©BEYKENT UNIVERSITY
ADDITIVES FOR HIGH QUALITY
GASOLINE PRODUCTION
Hisham BAMUFLAH*, Salah A. ABDULLAH* and Yavuz
YORULMAZ
***Chemical Engineering Dept. King Abdulaziz University, Jeddah Saudi Arabia
**Chemical Engineering Dept. Beykent University, İstanbul, Turkey, e-mail:yavuzyorulmaz@beykent.edu.tr
Received: 10 June 2007, Revised: 15 September 2007, Accepted: 29 December 2007
ABSTRACT
Gasolines are liquid fuels produced by refining operations for transport purposes. In this study, gasoline properties are studied for ideal purposes and compared and compared with each other for different additives. The octane value, RVP, IBP, 10%, 30%, 50%, 70% and FBP vaporization temperatures were the main properties studied and their predictions for a blend from its components were the utmost importance. The correlations obtained from the actual blending data by multiple regressional ana-lysis produced better results as compared to the ones obtained from the classical methods.
Key Words: Gasoline, octane number, Reid vapor pressure, blend,
multiple regressional analysis.
YÜKSEK DEĞERLİ (OKTAN) BENZİN
ÜRETİMİNDE KATKI MADDELERİ
ÖZET
Benzinler sıvı yakıtlardan olup, taşınımda kullanılmak amacıyla rafineri işlemlerin de üretilirler. Bu çalışmada en iyi kullanımları için, benzin özellikleri çalışılmış ve farklı katkı maddeleri için elde edilen değerler ile kıyaslamalar yapılmıştır. Oktan sayısı, RVP, IBP, 10%, 30%, 50%, 70%, ve FBP buharlaşma sıcaklıkları başlıca çalışılan özellikler olup ve bir karışım için bileşenlerin verilerinden bu değerlerin saptanması en büyük
amaç idi. Multiple regression analiz yöntemi ile bileşenlerin gerçek değerlerinden elde edilenkorrelasyon bağlantıları, klasik yöntemler ile elde edilenlerden daha iyi neticeler verdiği görülmüştür.
Anahtar Kelimeler: Benzin, oktan sayısı, Reid buhar basıncı, paçal,
multiple regression analiz.
1. INTRODUCTION
Liquid fuels are much easier to handle than solids and more convenient to store than gases or electricity. Motor gasoline is made up of a number of components depending on the complexity of the refinery. The gasolines are colorless mixtures of volatile hydrocarbons obtained from liquid petroleum fractions which boil within the temperature range of about
3 0 C - 2 0 0 C and prepared by blending various liquid stocks obtained in
refining operations. This number ranges from as low as a few to as many as fifteen.
Volatility is the tendency of liquid to evaporate or change from liquid to gaseous state. It depends on the nature of the liquid and its storage temperature. Reid vapor pressure (RVP) is the vapor pressure of the gasoline at about 3 8 C . The volatility of gasoline must be carefully
balanced to provide the optimum compromise among performance features that depend on the vaporization behaviour,[1].
Octane number is defined as the percentage by volume of iso-octane that must be mixed with normal heptane to match the the knock intensity of the fuel undergoing testing. There are two recognized laboratory engine test methods for evaluating the antiknock performance of motor fuels, namely the research and motor method . Octane value of a gasoline is one of the most important properties to reflect idea about smooth and proper fuel combustion in gasoline type engines. For this reason, finished product gasolines have to meet certain octane value standards and todays gasoline must meet certain specifications,[2] and [3].
2. THEORY
Gasoline blends prepared usually can not meet the required octane value as they are. For this reason additives methyl ethyl lead (MEL), tetra ethyl lead (TEL) are used as octane booster. The domestic gasoline octane and lead content are currently set at 95 RON (minimum) and 0.4 grams per liter (maximum). The non-linear effect of gasoline composition on octane
rating is of great importance in planning refinery operations. For the required component properties from the early studies are the volume percent olefins, the octane rating for the given test method and TEL concentrations for the components having low concentrations of sulfur and sulfur compounds. The octane rating of a blend given composition is predicted by the following equation , [4].
£ VD
t(R + CP)
£
VD>
Where, Vi is the volume of component i added to blend , Pi is the olefin difference, Ui -Umix, volume percent, Di is the A weighing factor, given
- APt
as Di= (APi)/(1-e ' ), Ri is octane rating of component i by the given test method, C is 0.130 for research octanes and 0.097 for motor octanes and A = 0.01414 for research octanes and 0.0199 for motor octanes. TEL remains an economic antiknock additive to produce gasolines and it basically
increases the gasoline resistance to engine knocking which permits the addition of greater quantities of lower octane components like naptha and FCCU gasoline into gasoline pool.
However, since the 1970's, environmental policies and regulations in the world major markets have put pressure on the refiners to phase out leaded gasoline to curb excessive emission of hydrocarbons, NOx and COx. Lead poisons the catalytic converters in the exaust system of automobiles and makes the converters ineffective for reducing the emissions of pollutants produced by engines, [5].
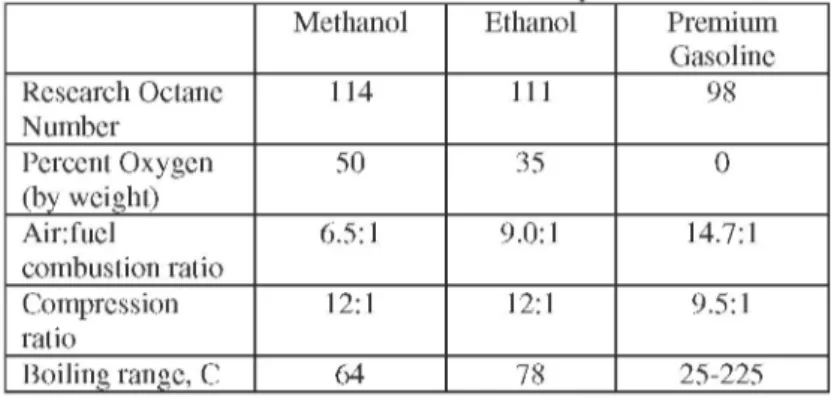
For some time alcohols appeared a as a fuel in the form of a blend or gasoline extender for partly or complete replacements for gasoline. The two alcohols that have received most attention are methanol and ethanol being convenient end products from biomass. Alcohols are composed of carbon, hydrogen and oxygen and are described as oxygenated fuels being different from hydrocarbons. The oxygen has the disadvantage that it adds nothing to energy content but increases the weight. The good combustion qualities of methanol and ethanol with their suitability as spark ignition fuels are shown by their high research octane in Table1. The high octane number is an indication of a fuel's combustion efficiency in a spark ignition engine and enables alcohols to be used in engines with
higher compression ratios. This together with their wider flammability limits which allows for clean burning gives rise to more efficient combustion.
Table 1. Alcohols and Gasoline Fuels Properties
Methanol Ethanol Premium Gasoline Research Octane 114 111 98 Number Percent Oxygen 50 35 0 (by weight) Air:fuel 6.5:1 9.0:1 14.7:1 combustion ratio Compression 12:1 12:1 9.5:1 ratio Boiling range, C 64 78 25-225 Gasoline engines the energy contained in the alcohol can be used that much more effectively than the energy contained in gasoline. However, alcohols are much less energy intensive than gasoline: one liter of ethanol contains 40 per cent less energy and one liter of methanol 55 percent less energy than a liter of gasoline. Therefore, even with the higher thermal efficiency the net result is an increase in the volume of fuel required per kilometer travelled. The handling of alcohols has to take into considerations that they are corrosive to certain metal and plastics which could be found in the distribution network and vehicle fuel systems. Methanol has the additional hazard of being extremely toxic and its skin contact mus be avoided. There isn't any major lubrication problems with ethanol but methanol may cause increased wear to parts of the engine, rusting and the formation of deposits in the inlet system and consequently require special lubricants and additives. Inadvertent ingestion of methanol, such as when one siphons fuel from a vehicle could pose more of a threat than gasoline because of color and odor lacks.
There is continual competition between the use of toluene as gasoline additive and main raw material for petrochemical industry. Most of the time toluene's market price will be close to its value as a gasoline blending component. Blending value is expected to increase steadily due to octane its octane capability. Supplies of toluene for gasoline will be relatively constant and can not be expected to play major factor in solving the octane shortage, [6].
The other possible gasoline blending component has received a great deal of publicity is methyl tertiary butyl ether (MTBE). Much of data refers to its effect on the octane number but not much on its effect on gasoline volatility. Generally, the addition of MTBE increases gasoline volatility to acceptable degree but no significant effect. However MTBE can mix with hydrocarbons by any percentage and does not cause the characteristics of separation layer. Its research and motor octane numbers are upto 117 and 101 respectively. It does not affect on the material and easy to transfer from place to place. When it is added to gasoline it reduces the amount of carbon monoxide and hydrocarbons. Generally it can be mixed with gasoline by 10-11% (by volume) and sold under the trade name 'gasohol'.
The other options involve blending high octane components are ETBE (ethyl tertiary butyl ether) and TAME (tertiary amyl methyl ether). These organic compounds contain oxygen and their use has increased in order to reduce emissions of engine pollutants. The addition of oxygenates raises the combustion temperature in the engine and improves its efficiency with low RVP characteristics.. Their comparative stydies are given in literature, [7].
3. PROCEDURE AND DISCUSSION OF RESULTS
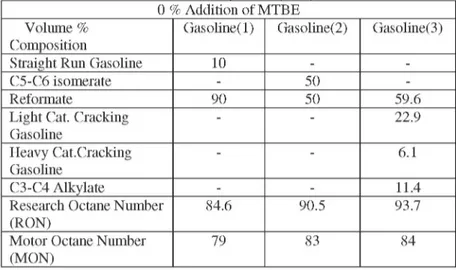
The effects of various amounts of methyl ter-butyl ether to car gasolines are studied and the results are given in Table 2, [8].
Table 2. The Effects of Methyl Tertiary Butyl Ether to Car Gasolines 0 % Addition of MTBE
Volume % Gasoline(l) Gasoline(2) Gasoline(3) Composition
Straight Run Gasoline 10 -
-C5-C6 isomerate - 50
-Reformate 90 50 59.6
Light Cat. Cracking Gasoline - - 22.9 Heavy Cat.Cracking Gasoline - - 6.1 C3-C4 Alkylate - - 11.4
Research Octane Number 84.6 90.5 93.7 (RON)
Motor Octane Number 79 83 84 (MON)
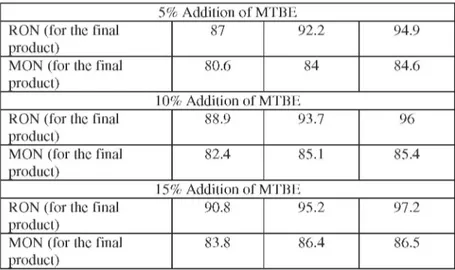
5% Addition of MTBE
RON (for the final 87 92.2 94.9 product)
MON (for the final 80.6 84 84.6 product)
10% Addition of MTBE
RON (for the final 88.9 93.7 96 product)
MON (for the final 82.4 85.1 85.4 product)
15% Addition of MTBE
RON (for the final 90.8 95.2 97.2 product)
MON (for the final 83.8 86.4 86.5 product)
This project work aimed at the premium gasoline produced at Jeddah Oil Refinery in Saudi Arabia and prediction of its Reid Vapor Pressure and temperatures at given percent vaporized from components distillation and composition data supplied by the same institution. The correlations established using a multiple regression computer program to correlate the blend RVP and percent vaporized temperature using the data of the blend components which are given in Table 3, [12].
Table 3. Components Data For Gasoline Blends
Properties LSR PLATFORMATE FCCU n-BUTANE Sp. Gr. @60 F 0.673 0.762 0.738 0.574 API 78.8 54.1 38.8 113.1 RVP, bar (psig) 0.72(10.5) 0.62(9.0) 0.62(9.0) 3.42(48.6) Sulfur, %wt 0.027 0.0 0.16 --Distillation, C IBP 36 39 37 --10% Vaporized 49 61 55 --50% Vaporized 69 122 107 --90% Vaporized 91 165 193 --FBP 113 200 220
--Recovery, % 98 97.3 97.7 --Residue, % 0.7 1.3 1.3 --Loss, % 1.3 1.4 1.0 --RON, Clear 63 87 89 92
The dependent variable was denoted as RVPWend and the independent
variables were the RVP values for each component times the volume percent of each component. The Table 5 shows the results of correlation between the RVP of blend and the RVP of its components. It was observed that a good fit results with a multiple correlation of 0.988%. Table 5 also shows that the percent error is very small with a minimum value of 0.09%, a maximum of 3.18% and average value of 1.105%. Therefore the following equation is concluded for correlated RVP of a premium gasoline from its components:
RVPblend = 6.097+( 1.749 (RVP1 X V1))+(1.285 (RVP2 X V2))+(1.97
((RVP3 XV3)) +(1.16 (RVP4 X V4))
Where; RVPi: Reid vapor pressure of component 'i'and Vi : Volume percent of component 'i' the results obtained from our developed correlation can be compared with those from classical method which involved the use of index tables. As it is seen from Table 4, our correlation results with low average percent error.
| Table 4._ Comparison of Estimated RVP blend With Experimental Values Observed Points OBSERVED ESTIMATED % DEVIATION
1 9.20 9.13 0.76 2 10.0 9.94 0.60 3 10.80 10.76 0.37 4 11.60 11.59 0.09 5 9.0 8.96 0.44 6 10.40 10.5 -1.63 7 8.50 8.59 -1.06 8 8.50 8.77 -3.18 9 8.90 9.05 -1.69 10 9.00 9.04 -0.44 11 9.30 9.28 0.22 12 9.20 8.98 2.39 13 9.30 9.28 0.22 14 9.20 8.98 2.39
Average Absolute Deviation = 1.105313
As for IBP equation for premium gasoline the following equation is obtained:
IBP blend = 47.307 - 22.330 P(1)3 + 12.045 P(2)3 _ 1280.636 P(3)3
-48.925(3) P(1)+P(2)2-112.106 (3)P(1) P(3)2+36.412 (3)
P(2)P(1)2-7.827(3)P(3)P(1)2
where: P(1) = IBP(1)0 2 9X VOL(1)
P(2)= IBP(2)0 2 9X VOL(2)
P(3)= IBP(3)0 29X VOL(3)
IBP(1) = Initial Boiling Point of FCCU gasoline IBP(2) = Initial Boiling Point of PLATFORMATE IBP(3) = Initial Boiling Point of LSR naptha
VOL(1) = Volume Percent of FCCU gasoline VOL(2) = Volume Percent of PLATFORMATE VOL(3) = Volume Percent of LSR naptha
In a similar way T10% b l e n d is correlated to T10% of components as
that of IBPblend. The same thing applies to Ts0% blend and T90% blend
temperatures. T10% b l e n d = -7.489 + 1.531 x X(1)3-21.803 x X(2)3- 381.947 x X(3)3 + 8.8360 x 3 x X(1) x X(2)2 + 37.000 x X(1) x X(3)2 _ 1.646 x 3 x X(2) x X(1)2 -0.229 x 3 X(3) x X(1)2 Where: X(1) = T10%(1)0 35x VOL(1) X(2) = T10%(2)a35x VOL(2) X(3) = T10%(3)a35x VOL(3)
T10%(1) = 10% distillation temperature of FCCU gasoline
T10%(2) = 10% distillation temperature of PLATFORMATE
T10%(3) = 10% distillation temperature of LSR naptha
T50% blend = 111.679 _ 60.374 x Y(1)3 + 229.727 x Y(2)3- 1706.069
x Y(3)3 -184.203 x Y(1) x Y(2)2 + 143.531 x 3 x Y(1) x Y(3)2 + 111.476
x 3 x Y(2) x Y(1)2 _ 10.930 x 3 x Y(3) x Y(1)2
Where; Y(1) = T50% (1)0 2 1 x VOL(1)
Y(2) = T50% (2)0.21 x VOL(2)
Y(3) = T50% ( 3 f2 1 x VOL(3)
T50%(1) = 50% distillation temperature of FCCU gasoline
T50%(2) = 50% distillation temperature of PLATFORMATE
T50%(3) = 50% distillation temperature of LSR naptha
T90% b l e nd = 151.110 - 37.251 x Z(1)3 + 500.205 x Z(2)3 + 790.175 x Z(3)3 -230.511x3 x Z(1) x Z(2)2 _ 66.579 x 3 x Z(1) x Z(3)2 + 101.838 x 3 x Z(2) x Z(1)2 + 14.964 x 3 x Z(3) x Z(1)2 Where; Z(1) = T90%(1)015 x VOL(1) Z(2)= T90%(2)0.15 x VOL(2) Z(3)= T90% (3)0.15 x VOL(3)
T90% (1) = 90% distillation temperature of FCCU gasoline
T90% (2) = 90% distillation temperature of PLATFORMATE
T90% (3) = 90% distillation temperature of LSR naptha
As for FBP value of the blend, the following equation has been obtained: FBPblend= 226.567 -135.593 x R(1)3 + 2411.704 x R(2)3 + 2204.347 x R(3)3 -930.966 x 3 x R(1) x R(2)2 - 148.257 x 3 x R(1) x R(3)2 + 356.208 x 3 R(2) x R(1)2 + 6.511 x 3 x R(3) x R(1)2 Where; R(1) = FBP(1)0 3 0x VOL(1) R(2) = FBP(2)0 30x VOL(2) R(3) = FBP(3)0 30x VOL(3)
FBP(1) = Final boiling point of FCCU gasoline FBP(2) = Final boiling point of PLATFORMATE FBP(3) = Final boiling point of LSR naptha
4. CONCLUSION
The effects of Reid vapor pressure and distillation characteristics on gasoline production are studied with actual cases. Restricting the lead content of gasoline causes changes in gasoline properties and may necessitate a change in specifications. Simple classical methods are not very conducive for RVP and distillation temperatures calculations from components data. Correlations produced from multiple regression analysis method are more indicative and accurate in calculating blend properties for each
refinery.
ACKNOWLEGMENT
W e extend our appreciation to Jeddah Oil Refinery for their valuable contribution to our research Project.
REFERENCES
[1]. William A. Gruse and Donald R. Stevens, Chemical Technology of Petroleum, McGraw -Hill Book Company Inc. New York, 1960.
[2]. Grayson M., Encyclopedia of Chemical Technology, Vol.3, John Wiley & Sons Inc., 1978.
[3]. The Story of Gasoline, Ethyl S.A., 100, Park Avenue, New York, U.S.A. [4]. Stewart E., Warren, Predict Octanes for Gasoline Blends, Petroleum Refiner Dec., 1959.
[5]. Shell Briefing Service, Alternative Road Transport Fuels, No 5, 1982. [6]. Dosher R. John, Toluene: Octanes or Chemicals, Hydrocarbon Processing, May1979.
[7]. Peter Dartnell and Kenneth Campbell, The Oil and Gas Journal, The Octet Co.Ltd England, Nov. 13, 1978.
[8]. Al-Hamid K., A., Options for Unladed Gasoline Scenario, Saudi Journal of Science, Vol. 4, Riyadh, Saudi Arabia, 1986.
[9]. Kirk-Othmar ncyclopedia of Chemical Technology, Third Ed. Vol.14, John Wiley & Sons Publishing Company, 1981.
[10]. Clberson S., F., Yepson G.,L., and Bartholomev, 'Refiners Mull No-Lead Options', Oil & Gas Journal, Vol.83, pp. 108-114, March 18, 1985.
[11]. Sharman, J., 'Fuel Oxygenates Demand Outspaces Supply, Chemical Engineering, pp. 57-58, October, 1979.
[12]. Jeddah Oil Refinery Values for Gasoline Components, Jeddah, S. Arabia, 1988.