This article was downloaded by: [ Karamanoglu Mehmetbey Uni] On: 10 January 2013, At: 05:21
Publisher: Taylor & Francis
Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK
Synthesis and Reactivity in Inorganic, Metal-Organic,
and Nano-Metal Chemistry
Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/lsrt20
Measurement and Properties of the Oxide Ionic
Conductivity of
β- and δ-Phases in the Binary (Bi
2
O
3
)
1-x
(Tb
4
O
7
)
x
System
Nilgun Ozpozan Kalaycioglu a & Esra Çırçır b a
Department of Chemistry, Faculty of Science, Erciyes University, Kayseri, Turkey b
Department of Materials Science and Engineering, Faculty of Engineering, Karamanoglu Mehmetbey University, Karaman, 70200, Turkey
Accepted author version posted online: 14 Mar 2012.Version of record first published: 24 Apr 2012.
To cite this article: Nilgun Ozpozan Kalaycioglu & Esra Çırçır (2012): Measurement and Properties of the Oxide Ionic
Conductivity of β- and δ-Phases in the Binary (Bi2O3)1-x (Tb4O7) x System, Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry, 42:3, 398-401
To link to this article: http://dx.doi.org/10.1080/15533174.2011.611565
PLEASE SCROLL DOWN FOR ARTICLE
Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
ISSN: 1553-3174 print / 1553-3182 online DOI: 10.1080/15533174.2011.611565
Measurement and Properties of the Oxide Ionic
Conductivity of
- and ␦-Phases in the Binary
(Bi
2
O
3
)
1
−x
(Tb
4
O
7
)
x
System
Nilgun Ozpozan Kalaycioglu
1and Esra C
¸ ırc¸ır
21Department of Chemistry, Faculty of Science, Erciyes University, Kayseri, Turkey
2Department of Materials Science and Engineering, Faculty of Engineering, Karamanoglu Mehmetbey University, Karaman, 70200, Turkey.
The total conductivity (σT) in theβ-phase and δ-Bi2O3doped
with Tb4O7system was measured in the composition range between
1 and 30 mol% Tb4O7 at different temperatures. According to
the DTA/TG results, this tetragonal type solid solution was stable up to about∼740◦C, and the solubility limit was found at ∼5 mol% Tb4O7in theβ-phase; this fcc type solid solution was stable
up to about∼740◦C, and the solubility limit was found at∼30 mol% Tb4O7in theδ-phase. All phases showed predominant oxide
ionic conduction. It has been proposed thatβ- and δ-Bi2O3phases
contain a large number of oxide anion vacancies and incorporated terbium cations at tetrahedral sites that affect the oxygen sublattice of the crystal structure.
Keywords bismuth oxide, oxygen ionic conductivity, terbium oxide
INTRODUCTION
Solid electrolytes are the most important components of solid-state electrochemical devices, which are becoming in-creasingly important for applications in energy conversion, chemical processing, sensing, and combustion control. Bismuth oxide systems exhibit high oxide ionic conductivity and have been proposed as good electrolyte materials for applications such as solid oxide fuel cell and oxygen sensors. However, due to their instability under low oxygen partial pressure con-ditions there has been difficulty in developing these materials as alternative electrolyte materials compared to state-of-the-art cubic stabilized zirconia electrolyte. Bismuth oxide and doped bismuth oxide systems exhibit a complex array depending on dopant concentration, temperature, and atmosphere.[1–5]
Six polymorphs of Bi2O3 have been reported in the
litera-ture: monoclinicα-Bi2O3, tetragonalβ-Bi2O3, cubic (bcc)γ
-Bi2O3, cubic (fcc)δ-Bi2O3, orthorhombicε-Bi2O3, and triclinic
Received 25 October 2010; accepted 4 August 2011. This work was supported by Erciyes University (EUBAP). Address correspondence to Esra C¸ ırc¸ır, Department of Chemistry, Faculty of Science, Erciyes University, Kayseri 38039, Turkey. E-mail: esracircir@gmail.com
ω-Bi2O3 phases. The phase transition from the monoclinic
α-phase, to the high-temperature cubicδ-phase occurred at ap-proximately 730◦C. Theδ-phase was also found to be stable up to its melting point of approximately 825◦C. On cooling, theδ-phase from high temperature to room temperature, a large hysteresis was observed, with the possible occurrence of two intermediate metastable phases: the tetragonalβ-phase or the bcc g-phase. The tetragonalβ-phase occurred at around 650◦C on cooling, while the g-phase formed at around 640◦C.[6–8]
β- and δ-Bi2O3 phases display oxygen ionic conductivity
properties and can be stabilized by doping with small amounts of other oxides.[9–17]This study examined the structure of β-andδ-Bi2O3phases doped with Tb4O7.[18]
In the present work, we conducted tests on the electrical and thermal properties ofβ- and δ-Bi2O3phases doped with Tb4O7. EXPERIMENTAL
The powder samples were synthesized by the solid-state reaction method. According to the nominal composition (Bi2O3)1−x(Tb4O7)x (x = 0.01–0.30), appropriate amounts
of the starting materialsα-Bi2O3 and Tb4O7 were thoroughly
mixed and homogenized in an agate mortar. The mixtures were heat treated from 600◦C to 950◦C, increasing in steps of 50◦C. According to X-ray powder diffraction data, we showed that samples of tetragonalβ-Bi2O3 were obtained by doping with
Tb4O7according to the formula of (Bi2O3)1−x(Tb4O7)x when x
was 0.01≤ x ≤ 0.05 and the fcc δ-Bi2O3samples were obtained,
when x= 0.06–0.10, 0.13–0.30.[18]
Thermal measurements were made by using a simultaneous DTA/TG system (Shimadzu FC-60 type, Japan). The samples of
β- and δ-Bi2O3doped with Tb4O7were heated at a rate of 10◦C
min−1 from room temperature to 830◦C. Measurements were made in a 60 mL min−1nitrogen atmosphere using a platinum sample holder and anα-Al2O3inert reference substance.
The total electrical conductivity (σT) measurements were
made on samples pelletized (diameter 10 mm, thickness ∼1 mm) using a four-probe dc method in the temperature range of 100–800◦C. To reduce contact resistance, fine platinum wires
398
CONDUCTIVITY OF (BI2O3)1–x(TB4O7)x 399
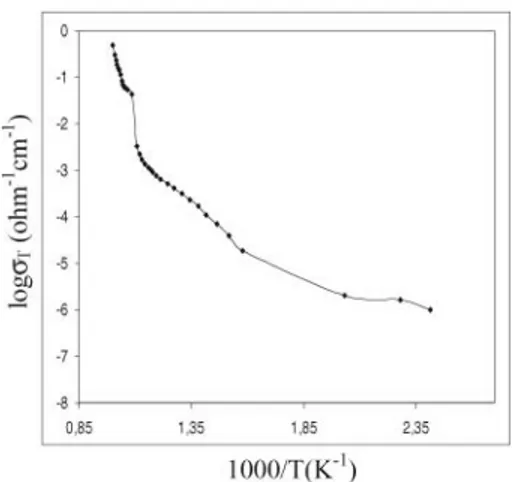
FIG. 1. Electrical conductivity plot ofβ-Bi2O3doped with 3 mol% Tb4O7.
were attached directly to the surface of the samples. The con-tacts were positioned symmetrically with respect to the center of the circular pellet, and the contact separations were 0.2 cm. The temperature in the furnace was increased in 20◦C steps in air atmosphere initially, and near the phase transition, the increase
in temperature was in 5◦C steps. During the measurements the sample temperature was determined by a thermocouple posi-tioned 5 mm away from the sample. All data were made by a Keithley 2400 source meter and a Keithley 2700 electrometer (USA), which are controlled by computer.
RESULTS
-Bi2O3Doped With Tb4O7
In Figure 1 the electrical conductivity plot ofβ-Bi2O3doped
with 3 mol% Tb4O7content are presented, and theσTplots for
the other β-Bi2O3 phases are quite similar. These data were
obtained during a repeated heating runs at a constant heat-ing rate in air. The electrical conductivity of β-Bi2O3 doped
with 1–5 mol% Tb4O7 increased with increasing temperature
up to∼620◦C. Beyond this temperature conductivity increased sharply up to about 665◦C. The reason for the sharp increase in conductivity was the phase transition and an alteration in the crystal structure possibly causing a change in the conductivity mechanism. The structural disorder during transformation may also contribute to the improvement of ionic conductivity. The
1000/T(K-1) log σT (ohm -1 cm -1 ) -8 -7 -6 -5 -4 -3 -2 -1 0 0,85 1,35 1,85 2,35 1000/T(K-1) log σT (ohm -1 cm -1 ) -8 -7 -6 -5 -4 -3 -2 -1 0 0,85 1,35 1,85 2,35 1000/T(K-1) log σT (ohm -1 cm -1 ) -8 -7 -6 -5 -4 -3 -2 -1 0 0,85 1,35 1,85 2,35
FIG. 2. Electrical conductivity plot ofδ -Bi2O3: (a) doped with 6 mol% Tb4O7, (b) doped with 14 mol% Tb4O7, (c) doped with 13 mol% Tb4O7.
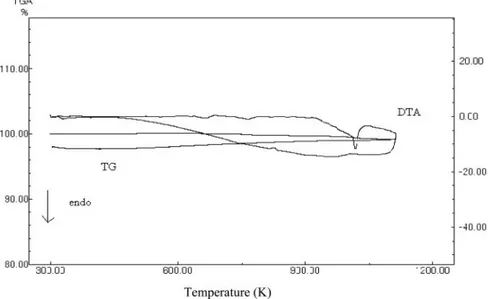
FIG. 3. DTA/TG diagrams ofδ -Bi2O3phase doped with 13 mol% Tb4O7.
β-δ phase transition for pure β-Bi2O3at a temperature of about
660–670◦C was reported using DTA thermal analysis and con-ductivity change graphs,[7,8]and experimental results showed
that theδ-Bi2O3 phase exhibited higher conductivity than the β-Bi2O3phase.
␦-Bi2O3Doped With Tb4O7
Electrical conductivity plots versusδ-Bi2O3samples doped
with 6, 13, and 14 mol% Tb4O7 concentrations contents are
given in Figure 2. The graphic curves of the otherδ-Bi2O3
sam-ples were quite similar to the curves the of samsam-ples given in this figure. As seen in the figures, the conductivity of all the sam-ples increases with increasing temperature up to 720◦C. Beyond this temperature conductivity increased rapidly up to∼810◦C. A marked increase in conductivity between 720–810◦C was considered to be the phase transition toδ-Bi2O3. The DTA/TG
measurements also suggested that a polymorphic transition took place, and the endothermic phase transition was observed on the DTA curve at about the same temperature (Figure 3). As can be seen in Figure 3, the transition temperature is∼740.0◦C, which was determined by DTA; the transition temperature in the con-ductivity versus temperature graph is in the range of 720–810◦C. The results showed thatδ-Bi2O3had high electrical
conduc-tivity; at the high temperature the conductivity was mainly ionic, with oxide ions being the main charge carrier.
DISCUSSION
The experimental results showed that in our samples the oxygen lattice points of theβ-phase and δ-Bi2O3 doped with
Tb4O7were not completely occupied with oxygen ions. If the
oxygen sublattice was fully occupied by O2−ions, The Tb4O7
doped β- and δ-Bi2O3 phases would not show such a high
degree of electrical conductivity. Some of the oxygen lattice
points located around the tetrahedral sites may have been vacant, forming an oxygen vacancy. These oxygen vacancies were filled randomly with neighboring oxygen ions at an increasing rate with increasing temperature. Jumping oxygen ions left their former sites vacant, thus another vacancy was formed; because this process was random, the total oxygen flow was zero in any direction without an applied electric field.
The conductivity of all samples increased with increasing temperature. It was proposed that this was related to ionic mo-bility, which rises with increasing temperature. At elevated tem-peratures, the thermal vibrational energy of the ions increased causing a higher oxygen ion jumping rate. Although oxygen va-cancies were present in the crystal structure at low temperatures (below 200◦C), the thermal energy of the anions was not high enough for them to jump out of their lowest energy positions. Thermal vibrations may also have assisted the jumping process briefly by either shortening the jumping distance or by widening the jumping channels through the crystal.
CONCLUSIONS
The β- and δ-phases in the (Bi2O3)1−x(Tb4O7)x (x =
0.01–0.30) binary oxide compounds possessing oxygen ionic conductivity were synthesized. The jumps, observed in the con-ductivity curves, indicated phase transitions. The concon-ductivity of these materials increased with dopant. The reason for this was the introduction of more lattice defects due to the increase in vacant lattice points.
The high ionic conductivity in β- and δ-Bi2O3 phases
supports the view that there is an average occupation of oxide ions in oxygen lattice sites that can move from site to site through the bismuth sublattice. The sample with the highest conductivity of –0.658 cm−1 at 810◦C was the
δ-phase of the (Bi2O3)0.87(Tb4O7)0.13and that with the highest
CONDUCTIVITY OF (BI2O3)1–x(TB4O7)x 401
conductivity of –0.299 cm−1 at 730◦C was theβ-phase of the (Bi2O3)0.97(Tb4O7)0.03.
REFERENCES
1. Arora, N.; Deo, G.; Wachs, I.E.; Hirt, A.M. Surfaces aspects of bismuth-metal oxide catalysts. J. Catal. 1996, 159, 1–13.
2. Shokr, Kh.E.; Wakkad, M.M.; Abd El-Ghanny, H. A.; Ali, H.M. Sb-doping
effects on optical and electrical parameters of SnO2films. J. Phys. Chem.
Solids. 2000, 61, 75.
3. Lybye, D.F.; Poulsen, W.; Mogensen, M. Conductivity of A- and B-site
doped LaAlO3, LaGaO3, LaScO3and LaInO3perovskites. Solid State
Ion-ics, 2000, 128, 91–103.
4. Bernik, S.; Ai, B.Presented at 33rd Int. Cong. on Microelectronics, Devices and Materials, Slovenia, September 24–26 1997; p. 87.
5. Goff, J.P.; Hayes, W.; Hulls, S.; Hutchings M.T.; Clausen, K.N. Defect structure of yttria-stabilized zirconia and its influence on the ionic conduc-tivity at elevated temperatures. Phys. Rev. B. 1999, 59, 14202–14219. 6. Yaremchenko, A.A.; Kharton, V.V.; Naumovich, E.N.; Tonoyan, A.A.
Sta-bility of δ-Bi2O3-based solid electrolytes. Mater. Res. Bull. 2000, 35,
515–520.
7. Harwig, H.A. On the structure of bismuthsesquioxide: theα, β, γ and δ
phase. Anorg. Allg. Chem. 1978, 444, 151–166.
8. Harwig, H.A.; Gerards, A.G. The polymorphism of bismuth sesquioxide. Thermochim. Acta. 1979, 28, 121–131.
9. Sammes, N.M.; Tompestt, G.A.; Nafe, H.; Aldinger, F. Bismuth based oxide electrolytes: structure and ionic conductivity. J. Eur. Ceram. Soc. 1999, 19, 1801–1826.
10. Cahen, H.T.; Van Den Belt, T.G. M.; De Wit J.H. W.; Broers, G.H. J. The
electrical conductivity ofδ –Bi203stabilized by isovalent rare-earth oxides
R2O3. Solid State Ionics, 1980, 1, 411–423.
11. Takahashi, T.; Esaka, T.; Iwahara, H. Oxide ion conduction in the sintered
oxides of MoO3-doped Bi2O3. J. Appl. Electrochem. 1977, 7, 31–44.
12. Harwig, H.A.; Gerards, A.G. Electrical properties of the: theα, β, γ and δ
phases of bismuthsesquioxide, J. Solid State Chem. 1978, 26, 265–274. 13. Takahashi, T.; Iwahara, H.; Nagai, Y. High oxide ion conduction in sintered
bismuth oxide containing strontium oxide, calcium oxide, or lanthanum oxide. J. Appl. Electrochem. 1972, 2, 97–104.
14. Miyayama, M.; Katsuta, S.; Suenaga, Y.; Yanagida, H. Electrical conduction
inβ-Bi2O3doped with Sb2O3. J. Am. Ceram. Soc. 1982, 66, 585–588.
15. Chiodelli, G.; Magistris, A.; Spinolo, G.; Tomasi, C.; Antonucci, V.; Gior-dano, N. Electrical properties in the Bi-rich part of the Bi, Mo/O system. Solid State Ionics 1994, 74, 37–45.
16. Mori, T.; Ikegamit, T.; Yamamura, H.; Atake, T. Improvement of electrical
conductivity in fluorite related Y2O3and fluorite CeO2systems based on a
unique effective index. J. Therm. Anal. Cal. 1999, 57, 831–838.
17. Miyayama, M.; Yanagida, H. Oxygen ion conduction in Bi2O3doped with
Sb2O3. J. Mater. Sci. 1986, 21, 1233–1236.
18. C¸ ırc¸ır, E. Synthesis and characterisation of Tb4O7doped polymorphs of
Bi2O3. M.Sc. Thesis, Erciyes University, Kayseri, Turkey, 2006.