https://doi.org/10.1007/s10157-019-01782-x
ORIGINAL ARTICLE
Nondipping heart rate and associated factors in patients with chronic
kidney disease
Zeynep Biyik1 · Yasemin Coskun Yavuz1 · Lütfullah Altintepe1 · Gulperi Celik1 · Ibrahim Guney2 · Sevıl Fısekcı Oktar3
Received: 5 April 2019 / Accepted: 16 August 2019 / Published online: 3 September 2019 © Japanese Society of Nephrology 2019
Abstract
Background Nondipping heart rate (NHR) is a condition reported to be associated with cardiovascular events and cardio-vascular mortality recently. We aimed to search whether there is difference among hypertensive patients with and without chronic kidney disease (CKD) in terms of NHR pattern and the factors associated with NHR in patients with CKD.
Methods The study included 133 hypertensive patients with normal kidney functions, 97 hypertensive patients with pre-dialysis CKD, and 31 hypertensive hemopre-dialysis patients. Heart rate, blood pressure and pulse wave velocity (PWV) were measured by 24-h ambulatory blood pressure monitorization. NHR was defined as a decrease of less than 10% at night mean heart rate when compared with daytime values.
Results NHR pattern was established as 26.3% in non-CKD hypertensive group, 43.3% in predialysis group and 77.4% in dialysis group. Among patients with CKD, when NHR group was compared with dipper heart rate group, it was seen that they were at older age, there were higher prevalence of diabetes mellitus and more female sex, and while the value of urea, creatinine, phosphorus, intact parathyroid hormone, and PWV were significantly higher, the value of hemoglobin, albumin and calcium were significantly lower. By multivariate analysis, hemoglobin [odds ratio (OR) 0.661; 95% CI 0.541–0.806; p < 0.001] and PWV (OR 1.433; 95% CI 1.107–1.853; p = 0.006) were established as independent determinants of NHR pattern.
Conclusions NHR pattern is significantly more frequently seen in hypertensive CKD patients than in hypertensive patients with non-CKD. Anemia and increased arterial stiffness are seen independently associated with NHR in CKD patients.
Keywords Chronic kidney disease · Nondipping heart rate · Hypertension
Introduction
Cardiovascular deaths are the most common cause of mor-tality in both hemodialysis patients and predialysis patients with chronic kidney disease (CKD). Of predialysis CKD patients, 45% die before developing an end-stage renal insuf-ficiency [1]. The exact cause of the high cardiovascular mor-tality in this patient group has not been clearly established,
yet. However, the presence of a high heart rate is suggested to be an important risk factor.
In the general population, the presence of a higher rest-ing heart rate has been shown to be a predictor for mortality [2]. In a study including 460 patients with CKD, having a glomerular filtration rate of < 60 mL/min, the presence of a high resting heart rate has been found to be associated with increased risk for cardiovascular events and mortality [3].
Similar to blood pressure, heart rate can also show fluc-tuations during the day. While heart rate increases in the morning as a consequence of neurohumoral activation, at night, both blood pressure and heart rate normally decrease by 10–20% due to the effect of the parasympathetic sys-tem, which becomes more dominant nocturnally [4]. The circadian rhythm, nondipping heart rate (NHR), in which this decrease is not seen, reflects that there is an insufficient reduction in sympathetic activity at night and is associated with increased cardiovascular events [5].
* Zeynep Biyik
drzeynepbiyik@gmail.com
1 Department of Internal Medicine, Division of Nephrology, Selcuk University Faculty of Medicine, Selçuklu, Konya, Turkey
2 Department of Nephrology, Konya Education and Research Hospital, Meram, Konya, Turkey
Nondipper blood pressure is currently considered a car-diovascular risk factor, but nondipping heart rate is over-looked. In a study conducted in the general population involving 1444 patients, who were followed up for 12 years, day–night heart rate dip ratio was found to be associated with all-cause mortality. In addition, neither day nor night heart rate was found to be associated with cardiovascular mortality in this study [6]. In another study, Cuspidi et al. followed up 2021 patients in the general population for 148 months and showed that NHR predicted fatal and non-fatal cardiovascular events independent of some confound-ing factors [7].
Similar findings have been reported in the literature in the hypertensive patient population. Eguchi et al. reported that in a hypertensive patient population, they evaluated NHR with ambulatory blood pressure monitorization (ABPM) demonstrating that it was a predictor of cardiovascular events and that it was associated with a 2.4-fold increase in the risk of developing cardiovascular disease [8]. In the diabetic patient population, the NHR was found to be inde-pendently related to the albumin–creatinine ratio and the platelet count [9].
Resting heart rate or nocturnal heart rate was evaluated in the studies conducted more frequently on CKD patients [3,
10, 11]. However, to the best of our knowledge, there are no studies in the literature evaluating the prevalence of NHR in patients with CKD. In our study, we aimed to investigate the prevalence of nondipping heart rate and its associated factors in both the predialysis CKD patients and in hemo-dialysis patients.
Materials and methods
Patients
We conducted a cross-sectional study. Patients, who were admitted to the nephrology outpatient clinic of Selcuk Uni-versity Medical Faculty in the period between January 2017 and January 2019, and who underwent a 24-h blood pres-sure monitoring were screened retrospectively. Patients with normal blood pressure were excluded from the study based on the results of 24-h ABPM. Patients taking antihyperten-sive drugs and patients with a mean systolic blood pressure (SBP) equal to or above 130 mmHg or a mean diastolic blood pressure (DBP) equal to or above 80 mmHg based on the results of ABPM were included in the study.
Evaluation methods
For the 24-h blood pressure and pulse wave velocity (PWV) measurements of the patients, a brachial cuff-based oscillometric device Mobil-O-Graph (IEM, Stolberg,
Germany) was used. This device has already been tested and validated with both invasive and noninvasive meas-urements in different populations including end-stage renal disease (ESRD) [12, 13]. In the literature, the pulse rate obtained from oscillometric devices has been dem-onstrated to approximate the accuracy and reliability of measures obtained by the available standard methods [14]. The patients included in our study had their blood pres-sure and heart rate meapres-surements with this device, per-formed at 15-min intervals during the day (08:00–24:00) and at 30-min intervals during the night (24:00–08:00). These measurements were used for deriving the mean sys-tolic blood pressure, the mean diassys-tolic blood pressure, the mean arterial blood pressure, and the pulse pressure analyzes during the day, the night, and during the 24 h in a day and to derive the mean heart rate during the day and night. Patients having valid measurements less than 80% were excluded from the study. In addition, pregnant women, patients taking drugs that can affect the heart rate (beta blockers or nondihydropyridine calcium channel blockers, digitalis, beta agonist bronchodilators); patients having any of the following conditions including hypothy-roidism or hyperthyhypothy-roidism, arrhythmia, and acute kidney injury in the last 3 months; renal transplant patients, and patients with missing data were excluded from the study. Among the eligible patients, the following were excluded including 3 pregnant women, 112 patients regularly taking drugs with a potential to affect the heart rate, 26 patients with thyroid gland dysfunction, 15 patients with arrhyth-mia, 5 patients with acute kidney injury developing in the last three months, 4 renal transplant patients, 25 patients with missing data, and 34 patients with invalid ABPM. As a result, a total of 261 patients were included in the study.
History of comorbid diseases, regularly used medica-tions, levels of hemoglobin, levels of glucose after an over-night fasting, the levels of lipids, urea, creatinine, albumin, uric acid, calcium, phosphorus, intact parathyroid hormone (iPTH) in the blood and the levels of spot protein and spot creatinine in the urine were obtained from the medical records of the patients. The body mass index (BMI) of the patients was calculated with the following formula: weight/ height2 (kg/m2).
All hemodialysis patients underwent hemodialysis three days a week for 4 h at a blood flow rate of 250–300 mL/ min with a dialysate flow of 500 mL/min. The hemodialysis patients were included in the study if they had undergone hemodialysis at least in the last 3 months before the start of the study period. In hemodialysis patients, ABPM val-ues on the days of the week other than the days of dialysis were included in the study to exclude the potential effects of dialysis and fluid removal on the heart rate. The blood levels of the parameters collected from the patients prior to the dialysis sessions were included in the study.
The estimated glomerular filtration rate (eGFR) measure-ments of the patients were calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) for-mula [15]. Nondipper heart rate was defined as the result-ing value of < 0.1 derived from the formula: (daytime heart rate − nighttime heart rate)/daytime heart rate, according to a previous study. Nondipper blood pressure was defined as the resulting value of < 0.1 derived from the formula: (daytime systolic blood pressure − nighttime systolic blood pressure)/daytime systolic blood pressure, according to a previous study [16]. All hypertensive patients were divided into groups according to their renal functions. The KDIGO (Kidney Disease Improving Global Outcomes) 2012 clini-cal practice guideline was used to define and stage CKD [17]. CKD is defined as abnormalities in kidney structure or function, present for 3 months, with implications for health. Having one or more of the following criteria for more than 3 months was accepted as CKD: GFR of < 60 mL/min per 1.73m2 or albumin-to-creatinine ratio ≥ 30 mg/g or
abnor-malities detected by histological examination or structural abnormalities detected by imaging methods or urine sedi-ment abnormalities or abnormalities associated with tubular disorders including abnormal levels of electrolytes.
The hypertensive patients were divided into three groups as patients with normal renal function, predialysis CKD patients, and hemodialysis patients. In these study groups, the daytime heart rate, the nighttime heart rate, and the 24-h heart rate were measured with ABPM and the results were compared. Furthermore, NHR ratios and nondipper hyper-tension ratios were compared.
Statistical analysis
Kolmogorov–Smirnov test was used to test the normal distribution. Continuous variables were expressed as mean ± standard deviation, whereas non-normally distrib-uted continuous variables were expressed as median (inter-quartile range). Categorical data were presented in percent-ages. For the statistical analyses, the Chi-square test was used for analyzing the categorical variables, the t test or one way analysis of variance was used for analyzing the continuous variables, and the Mann–Whitney U test or the Kruskal–Wallis test was used for analyzing the non-nor-mally distributed data. The Jonckheere–Terpstra trend test (multiple-group comparisons) was used for comparing the non-normally distributed numerical data and intergroup dif-ferences. The Cochran–Armitage trend test (multiple-group comparisons) was used for comparing the categorical vari-ables. To analyze the correlation among the variables, the Pearson’s correlation analysis was used for the parametric data and the Spearman’s correlation analysis was used for the nonparametric data. Multivariate regression analysis was performed to determine the independent predictors of
developing a nondipping heart rate. To perform this sis, variables with a p value of < 0.1 in the univariate analy-sis were included in the multivariate analyanaly-sis. A p value of < 0.05 was considered to be significant. SPSS 22 (IBM Corp., SPSS, NY, USA) program was used for statistical analysis.
Results
A total of 261 hypertensive patients who met the eligibility criteria were included in the study. Of these, 133 had nor-mal functioning of the kidneys, 97 were predialysis CKD patients, and 31 underwent hemodialysis. Of the 97 predi-alysis patients, 22 patients were classified as CKD stage 1, 32 patients were classified as CKD stage 2, 18 patients as CKD stage 3, 15 patients as CKD stage 4, and 10 patients as CKD stage 5. The study population was composed of males at a rate of 52.8%. The rate of diabetic patients in the study population was 25.6%. The mean age in the study population was 51.3 ± 14.2 years. There were no differences in gender distribution, the mean age, and the distribution of diabetic patients among the groups. Demographic and laboratory features of the patients by the groups are shown in Table 1.
The mean daytime heart rate was 78.7 ± 10.6 in the non-CKD hypertension group, 80.7 ± 11.8 in the pre-dialysis group, and 84.8 ± 11.2 in the hemodialysis group. The mean night heart rate was 67.6 ± 9.4 in the non-CKD hypertension group, 71.6 ± 11.6 in the predialysis group, and 80.1 ± 11.9 in the hemodialysis group. As the renal function worsened, both the daytime (p = 0.020) and the nighttime heart rate (p < 0.001) were significantly increased.
The proportion of the patients showing an NHR pat-tern was 26.3% among the non-CKD hypertensive patients, 43.3% in predialysis CKD group, and 77.4% in the hemodi-alysis group. The prevalence of the NHR pattern was signifi-cantly increased as renal failure progressed (Fig. 1). How-ever, this was not the case for nondipping hypertension. The proportion of the patients with nondipping hypertension was 69.9% among the non-CKD hypertensive patients, 73.2% in the predialysis CKD group, and 83.9% in the hemodialysis group. No significant differences in the prevalence of non-dipping hypertension were found among these three groups (p = 0.143).
A correlation analysis was performed to determine the parameters associated with nocturnal heart rate decline. Nocturnal heart rate decline demonstrated a significantly negative correlation with the age (r = − 0.230, p < 0.001), and the levels of urea (r = − 0.334, p < 0.002), creatinine (r = − 0.237, p < 0.001), phosphorus (r = − 0.224, p = 0.001), urine protein/creatinine ratio (r = − 255, p = 0.001), PWV (r = − 0.216, p = 0.001), and iPTH (r = − 0.331, p < 0.001); whereas it showed a significantly positive correlation
with the levels of albumin (r = 0.363, p < 0.001), calcium (r = 0.316, p < 0.001), and hemoglobin (r = 0.386, p < 0.001). We assigned the CKD patients (predialysis and hemodial-ysis patients) in our study to two groups as the patients with a dipping heart rate (DHR) in one group and the patients with NHR in the other. The NHR group was at an older age (57.7 ± 15.8 years vs. 47.5 ± 13.5 years; p < 0.001), had a higher prevalence of diabetes mellitus (36.3% vs. 17.7%; p = 0.006), and a high rate of female patients (48.5% vs. 32.2%) compared to the DHR group. In the NHR group, the nighttime SBP (133.8 ± 21.3 vs. 126.8 ± 16.7; p = 0.041) and the nighttime heart rate (78.5 ± 11.9 vs. 68.5 ± 10.2; p < 0.001) were significantly higher compared to the DHR group. The analysis of the laboratory test results dem-onstrated that the levels of urea (p < 0.001), creatinine
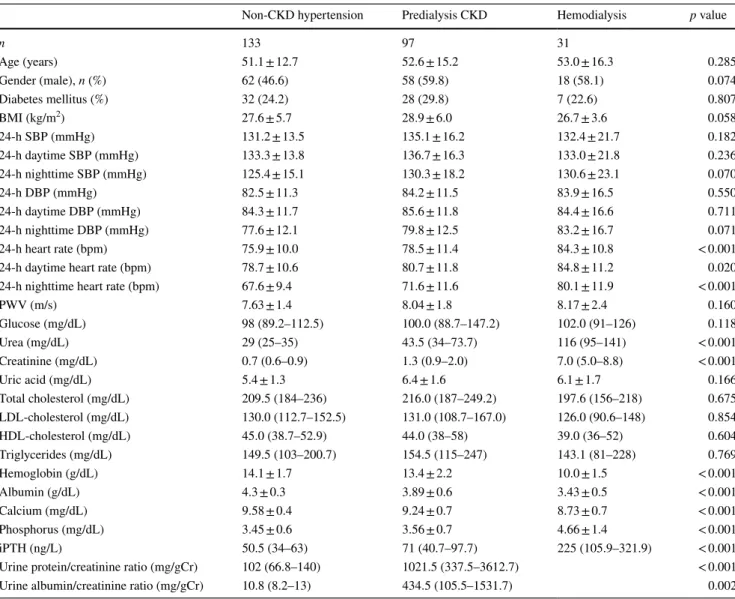
Table 1 Demographic and laboratory data of the study population
Data are presented as mean ± standard deviation or median (interquartile range) or n (%)
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, iPTH intact parathyroid hormon, LDL low-density lipoprotein, HDL high-density lipoprotein, PWV pulse wave velocity
Non-CKD hypertension Predialysis CKD Hemodialysis p value
n 133 97 31 Age (years) 51.1 ± 12.7 52.6 ± 15.2 53.0 ± 16.3 0.285 Gender (male), n (%) 62 (46.6) 58 (59.8) 18 (58.1) 0.074 Diabetes mellitus (%) 32 (24.2) 28 (29.8) 7 (22.6) 0.807 BMI (kg/m2) 27.6 ± 5.7 28.9 ± 6.0 26.7 ± 3.6 0.058 24-h SBP (mmHg) 131.2 ± 13.5 135.1 ± 16.2 132.4 ± 21.7 0.182 24-h daytime SBP (mmHg) 133.3 ± 13.8 136.7 ± 16.3 133.0 ± 21.8 0.236 24-h nighttime SBP (mmHg) 125.4 ± 15.1 130.3 ± 18.2 130.6 ± 23.1 0.070 24-h DBP (mmHg) 82.5 ± 11.3 84.2 ± 11.5 83.9 ± 16.5 0.550 24-h daytime DBP (mmHg) 84.3 ± 11.7 85.6 ± 11.8 84.4 ± 16.6 0.711 24-h nighttime DBP (mmHg) 77.6 ± 12.1 79.8 ± 12.5 83.2 ± 16.7 0.071 24-h heart rate (bpm) 75.9 ± 10.0 78.5 ± 11.4 84.3 ± 10.8 < 0.001
24-h daytime heart rate (bpm) 78.7 ± 10.6 80.7 ± 11.8 84.8 ± 11.2 0.020
24-h nighttime heart rate (bpm) 67.6 ± 9.4 71.6 ± 11.6 80.1 ± 11.9 < 0.001
PWV (m/s) 7.63 ± 1.4 8.04 ± 1.8 8.17 ± 2.4 0.160 Glucose (mg/dL) 98 (89.2–112.5) 100.0 (88.7–147.2) 102.0 (91–126) 0.118 Urea (mg/dL) 29 (25–35) 43.5 (34–73.7) 116 (95–141) < 0.001 Creatinine (mg/dL) 0.7 (0.6–0.9) 1.3 (0.9–2.0) 7.0 (5.0–8.8) < 0.001 Uric acid (mg/dL) 5.4 ± 1.3 6.4 ± 1.6 6.1 ± 1.7 0.166 Total cholesterol (mg/dL) 209.5 (184–236) 216.0 (187–249.2) 197.6 (156–218) 0.675 LDL-cholesterol (mg/dL) 130.0 (112.7–152.5) 131.0 (108.7–167.0) 126.0 (90.6–148) 0.854 HDL-cholesterol (mg/dL) 45.0 (38.7–52.9) 44.0 (38–58) 39.0 (36–52) 0.604 Triglycerides (mg/dL) 149.5 (103–200.7) 154.5 (115–247) 143.1 (81–228) 0.769 Hemoglobin (g/dL) 14.1 ± 1.7 13.4 ± 2.2 10.0 ± 1.5 < 0.001 Albumin (g/dL) 4.3 ± 0.3 3.89 ± 0.6 3.43 ± 0.5 < 0.001 Calcium (mg/dL) 9.58 ± 0.4 9.24 ± 0.7 8.73 ± 0.7 < 0.001 Phosphorus (mg/dL) 3.45 ± 0.6 3.56 ± 0.7 4.66 ± 1.4 < 0.001 iPTH (ng/L) 50.5 (34–63) 71 (40.7–97.7) 225 (105.9–321.9) < 0.001
Urine protein/creatinine ratio (mg/gCr) 102 (66.8–140) 1021.5 (337.5–3612.7) < 0.001
Urine albumin/creatinine ratio (mg/gCr) 10.8 (8.2–13) 434.5 (105.5–1531.7) 0.002
(p < 0.001), phosphorus (p = 0.026), iPTH (p < 0.001), and the urine protein/creatinine ratio (p = 0.001) were sig-nificantly higher in the NHR group, while the levels of hemoglobin (p < 0.001), albumin (p < 0.001), and calcium (p < 0.001) were significantly lower (Table 2).
Univariable and multivariable adjusted regression analyses were performed to determine the independent determinants of the NHR pattern in CKD patients. In uni-variable logistic regression analysis age, the diagnosis of
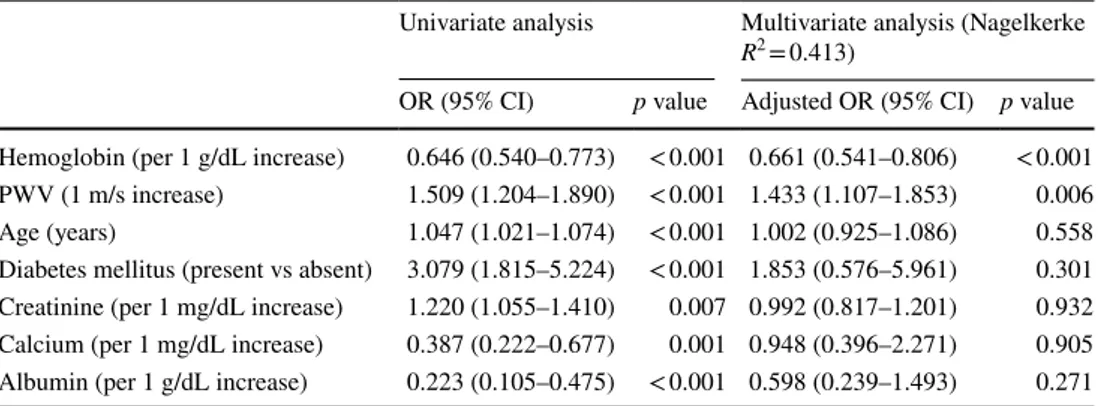
diabetes mellitus, and the levels of creatinine, calcium, albumin, and hemoglobin, and PWV and were all found to be associated with presence of NHR pattern in CKD patients. These variables were then used to construct a multivariate model. The results of the multivariable adjusted regression analysis demonstrated that the levels of hemoglobin (odds ratio [OR] 0.661; 95% CI 0.541–0.806; p < 0.001) and PWV (OR 1.433; 95% CI 1.107–1.853; p = 0.006) were the independent predictors of the NHR pattern (Table 3).
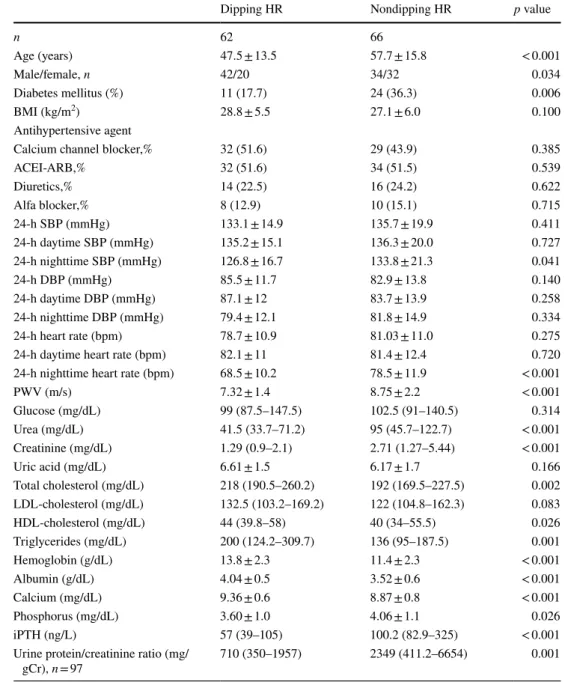
Table 2 Characteristics of hypertensive patients with chronic kidney disease according to dipping and nondipping heart rate pattern
Data are presented as mean ± standard deviation or median (interquartile range) or n (%)
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, iPTH intact parathyroid hormon, LDL low-density lipoprotein, HDL high-density lipoprotein, ACEI angiotensin converting enzyme inhibitors, ARB angiotensin II receptor blockers, PWV pulse wave velocity, HR heart rate
Dipping HR Nondipping HR p value
n 62 66 Age (years) 47.5 ± 13.5 57.7 ± 15.8 < 0.001 Male/female, n 42/20 34/32 0.034 Diabetes mellitus (%) 11 (17.7) 24 (36.3) 0.006 BMI (kg/m2) 28.8 ± 5.5 27.1 ± 6.0 0.100 Antihypertensive agent
Calcium channel blocker,% 32 (51.6) 29 (43.9) 0.385
ACEI-ARB,% 32 (51.6) 34 (51.5) 0.539 Diuretics,% 14 (22.5) 16 (24.2) 0.622 Alfa blocker,% 8 (12.9) 10 (15.1) 0.715 24-h SBP (mmHg) 133.1 ± 14.9 135.7 ± 19.9 0.411 24-h daytime SBP (mmHg) 135.2 ± 15.1 136.3 ± 20.0 0.727 24-h nighttime SBP (mmHg) 126.8 ± 16.7 133.8 ± 21.3 0.041 24-h DBP (mmHg) 85.5 ± 11.7 82.9 ± 13.8 0.140 24-h daytime DBP (mmHg) 87.1 ± 12 83.7 ± 13.9 0.258 24-h nighttime DBP (mmHg) 79.4 ± 12.1 81.8 ± 14.9 0.334 24-h heart rate (bpm) 78.7 ± 10.9 81.03 ± 11.0 0.275
24-h daytime heart rate (bpm) 82.1 ± 11 81.4 ± 12.4 0.720
24-h nighttime heart rate (bpm) 68.5 ± 10.2 78.5 ± 11.9 < 0.001
PWV (m/s) 7.32 ± 1.4 8.75 ± 2.2 < 0.001 Glucose (mg/dL) 99 (87.5–147.5) 102.5 (91–140.5) 0.314 Urea (mg/dL) 41.5 (33.7–71.2) 95 (45.7–122.7) < 0.001 Creatinine (mg/dL) 1.29 (0.9–2.1) 2.71 (1.27–5.44) < 0.001 Uric acid (mg/dL) 6.61 ± 1.5 6.17 ± 1.7 0.166 Total cholesterol (mg/dL) 218 (190.5–260.2) 192 (169.5–227.5) 0.002 LDL-cholesterol (mg/dL) 132.5 (103.2–169.2) 122 (104.8–162.3) 0.083 HDL-cholesterol (mg/dL) 44 (39.8–58) 40 (34–55.5) 0.026 Triglycerides (mg/dL) 200 (124.2–309.7) 136 (95–187.5) 0.001 Hemoglobin (g/dL) 13.8 ± 2.3 11.4 ± 2.3 < 0.001 Albumin (g/dL) 4.04 ± 0.5 3.52 ± 0.6 < 0.001 Calcium (mg/dL) 9.36 ± 0.6 8.87 ± 0.8 < 0.001 Phosphorus (mg/dL) 3.60 ± 1.0 4.06 ± 1.1 0.026 iPTH (ng/L) 57 (39–105) 100.2 (82.9–325) < 0.001
Urine protein/creatinine ratio (mg/
Discussion
We found in this study that nondipping heart rate was sig-nificantly higher in the hypertensive patients with CKD compared to the hypertensive patients with normal renal function, and that the prevalence increased as the severity of the renal dysfunction advanced. While the NHR pat-tern was reported to be 14% in the general population [7], this prevalence was reported to be 22.5% in the J-HOP study including high-risk hypertensive patients [18]. Ver-decchia et al. found a 22.4% prevalence in untreated but non-complicated essential hypertension patients [19]. In a study on older age (the mean age 67 years) hypertensive patients, including a higher proportion of diabetic patients (44%), a high NHR prevalence (40.5%) was reported [8].
In our study, the study group consisting of the middle-aged hypertensive patients with normal renal function, we found the prevalence of an NHR pattern as 26.3%, consist-ent with previous studies. We found this prevalence to be 43.3% in the hypertensive predialysis patients and 77.4% in the hypertensive hemodialysis patients. Arterial stiff-ness occurs prior to the manifestation of atherosclerosis and is considered as a risk factor for developing athero-sclerosis [3]. PWV is used as a parameter for evaluating the arterial stiffness. In our study, we found that the NHR pattern was independently associated with the PWV in the CKD patients. A high heart rate may have direct athero-genic effects on arteries by increasing the wall stress [20]. A high heart rate initially leads to endothelial dysfunction creating intense levels of stress in the endothelium. Later, the increased mechanical load and shear stress induce vascular stiffening by stimulating the growth of vascu-lar smooth muscle cells and collagen production [10]. It is thought that an increase in the heart rate and changes in the arterial wall viscoelasticity may cause increased arterial stiffness [21]. In parallel with our findings, it was reported that the PWV increased by 0.3 per every 10-beat increase in the heart rate [22]. Furthermore, a high heart rate during the sleep was found to be significantly
associated with increased arterial stiffness in predialysis CKD patients [10].
In our study, another parameter that was independently associated with the NHR pattern was the hemoglobin levels. As a physiological response to anemia, the heart rate, the stroke volume, and the cardiac output increases. Tachycar-dia, which is common in patients with anemia, reflects the increased sympathetic activity [23]. Therefore, it is expected that the NHR pattern will be higher in anemic patients. In previous studies on patients with stable coronary artery dis-ease, a low heart rate variability was found to be related to anemia [24]. Yokusoglu et al. also reported that a failure in the heart rate variability in anemic patients might occur due to increased sympathetic and decreased parasympathetic activity [25].
In our study, the calcium levels were significantly lower, whereas, the phosphorus and iPTH levels were significantly higher in the CKD patients in the NHR group although they were not independently related. Zhang et al. found that the heart rate variability indices were lower in the end-stage renal disease patients compared to the healthy controls, and reported that this was independently associated with the serum calcium, phosphorus, and PTH levels [26]. They suggested that disturbances in the mineral metabolism may affect cardiac autonomic neuropathy. A similar situation has also been reported for blood pressure. The nondipper and the reverse dipper blood pressure were reported to be inde-pendently related to high CaxP (calcium and phosphorus product) and PTH levels in CKD patients [27]. Although the underlying mechanism is not well known, abnormal mineral metabolism may also contribute to the increased NHR rates in CKD patients.
When the nondipping and dipping heart rate groups shown in Table 2 were compared, the levels of creatinine were found to be higher in the nondipping heart rate group. In addition, as the renal functions got worse, the rate of NHR was observed to increase significantly. All of these factors lead to the suggestion that the presence of renal dysfunction contributes to developing an NHR pattern.
Table 3 Univariate and multivariate regression analysis for determinants of nondipper heart rate pattern
OR odds ratio, PWV pulse wave velocity
Univariate analysis Multivariate analysis (Nagelkerke R2 = 0.413)
OR (95% CI) p value Adjusted OR (95% CI) p value Hemoglobin (per 1 g/dL increase) 0.646 (0.540–0.773) < 0.001 0.661 (0.541–0.806) < 0.001 PWV (1 m/s increase) 1.509 (1.204–1.890) < 0.001 1.433 (1.107–1.853) 0.006 Age (years) 1.047 (1.021–1.074) < 0.001 1.002 (0.925–1.086) 0.558 Diabetes mellitus (present vs absent) 3.079 (1.815–5.224) < 0.001 1.853 (0.576–5.961) 0.301 Creatinine (per 1 mg/dL increase) 1.220 (1.055–1.410) 0.007 0.992 (0.817–1.201) 0.932 Calcium (per 1 mg/dL increase) 0.387 (0.222–0.677) 0.001 0.948 (0.396–2.271) 0.905 Albumin (per 1 g/dL increase) 0.223 (0.105–0.475) < 0.001 0.598 (0.239–1.493) 0.271
The univariate analysis demonstrated that the levels of cre-atinine were found to be significantly related with NHR. However, in the logistic regression analysis, the levels of creatinine were not found out to be independently related with NHR.
The studies investigating the relationship between renal dysfunction and heart rate is limited in the literature. Kanaoka et al. studied the relationship of the renal function parameters with ambulatory blood pressure values and heart rate in hypertensive CKD patients. A 24-h and nighttime HR variability was found to be positively correlated with eGFR, while the nighttime HR was found to be negatively corre-lated with eGFR. As a result, the ambulatory blood pressure and the heart rate profile were found to be closely associated with the worsening in renal functions [28]. In another study performed on patients with CKD, the heart rate variability (HRV) was evaluated, resulting in the following observation that the HRV was low in late stage CKD patients due to car-diac autonomous neuropathy. Moreover, a lower rate of HRV was found to be associated with a high risk of cardiovascular disease and a high risk of progression to ESRD. Therefore, it was thought that the HRV may have a role in developing a cardiorenal syndrome and progression in CKD [29].
There was a bidirectional interaction between cardiovas-cular system and renal system. A primary dysfunction in one of them affects the functions of the other. Studies reporting a causal relationship between the cardiac autonomous neu-ropathy and renal impairment are available in the literature. As renal dysfunction can cause cardiac autonomous neurop-athy, reverse conditions may also occur. The Atherosclerosis Risk in Communities (ARIC) Study followed up more than 13,000 patients for 16 years evaluating the measurements of the resting heart rate and the heart rate variability. A low HRV was found to be associated with subsequent develop-ment of ESRD and CKD in association with hospitalization [30]. Similarly, when 1117 patients with type 2 diabetes mellitus without CKD were observed during a period of 9.6 years, the presence of cardiac autonomous neuropathy was found to be an independent prognostic factor for future development of CKD [31]. All these findings support the close relationship of renal dysfunction with cardiac autono-mous neuropathy.
Our study has some limitations. The number of patients is relatively low. Due to the retrospective nature of the study, we could not evaluate the factors that might affect the heart rate, such as physical activity and sleep quality. In addi-tion, although we have taken the potential confounding fac-tors into account, we may not be able to define any pos-sible effects of some other factors on heart rate. Although the NHR pattern is known to be associated with adverse cardiovascular events, the studies in the literature report contradicting results about the benefits of a pharmacologi-cally reduced heart rate [32]. This potential treatment goal
is ignored by clinicians, and prospective studies are needed on this issue.
As a result, the prevalence of NHR is high in this group of patients with high risk of cardiovascular disease and should not be overlooked. Prospective studies are needed to assess whether this pattern can be corrected by treatment and whether resolution of NHR will be effective in reducing cardiovascular events.
Compliance with ethical standards
Conflict of interest The authors have declared that no conflict of inter-est exists.
Ethics standards The study was approved by the Ethics Advisory Com-mittee of the Selcuk University (approval number: 201912).
Informed consent Informed consent was not required due to the
retro-spective nature of the study.
References
1. Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longi-tudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–63.
2. Benetos A, Rudnichi A, Thomas F, Safar M, Guize L. Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension. 1999;33(1):44–52.
3. Beddhu S, Nigwekar SU, Ma X, Greene T. Associations of rest-ing heart rate with insulin resistance, cardiovascular events and mortality in chronic kidney disease. Nephrol Dial Transplant. 2009;24(8):2482–8.
4. Kantharia BK. Non-dipping heart rate, microalbuminuria and thrombocytosis in type 2 diabetes mellitus: can we connect the dots? Cardiology. 2014;129(1):25–7.
5. Baka T, Simko F. Nondipping heart rate: a neglected cardiovas-cular risk factor based on autonomic imbalance? Auton Neurosci. 2018;210:83–4.
6. Hozawa A, Inoue R, Ohkubo T, Kikuya M, Metoki H, Asay-ama K, et al. Predictive value of ambulatory heart rate in the Japanese general population: the Ohasama study. J Hypertens. 2008;26(8):1571–6.
7. Cuspidi C, Facchetti R, Bombelli M, Sala C, Tadic M, Grassi G, et al. Night-time heart rate nondipping: clinical and prog-nostic significance in the general population. J Hypertens. 2018;36(6):1311–7.
8. Eguchi K, Hoshide S, Ishikawa J, Pickering TG, Schwartz JE, Shimada K, et al. Nocturnal nondipping of heart rate predicts cardiovascular events in hypertensive patients. J Hypertens. 2009;27(11):2265–70.
9. Magri CJ, Xuereb RG, Fava S. Non-dipping heart rate and microalbuminuria in type 2 diabetes mellitus. Cardiology. 2014;129(1):28–35.
10. Bai Y, Xiao H, Liu Z, Huang X, Tian X, Wang T, et al. Increased night heart rate is associated with worse large artery elasticity in chronic kidney disease patients. Int Urol Nephrol. 2013;45(6):1621–7.
11. Zhang J, Wen R, Yin J, Zhu Y, Lin L, Ye Z, et al. Nocturnal pulse rate correlated with ambulatory blood pressure and target organ damage in patients with chronic kidney disease. J Clin Hypertens (Greenwich). 2019;21(1):77–87.
12. Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T. Oscillometric estimation of aortic pulse wave velocity: compar-ison with intra-aortic catheter measurements. Blood Press Monit. 2013;18(3):173–6.
13. Sarafidis PA, Georgianos PI, Karpetas A, Bikos A, Korelidou L, Tersi M, et al. Evaluation of a novel brachial cuff-based oscillo-metric method for estimating central systolic pressure in hemodi-alysis patients. Am J Nephrol. 2014;40(3):242–50.
14. Latman NS, Latman A. Evaluation of instruments for noninvasive blood pressure monitoring of the wrist. Biomed Instrum Technol. 1997;31(1):63–8.
15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feld-man HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
16. Cuspidi C, Meani S, Negri F, Valerio C, Sala C, Mancia G. Is blunted heart rate decrease at night associated with prevalent organ damage in essential hypertension? Blood Press Monit. 2011;16(1):16–211.
17. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–30.
18. Oba Y, Kabutoya T, Hoshide S, Eguchi K, Kario K. Asso-ciation between nondipper pulse rate and measures of cardiac overload: the J-HOP Study. J Clin Hypertens (Greenwich). 2017;19(4):402–9.
19. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Telera MP, Pede S, et al. Adverse prognostic value of a blunted circadian rhythm of heart rate in essential hypertension. J Hypertens. 1998;16(9):1335–433.
20. Palatini P, Julius S. Heart rate and the cardiovascular risk. J Hypertens. 1997;15(1):3–17.
21. Tan I, Butlin M, Spronck B, Xiao H, Avolio A. Effect of heart rate on arterial stiffness as assessed by pulse wave velocity. Curr Hypertens Rev. 2018;14(2):107–22.
22. Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39(6):1083–7.
23. Tuncer M, Gunes Y, Guntekin U, Gumrukcuoglu HA, Eryonucu B, Guler N, et al (2009) Heart rate variability in patients with iron deficiency anemia. Arq Bras Cardiol. 92 (5):368–71, 85–8, 400–3. 24. Gehi A, Ix J, Shlipak M, Pipkin SS, Whooley MA. Relation of
anemia to low heart rate variability in patients with coronary heart disease (from the Heart and Soul study). Am J Cardiol. 2005;95(12):1474–7.
25. Yokusoglu M, Nevruz O, Baysan O, Uzun M, Demirkol S, Avcu F, et al. The altered autonomic nervous system activity in iron deficiency anemia. Tohoku J Exp Med. 2007;212(4):397–402. 26. Zhang L, Yang S, Chen J, Ma J, Ren Y. Associations of
parathy-roid hormone levels and mineral parameters with heart rate vari-ability in patients with end-stage renal disease. Int Urol Nephrol. 2017;49(6):1079–85.
27. Oh YK, Chin HJ, Ahn SY, An JN, Lee JP, Lim CS, et al. Discrep-ancies in clinic and ambulatory blood pressure in Korean chronic kidney disease patients. J Korean Med Sci. 2017;32(5):772–81. 28. Kanaoka T, Tamura K, Ohsawa M, Yanagi M, Haku S, Wakui
H, et al. Relationship of ambulatory blood pressure and the heart rate profile with renal function parameters in hyperten-sive patients with chronic kidney disease. Clin Exp Hypertens. 2012;34(4):264–9.
29. Chandra P, Sands RL, Gillespie BW, et al. Predictors of heart rate variability and its prognostic significance in chronic kidney disease. Nephrol Dial Transplant. 2012;27:700–9.
30. Orlov S, Cherney DZ, Pop-Busui R, Lovblom LE, Ficociello LH, Smiles AM, et al. cardiac autonomic neuropathy and early pro-gressive renal decline in patients with nonmacroalbuminuric Type 1 diabetes. Clin J Am Soc Nephrol. 2015;10(7):1136–44. 31. Yun JS, Ahn YB, Song KH, Yoo KD, Kim HW, Park YM,
et al. The association between abnormal heart rate variability and new onset of chronic kidney disease in patients with type 2 diabetes: a ten-year follow-up study. Diabetes Res Clin Pract. 2015;108(1):31–7.
32. Messerli FH, Rimoldi SF, Bangalore S, Bavishi C, Laurent S. When an increase in central systolic pressure overrides the bene-fits of heart rate lowering. J Am Coll Cardiol. 2016;68(7):754–62.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.