day. The
production of free proline was determined to be associated with flavour Swiss cheese and attributed to the metabolic activity or the propionibacterium in some studies 70, 71. Such results are
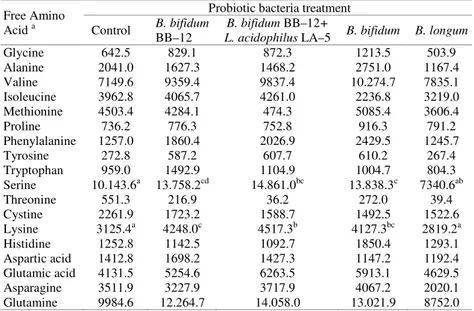
convenient with our study. Free methionine increase in ripening period may also increase the rate of sulphur volatile flavour compounds. Free amino acids may exhibit various taste characteristics depending on their side chains. When the effects of the presence of probiotic bacteria (Table 3) on free amino acid rate were taken into consideration, only the effects of lysine and serine were found to be significant (p<0.05). It was found when considered 5 sample types treated with 4 different probiotic bacteria contents (Table 4) that free amino acid rate exhibited a higher increase in 4 bifidobacteria added cheese curd slurries in 60-day ripening period compared to B. bifidum BB–12+L. acidophilus LA–5, B. bifidum and the B. longum, B. bifidum BB–12 and control sample. In Beyaz cheese curd slurries, proteolytic activity
Beyaz Cheese Samples Ripening time (days)
Free fatty acids (%) Butiric (C4) Caproic (C6 ) Caprilic (C8) Capric (C10) A 2 0.56±0.63 1.01±0.70 0.21±0.16 2.29±0.32 60 0.53±0.32 1.61±0.52 0.21±0.21 2.85±0.24 B 2 0.53±0.56 1.74±0.34 0.22±0.10 2.51±0.32 60 0.52±0.35 1.67±0.43 0.16±0.16 2.57±0.22 C 2 0.58±0.50 1.64±0.56 0.17±0.39 2.29±0.30 60 0.52±0.42 1.36±0.17 0.15±0.25 1.92±0.24 D 2 0.54±0.28 1.37±0.25 0.13±0.13 1.82±0.43 60 0.50±0.45 1.51±0.41 0.15±0.34 2.20±0.29 E 2 0.55±0.29 1.38±0.53 0.14±0.18 2.10±0.50 60 0.52±0.64 1.29±0.59 0.13±0.31 1.97±0.21
Table 2. Free fatty acids (FFAs) composition of probiotic Beyaz
cheeses during storage.
Free Amino Acid a
Ripening Time (days)
2 15 30 60 Glycine 216.9a 936.1b 1461.2b 1744.0c Alanine 321.2a 2310.5b 3159.4c 3865.9c Valine 125.6a 12.260.7b 17.100.2c 19.213.6d Isoleucine 78.3a 5114.0b 6635.8b 7064.0b Methionine 115.6a 5291.7b 8276.9c 8954.5cd Proline 295.7a 291.6c 715.3b 827.9d Phenylalanine 139.5a 1578.2b 3891.4c 3759.4b Tyrosine 179.3a 154.3a 1205.1b 951.6c Tryptophan 357.6a 914.8b 1890.3c 2162.0c Serine 1424.2a 17.100.2b 17.383.6b 18.940.9b Threonine 175.9 233.9 321.8 357.6 Cystine 1582.7 1374.0 959.2 925.4 Lysine 479.6a 5170.3b 7532.9c 5333.7b Histidine 301.4a 1251.7b 2413.6c 3100.9c Aspartic acid 92.5a 1621.2b 2258.0c 2937.4c Glutamic acid 318.6a 6413.4b 9475.3d 9978.1b Asparagine 392.1a 4720.1b 5465.8b 5873.6d Glutamine 1729.8a 14.396.1b 20.417.1b 22.645.2b
Table 3. Effect of ripening time on free amino acid
contents in Beyaz cheese.
a:Values are given in nmoles g-1 cheese slurry and are means of triplicate analyses.
Free Amino Acid a
Probiotic bacteria treatment Control B. bifidum
BB–12
B. bifidum BB–12+
L. acidophilus LA–5 B. bifidum B. longum
Glycine 642.5 829.1 872.3 1213.5 503.9 Alanine 2041.0 1627.3 1468.2 2751.0 1167.4 Valine 7149.6 9359.4 9837.4 10.274.7 7835.1 Isoleucine 3962.8 4065.7 4261.0 2236.8 3219.0 Methionine 4503.4 4284.1 474.3 5085.4 3606.4 Proline 736.2 776.3 752.8 916.3 791.2 Phenylalanine 1257.0 1860.4 2026.9 2429.5 1245.7 Tyrosine 272.8 587.2 607.7 610.2 267.4 Tryptophan 959.0 1492.9 1104.9 1004.7 804.3 Serine 10.143.6a 13.758.2cd 14.861.0bc 13.838.3c 7340.6ab Threonine 551.3 216.9 36.2 272.0 39.4 Cystine 2261.9 1723.2 1588.7 1492.5 1522.6 Lysine 3125.4a 4248.0c 4517.3b 4127.3bc 2819.2a Histidine 1252.8 1142.5 1092.7 1850.4 1293.1 Aspartic acid 1412.8 1698.2 1427.3 1147.2 1192.4 Glutamic acid 4131.5 5254.6 6263.5 5913.1 4629.5 Asparagine 3511.9 3227.9 3717.9 4067.2 2020.1 Glutamine 9984.6 12.264.7 14.058.0 13.021.9 8752.0
a:Values are given in nmoles g-1 cheese slurry and are means of triplicate analyses.
Table 4. Effect of probiotic bacteria treatment on free amino acid contents in
Beyaz cheese samples.
increased with the free amino acid content when bifidobacteria is presented in the media. Bergamini et al. 2 stated that compared to
control samples larger rate of free amino acid in probiotic bacteria added semi-hard cheese samples may be caused by higher proteolytic activity of the samples. These findings are convenient with the results of the present study.
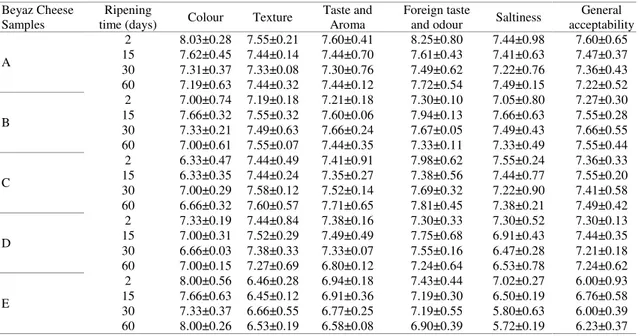
Sensory analysis: Table 5 shows the mean scores given to the sensory parameters for probiotic cheese samples during their storage period. Panellists gave nine points to all probiotic cheese groups and the results of sensory analysis indicated that C cheese batch (B. bifidum BB-12 and L. acidophilus LA-5) received the highest sensory scores. E samples were given the lowest score for saltiness parameter (5.72), which might have been due to high salt concentration of E probiotic cheese. Previous studies (e.g. 26, 33, 72, 73) reported no negative effects on sensorial properties of
probiotic cheese. The results of sensory analysis in the present study showed that there might have been a common effect between the test culture mix and commercial starter culture and the combination of test probiotic culture and commercial starter culture might be suggested to have positive effects on the sensory characteristics of Beyaz cheese.
Conclusions
Objective of this study was to investigate chemical and organoleptic characteristics of probiotic bacteria added Beyaz cheese. In B. bifidum BB-12+L. acidophilus LA-5+lactic culture cheese samples, increase in FFA was found to be higher than that in probiotics added cheese samples at the end of ripening process. It was thought by considering the increased content of some free amino acids in cheese samples that when probiotic bacteria were added in cheese samples, they might have accelerated the ripening process. It may be concluded from the results of the study that Beyaz cheese bears the ability of being one of the most suitable tools, which can be used to vehicle for delivering the tested strains of probiotic bacteria via human diet.
Acknowledgements
This work was supported financially by the Atatürk University Research fund (Project No: 2008/64).
References
1Saarela, M., Lähteenmäki, L., Crittenden, R., Salminen, S. and
Mattila-Sandholm,T. 2002. Gut bacteria and health foods - the European perspective. Int. J. Food Microbiol. 78(1):99–117.
2Bergamini, C. V., Hynes, E. R. and Zalazar, C. A. 2006. Influence of
probiotic bacteria on the proteolysis profiles of a semi-hard cheese. Int. Dairy J. 16(8):856–866.
3Playne, M. J., Bennett, L. E. and Smithers, G. W. 2003. Functional dairy
foods and ingredients. Aust. J. Dairy Tech. 58(3):242–264.
4Fuller, R. 1989. Probiotics in man and animals. J. Appl. Bacteriol. 66:365–
378.
5Mattila-Sandholm, T., Myllärinen, P., Crittenden, R., Mogensen, G.,
Fonden, R. and Saarela, M. 2002. Technological challenges for future probiotic foods. Int. Dairy J. 12:173–182.
6FAO/WHO 2001. Evaluation of Health and Nutritional Properties of
Powder Milk with Live Lactic Acid Bacteria. Report of FAO/WHO Expert Consultation 1-4 October, pp. 1-4.
7Gupta, A., Mann, B., Kumar, R. and Sangwan, R. B. 2009. Antioxidant
activity of Cheddar cheeses at different stages of ripening. Int. J. Dairy Sci. 62:339-347.
8Pitino, I., Randazzo, C. L., Cross, K. L., Parker, M. L., Bisignano, C.,
Wickham, M. S. J., Mandalari, G. and Caggia, C. 2012. Survival of Lactobacillus rhamnosus strains inoculated in cheese matrix during simulated human digestion. Food Microbiol. 31:57–63.
9Solieri, L., Bianchi, A., Mottolese, G., Lemmetti, F. and Giudici, P.
2014. Tailoring the probiotic potential of non-starter Lactobacillus strains from ripened Parmigiano Reggiano cheese by in vitro screening and principal component analysis. Food Microbiol. 38:240-249.
10Boylston, T. D., Vinderola, C. G., Ghoddusi, H. B. and Reinheimer, J.
A. 2004. Incorporation of bifidobacteria into cheeses: Challenges and rewards. Int. Dairy J. 14:375–387.
11Djoussé, L., Pankow, J. S., Hunt, S. C., Heiss, G., Province, M. A.,
Kabagambe, E. K. and Ellison, R. C. 2006. Influence of saturated fat and linolenic acid on the association between intake of dairy products and blood pressure. Hypertension 48:335–341.
12Engberink, M. F., Hendriksen, M. A. H., Schouten, E. G., van Rooij, F.
J. A., Hofman, A., Witteman, J. C. M. and Geleijnse, J. M. 2009. Inverse association between dairy intake and hypertension: The Rotterdam Study. Am. J. Clin. Nutr. 89:1877–1883.
13Levitan, E. B., Wolk, A. and Mittleman, M. A. 2009. Consistency with
the DASH diet and incidence of heart failure. Ann. Intern. Med. 169:851–857.
14Toledo, E., Delgado-Rodríguez, M., Estruch, R., Salas-Salvadó, J.,
Corella, D., Gomez-Gracia, E., Fiol, M., Lamuela-Raventós, R.M., Schröder, H., Arós, F., Ros, E., Ruíz-Gutiérrez, V., Lapetra, J., Conde-Herrera, M., Sáez, G., Vinyoles, E. and Martínez-González, M.A. 2009. Low-fat dairy products and blood pressure: Follow-up of 2290 older persons at high cardiovascular risk participating in the Predimed study. Br. J. Nutr. 101:59–67.
15Songisepp, E., Rätsep, M., Shkut, E., Kõljalg, S., Truusalu, K.,
Stsepetova, J., Smidt, I., Kolk, H., Zagura, M. and Mikelsaar, M. 2012. Safety of a probiotic cheese containing Lactobacillus plantarum Tensia according to a variety of health indices in different age groups. J. Dairy Sci. 95:5495–5509.
16Tulini, F. L., Winkelströter, L. K. and De Martinis, E. C. 2013.
Identification and evaluation of the probiotic potential of Lactobacillus paraplantarum FT259, a bacteriocinogenic strain isolated from Brazilian semi-hard artisanal cheese. Anaerobe 22:57-63.
17Ishibashi, N. and Shimamura, S. 1993. Bifidobacteria: Research and
development in Japan. Food Technol. 47:126-135.
18Abadía-García, L., Cardador, A., Campo, S. T. M., Arvízu, S. M.,
Castaño-Tostado, E., Regalado-González, C., García-Almendarez, B. and Amaya-Llano, S.L. 2013. Influence of probiotic strains added to cottage cheese on generation of potentially antioxidant peptides, anti-listerial activity, and survival of probiotic microorganisms in simulated gastrointestinal conditions. Int. Dairy J. 33:191-197.
19Corbo, M. R., Albenzio, M., de Angelis, M., Sevi, A. and Gobbetti, M.
2001. Microbiological and biochemical properties of Canestrato Pugliese hard cheese supplemented with bifidobacteria. J. Dairy Sci. 84:551– 561.
20Reid, A. A., Champagne, C. P., Gardner, N., Fustier, P. and Vuillemard,
J. C. 2007. Survival in food systems of Lactobacillus rhamnosus R011 microentrapped in whey protein gel particles. J. Food Sci. 72:M31-M37.
21Heller, K. J. 2001. Probiotic bacteria in fermented foods: Product
characteristics and starter organisms. Am. J. Clin. Nut. 73(suppl.):374– 379.
22Bergamini, C. V., Hynes, E. R., Quiberoni, A., Suárez, V. B. and Zalazar,
C. A. 2005. Probiotic bacteria as adjunct starters: Influence of the addition methodology on their survival in a semi-hard Argentinean cheese. Food Res. Int. 38:597–604.
23Souza, C. H. B. and Saad, S. M. I. 2009. Viability of Lactobacillus
acidophilus LA–5 added solely or in co-culture with a yoghurt starter Beyaz Cheese
Samples
Ripening
time (days) Colour Texture
Taste and Aroma
Foreign taste
and odour Saltiness
General acceptability A 2 8.03±0.28 7.55±0.21 7.60±0.41 8.25±0.80 7.44±0.98 7.60±0.65 15 7.62±0.45 7.44±0.14 7.44±0.70 7.61±0.43 7.41±0.63 7.47±0.37 30 7.31±0.37 7.33±0.08 7.30±0.76 7.49±0.62 7.22±0.76 7.36±0.43 60 7.19±0.63 7.44±0.32 7.44±0.12 7.72±0.54 7.49±0.15 7.22±0.52 B 2 7.00±0.74 7.19±0.18 7.21±0.18 7.30±0.10 7.05±0.80 7.27±0.30 15 7.66±0.32 7.55±0.32 7.60±0.06 7.94±0.13 7.66±0.63 7.55±0.28 30 7.33±0.21 7.49±0.63 7.66±0.24 7.67±0.05 7.49±0.43 7.66±0.55 60 7.00±0.61 7.55±0.07 7.44±0.35 7.33±0.11 7.33±0.49 7.55±0.44 C 2 6.33±0.47 7.44±0.49 7.41±0.91 7.98±0.62 7.55±0.24 7.36±0.33 15 6.33±0.35 7.44±0.24 7.35±0.27 7.38±0.56 7.44±0.77 7.55±0.20 30 7.00±0.29 7.58±0.12 7.52±0.14 7.69±0.32 7.22±0.90 7.41±0.58 60 6.66±0.32 7.60±0.57 7.71±0.65 7.81±0.45 7.38±0.21 7.49±0.42 D 2 7.33±0.19 7.44±0.84 7.38±0.16 7.30±0.33 7.30±0.52 7.30±0.13 15 7.00±0.31 7.52±0.29 7.49±0.49 7.75±0.68 6.91±0.43 7.44±0.35 30 6.66±0.03 7.38±0.33 7.33±0.07 7.55±0.16 6.47±0.28 7.21±0.18 60 7.00±0.15 7.27±0.69 6.80±0.12 7.24±0.64 6.53±0.78 7.24±0.62 E 2 8.00±0.56 6.46±0.28 6.94±0.18 7.43±0.44 7.02±0.27 6.00±0.93 15 7.66±0.63 6.45±0.12 6.91±0.36 7.19±0.30 6.50±0.19 6.76±0.58 30 7.33±0.37 6.66±0.55 6.77±0.25 7.19±0.55 5.80±0.63 6.00±0.39 60 8.00±0.26 6.53±0.19 6.58±0.08 6.90±0.39 5.72±0.19 6.23±0.37
Table 5. Results of sensory analysis of probiotic Beyaz cheese samples for 60 days.
culture and implications on physico-chemical and related properties of Minas fresh cheese during storage. Lebensmittel-Wissenschaft und -Technologie 42:633–640.
24Madureira, A. R., Soares, J. C., Pintado, M. E., Gomes, A. M. P.,
Freitas, A. C. and Malcata, F. X. 2008. Sweet whey cheese matrices inoculated with the probiotic strain Lactobacillus paracasei LAFTI®
L26. Dairy Sci. Tech. 88:649–665.
25Cruz, A. G., Buriti, F. C. A., Souza, C. H. B., Faria, J. A. F. and Saad, S.
M. I. 2009. Probiotic cheese: Health benefits, technological and stability aspects. Trends Food Sci.Tech. 20:344-354.
26Kilic, G. B., Kuleasan, H., Eralp, I. and Karahan, A. G. 2009.
Manufacture of Beyaz cheese added with probiotic strains. Lebensmittel-Wissenschaft und -Technologie 42:1003–1008.
27Rodrigues, D., Rocha-Santos, T. A. P., Gomes, A. M., Goodfellow, B.
J. and Freitas, A. C. 2012. Lipolysis in probiotic and synbiotic cheese: The influence of probiotic bacteria, prebiotic compounds and ripening time on free fatty acid profiles. Food Chem. 131:1414–1421.
28Gomes, A., Malcata, F., Klaver, F. and Grande, H. 1995. Incorporation
and strain Ki in a cheese product. Nederlands Melk en Zuiveltijdschrift J. 49:71–95.
29Gardiner, G., Ross, R. P., Collins, J. K., Fitzgerald, G. and Stanton, C.
1998. Develpoment of a probiotic cheddar cheese containing human-derived Lactobacillus paracasei strains. Appl. Microbiol. Biotechnol. 64:2192–2199.
30Songisepp, E., Kullisaar, T., Hutt, P., Elias, P., Brilene, T., Zilmer, M.
and Mikelsaar, M. 2004. A new probiotic cheese with antioxidative and antimicrobial activity. J. Dairy Sci. 87:2017–2023.
31Ross, R., Fitzgerald, G., Collins, J. K., Sullivan, G. C. and Stanton, C.
2005. Process of manufacture of probiotic cheese. Enterprise Ireland and Teagasc, The Agriculture and Food Development Authority, Assignee. US Pat. No. 6872411. Accessed July 20: 2011. http:// www.freepatentsonline.com/6872411.html.
32Ibrahim, F., Ruvio, S., Granlund, L., Salminen, S., Viitanen, M. and
Ouwehand, A. C. 2010. Probiotics and immunosenescence: Cheese as a carrier. FEMS Immunol. Med. Microbiol. 59:53–59.
33Stanton, C., Gardiner, G., Lynch, P. B., Collins, J. K., Fitzgerald, G. and
Ross, R. P. 1998. Probiotic cheese. Int. Dairy J. 8:491–496.
34McSweeney, P. L. H. and Sousa, M. J. 2000. Biochemical pathways
for the production of flavour compounds in cheeses during ripening: A review. Lait 80:293–324.
35Desjardins, M. L., Roy, D. and Goulet, J. 1990. Growth of bifidobacteria
and their enzyme profiles. J. Dairy Sci. 73:299–307.
36Peterson, S. D., Marshall, R. T. and Heymann, H. 1990. Peptidase
profiling of lactobacilli associated with cheddar cheese and its application to identification and selection of strains for cheese-ripening studies. J. Dairy Sci. 73:1454–1464.
37Habibi-Najafi, M. B. and Lee, B. H. 1994. Proline-specific peptidases
of Lactobacillus casei subspecies. J. Dairy Sci. 77:385–392.
38Williams, A. G. and Banks, J. M. 1997. Proteolytic and other hydrolytic
enzyme activities in non-starter lactic acid bacteria (NSLAB) isolated from Cheddar cheese manufactured in the United Kingdom. Int. Dairy J. 7:763–774.
39Shihata, A. and Shah, N. P. 2000. Proteolytic profiles of yoghurt and
probiotic bacteria. Int. Dairy J. 10:401–408.
40Bergamini, C. V., Hynes, E. R., Palma, S. B., Sabbag, N. G. and Zalazar,
C. A. 2009. Proteolytic activity of three probiotic strains in semi-hard cheese as single and mixed cultures: Lactobacillus acidophilus, Lactobacillus paracasei and Bifidobacterium lactis. Int. Dairy J. 19:467–475.
41Coskun, H. and Ondul, E. 2004. Free fatty acid accumulation by
mesophilic lactic acid bacteria in cold stored milk. J. Microbiol. 42:133– 138.
42Kurmann, J. A. 1988. Starters of fermented milks: Starters with selected
intestinal bacteria. International Dairy Federation Bulletin 227:41–45.
43Yadav, H., Jain, S. and Sinha, P. R. 2007. Production of free fatty acids
and conjugated linoleic acid in probiotic dahi containing Lactobacillus
acidophilus and Lactobacillus casei during fermentation and storage. Int. Dairy J. 17:1006–1010.
44Martensson, O., Öste, R. and Holst, O. 2002. The effect of yoghurt
culture on the survival of probiotic bacteria in oat-based, non-dairy products. Food Res. Int. 35:775–784.
45Demirci, M. and Şimşek, O. 1997. Milk Processing Technology. Hasad
Publication, Istanbul, 246 p.
46Kurt, A., CakmakcÏ, S. and Caglar, A. 2007. Milk and Products
Inspection and Analysis Methods Guide. Atatürk University Agricultural Faculty Publication No. 252, Erzurum, Turkey, 254 p.
47Savello, P. A., Ernstrom, C. A. and Kalab, M. 1989. Microstructure and
meltability of model process cheese made with rennet and acid casein. J. Dairy Sci. 72(1):11.
48Polychroniadou, A., Michaelidou, A. and Paschaloudis, N. 1999. Effect
of time, temperature and extraction method on the trichloroacetic acid-soluble nitrogen of cheese. Int. Dairy J. 9(8):559–568.
49IDF (International Dairy Federation) 1993. Standard Method 20B:
Milk. Determination of nitrogen content. IDF, Brussels, Belgium, 235p.
50Akalın, A. S., Kınık, Ö. and Gönc, S. 1998. Researches on fatty acid
composition of some cheese variaties in İzmir market. Journal of Food 23(5):357–363.
51Standara, S., Veselá, M. and Drdák, M. 2000. Determination of biogenic
amines in cheese by ion exchange chromatography. Nahrung 44:28– 31.
52Lyne, J. 1995. Improving cheese flavour. 4th Cheese Symposium, National
Dairy Products Research Centre, Moorepark,Fermory Co. Cork, pp. 46–50.
53Yildiz, N. and Bircan, H. 1994. Research and Experimental Methods.
Agriculture Faculty, Atatürk University, Turkey.
54Kindstedt, P. S., Rowney, M. and Roupas, P. 1999. Technology,
biochemistry and functionality of pasta filata/pizza cheese. In Law, B. A. (ed.). Technology of Cheese Making. CRC, Boca Raton, pp. 193– 221.
55Buriti, F. C. A., Rocha, J. S., Assis, E. G. and Saad, S. M. I. 2005.
Probiotic potential of Minas fresh cheese prepared with the addition of Lactobacillus paracasei. Lebensmittel-Wissenschaft und Technologie 38:173–180.
56Özdemir, S. 1990. Can Be Kept with Sheep’s Milk is Possibilities of
Hydrogen Peroxide and Potassium Sorbate, and the Addition of Starter Cultures to Milk, Some Quality Criteria of Fresh and Ripened White Cheese. PhD thesis, Erzurum, Turkey, 116 p.
57Tayar, M. 1995. Changes in the microbiological and chemical
characteristics of Turkish white cheese during ripening. Journal of Food 20(2):97–101.
58Atasever, M., Ceylan, Z. G. and Alişarlı, M. 2002. Changes in the
sensory, microbiological properties of Turkish white cheese during ripening. Acta Alimentaria Budapest 31(4):319–326.
59Topcu, A. and Saldamlı, I. 2006. Proteolytical, chemical, textural and
sensorial changes during the ripening of Turkish white cheese made of pasteurized cows’ milk. Int. J. Food Prop. 9(4):665–678.
60Öksüz, O., Arıcı, M., Kurultay, S. and Gümüs, T. 2004. Incidence of
Escherichia coli O157 in raw milk and white pilce cheese manifactured from raw milk in Turkey. Food Control 15:453–456.
61Metin, M. 1998. Milk Technology. Ege University Printing Office,
439 p.
62Cambaztepe, F., Cakmakci, S. and Dagdemir, E. 2009. Effect of some
technological parameters on microbiological, chemical and sensory qualities of Civil cheese during ripening. Int. J. Dairy Sci. 62(4):541– 548.
63Abd El-Salam, M. H., Alichanidis, E. and Zerfiridis, G. K. 1993. Domiati
and Feta type cheeses. In Fox, P. F. (ed.). Cheese: Chemistry, Physics and Microbiology. Chapman and Hall, London, UK, pp. 301–335
64Hayaloğlu, A. A. 2003. Starter Kültür Olarak Kullanılan Bazı
Lactococcus Sujlarının Beyaz peynirin Özellikleri ve Olgunlaşmaları Üzerine Etkileri. Doktora Tezi. Ç.Ü. Fen Bil. Enst. Gıda Müh. ABD, Adana, 170 p. (in Turkish).
Journal of Food, Agriculture & Environment, Vol.12 (3&4), July-October 2014 39
65Ong, L. and Shah, N. P. 2009. Probiotic Cheddar cheese: Influence of
ripening temperatures on survival of probiotic microorganisms, cheese composition and organic acid profiles. Lebensmittel-Wissenschaft und Technologie 42:1260–1268.
66Ong, L., Henriksson, A. and Shah, N. P. 2006. Development of probiotic
Cheddar cheese containing Lactobacillus acidophilus, Lb. casei, Lb. paracasei and Bifidobacterium spp. and the influence of these bacteria on proteolytic patterns and production of organic acid. Int. Dairy J. 16:446–456.
67Prandini, A., Sigolo, S., Tansini, G., Brogna, N. and Piva, G. 2007.
Different level of conjugated linoleic acid (CLA) in dairy products from Italy. J. Food Compos. Ana. 20:472–479.
68El Soda, M., Law, J., Tsakalidou, E. and Kalantzopoulos, G. 1995.
Lipolytic activity of cheese related microorganisms and its impact on cheese flavour. In Charalambous, G. (ed.). Food Flavours, Generation, Analysis and Process Influence. Elsevier Science, Amsterdam, The Netherlands, pp. 212–217.
69Kaur, I. P., Chopra, K. and Saini, A. 2002. Probiotics: Potential
pharmaceutical applications. Eur. J. Pharmaceut. Sci. 15:1–9.
70Langsrud, T., Reinbold, G. W. and Hammond, E. G. 1977. Proline
production by Propionibacterium shermanii P59. J. Dairy Sci. 60(1):16-23.
71Montoya, D., Boylston, T. D. and Mendonca, A. 2009. Preliminary
screening of Bifidobacteria spp. and Pediococcus acidilactici in a Swiss cheese curd slurry model system: Impact on microbial viability and flavor characteristics. Int. Dairy J. 19:605–611.
72Gardiner, G. E., Bouchier, P., O’Sullivan, E., Kelly, J., Collins, J. K.,
Fitzgerald, G., Ross, R.P. and Stanton C. 2002. A spray-dried culture for probiotic Cheddar cheese manufacture. Int. Dairy J. 12:749–756.
73Dabevska-Kostoska, M., Kuzmanova, S. and Winkelhausen, E. 2010.
A traditional brined white cheese enriched with probiotic bacteria Lactobacillus casei. Traditional Foods from Adriatic to Caucasus, 2010 April 15-17, Tekirdağ, Turkey, pp. 213–215.