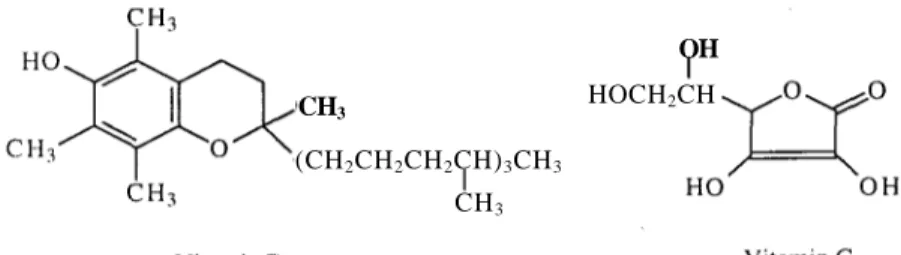RADIOPROTECTIVE AGENTS IN THE TREATMENT OF CANCER AND THE ROLE OF AMIFOSTINE
KANSER TEDAVİSİNDE RADYOPROTEKTİF BİLEŞİKLER VE AMİFOSTİNİN ROLÜ
Sibel SÜZEN*
* Ankara University, Faculty of Pharmacy, Department of Pharmaceutical Chemistry, 06100, Tandoğan, Ankara, Turkey
ABSTRACT
Recently, there has been an increase in the interest in research on radioprotective agents. The aim is to achieve preferential protection of normal tissues against injury inflicted by ionising radiation or chemotherapy agents used to treat tumours. There are several classes of radioprotective compounds are present and still under investigation. Among these agents, commonly the sulfydryl-containing amifostine (EthyolR) is currently the most widely used. Amifostine is a potent radioprotector of normal tissues and protect against radiation carcinogenesis. To be successful in tumor therapy, developing of new radioprotective compounds is urgently needed considering the amifostine is the only one effective compound in use in cancer treatment so far.
Key Words: Radioprotectors, Amifostine, Cancer, Ionising radiation ÖZET
Son yıllarda radyoprotektif bileşikler ile ilgili araştırmalara artan bir ilgi başlamıştır. Amaç tümör tedavisinde kullanılan iyonize radyasyon ve kemoterapötik bileşiklerin oluşturacakları hasarlardan normal dokuların korunmasıdır. Hali hazırda çeşitli kimyasal gruplara ait radyoprotektif bileşikler mevcuttur ve yoğun araştırmalar yapılmaktadır. Bu bileşikler arasında son zamanlarda en geniş kullanım alanına sahip olanı sülfidril grubu içeren amifostin (EthyolR) dir. Amifostin normal dokular için etkili bir radyoprotektör bileşiktir ve radyasyon karsinojenezisine karşı koruma sağlar. Amifostinin kanser tedavisinde şimdiye kadar kullanılan tek etkin radyoprotektif bileşik olduğu düşünülürse, tümör tedavisinde başarılı olabilmek için yeni radyoprotektif bileşiklerin geliştirilmesi acilen gerekmektedir.
INTRODUCTION
Over the recent years, as an understanding of radiation chemistry has developed, it has been possible to develop compounds which modify the initial radiochemical event. The recognition by Gray et al (1) in 1953 that oxygen had a major effect on radiosensitivity has led to extensive investigations into the potential for this factor in tumour therapy. Membranes and DNA are critical targets for ionizing radiation and may be major sites for the development of the oxygen effects (2). Explanations of this effect have previously emphasised the involvement of reactive oxygen species such as the superoxide anion and the hydroxyl radical to explain some of the cytotoxic effects of ionizing radiations (3). Free radicals are highly reactive molecular species. They react with almost every type of cellular molecules, causing metabolic disturbance, cell injury and even cell death (4). Oxygen radicals are involved in tumour promotion and causative agents of the deleterious effects of ionizing radiation (5).
Radiotherapy and chemotherapy are traditional cancer treatments, that increases DNA damage in tumour cells. Due to higher replication rate of tumour cells DNA repair mechanisms counteract the effects of radiation and chemotherapy treatment by promoting the replication of the tumour cells. It may be possible to prevent this process by inhibiting the DNA repair mechanism by radioprotective compounds.
Radiation damage to the heamatopoietic system is considered as the most commonly encountered disorders in radiation sickness. Reduction in the number of blood cells and damage to the bone marrow with resulting anaemia and haemorrhage have been profoundly observed (6). Ionizing radiation, through production of oxygen radical species, can result in DNA damage, especially by forming thymidine hydroperoxyde (7).
It has been suggested that various chemical structures may protect against the acute cell and tissue toxicities and delayed carcinogenesis that are induced by radiation via generation of free radicals and various ionic species (8)
RADIOPROTECTIVE AGENTS IN MEDICINE
Current multidisciplinary research in the field of radioprotection involves all aspects of basic and clinical research ranging from the subatomic mechanisms of free radical formation, macromolecular and intracellular radiation-induced alterations, biochemical and physiological mechanisms to the clinical management of radiation casualties (9). Radioprotective agents, although widely studied in the past four decades and including several thousand agents, have
Misonidazole KIN-1800 Figure 1. Some radioprotective nitroimidazole derivatives
SULFHYDRYL-CONTAINING COMPOUNDS AND THE REPAIR OF FREE RADICALS Protecting against ionizing radiation by sulfhydryl-containing compounds was first described by Patt et al in the late 1940s (14). Radiation-produced radicals of cellular macromolecules may be repaired by hydrogen atom transfer from thiols. Some new drugs such as 2-aminoethylisothiouronium and cysteamine HC1 have been introduced as potent radioprotectors (15,16,17). Various antioxidants such as sodium L-ascorbate, sodium 5,6-benzylidene-L-ascorbate, gallic acid and caffeic acid (Figure 2), induced the apoptotic cell death, increased the oxidation potential in the culture medium (18).
H2NCH2CH2.HC1 (HO)2C6H3CH=CHCOOH (HO)3C6H2COOH Cysteamine HC1 Caffeic acid Gallic acid
Figure 2. Cysteamine HC1 and radioprotective acid derivatives
not reached the level of providing the field of medicine with an agent that conforms to all criteria of an optimal radioprotection, including effectiveness, toxicity, availability, specificity and tolerance (10).
NITROIMIDAZOLES
The first group of compounds which proved to be sufficiently nontoxic for in vivo use and which sensitized hypoxic mammalian cells were the nitroimidazoles (figure 1). These compounds have now received extensive clinical testing throughout the world (11,12). Most hypoxic cell radiosensitizers were designed to improve the radiosensitizing activity of misonidazole, primarily by modifying the side chains (13) such as KIN-1800.
All cells contain varying degrees of non-protein sulfhydryls which act to protect DNA. As the level of these compounds is reduced to low concentrations either through chemical suppressers such as N-ethylmaleimide or through synthesis blockers such as buthionine-SR-sulfoximine, sensitization of the cell occurs (19). Cellular radiosensitivity is affected by the presence of endogenous intracellular thiols. Oxydation of these thiols by diamide (20,21) renders the cells more sensitive. Monoethyl and diethyl esters of glutathione had the capacity to provide some protection of normal and buthionine sulfoxime pretreated cells against X-irridation (22).
Ionising radiation induces multiple biological effects through direct interaction with DNA or through formation of free radical species. It has been shown that small doses of hydrogen peroxide can induce protection against subsequent doses of ionizing radiation, which supports the hypothesis that oxidative species or single-strand breaks function as inducers (23,24). Ionizing radiation increases oxidative stress followed by glutathione depletion as a result of enchanced detoxification of hydrogen peroxide by glutathion peroxidase (25). Diethyl maleate reacting with glutathione and buthionine sulfoximine inhibiting glutathione synthesis, cause radiosensitization (26).
The original finding that substitution of a halogenated uridine for thimidine in DNA would sensitize to radiation (27). The degree of sensitization depends on the percentage of DNA which is replaced by the thymidine analogue. The bromenated and iodinated compounds have proved most efficacious.
It has been shown in several systems, including plateau-phase cultured cells and tumours in vivo, that increased survival will occur under certain conditions after radiation exposure (Table 1) (28,29,).
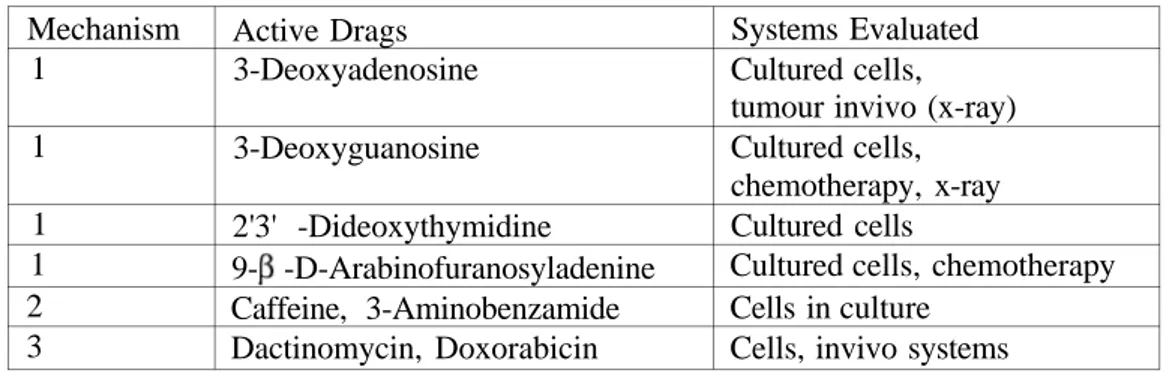
Table 1: Alteration of intracellular nucleotide pool, 2: Inhibition of poly synthesis, 3: Modification of damage Mechanism 1 1 1 1 2 3 Active Drags 3-Deoxyadenosine 3-Deoxyguanosine 2'3' -Dideoxythymidine 9- -D-Arabinofuranosyladenine Caffeine, 3-Aminobenzamide Dactinomycin, Doxorabicin Systems Evaluated Cultured cells, tumour invivo (x-ray) Cultured cells, chemotherapy, x-ray Cultured cells
Cultured cells, chemotherapy Cells in culture
R A D I O P R O T E C T I V E D E H Y D R O A L A N I N E S
Olefines such as dehydroalanines have radioprotective effect (Figure 3). Olefines which are substituted at the geminal carbon atom by both an electron-donating and an-electron-withdrawing group, have been shown to inactive free radicals by forming stabilized free radical adducts. N-(Acylaryl)-dehydroalanine derivatives called AD-compounds and react with and scavenge mainly superoxide radical and hydroxyl radical. The o-methoxyphenylacetyl dehydroalanine derivative, indexed as AD-20, protects mice against damage resulting from total body X-radiaditon (30).
Figure 3. Possible reactions of AD-compounds with free radicals. (I) Radical addition to the
C=C. (II) Hydrogen abstraction from the proradical site. (III) Aromatic hydroxylation. COOH A COOH COOH A A NHR NHR +B I) II) A + RC-CH2-Ph RC-CH—Ph NHR 2 A COOH B NHR Ph Ph CR CH CH RC AH +B RC CH B Ph III) HO + R R OH H R H + H2O R OH + R R
OTHER IMPORTANT RADIOPROTECTIVE COMPOUNDS
-Carotene is known to offer some protection against radiation induced toxicity. Squalene is a 30 carbon chain with 6 double bonds that is structurally similar to -carotene. Squalene also reduces radiation induced damage by its antioxidant activities and stabilised oxygen radicals (31,32). Vitamins C and E have generated a great deal of interest for a wide range of protective effects in biological systems (Figure 4) (33). They are known to be synergistic antioxidants in vitro (34). The role of vitamin C against radiation damage is not only in the initial stages of radical scavenging but also in cellular redox processes mediated by glutathione (35).
Figure 4. Radioprotective vitamins
Polyamines such as spermidine (36) have radioprotective effects by scavenging of hydroxyl radicals in the bulk and reduced accessibility of the attack sites in the condensed structures induced by spermine or spermidine (Figure 5).
H2N— (CH2)3-NH—(CH2)4-NH-(CH2)3-NH2 H2N-(CH2)4-NH-(CH2)3-NH2
Spermine Spermidine Figure 5. Some polyamine radioprotective compounds
AMIFOSTINE FOR PROTECTION FROM ANTINEOPLASTIC DRUG TOXICITY During the initial radiochemical events the presence of sulfhydryl compounds can reduce the permanent lesions in DNA. It is highly likely that endogenous sulfhydryls cause natural radioprotection and that the exogenous sulfhydryls can cause further protection through repair of free radicals in DNA as well as neutralization of free radicals formed in cellular water
CH3
(CH2CH2CH2CH)3CH3
CH3
OH HOCH2CH
(37). Some of the most potent compounds are less toxic by covering the sulfhydryl group with a phosphate group.
The most effective radioprotective agent was developed in the 1959-1972 U.S. Army Program was S-[2-[3-aminopropylamino]ethyl]dihydrogen phosphorothioate (WR2721, Amifostine, E t h y o lR) . T h i s compound is the phosphorothioate derivative of 2-[(3-aminopropyl)amino]ethanethiol (WR1065). WR1065 has been shown to be active in radioprotection and it is believed that WR2721 serves as a prodrug which releases WR1065 in tissue through the action of phosphatase enzymes (Figure 6) (38,39).
H2N—(CH3)2-NH—CH2-CH2-SPO3H2 WR2721 Phosphotase enzymes H2N—(CH3)2-NH—CH2-CH2-SH WR1065 Figure 6. Dephosphorylation of WR2127
Normal cells are constantly subjected to both external (UV rays, carsinogens) and internal (metabolic by-products) assaults which cause damage, or genetic lesions, to the DNA molecule. Because the cells structural integrity is dependent on its ability to read and translate DNA, reparing DNA damage is essential to the cells survival. It may be possible to prevent this DNA damage by inhibiting The DNA repair mechanism with a compound such as amifostine. Considering the mechanism of action, amifostine is a prodrug converted by alkaline phosphatase to the active sulfhydryl compound WR-1065. WR-1065 protects normal cells by scavenging free radicals, donating hydrogen ions to free radicals, depleting oxygen, and binding to active derivatives of antineoplastic agents (40). The immediate conversion of amifostine to WR-1065, radioprotection starts by scavenging of hydroxyl ion and by chemical DNA repair (by hydrogen donation from its SH function) (39). Comparison the radioprotective activity of the compounds have free SH groups to compounds have no free SH group such as cystine (which has disulfide bonds), shows that free SH group plays a significant role in protecting the cells from antioxidant-induced cytotoxicity by hydrogen atom transfer reaction to free radicals (18).
A major mechanism underlying the radioprotective effect of WR2721 is the scavenging highly reactive free radicals induced by ionizing radiation (41). Since damage inflicted by free radicals is a major event responsible not only for killing mammalian cells by radiation but also for malignant transformation of these cells (42).
The preclinical evaluation of amifostine confirms selective protection of normal tissues against toxicity due to radiation therapy and alkylating agent chemotherapy (43). In a broad range of phase II and III clinical studies, amifostine has been shown to substantially reduce anticancer drug-induced toxicities (44) and a novel clinical application of the drug could be in its use to protect against radiation therapy induced genotoxic damage to normal cells (45). The potential value of such an agent includes reducing treatment related toxicity and the opportunity for radiation dose escalation in the curative treatment of cancer (46). The selectivity of amifostine for normal tissue is hypothesised to be a result of the decreased vascularity of tumours, decreased activity of alkaline phosphatase in tumour cells, and pH dependence of WR1065 uptake (40). On the other hand cell type differences in chromatin organisation and DNA-drug associations could play a role in the selective radioprotection as well as the pH value differences between the normal and the tumor cells.
The first evaluation of WR2127 using the iv compound was carried out in Japan (47). Then the results suggested that WR1065 which is the dephosphorylated, free thiol active metabolite of WR2721, may enable tissue recover from irridation by promoting the replication of endothelial cells, possibly by mechanisms independent of glutathion (48).
Significant side effects related to amifostine include nausea, vomiting and hypotension which can be ameliorated with appropriate premedication and combination with other drugs such as mitomycin and vinblastine (49).
CONCLUSION
A better understanding of the nature of cancer and an improvement in technical capabilities have contributed to the advent of radiation therapy as a viable treatment option for cancer patients (50). Chemical radiation modification has reached a high level of clinical investigation. Amifostine is a cytoprotective agent in patients with breast, bladder, cervix, head and neck, small cell and non-small cell lung, ovarian, and rectal cancers, as well as melanoma,
pediatric sarcomas, and lymphomas, including Hodgkin's disease (43,49). Amifostine has shown promise in protecting non-malignant cells from the toxic effects of antineoplastics, apparently without compromising toxicity against cancer cells. The biggest advantage of Ethyol is that it shows protection against whole-body irridation, and myelo and nephrotoxicity of cytotoxic agents, does not reduce the antineoplastic activity and has minor side effects when compared with the other radioprotector compounds. The disadvantage of Ethyol is that only present in iv form and has to be taken approximately 20 minutes before the radiation therapy. Ethyol is already in use in the U.S.A and under investigation by Turkish outorities.
REFERENCES
1. Gray, L.H., Conger, A.D. and Ebert, M. "The concentration of oxygen dissolved in tissues at the time of irridation as a factor in radiotherapy", Br. J. Radiol., 26, 638-648 (1953). 2. McLennan, G., Oberley, L.W. and Author, A.P. "The role of oxygen derived free radicals
in radiation-induced damage and death of nondividing eucaryotic cells", Radiat. Res., 84, 122-132(1980).
3. Brown, J.M. "Sensitizers and protectors in radiotherapy", Cancer, 55, 2222-2228 (1985). 4. Slater, T.F. "Free radical mechanisms in tissue injury", Biochem. J., 222, 1-15 (1984). 5. Cerutti, P.A. "Prooxidant states and tumor promotion", Science, 227, 375-381 (1985).
6. Shaheen, A.A. and Hassan, S.M. "Radioprotection of whole body", Strahlenter. Onkol., 167,498-501 (1991).
7. Buc-Calderon, P. and Roberfroid, M. "Increase in the survival time of mice exposed to ionizing radiation by a new class of free radical scavengers", Experientia, 46, 708-710 (1990).
8. Storm, H.M., Oh, S.Y., Kimler, B.F. and Norton, S. "Radioprotection of mice by dietary squalene", Lipids, 28, 555-559 (1993).
9. Durakovi, I. "Radioprotective agents in medicine", Arh. Big. Rada. Toksikol., 44, 331-354, (1993).
10. Vanbeckevoort, D., Ponette, E. and Beart, A.L. "The evolution of radioprotection", J.
Belge. Radiol., 78, 377-381 (1995).
11. Stratford, I.J. "Mechanisms of hypoxic cell radiosensitization and the development of new sensitizers", Int. J. Radiat. Oncol. Biol. Phys., 8, 391-398 (1982).
12. Hahn, SM., Sullivan, F.J., DeLuca, A.M., Krishna, C M . , Wersto, N., Russo, A. and
Mitchell, J.B. "Evolution of tempol radioprotection in a murine tumor model", Free Radic. Biol. Med., 22, 1211-1216 (1997).
13. Hori, H., Nagasawa, H. and Terada, H., Advances in Enviromental Sciences and Technology, John Wiley, New York, 28, 425-443 (1994).
14. Patt, H.M., Tyree, E.B. and Straube, R.L. "Cysteine protection against x-irridation",
Science, 110, 213-214 (1949).
15. Mazur, L. "Effects of sulphur containing compounds and x-rays on the mouse erytropoietic system assayed by in vivo peripheral blood micronucleus test", Strahlenter Onkol, 172, 25-29(1996).
16. Narra, V.R., Harapanhalli, R.S., Goddu, S.M., Howell, R.W. and Rao, D.V. "Radioprotection against biological effects of internal radionucleides in vivo by S-(2-aminoethyl)isothiouronium bromide hydrobromide", J. Nucl. Med., 36, 259-266 (1995). 17. Biscay, P., Lespinasse, F., Oiry, J., Huczkowski, J., Imbach, J., Malaise, E.P. and
Guichard, M. "Radiobiological evaluation of a newly synthesised cysteamine derivative", Int. Radiat. Oncol. Biol. Phys., 12, 1469-1473 (1986).
18. Satoh, K. and Sakagami, H. "Effect of cystaine, N-acetyl-L-cysteine and glutathione on cytotoxic activity of antioxidants", Anticancer Res., 17, 2175-2180 (1997).
19. Bump, E.A., Yu, N.Y. and Brown, J.M. "Radiosensitization of hypoxic tumor cells by depleting of intracellular glutathione", Science, 217, 544-545 (1982).
20. Harris, J.W. and Power, J.A "A new sensitizer for anoxic cells", Radiation Research. 56, 97-109(1973).
21. Vos, O., Budke, L. and Grant, G.A. "Radiosensitization of mammalian cells by diamide",
Int. J. Radiat. Biol, 29, 513-522 (1976).
22. Vos. O. and Roos-Verhey, W.S.D. "Radioprotection by glutathione esters and cysteamine in normal and glutathione-depleted mammalian cells", Int. J. Radiat. Biol, 53, 273-281 (1988).
23. Kim, S.G., Nam, S.Y., Kim, C.W., Kim, J.H., Cho, C.K. and Yoo, S.Y. "Enhancement of radiation-inducible hepatic glutathion-S-transferases", Mol. Pharmacol, 51, 225-233 (1996).
24. Van Buul, P.P., Van Duyn, G.A., deRooij, D.G. and Sankaranarayanan, K. "Differential radioprotective effects of misoprostol in DNA repair-proficient and deficient or radiosensitivite cell systems", Int. Radiat. Biol, 71, 259-264 (1997).
25. Daniel, V. "Glutathion S-transferases", Crit. Rev. Biochem. Mol. Biol, 28, 173-207 (1993). 26. Schans, G.P., Vos, O., Roos-Verhey, W.S.D. and Lohman, P.H.M. "The influence of
oxygen on the induction of radiation damage in mammalian cells after sensitization by intracellular glutathion depletion", Int. J. Radiat. Biol, 50,453-465 (1986).
27. Djordjevic, B. and Szbalski, W. "Genetic of human cell lines", J. Exp. Med., 112, 509-531 (1960).
28. Phillips, R.A. and Tomlach, L.J. "Repair of potentially lethal damage in x-irradiated HeLa cells", Radiat. Res., 29, 414-432 (1966).
29. Young, H.K., Floyd, R.A., Maidt, M.L. and Dynlacht, J.R. "Evaluation of nitrone spin-trapping agents as radioprotectors", Radiat. Res., 146, 227-231 (1996).
30. Vo, T.K.O., Fischer, S.M. and Slaga, T.J. "Effects of N-acyl dehydroalanines on phorbol ester elicited tumor development and other events in mouse skin", Cancer Lett., 60, 25-32 (1991).
31. Sharan, R.N., Alam, A., Chakraborty, S., Saikia, J.R. and Srivastava, P.N. "2-Mercaptopropionylglycine affords enchanced radioprotection after a liposome encapsulation", J. Radiat. Res., 36, 31-37 (1995).
32. Hsu, H.Y., Yang, J.J., Lin, S.Y. and Lin, C.C. "Comparisons of geniposodic acid and geniposide on antitumor and radioprotection after sublethal irridation", Cancer Lett., 113, 31-37(1997).
33. Packer, L. "Protective role of vitamine E in biological systems", Am. J. Clinical Nutr., 53, 1050S-1055S (1991).
34. Srinivasan, V. and Weiss, J.F. "Radioprotection by vitamin E", Int. J. Radiat. Oncology
Biol Phys.. 23, 841-845 (1992).
35. Harapanhalli, R.S., Yghmai, V., Giuliani, D., Howell, R.W. and Rao, D.V. "Antioxidant effects of vitamin C in mice following x-radiation", Res. Commun. Mol. Pathol
Pharmacol, 94, 271-287 (1996).
36. Spotheim, M.M., Ruiz, S., Sabattier, R. and Charlier, M. "Radioprotection of DNA by polyamines", Int. J. Radiat. Biol, 68, 571-577 (1995).
37. Phillips, T.L. and Wasserman, T.H. "Promise of radiosensitizers and radioprotectors in the treatment of human cancer", Cancer Treatment Peports, 68, 291-302 (1984).
38. Carroll, F.I., Gopinathan, M.B. and Philip, A. "S-[2-[(2'-Carbamoylethyl) amino]ethyl]phosphorothioate and related compounds as potential antiradiation agents", J.
Med. Chem., 33, 2501-2508 (1990).
39. Savoye, C, Swenberg, C, Hugot, S., Sy, D., Sabattier, R., Charlier, M. and Spotheim, M.M. "Thiol and disulphide, two metabolites of the drug ethyol protect DNA against fast neutron induced strand breakage", Int. J. Radiat. Biol., 71, 193-202 (1997).
40. Foster N.J.A and Siden, R. "Amifostine for protection from antineoplastic drug toxicity",
Am. J. Health Syst. Pharm., 54, 787-800 (1997).
41. Phillips, T.L. "Rationale for initial clinical trials and future development of radioprotectors", Cancer Clin. Trials, 3, 165-173 (1980).
42. Greenstock, C.L. "Redox process in radiation biology and cancer", Radiat. Res., 86, 196-211 (1981).
43. Schuchter, L.M. "Guidelines for the administration of amifostine", Semin. Onkol, 23, 40-43(1996).
44. Alberts, D.S. and Bleyer, W.A. "Future development of amifostine in cancer treatment",
Semin. Onkol., 23, 90-99 (1996).
45. Mazur, L. "Anticlastogenic effect of S-2-(3-aminopropylamino)ethylphosphoro thioic acid against x-rays in mice", Phisiol. Res., 45, 59-63 (1996).
46. Tannehill, S.P. and Mehta, M.P. "Amifostine and radiation therapy", Semin. Oncol, 23, 69-77(1996).
47. Tanaka, Y. and Sugahara, T. "Clinical experience of chemical radiation protection in tumor radiotherapy in Japan" In Radiation Sensitizers, Brandy L. (ed.), Masson Publishers, New York, p 421-425, (1979).
48. Rubin, D.B., Drab, E.A., Kang, H.J., Baumann, F.E. and Blazek, E.R. "WR1065 and radioprotection of vascular endothelial cells", Radiat. Res., 145, 210-216 (1996).
49. Kusenda, Z., Kerger, J., Awada, A., Geurs, F., Van Vreckem, A., Habboubi, N. and Piccart, M.J. "Mitomycin C and vinblastine in combination with amifostine in metastatic breast cancer", Support Care Cancer, 5, 414-416 (1997).
50. Kinsella, T.J. "Radiosensitization and cell kinetics", Seminars in Oncology. 19, 41-47 (1994).