EXPERIMENTAL STUDY
Protective effect of betaine against skeleton muscle apoptosis
in rats induced by chronic alcohol and statin consumption
Oglakci Ilhan A
1, Ozkoc M
2, Kusat Ol K
3, Karimkhani H
4, Senturk H
5, Burukoglu D
6, Kanbak G
2Cankiri Karatekin University, Eldivan Vocational School of Health Services, Cankiri, Turkey. ayseguloi@karatekin.edu.tr
ABSTRACT
AIM: The aim of the present study was to investigate the effect of apoptosis on rat skeletal muscle caused by chronic alcohol and statin consumption with modifi ed liquid diet and to elucidate protective effects of betaine supplementation.
METHODS: TNF-α (tumor necrosis factor), NF-kB (Nuclear Factor kappa B), cytochrome c and caspase-3 levels with or without betaine treatment in alcohol and/or statin-induced skeleton muscle apoptosis rats as well as in controls were measured in serum and tissue. Histologic examinations of the muscle tissues were also performed.
RESULTS: In our study, betaine treated treatment groups we found that calpain and caspase activities and cytokine c release were decreased caused by alcohol, statin and more importantly alcohol+statin group and TNF and NF-kB levels were also close to the levels of control group. Similarly, signifi cant improvements have been observed in our morphological and histological examination results also supporting our biochemical data. CONCLUSION: We found that combined consumption of ethanol and statin is capable of triggering apoptotic cell death in rat muscles more than the consumption of only alcohol or only statin. Betaine was able to reduced this muscle cell death induced by alcohol and/or statin consumption (Tab. 4, Fig. 4, Ref. 43). Text in PDF www.elis.sk
KEY WORDS: alcohol, statin, rat, muscle, apoptosis.
1Cankiri Karatekin University,Eldivan Vocational School of Health
Ser-vices, Çankiri, Turkey, 2Eskisehir Osmangazi University, Faculty of
Medi-cine, Department of Biochemistry, Eskisehir, Turkey, 3Turkish Health of
Ministry, Turkish Medicines and Medical Devices Agency, Ankara, Tur-key, 4Istanbul Medipol University, Faculty of Medicine, Department of
Biochemistry, Istanbul, Turkey, 5Eskisehir Osmangazi University, Science
and Arts Faculty, Department of Biology, Eskisehir, Turkey, and 6
Eskise-hir Osmangazi University, Faculty of Medicine, Department of Anatomy, Eskisehir Turkey
Address for correspondence: A. Oglakci Ilhan, Cankiri Karatekin
Uni-versity, Eldivan Vocational School of Health Services, Cankiri, Turkey. Phone: +905532040394
Acknowledgements: This work was supported by TUBITAK 114S241
project.
Abbreviations: ADP: Adenosine diphosphate, AMC: 7-amino-4-methyl coumarin, ATP: Adenosine triphosphate, CK: Keratin Kinase, JNK: Jun-N-terminal kinases, MLD: Modifi ed Liquid Diet, NF: Nuclear Factor, NF-kB: Nuclear Factor kappa B, pNA: p-Nitroaniline, SAH: S-adenosylhomocysteine, SAM: S-adeno-sylmethionine, TNF-α: Tumor Necrosis Factor
Introduction
Primary disease of skeletal muscle is expressed in terms of myopathy. Myopathies may have genetic, infectious, autoimmune and toxic reason. Toxic myopathies can develop depending on
using drugs and alcohol. Alcohol can lead to acute and chronic myopathy depending on the period of use (1, 2). There are many medicines that are held responsible for the development of toxic myopathy. Statin derivative drugs that are used in the treatment of hypercholesterolaemia cause the development of myopathy (3). Although the mechanisms of statin induced myopathy is not fully understood, several theories have been proposed by researchers. These include alteration of the level of ubiquinone or alteration of the sarcolemic cholesterol content or proteolysis and induction of apoptosis. The most important group of membrane receptors involved in apoptosis is actively induced by several structurally related receptors, called death receptors. The best known are TNF-alpha and Fas receptors (4). TNF-α is caused by apoptosis with 3 important signaling pathways. The fi rst pathway triggers apoptosis by triggering caspases through the FADD-associated death zone (FADD). The second pathway activates Jun-N-terminal kinases (JNK) and transcription factor AP-1, again leading to apopto-sis. In the third pathway, it activates NF-κB, the main mediator in the control of TNF-α transcription. TNF-α is important in the activation and translocation of NF-κB in the skeleton (5). NF-kB increases the expression of several members of the Bcl 2 family that inhibits apoptosis by inhibiting cytochrome c release and mitochondrial permeability (6). The Bcl-2 (antiapoptotic protein) family is the most important group in the control of the apoptotic cascade and has a large number of squamous cells. Bcl-2 protect mitochondria membrane permeability. It inhibits apoptosis by
in-hibiting proapoptotic proteins (Bax and Bad) (7). Bax protein is a proapoptotic protein and has an important role in the induction of apoptosis (8). In healthy cells Bax is found in cytosol. Apop-totic stimulation leads to cytosolic bax to mitochondria and, as a result of various changes, hydrophobic C-terminus of Bax is re-leased and causes cytochrome c release (7). Rere-leased cytochrome c plays an important role for caspase-3 activation in apoptotic cell death. Activation of the cofactor nucleotide triphosphate (d-ATP and ATP) activates cytochrome c and apaf-1 to activate procas-pase-9. Activated caspase-9 also activates caspase-3 and triggers the other caspase cascade (9). The release of Bax is stimulated by the calpain, resulting in the release of cytochrome c (7). Cal-pains are calcium-dependent thiol-proteases (10). CalCal-pains are proteases involved in cell skeleton and signal transduction. They are also involved in other physiological and pathophysiological processes such as cell cycle regulation, apoptosis, infl ammation, ischemia, muscular dystrophy, cataractogenesis, Alzheimer’s and Parkinson’s diseases (11). Activated calpains promote apoptosis by causing caspase 9 and 3 activation (7).
Skeletal myopathy is observed in 30 % to 50 % of high-dose chronic alcohol consumers and it has been related to a direct dose-dependent toxic effect of ethanol. However, its pathogenesis has not been clearly established (12). Apoptosis may be responsible for the progressive intrinsic contractile dysfunction of skeletal myo-cytes and progressive myocyte loss in alcoholic myopathy (13). Ethanol may activate apoptosis through diverse mechanisms, with the increase in TNF, activation of nuclear-factor-(NF), activation of mitochondrial caspases and disruption of intracellular (Ca2+) transients being the most frequently implicated (12). The change in intracellular calcium level triggers pathways that cause apoptosis. It is thought that calcium plays a role in dephosphorylation of Bad which affects cytochrome c release. Similarly, (Ca2+) -dependent cysteine protease calpain, providing Bax cleavage and it causes Bax to perform its proapoptotic activity. Furthermore, (Ca2+) has been implicated in the release of cytochrome c by affecting the mitochondrial membrane transition pores during apoptosis (14). Cytochrome c can initiate the activation cascade of caspases once it is released into the cytosol and lead to apoptosis (15).
Natural products have been the starting point for the discovery of many modern medicines. Betaine was fi rst discovered in the sugar beet (Beta vulgaris) juice in the 19th century and is known to be present in some other organisms (16). Betaine, a molecular chaperone task, is an inhibitor of oxidative stress and mitochon-drial damage (17). It is the methyl source of the transmethylation reactions used in many biochemical pathways, thus providing single carbon units to be used in the formation of methionine and cholines necessary for optimal nutrition (16). The conversion of homocysteine to methionine is necessary for the maintenance of the level of methionine, the detoxifi cation of homocysteine and the production of S-adenosylmethionine (SAM). Ethanol con-sumption increases the rate of S-adenosylhomocysteine (SAH) in liver cells (19). Betaine reduces the rate of increasing SAH (17). In recent years, an increase in the number of intracellular SAHs has been reported with increasing apoptosis in many different cell types (18, 19).
In our study, supported by Tübitak 1002 support program, the role of statin use on skeletal muscle apoptotic cell death and the protective role of betaine in chronic alcohol consumption were investigated biochemically and histologically. Orally, betaine treat-ment has been shown to reduce liver necrosis, liver fat accumu-lation, apoptosis induced in various forms. A similar result was aimed at seeing when chronic alcohol use and statin use coexist. In this context; as a result of chronic alcohol use TNF-α, NF-kB, cytochrome c and caspase-3 levels as markers of skeletal apoptosis and statin consumption in rat muscle tissues were determined from cysteine proteases known to be involved in triggering apoptosis. CK enzyme levels, which are muscle injury indicators in serum of rats, were measured. Bcl-2 and Bax levels were determined by immunohistochemical analysis, TUNEL method and light micro-scopy. The results obtained here provide us with the fi rst informa-tion in the literature about whether there is a protective role for betaine on the use of statin along with chronic alcohol consump-tion and will shed light on the development of different treatment strategies for the use of stain during chronic alcohol use. Materials and methods
Materials
56 adult male Sprague-Dawley rats weighting 160–195 g were obtained from TICAM (Medical and Surgical Experimental Research Center, Eskisehir-TURKEY) and housed in polycar-bonate cages in a room with controlled temperature (22 ± 2 DC), humidity (50 ± 5 %), and a 12 hour cycle of light and dark (07:00 AM-07:00 PM light). The rats were placed in separate cages and acclimated to laboratory conditions for one week before starting the experiments. The rats were randomized into 7 groups, each consisting of 8 animals.
Control group (n = 8): Received isocaloric modifi ed liquid diet (MLD)
Alcohol group (n = 8): Received MLD solution with ethanol Statin group (n = 8): Received isocaloric MLD and 10 mg/kg
atorvastatin
Alcohol+statin group (n = 8): Received MLD solution with etha-nol and 10 mg /kg atorvastatin
Alcohol+betaine group (n = 8): Received MLD solution with etha-nol and betaine (1 % w/v).
Statin+betaine group (n = 8): Received isocaloric MLD, 10 mg/kg atorvastatin and betaine (1 % w/v)
Alcohol+statin+betaine group (n = 8): Received MLD solution with ethanol, 10 mg/kg atorvastatin and betaine (%1 w/v) The MLD comprised 925 ml low fat cows’ milk (Sütaş, Es-kisehir, Turkey), from 25 to 75 ml alcohol (96.5 % v/v ethanol; Merck Millipore, Darmstadt, Germany) and 17 g sucrose (Merck Millipore). The caloric content of the diet was 1,007 kcal/l. The weight of the rats was recorded every day and the daily alcohol intake was also measured. The MLD was prepared daily and given to the animals while fresh. Extra chow or water were not available during the experimental period. At the beginning of the study, the rats were given MLD without alcohol for 2 days. Then, a liquid diet with 2.4 % alcohol was administered for 3 days. The
alco-hol concentration was increased to 4.8 % during the following 4 days and fi nally reached 7.2 % over the next 21 days. Isocaloric modifi ed liquid diet containing sucrose as a caloric substitute for alcohol (96 g sucrose and 75 ml cows’ milk replaced 60.75 g or 75 ml ethanol) (20, 21).
10 mg / kg atorvastatin was dissolved in 0.9 % NaCl by a mag-netic stirrer and given oral gavage for 30 days at the same time.
The rats were anesthetized (Ketamine / Xylazine 3: 1, 1.32 mg / kg intraperitoneal) and blood was taken from their hearts. Skele-tal muscle was surgically removed. Tissues used for biochemi-cal studies were frozen and kept at –80 °C until they were tested. For histological studies, the tissues were fi xed in neutral form for 24–48 hours. Routine histologic follow-up of tissues was done af-ter fi xation. All experiments were carried out in accordance with institutional guidelines for animal welfare (Eskisehir Osmangazi University Animal Care and Use Committee, Eskisehir, Turkey) and were approved by the Ethics Committee of the Medical and Surgical Experimental Research Center of Osmangazi University Methods
Measurement of CK Activity: Serum was measured using the CK Elisa kit (MAK116, Sigma-Aldrich).
Measurement of TNF-α Levels: Tissue TNF-a levels were measured using a quantitative ELISA test kit (RAB0479, Sigma-Aldrich).
Measurement of NF kB Levels: Tissue NF kB levels were mea-sured using a quantitative ELISA test kit (CSB-E13148r, Cusabio). Measurement of Caspase-3 Activities: Tissue samples were homogenized (1/10 w/v) in lysis buffer (250 mM HEPES, pH 7.4, 1 % CHAPS, 50 mM DTT, 10 mM EDTA) and then centrifuged for 20 min at 3,000 rpm and 4 °C. Supernatants were collected and centrifuged twice at 14,000 rpm for 15 min (22). Commercial assay kit (Sigma CASP-3-C) was used to measure caspase-3 ac-tivities in supernatant fractions. Results were expressed in μmol pNA/minute.
Measurement of Cytochrome-c Release: Cytochrome c levels were measured in both cytosolic and mitochondrial fractions us-ing a commercial assay kit (R&D Systems MCTC0) and cytosolic fraction/mitochondrial fraction ratios were used as indicators of mitochondrial damage (23, 24).
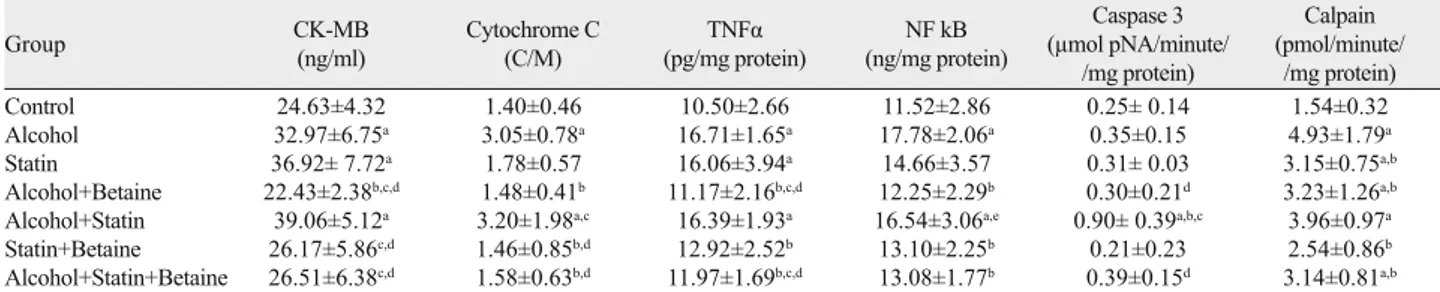
Calpain Activity Assay: The difference between measurement rates in calcium free and calcium containing assay buffer was used to determine calpain activity. Calpain activity was determined as the difference between the calcium-dependent fl uorescence and the Group CK-MB(ng/ml) Cytochrome C (C/M) (pg/mg protein)TNFα (ng/mg protein)NF kB
Caspase 3 (μmol pNA/minute/ /mg protein) Calpain (pmol/minute/ /mg protein) Control 24.63±4.32 1.40±0.46 10.50±2.66 11.52±2.86 0.25± 0.14 1.54±0.32 Alcohol 32.97±6.75a 3.05±0.78a 16.71±1.65a 17.78±2.06a 0.35±0.15 4.93±1.79a Statin 36.92± 7.72a 1.78±0.57 16.06±3.94a 14.66±3.57 0.31± 0.03 3.15±0.75a,b Alcohol+Betaine 22.43±2.38b,c,d 1.48±0.41b 11.17±2.16b,c,d 12.25±2.29b 0.30±0.21d 3.23±1.26a,b
Alcohol+Statin 39.06±5.12a 3.20±1.98a,c 16.39±1.93a 16.54±3.06a,e 0.90± 0.39a,b,c 3.96±0.97a
Statin+Betaine 26.17±5.86c,d 1.46±0.85b,d 12.92±2.52b 13.10±2.25b 0.21±0.23 2.54±0.86b
Alcohol+Statin+Betaine 26.51±6.38c,d 1.58±0.63b,d 11.97±1.69b,c,d 13.08±1.77b 0.39±0.15d 3.14±0.81a,b
Distribution of creatine kinase, cytochrome c, TNFα, NF Kb, caspase3 and calpain levels in rat groups. The results are expressed as mean ± standard deviation. a: Signifi -cant difference from control group (p < 0.05), b: Signifi -cant difference from alcohol group (p < 0.05), c: Signifi -cant difference from statin group (p < 0.05), d: Signifi -cant difference from alcohol+statin group (p < 0.05), d: Signifi cant difference from alcohol+betaine group (p < 0.05)
Tab. 1. Biochemical results of groups.
Groups (median 25–75%)
Necrosis Myofi brillar degeneration Nuclear proliferation Nuclear hypertrophy Edema Control (0.00–0.00)0.00 (0.00–1.00)0.20 (0.00–0.00)0.00 (0.00–1.00)0.10 (0.00–0.00)0.00 Alcohol (1.00–2.00)1.25 a (1.00–2.00)1.30 a (1.00–2.00)1.40 a (1.00–2.00)1.30 a (1.00–2.00)1.30 a Statin (1.00–2.00)1.25 a (1.00–2.00)1.40 a (1.00–2.00)1.30 a (1.00–2.00)1.20 a (1.00–2.00)1.40 a Alcohol+Statin 2.80 (2.00–3.00)a, b 2.70 (2.00–3.00)a, b 2.90 (2.00–3.00)a, b 2.70 (2.00–3.00)a, b 2.80 (2.00–3.00)a, b Alcohol+Betaine (0.00–1.00)0.75 a, c 0.60 (0.00–1.00)b 0.80 (0.00–1.00)a, b, c 1.00 (0.00–2.00)a, c 0.80 (0.00–2.00)a, c Statin+Betaine (0.00–1.00)0.50 a, b (0.00–1.00)0.80 (0.00–1.00)0.80 a (0.00–1.00)0.80 a (0.00–2.00)0.80 a Alcohol+Statin+Betaine (0.00–1.00)0.25 b, c, d (80.00–1.00)0.20 b, c, d (0.00–1.00)0.10 b, c, d (0.00–1.00)0.30 b, c, d (0.00–1.00)0.20 b, c, d
Light microscopic evaluations: (0) no injury. (1) mild. (2) moderate and (3) severe. a: Statistical difference compared with control group p < 0.05. b: Statistical difference com-pared with alcohol group p < 0.05. c: Statistical difference comcom-pared with alcohol+statin groups p < 0.05. d: Statistical difference comcom-pared with alcohol+betaine groups p < 0.05
noncalcium-dependent fl uorescence. A 7-amino-4-methyl couma-rin (AMC) standard curve was constructed. Calpain activity was expressed as pm of AMC released per minute of incubation time per minute of total protein (25).
Histological analysis
At the end of the experimental period all the groups were sac-rifi ced with anesthesia and muscle specimens of rats were fi xed in 10 % formalin fi xative for 48 hours in order to be able to perform
his-Fig. 1. Distribution of creatine kinase, cytochrome c, TNF-α, NF Kb, caspase-3 and calpain levels in rat groups. The results are expressed as mean ± standard deviation in eight rat of each groups. a*: Compared with control group p<0.047, a**: Compared with control group p<0.010, a***: Compared with control group p<0.001, b*: Compared with alcohol group p<0.047, b**: Compared with alcohol group p<0.010, b***: Compared with alcohol group p<0.001, c*: Compared with statin group p<0.047, c**: Compared with statin group p<0.010, c***: Com-pared with statin group p<0.001, d*: ComCom-pared with alcohol+statin group p<0.047, d**: ComCom-pared with alcohol+statin group p<0.010, d***: Compared with alcohol+statin group p<0.001, e*: Compared with alcohol+betain group p<0.047
tological examinations at light microscopic level. The samples were subjected to routine procedures, were processed into paraffi ded blocks. 5 μm-sections were obtained from each paraffi n-embed-ded block, and these sections were stained with Hematoxylin Eosin and examined under Olympus BH-2 microscope and photographed. In addition, sections were taken for TUNEL staining to determine apoptotic cells from all muscle tissue specimens. The paraffi ns of
the preparations were melted at 60 degrees for 1 hour. Then TUNEL staining technique was applied to muscle tissue specimens. Immunohistochemically, sections were taken from the same muscle tissue samples and Bcl-2 and Bax levels were measured. Statistical analysis
SPSS software, version 15.0 for Win-dows (SPSS, Inc., Chicago, IL, USA) was used for the statistical analysis of biochem-ical data. In order to assess differences be-tween groups, one-way analysis of variance (ANOVA) and Tukey’s multiple compari-son test were used. Results are presented as mean ± standard deviation and p<0.05 was considered to indicate a statistically signifi cant result.
Results
Biochemistry results
CK levels: Serum CK levels in the al-cohol (p < 0.047), statin (p < 0.010) and alcohol+statin (p < 0.001) groups were significantly increased compared with the control group. The CK levels in the alcohol+betaine group exhibited a signifi -cant reduction compared with the alcohol, statin and alcohol+statin group (respec-tively p < 0.010, p < 0.001, p < 0.001). The CK levels in the statin+betaine and alcohol+statin+betaine group were de-creased compared with the statin group (p < 0.010).The CK levels of the statin+betaine and alcohol+statin+betaine were found to be signifi cantly lower compared with alcohol+statin group (p < 0.001) (Tab. 1).
c release: Cytochrome-c release in the alCytochrome-cohol, statin and alcohol+statin groups was increased com-pared with the control group and this in-crease was statistically signifi cant only for the alcohol+statin group (p < 0.001). In the alcohol+statin group it was signifi cantly in-creased compared with the statin group (p < 0.001). The release cytochrome c levels of the statin+betaine group were found to be lower compared with alcohol and alcohol+statin group (respec-tively p < 0.047, p < 0.010). The release cytochrome c levels in the alcohol+statin+betaine group exhibited a signifi cant reduction compared with the statin and alcohol+statin group (respectively p < 0.010, p < 0.001) (Tab. 1).
TNF-α Levels: TNF- α levels in the alcohol, statin and alcohol+statin groups were signifi cantly increased compared
Fig. 2. Light microscope results in rat groups (a-g). a) Control group, the overall appearance. Located peripheral nuclei →), (HE, scale bar: 100 μm, X20, scale bar: 50.0 μm, X40). b) Alcohol group: (*) partial edema, (►) vacuolization, (→) nuclear hypertrophy (HE, scale bar: 100 μm, X20, scale bar: 50.0 μm, X40). c) Statin group: (►) partial edema, (→) nuclear hypertrophy (HE, scale bar: 100 μm, X20, scale bar: 50.0 μm, X40). d) Alcohol+statin group: ►) necrotic myofi brils, (→) nuclear proliferation (HE, scale bar: 100 μm, X20, scale bar: 50.0 μm, X40).
a
b
c
with the control group (p < 0.001). The TNF-α levels of the alcohol+betaine group were found to be lower compared with alcohol, statin and alcohol+statin group (respectively p < 0.001, p < 0.010, p < 0.047). In the statin+betaine group the levels were decreased compared with the alcohol group (p < 0.047). The TNF- α levels in the alcohol+statin+betaine group exhibited a signifi cant reduction compared with the alcohol, statin and alcohol+statin group (respectively p < 0.010, p < 0.047, p < 0.048) (Tab. 1).
NF-kB Levels: Compared with the control group, NF kB levels in the alcohol and alcohol+statin group were found to be statisti-cally signifi cantly higher (respectively p < 0.001, p < 0.010). In the alcohol+betaine, statin+betaine, alcohol+statin+betaine groups the levels were found to be decreased compared with the alcohol group and this decreased was statistically signifi cant (p < 0.010). The NF kB levels in the alcohol+statin group exhibited a signifi cant incre-ment compared with the alcohol+betaine group (p < 0.047) (Tab. 1). Caspase 3 enzyme activity: When the caspase 3 activities of the groups were assessed signifi cantly, it was seen that the
caspase 3 activity of alcohol+statin group statistically showed a signifi cant increase compared to the control group (p < 0.001). Caspase 3 activities of alcohol+statin group were statistically signifi cantly increased compared with the alcohol and statin groups (p < 0.001). The caspase 3 en-zyme activities of the alcohol+betaine and alcohol+statin+betaine groups were found to be statistically lower compared with alcohol+statin group (p < 0.001) (Tab. 1).
Calpain activity: Compared with the control group, calpain activities in the alcohol, alcohol+statin, statin and alcohol+betaine group were found to be statistically signifi cantly higher (respec-tively p < 0.001, p < 0.001, p < 0.047, p < 0.047). In the statin, alcohol+betaine and alcohol+statin+betaine groups the levels were found to be statistically de-creased compared with the alcohol group (p < 0.047). The calpain activity of the statin+betaine group was found to be statis-tically lower compared with alcohol group (p < 0.001) (Tab. 1).
Histological results
Light microscope evaluation: Light microscopy results are shown in Figure 1. In light microscope evaluation, samples were scored according to the presence of necrosis, myofi brillar degeneration, nucle-ar proliferation, nuclenucle-ar hypertrophy and edema. Obtained data was compared sta-tistically. According to the data, necrosis rate was quite high in alcohol, statin and alcohol+statin groups compared to control group and this increase was signifi cant (p < 0.001). Necrosis rate was moderately high in statin+betaine and alcohol+betaine group compared to control group (p < 0.046). Necrosis in the alcohol + statin group was signifi cantly increased compared to alcohol and statin groups. Necrosis rate was quite signifi cantly decreased in alcohol+statin+betain group compared to alcohol and statin groups. Necrosis in the statin+betaine group was signifi cantly reduced compared to statin groups (p < 0.046). Necrosis rate was quite high in alcohol+statin group compared to statin+betaine and alcohol+betaine group and this increase was signifi cant (p < 0.001) (Tab. 2).
Myofibrillar degeneration in the alcohol, statin and alcohol+statin groups were increased quite signifi cantly com-pared to control group (p < 0.001). Myofi brillar degeneration in the alcohol+statin group was increased quite signifi cantly (p < 0.001), alcohol+betaine and alcohol+statin+betaine groups were quite signifi cantly decreased compared with alcohol group (p < 0.001). Myofi brillar degeneration in the alcohol+statin group was quite
Fig. 2. Light microscope results in rat groups (a-g). e) Alcohol+betaine group: (→) Located peripheral nuclei (HE, scale bar: 100 μm, X20, scale bar: 50.0 μm, X40). f) Statin+betaine group: (→) Located peripheral nuclei (HE, scale bar: 100 μm, X20, scale bar: 50.0 μm, X40). g) Alcohol+statin+betaine group: (→) Located peripheral nuclei (HE, scale bar: 100 μm, X20, scale bar: 50.0 μm, X40).
e
f
signifi cantly increased (p < 0.001), whereas in the alcohol+betaine and alcohol+statin+betaine signifi cantly decreased compared to statin group (respectively p < 0.010 and p < 0.001). Myofi brillar de-generation in the alcohol+statin+betaine group was decreased quite signifi cantly compared to alcohol+statin group (p < 0.001) (Tab. 2). Nuclear proliferation in myofi brills in the alcohol, statin, alcohol+statin and statin+betaine groups were increased quite sig-nifi cantly compared to control group (p < 0.001). Compared with alcohol group, nuclear proliferation was increased statistically sig-nifi cantly in alcohol+statin group (p < 0.001). A sigsig-nifi cant decrease
was seen in statin+betaine, alcohol+betaine and alcohol+statin+betaine groups (re-spectively p < 0.05, p < 0.05, p < 0.001). There was a signifi cant increase in the statin group compared to the alcohol+statin group (p < 0.001). This increase in proli-feration was decreased signifi cantly in the alcohol+statin+ betaine group (p < 0.001). A signifi cant decrease was observed in the alcohol+statin+ betaine group compared to the statin+betaine group (p <0.001). Compared with alcohol+statin group, a signifi -cant decrease was found in alcohol+betaine and alcohol+statin+betaine groups (p < 0.001) (Tab. 2).
Hypertrophy in alcohol, statin, statin+betaine, alcohol+statin and alcohol + betaine groups was increased quite sig-nifi cantly compared to control group (re-spectively p < 0.001, p < 0.001, p < 0.046, p < 0.001, p < 0.001). A signifi cant decrease was found in the alcohol+statin+betaine group (p < 0.001), whereas a signifi cant increase was observed in the alcohol+statin group compared to alcohol and statin groups (p < 0.001). Hypertrophy in the al-cohol + statin group was signifi cantly high-er than in the alcohol + betaine group (p < 0.001). Compared to alcohol + statin group, hypertrophy in the alcohol+statin+betaine groups was decreased statistically signifi -cantly (p < 0.046) (Tab. 2).
Edema in connective tissue surround-ing myofi brils of statin, alcohol and al-cohol + statin groups was quite statisti-cally higher than in the control group (p < 0.001). Statin + betaine and alcohol + be-taine groups showed a signifi cant increase in edema compared to the control group (p < 0.010) (Tab. 2).
Edema in alcohol + statin group was statistically signifi cantly higher than in the alcohol and statin groups (p < 0.001), but
Bax Control 0.20 (0.00–1.00) Alcohol 1.30 (1.00–2.00) a Statin 1.30 (1.00–2.00) a Alcohol+Statin 2.90(2.00–3.00) a.c.d Alcohol+Betaine 0.10 (0.00–1.00) c Statin+Betaine 0.80 (0.00–1.00) b Alcohol+Statin+Betaine 0.80 (0.00–1.00) b.e
a: Compared with control group p < 0.05, b: Compared with control group p < 0.05, c: Compared with alcohol group p < 0.05, d: Compared with statin+betaine group p < 0.05, e:Compared with alcohol+statin group p < 0.05
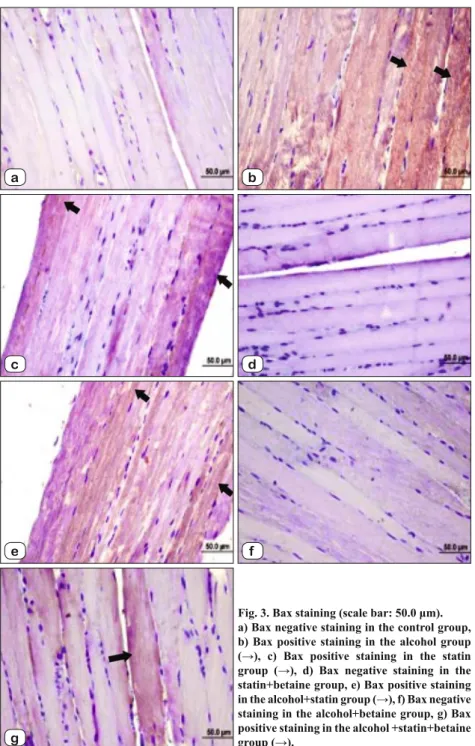
Tab. 3. Bax levels. Fig. 3. Bax staining (scale bar: 50.0 μm). a) Bax negative staining in the control group, b) Bax positive staining in the alcohol group (→), c) Bax positive staining in the statin group (→), d) Bax negative staining in the statin+betaine group, e) Bax positive staining in the alcohol+statin group (→), f) Bax negative staining in the alcohol+betaine group, g) Bax positive staining in the alcohol +statin+betaine group (→). a c e b d f g
this increase was signifi cantly reduced in the alcohol+statin+ betaine group (p < 0.001). The edema in the alcohol+statin group was signifi cantly higher than in the alcohol +betaine and alcohol+statin+betain groups (p < 0.001) (Tab. 2).
Bax Levels: When bax levels were examined in skeletal muscle specimens constituting all groups, there was a progressive increase in alcohol, statin, alcohol+statin groups (p < 0.001), a statistically signifi cant increase in statin+betaine and alcohol+statin+betaine groups (p < 0.046). There was a signifi cant increase in alcohol+statin group compared to alcohol group (p < 0.001), this increase was signifi cantly reduced in the alcohol+betaine group (p < 0.001). Bax levels in alcohol + statin group were higher than in the statin+ betaine group (p < 0.001). Statistically signifi cant reduction in the
alcohol+statin+betaine group was observed when compared with the alcohol + statin group (p < 0.001) (Tab. 3, Fig. 2).
Bcl-2 Levels: Bcl-2 levels were ex-amined in skeletal muscle samples con-stituting all groups and it was found low in alcohol and statin groups and high in alcohol+ statin+betaine group when com-pared with control group (p < 0.001). Bcl levels were high whereas it was low in alcohol + betaine group compared to al-cohol group (p < 0.001). Bcl-2 levels in alcohol+statin and statin+betaine groups were lower than in the statin group (p < 0.047). A signifi cant increase was seen in the alcohol+statin+betain group compared with the statin group (p < 0.001) (Tab. 4, Fig. 3).
TUNEL results: Skeletal muscle sam-ples were stained with TUNEL method and brown stained samples were evalu-ated as TUNEL (+). TUNEL values were increased in skeletal muscle myopathy caused by alcohol, statin and combine us-ing of alcohol+statin. Also this increased TUNEL values were decreased in the alcohol+statin+betaine group (Fig. 4). Discussion
Excessive alcohol consumption and alcohol-related problems are also a major public health problem and result in large proportions of mortality and morbidity. Many factors infl uence the development of alcohol-related problems (26, 27). One of the most common causes of myopathy is the use of drugs from the statin group (7). The most commonly used antihyperlipid-emic drugs are statins, HMG CoA reduc-tase inhibitors, due to theit high activity and low side effect profi le (28). Tiredness, weakness symptoms and myopathy are the most common side effect of statin drugs (7). It is estimated that at least 1.5 million a c e b d f g
Fig. 4. Bcl-2 staining (scale bar:50.0 μm). a) Bcl-2 positive staining in the control group (→). b) Bcl-2 negative staining in the alcohol group. c) Bcl-2 negative staining in the statin group. d) Bcl-2 positive intense staining in the betaine group (→). e) Bcl-2 negative staining in the alcohol+statin group. f) Bcl-2 positive light staining in the ethonol+betaine group (→). g) Bcl-2 positive staining in the alcohol + statin+betaine group (→). Bcl–2 Control 1.40 (1.00–2.00) Alcohol 0.20 (0.00–1.00) a Statin 0.10 (0.00–1.00) a Alcohol+Statin 0.80 (0.00–1.00) c Alcohol+Betaine 1.00 (0.00–2.00) b Statin+Betaine 0.80 (0.00–1.00) c Alcohol+Statin+Betaine 2.70 (2.00–3.00) a,b,c,d
a: Compared with control group p < 0.05, b: Compared with alcohol group p < 0.05, c: Compared with statin group p < 0.05, d: Compared with alcohol+statin+betaine group p < 0.05
people have muscle complaints related to statin use annually (29). An increase in creatine kinase levels biochemically is observed in statin-bound myotoxicity (30). In our study, creatinine kinase levels were increased in alcohol and statin treated groups. Pasternak and colleagues concluded that the combined use of ethanol and statin in the study was an increasing risk factor for myopathy (31). In paral-lel with this study, in our study the greatest increase in creatine ki-nase levels was also noted in the group in which alcohol and statin were given together. However, Studies have also shown normal creatine kinase levels in statin-dependent myotoxicity (32, 33). In our study creatine kinase levels were increased in the alcohol and statin given groups. In previous study, Pasternak and colleagues have argued that the combined use of ethanol and statin represents
an increased risk for the formation of myo-pathy (31). Paralleling this study, increase in creatine kinase levels was observed in combining of alcohol and statin group. It is known that long term alcohol use has an apoptosis-initiation effect by im-pairing mitochondrial permeability. In this way, cytochrome c is released into the cy-tosol and binds with Apaf-1 and activated caspase-9. The proteolytic activity of cas-pase-9 activates caspase-3 and apoptosis is initiated (34). In our study to determine the effect of statin and alcohol use on muscle, we measured the release of mitochondrial cytochrome c into the cytosol. We also measured the amount of cytochrome c re-maining in the mitochondria and estimated cytosol to mitochondria ratio. Because of this ratio, as the cytochrome c content de-creases in the mitochondrial fraction when the cytosol penetration increases, the ratio between them also increases remarkably. As in earlier studies, this rate represents mitochondrial damage (35). In our study this ratio increased in alcohol, statin, and alcohol + statin groups, but the highest in-crease was seen in alcohol + statin group. These results have also affected the re-sults of caspase-3, since cytosol-releasing cytochrome c is involved in the caspase-9 activation necessary for the activation of caspase-3 (36). In parallel with our cyto-chrome c results, the highest increase was in our alcohol + statin group. We attributed the highest cytrochrome c and caspase 3 results in the alcohol + statin group to the effect of apoptosis both using alcohol and statin separately. Human and rodent studies have shown that statin administration in-duced apoptosis in the skeleton (37, 38, 39). The use of statin causes an increase in cytosolic calcium resulting in a change in synthesis of isoprenoid. Increased levels of calcium also cause ac-tivation of the calpains. Calpains generate apoptosis by initiating caspase cascade (7). In our study, there was a statistically signifi cant increase in the statin group compared to the control group. Rajgopal et al. demonstrated that calpains are active in cell death caused by ethanol (40). In our study, calpain levels of alcohol and alcohol + statin group had a signifi cant increase compared to control group.
Studies have shown that there is a decrease in the ratio of Bcl2/ Bax that causes cytochrome c release in statin-induced apoptosis in skeletal muscle (7). Our results of our Bcl-2/Bax in our histo-logy fi ndings support this decrease. In another study; an increase in TUNEL values was observed in skeletal muscle myopathy caused by alcohol (41). a c e b d f g
Fig. 4. TUNEL results.
a) TUNEL negative staining in the control group, b) TUNEL positive staining in the al-cohol group (→), c) TUNEL positive staining in the statin group (→), d) TUNEL negative staining in the betain group, e) TUNEL posi-tive staining in the alcohol+statin group (→), f) TUNEL positive staining in the alcohol+betain group (→), g) TUNEL negative staining in the alcohol+statin+betain group.
Histological examinations of our study showed that, necrotic myofi brils and intense nuclear proliferation are evident in alcohol and statin groups, while partial edema, vacuolization, centrally located nuclei, nuclear proliferation in the myofi brils and ne-crotic myofi brils in the alcohol+statin group in association with these were shown previous studies. Alcohol +betaine+statin group showed decreased myofi bril damage and muscular cells in histo-logical structure near normal cells.
Betaine is lipotropic and passes through the lipid layer quickly when given externally and inhibits lipid peroxidation in cellular membranes. The addition of betaine to protect against necrotic damage is shown in a study by Kim et al (42). As in our previous study with betaine (43), in this study, betaine-treated treatment groups showed that calpain and caspase activities and cytokine c release were decreased caused by alcohol, statin and more im-portantly alcohol + statin, and TNF and NF kB levels were also close to the control group.
Similarly, signifi cant improvements have been observed in our morphological and histological examination results that are also supported by our biochemical data.
In conclusion, our fi ndings in this experimental study sug-gested that alcohol and statin combined consumption increase apoptotic cellular degeneration in the skeletal muscles and follow-ing betaine treatments can cause a decrease in this apoptotic dam-age. We have found protective effi cacy of betaine and believe that these fi ndings will be a stepping stone for future studies. Further studies are also needed about the possible effects of application of this supplement.
References
1. Yilmaz S. İlaca bağli miyopatiler. Uludağ Üniversitesi Tip Fakültesi
Dergisi. 2010; 34 (3): 129-133.
2. Alkiş K. Hiperkolesterolemi oluşturulmuş farelerde kefi rin ve statin
içerikli ilaçlarin kolesterol üzerinde etkilerinin araştirilmasi. Kafkas Üni-versitesi, Fen Bilimleri Enstitüsü, Biyoloji Anabilim Dali, Yüksek Lisans Tezi, Kars, 2009.
3. Öztürk GT. Atorvastatin kullanan hastalarda kas performasinin
izo-kinetik test ile değerlendirilmesi. Gazi Üniversitesi Tip Fakültesi Fizik-sel Tip Ve Rehabilitasyon Anabilim Dali, Uzmanlik Tezi, Ankara, 2010.
4. Solakoğlu Z. Apoptoz varliği ya da yokluğu bir hastalik nedeni. İstanbul
Üniversitesi, İstanbul Tip Fakültesi, Fizyoloji Anabilim Dali, İstanbul, 2010.
5. Liu L, Sakai T, Sano N, Fukui K. Nucling mediates apoptosis by
in-hibiting expression of galectin-3 through interference with nuclear factor kB signaling. Biochemical Journal 2004; 380: 31–41.
6. Yanmaz MT. Kolorektal kanserde epidermal büyüme faktörü reseptörü
ve nükleer factor kappa b ekspresyonunun prognoza etkisi. İstanbul Üniver-sitesi, Cerrahpaşa Tip Fakültesi, Tibbi Onkoloji Abd, Uzmanlik Tezi, 2006.
7. Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy.
American Journal of Physiology-Cell Physiology 2006; 291: 1208–1212.
8. Aslan A. Ratlarda azoksimetan uygulanarak oluşturulan kolorektal
kan-serde likopenin siklooksijenaz-2 (cox-2), kaspaz-3, kaspaz-9, bax, bcl-2, p53 proteinlerinin ekspresyonu ve DNA hasari üzerine etkisi. Firat Üni-versitesi Fen Bilimleri Enstitüsü, Doktora Tezi, 2011.
9. Binen M. Kalpain inhibitörü olan AK295‟ in siçanlarda renal
iskemi-reperfüzyon hasarinda kaspaz 3’e olan etkisinin araştirilmasi. İnönü Üni-versitesi Fen Bilimleri Enstitüsü, Yüksek Lisans Tezi Biyoloji Anabilim Dali Malatya, 2011.
10. Kar P, Samanta K, Shaikh S, Chowdhury A, Chakraborti T, Chakraborti S. Mitochondrial calpain system: An overview. Archives
Of Biochemistry And Biophysics 2010; 495:1-7.
11. Kuralay F, Çavdar Z. İnfl amatuar medyatörlere toplu bir bakiş. Genel
Tip Dergisi 2006; 16, 3, 143-152..
12. Fernández-Solà J, Nicolás JM, Fatjó F, García G, Sacanella E, Estruch R et al. Evidence of apoptosis in chronic alcoholic skeletal
my-opathy. Human Pathology 2003; 34, 12.
13. Fernandez-Solà J, Preedy VR, Lang CH, Gonzalez-Reimers E, Arno M, Lin JC. Molecular and cellular events in alcohol-induced muscle
disease. Alcoholism: Clinical and Experimental Research 2007; 31, 12.
14. Gill C, Mestril R, Smali A. Losing heart: the role of apoptosis in
heart disease--a novel therapeutic target?. The FASEB Journal 2002; 16 (2): 135-46.
15. Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: the
role of cytochrome c. Biochimica et Biophysica Acta 1998;1366:139-149.
16. Craig SA. Betaine in human nutrition. The American Journal of
Clini-cal Nutrition 2004; 80: 539–549.
17. Welch WC, Brown CR. Infl uence of molecular and chemical
chaper-ones on protein folding. Cell Stress Chaperchaper-ones 1996; 1: 109-115.
18. Ratter F, Germer M, Fischbach T, Schulze-Osthoff K, Peter ME, Dröge W et al. S-Adenosylhomocysteine as a physiological
modu-lator of apo-1-mediated apoptosis. International Immunology 1996; 8:1139–47.
19. Galan AI, Munoz ME, Jimenez R. S-Adenosylmethionine protects
against cyclosporin a-induced alterations in rat liver plasma membrane fl uidity and functions. Journal of Pharmacology and Experimental Thera-peutics 1999; 290: 774-781.
20. Baysan O, Kaptan K, Erinç K, Oztas Y, Coskun T, Kayir H et al.
Chronic heavy alcohol consumption is associated with decreased platelet aggregation in rats. The Tohoku Journal of Experimental Medicine 2005; 206: 85-90.
21. Erinç K, Barçin C, Ozsoy N,Oztaş E, Gül N, Sağ C et al. Effects of
chronic alcohol consumption on myocardial ischemia in rats. Pharmaco-logical Research 2003; 47:175–180.
22. Zovein A, Flowers-Ziegler J, Thamotharan S, Shin D, San-kar R, Nguyen K et al. Postnatal hypoxic-ischemic brain injury alters
mechanisms mediating neuronal glucose transport. American journal of physiology,Regulatory, integrative and comparative physiology 2004; 286 (2): 273-282.
23. Soeda J, Miyagawa S, Sano K, Masumoto J, Taniguchi S, Kawasaki S. Cytochrome c release into cytosol with subsequent caspase activation
during warm ischemia in rat live. American Journal of Physiology-Gas-trointestinal and Liver Physiology 2001; 281 (4): 1115-1123.
24. Grunnet LG, Aikin R, Tonnesen MF,Paraskevas S, Blaabjerg L, Størling J et al. Proinfl ammatory cytokines activate the intrinsic apoptotic
pathway in beta-cells. Diabetes 2009; 58, 8.
25. McDonald MC, Mota-Filipe H, Paul A, Cuzzocrea S, Abdelrahman M, Harwood S et al. Calpain inhibitor I reduces the activation of nuclear
factor-kb and organ injury/dysfunction in hemorrhagic shock. The FASEB Journal 2001; 15 (1): 171-186.
26. Nguyen VA, Le T, Tong M, Silbermann E, Gundogan F, M. de la Monte S et al. Impaired insulin/igf signaling in experimental
alcohol-related myopathy. Nutrients 2012; 4, 1058-1075.
27. Akvardar Y, Uçku R. Alkol kullanim sorunlari nasil önlenir? Alkol
kullanim bozukluklarinin tani ve tedavisinde kisa müdahale yaklaşimi. Anadolu Psikiyatri Dergisi 2010;11 (1): 51-59.
28. Etli M. Hemodiyaliz amaçli açilan arterio-venöz fi stüllerde gelişen
endotel disfonksiyonuna statinlerin etkisi. Süleyman Demirel Üniversitesi Tip Fakültesi, Uzmanlik Tezi Kalp Ve Damar Cerrahisi Anabilim Dali, Isparta, 2011.
29. Taşoğlu, Ö. Miyaljide D vitamini ve statin ilişkisi: Statin kullanan
hastalarda D vitamini eksikliği miyalji gelişimi içi bir risk faktörü müdür?. Hacettepe Üniversitesi Tip Fakültesi Fiziksel Tip Ve Rehabilitasyo Anabil-im Dali, Ankara, 2012.
30. Tomaszewski M, Stepien MK, Tomaszewska J, Czuczwar SJ.
Statin-induced myopathies. Pharmacological Reports 2011; 63 (4): 859-66.
31. Pasternak RC, SmithJr SC, C. Noel Bairey-Merz, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI clinical advisory on the use
and safety of statins. Circulation 2002; 106, 1024-1028.
32. Bennett WE, Drake AJ, Shakir KM. Reversible myopathy after statin
therapy in patients with normal creatine kinase levels. Annals of Internal Medicine 2003; 138, 436–437.
33. Phillips PS, Haas RH, Bannykh S. Statin associated myopathy with
normal creatine kinase levels. Annals of Internal Medicine 2002; 137, 581-585.
34. Higuchi H, Adachi M, Miura S, Gores GJ, Ishii H. The
mitochon-drial permeability transition contributes to acute ethanol-induced apoptosis in rat hepatocytes. Hepatology 2001; 34(2): 320–328.
35. Burgess DH, Svensson M, Dandrea T, Grönlund K, Hammarquist F, Orrenius S et al. Human Skeletal muscle cytosols are refractory to
cy-tochrome c dependent activation of type-II caspases and lack apaf-1. Cell Death Differ 1999; 6, 256–261.
36. Gill C, Mestril R, Samali A. Losing heart: The role of apoptosis in
heart disease—a novel therapeutic target. The FASEB Journal 2002; 16.
37. Kobayashi M, Kaido F, Kagawa T, Itagaki S, Hirano T, Iseki K.
Preventive effects of bicarbonate on cerivastatin-induced apoptosis. Inter-national Journal of Pharmaceutics 2007; 341, 181-188.
38. Kaufmann P, Török M, Zahno A, Waldhauser KM, Brecht K, Krähenbühl S. Toxicity of statins on rat skeletal muscle mitochondria.
Cellular and Molecular Life Sciences 2006; 63, 2415-2425.
39. Demyanets S, Kaun C, Pfaffenberger S, Hohensinner PJ, Rega G, Pamme J et al. Hydroxymethylglutaryl-coenzyme A reductase inhibitors
induce apoptosis in human cardiac myocytes in vitro. Biochemical Phar-macology 2002; 71, 1324-1330.
40. Rajgopal Y, Vemuri MC. Calpain activation and a-spectrin cleavage
in rat brain byethanol. Neuroscience Letters 2002; 321, 187–191.
41. Fernández-Solà J, Nicolás JM, Fatjó F, García G, Sacanella E, Estruch R et al. Evidence of apoptosis in chronic alcoholic skeletal
my-opathy. Human Pathology 2003; 34 (12): 1247-1252.
42. Kim SK, Kim YC. Effects of singly administered betaine on
hepa-totoxicity of chloroform in mice. Food and Chemical Toxicology 1998; 36, 655–661.
43. Ol KK, Kanbak G, Ilhan AO, Burukoglu D, Yücel F. The
investiga-tion of the prenatal and postnatal alcohol exposure-induced neurodegen-eration in rat brain: protection by betaine and/or omega-3. Childs Nerv Syst 2016; 32:467–474.
Received January 14, 2020. Accepted February 28, 2020.