Full Terms & Conditions of access and use can be found at
https://www.tandfonline.com/action/journalInformation?journalCode=cije20
ISSN: 0960-3123 (Print) 1369-1619 (Online) Journal homepage: https://www.tandfonline.com/loi/cije20
New pharmacological targets of three Asphodeline
species using in vitro and ex vivo models of
inflammation and oxidative stress
Gokhan Zengin, Marcello Locatelli, Claudio Ferrante, Luigi Menghini,
Giustino Orlando, Luigi Brunetti, Lucia Recinella, Annalisa Chiavaroli, Sheila
Leone, Lidia Leporini, Muhammad Zakariyyah Aumeeruddy & Mohamad
Fawzi Mahomoodally
To cite this article: Gokhan Zengin, Marcello Locatelli, Claudio Ferrante, Luigi Menghini, Giustino Orlando, Luigi Brunetti, Lucia Recinella, Annalisa Chiavaroli, Sheila Leone, Lidia Leporini, Muhammad Zakariyyah Aumeeruddy & Mohamad Fawzi Mahomoodally (2019) New pharmacological targets of three Asphodeline species using in�vitro and ex�vivo models of inflammation and oxidative stress, International Journal of Environmental Health Research, 29:5, 520-530, DOI: 10.1080/09603123.2018.1552930
To link to this article: https://doi.org/10.1080/09603123.2018.1552930
Published online: 05 Dec 2018. Submit your article to this journal
Article views: 153 View related articles
ARTICLE
New pharmacological targets of three Asphodeline species using
in vitro and ex vivo models of inflammation and oxidative stress
Gokhan Zengina, Marcello Locatelli b, Claudio Ferranteb, Luigi Menghinib, Giustino Orlandob,
Luigi Brunettib, Lucia Recinellab, Annalisa Chiavarolib, Sheila Leoneb, Lidia Leporinib,
Muhammad Zakariyyah Aumeeruddycand Mohamad Fawzi Mahomoodallyc
aDepartment of Biology, Faculty of Science, Selcuk University, Konya, Turkey;bDepartment of Pharmacy,
G. d’Annunzio University Chieti-Pescara, Chieti, Italy;cDepartment of Health Sciences, Faculty of Science,
University of Mauritius, Reduit, Mauritius
ABSTRACT
This study explored the efficacy of the methanolic extract of three Asphodeline species (A. damascena subsp. rugosa, A. tenuior subsp. tenui-flora var. tenuitenui-flora, and A. cilicica) to protect against hydrogen peroxide (H2O2)-induced lactate dehydrogenase (LDH) activity in HCT116 cells, and
also any protective effects against lipopolysaccharides (LPS)-induced nitrite levels, prostaglandin E2 (PGE2) and 8-iso-prostaglandin F2α (8-iso-PGF2α) levels, 5HIAA/5-HT ratio, tumor necrosis factor (TNF)-α and inter-leukin (IL)-6 gene expression in rat colon specimens. Interestingly, A. tenuior extract was most effective in improving the tested biomarkers, by reducing LDH activity and nitrite level. On the other hand, A. damascena was the only species able to blunt LPS-induced TNF-α gene expression in rat colon specimens. The presentfindings highlighted the protective effects of Asphodeline extracts via in vitro and ex vivo models of inflammation and oxidative stress, adding new insights to the pharmacological actions of these medicinal plant species.
Abbreviations: IBD: inflammatory bowel disease; LPS: lipopolysacchar-ide; LDH: lactate dehydrogenase; 5HIAA: 5-hydroxyindoleacetic acid; 5-HT: 5-hydroxytryptamine
ARTICLE HISTORY
Received 12 August 2018 Accepted 23 November 2018
KEYWORDS
Asphodeline; oxidative stress; inflammation; natural agents; pharmaceutical applications
Introduction
Oxidative stress is generated when the production of reactive oxygen species (ROS) exceeds the extent of cellular antioxidant defense (Zou et al.2018). Oxidative stress has been linked to a number of chronic diseases such as cancer, diabetes, aging, cardiovascular disease, neurodegenerative disease, and in flam-mation (Ismail et al.2017). Although inflammation is an important defense immune response which facilitates the clearance of invading pathogens, if uncontrolled, it can contribute to disease progression in some autoimmune and chronic inflammatory diseases (Zhu et al. 2018). After stimulation with lipopolysaccharides (LPS), viruses, and bacterial endotoxin, monocytes are able to differentiate to macrophages at the infection site followed by the release of inflammatory factors including nitric oxide (NO), prostaglandin E2 (PGE2), and other cytokines (Gioda et al. 2011; Lee and Bae2018). Therefore, the regulation of the level of these inflammatory factors has become vital for the treatment of inflammatory diseases (Han et al.2018). Currently prescribed drugs to treat various inflammatory
CONTACTGokhan Zengin gokhanzengin@selcuk.edu.tr Department of Biology, Faculty of Science, Selcuk University, Konya 42250, Turkey
2019, VOL. 29, NO. 5, 520–530
https://doi.org/10.1080/09603123.2018.1552930
diseases include indomethacin, aspirin, and ibuprofen. Nonetheless, many side effects including hepa-totoxicity and gastric trouble have been reported. Therefore, the development of safer and stronger anti-inflammatory drugs effects is essential (Kim et al.2018).
The genus Asphodeline (Family: Xanthorrhoeaceae) consist of 59 species names as recorded in The Plant List (http://www.theplantlist.org/); of these 17 are accepted species names, 37 synonyms, and 5 still unassessed. Asphodeline species may exist as rhizomatous or stoloniferous perennial plants, biennial plants, or annual herbs, and they occupied diverse habitats such as river banks, fallowfields, forest clearings, and rocky or clayey slopes (Zengin et al.2016a). Members of this genus are present in the southwest of Asia, mostly in Middle-Eastern countries and the Mediterranean region (Zengin et al.
2012). In Turkey, the genus contains 20 taxa, 12 taxa of which are endemic (Zengin et al.2016a), and are abundant especially in the mountains and steppes of inner Anatolia (Zengin et al.2012). They are known under the names Çiriş otu, Kiriş otu and Yayla çirişi (Zengin et al. 2016a). Moreover, in different regions of Turkey, several species are consumed in salads and their leaves are known to contain a good nutritional quality (Lazarova et al.2014).
Many species of the genus Asphodeline have significant applications in traditional medicines. For instance, A. tenuior root is used for wound healing (Altundag and Ozturk2011), A. taurica flower is used for shortness of breath and burn (Özdemir and Alpınar2015) while its root is used for wound care and eczema. Similarly, the root of the two species A. baytopiae and A. brevicaulis are used for wound care and to manage eczema (Sargin2015). In addition, A. baytopiae is also used as meal, mixture (with onion), cataplasm with rhizome powder, to treat intestinal spasms and rheumatism (Sargin et al. 2015). Also, decoction of the aerial part of A. lutea is used as a diuretic and against pains (Tuttolomondo et al.2014).
Scientific studies have validated the biological properties of a number of Asphodeline species. As part of a continuation of research on this genus, we aimed to evaluate the biological potential of three Asphodeline species: A. damascena subsp. rugosa, A. tenuior subsp. tenuiflora var. tenuiflora, and A. cilicica. It is to be noted that several previous studies have probed into their in vitro pharmacological properties. For instance, A. tenuior and several subspecies of A. damascena were found to possess antioxidant activities and enzyme inhibitory effects against acetyl cholinesterase, butyrylcholinesterase, α-amylase, α-glucosidase, and tyrosinase (Locatelli et al. 2017b). A. cilicica was also found to inhibit the aforementioned enzymes and was further studied for its inhibitory properties against elastase, collagenase, and hyaluronidase (Ilhan et al.
2016). As a further investigation of these species, the present study aimed to evaluate their in vitro biological effects on hydrogen peroxide-induced LDH activity in HCT116 cells, and also their protective effects against LPS-induced nitrite, prostaglandin E2 (PGE2) and 8-iso-prostaglandin F2α (8-iso-PGF2α) levels, 5HIAA/5-HT ratio and tumor necrosis factor (TNF)-α and interleukin (IL)-6 gene expression in rat colon specimens.
Material and methods
Plant materials and preparation of extracts
Asphodeline species were collected (at flowering stage) in Turkey regions. The information of these plants was reported below. Voucher specimens were deposited in KNYA Herbarium (Department of Biology, Selcuk University, Konya, Turkey). The powdered root samples (10 g) were macerated with 250 mL of methanol at room temperature for 24 h. The extracts were concentrated and then they were stored at +4°C in dark until analyses.
A. damascena (Boiss.) Baker subsp. rugosa E. Tuzlaci: Kayseri, between Yahyali and Sazak road, 1212 m, 38° 05ʹ 18″N, 35° 21ʹ 38″ E. (Endemic)
A. cilicica E. Tuzlaci: Adana, between Catalan and Aladag, 37° 27ʹ37″ N, 35° 20ʹ 12″ E, 1080 m (Endemic)
A. tenuior (Fischer) Ledeb. subsp. tenuiflora (C. Koch) E. Tuzlaci var. tenuiflora (Fischer) Ledeb.: Malatya: between Malatya and Darende road, 1003 m, 38° 30ʹ 40″ N, 37° 31ʹ 19″ E.
In vitro studies
HCT116 cells were cultured in DMEM (Euroclone) supplemented with 10% (v/v) heat-inactivated fetal bovine serum and 1.2% (v/v) penicillin G/streptomycin in 75 cm2tissue cultureflask (n = 5 individual culture flasks for each condition). The cultured cells were maintained in humidified incubator with 5% CO2at 37°C.
For cell differentiation, HCT116 cell suspension, at a density of 1 × 106cells/mL, were treated
with various concentrations (10, 50, and 100 ng/mL) of phorbol myristate acetate (PMA, Fluka) for 24 h or 48 h (induction phase). Thereafter, the PMA-treated cells were washed twice with ice-cold pH 7.4 phosphate buffer solution (PBS) to remove PMA and non-adherent cells, whereas the adherent cells were further maintained for 48 h (recovery phase). Morphology of cells was examined under an inverted phase-contrast microscope.
To assess the basal cytotoxicity of the methanolic Asphodeline extracts, a viability test was performed in 96 microwell plates, using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test. Cells were incubated with extracts (ranging concentration 10–1000 μg/ mL) for 24 h. 10 μL of MTT (5 mg/mL) was added to each well and incubated for 3 h. The formazan dye formed was extracted with dimethyl sulfoxide and absorbance recorded as pre-viously described (Menghini et al. 2016; Menghini et al. 2018). Effects on cell viability were
evaluated in comparison to untreated control group.
Lactate dehydrogenase (LDH) activity was measured by evaluating the consumption of NADH in 20 mM HEPES-K+(pH 7.2), 0.05% bovine serum albumin, 20 µM NADH and 2 mM pyruvate using a microplate reader (excitation 340 nm, emission 460 nm) according to manufacturer’s protocol (Sigma-Aldrich).
Ex vivo studies
Male adult Sprague-Dawley rats (200–250 g) were housed in Plexiglas cages (40 cm × 25 cm × 15 cm), two rats per cage, in climatized colony rooms (22 ± 1°C; 60% humidity), on a 12 h/12 h light/dark cycle (light phase: 07:00–19:00 h), with free access to tap water and food, 24 h/day throughout the study, with no fasting periods. Rats were fed a standard laboratory diet (3.5% fat, 63% carbohydrate, 14% protein, 19.5% other components without caloric value; 3.20 kcal/g). Housing conditions and experimentation procedures were strictly in accordance with the European Union ethical regulations on the care of animals for scientific research.
According to the recognized ethical principles of ‘Replacement, Refinement and Reduction of Animals in Research’, colon specimens were obtained as residual material from vehicle-treated rats randomized in our previous experiments approved by Local Ethical Committee (University ‘G. d’Annunzio’ of Chieti-Pescara) and Italian Health Ministry (Project N. 880 definitely approved by Italian Health Ministry on 24 August 2015). Rats were sacrificed by CO2 inhalation (100% CO2 at aflow rate of 20% of the chamber volume per min) and colon
specimens were immediately collected and maintained in humidified incubator with 5% CO2
at 37°C for 4 h, in RPMI buffer with added bacterial LPS (10 µg/mL) (incubation period). During the incubation period, tissues were treated with scalar sub-toxic concentrations of methanol Asphodeline extracts (100μg/mL). Tissue supernatants were collected and PGE2 and
8-iso-PGF2α levels (ng/mg wet tissue) were measured by radioimmunoassay (RIA), as
pre-viously reported (Chiavaroli et al. 2010, Verratti et al. 2011, Locatelli et al. 2018). Briefly,
specific anti-8-iso-PGF2α and anti-PGE2 were developed in the rabbit; the cross-reactivity
against other prostanoids is < 0.3%. 100 μL of prostaglandin standard or sample were incubated overnight at 4°C with the 3H-prostaglandin (3000 cpm/tube; NEN) and antibody (final dilution: 1:120,000), in a volume of 1.5 mL of 0.025 M phosphate buffer. Free and
antibody-bound prostaglandins were separated by the addition of 100 μL 5% bovine serum albumin and 100μL 3% charcoal suspension, followed by centrifuging for 10 min at 4000 g at 5°C and decanting off the supernatants into scintillation fluid (Ultima Gold™, Perkin Elmer) for β emission counting. The detection limit of the assay method is 0.6 pg/mL.
Additionally, tissue supernatant was assayed for nitrite determination by Griess assay, as previously described (Zengin et al. 2017). On the other hand, individual colon specimens were dissected and subjected to extractive procedures to evaluate 5-HT and 5HIAA (ng/mg wet tissue) as previously reported (Brunetti et al. 2014a, Ferrante et al. 2016). As regards to 5-HT analysis, tissues were homogenized in ice bath for 2 min with Potter-Elvehjem homogenizer in 1 mL of 0.05 N perchloric acid containing 0.004% sodium EDTA and 0.010% sodium bisulfite. Thereafter, samples were analyzed by HPLC coupled to electrochemical detection consisting of ESA Coulochem III detector equipped with ESA 5014B analytical cell.
Finally, TNFα and IL-6 gene expression was evaluated in rat colon as previously reported (Ferrante et al. 2017; Brunetti et al. 2014b). Total RNA was extracted from the cells using TRI Reagent (Sigma-Aldrich, St. Louis, MO), and 1 μg was reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Reactions were incubated in a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA) initially at 25°C for 10 min, then at 37°C for 120 min, andfinally at 85°C for 5 s. Gene expression was determined by quantitative real-time PCR using TaqMan probe-based chemistry (Applied Biosystems, Foster City, CA, USA). PCR primers and TaqMan probes were obtained from Applied Biosystems [Assays-on-Demand Gene Expression Products, Hs00174128_m1 for TNF-α; Rn01410330_m1 for IL-6; Hs99999909_m1 for hypoxanthine phosphoribosyl transferase (HPRT1)]. HPRT1 was used as the housekeeping gene. The real-time PCR was carried out in triplicate for each cDNA sample in relation to each of the investigated genes. Data were elaborated with the Sequence Detection System (SDS) software version 2.3 (Applied Biosystems, Foster City, CA, USA).
Statistical analysis. Statistical analysis was performed using GraphPad Prism version 5.01 for
Windows (GraphPad Software, San Diego, CA, USA). Means ± SEM were determined for each experimental group and analyzed using one way analysis of variance (ANOVA) followed by Newman-Keuls post hoc test. As for gene expression analysis, 1.00 (calibrator sample) was considered the theoretical mean for the comparison. Statistical significance was accepted at p < 0.05. As regards to the animals randomized for each experimental group, the number was calculated on the basis of the‘Resource Equation’ (Charan and Kantharia2013).
Results and discussion Pharmacological studies
We observed that the methanol extracts of all tested Asphodeline species (10–1000 μg/mL) exerted an inhibitory effect on HCT116 viability starting from the concentration of 150 μg/mL (data not shown). As previously reported, the high sensitivity of HCT116 line to cytotoxic effects of the herbal extracts (Locatelli et al. 2017a) could depend on the low grade of cell differentiation.
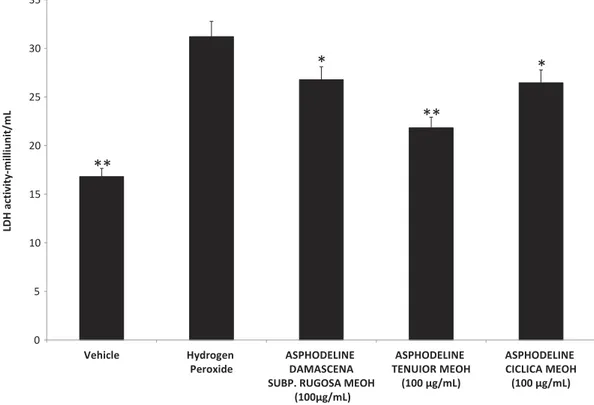
Considering the results of viability test, the following pharmacological tests were performed at the upper concentration tolerated by the cell line (150μg/mL). Particularly, we evaluated the effect of the methanolic Asphodeline extracts on LDH activity. The extracts displayed a significant reduc-tion on hydrogen peroxide-induced LDH activity (Figure 1). Hydrogen peroxide caused an increase in LDH activity (31.21 milliunit/mL) compared to the control group (16.81 milliunit/ mL). The Asphodeline extracts were able to counteract this increase, with the A. tenuior extract being most effective (21.83 milliunit/mL (30% reduction)) followed by A. cilicica (26.45 milliunit/ mL (15.3% reduction)) and A. damascenea (26.78 milliunit/mL (14.2% reduction)). LDH could be considered a marker of tissue damage, especially in the gut, and reduced LDH activity following
herbal extracts treatment has been related to protective effects in inflammatory bowel disease (IBDs) (Kannan and Guruvayoorappan2013, Nagarjun et al.2017). Additionally, LDH levels have been inversely related to wound healing process (Park et al.2015). In this context, ourfinding of reduced LDH activity in intestinal HCT116 cell line following treatment could support a potential application of Asphodeline extracts as protective agents in the gut.
To test this hypothesis, we performed a second set of experiments on rat colon specimens challenged with LPS, an experimental paradigm of IBD ex vivo (Menghini et al.2016). Our study revealed that all methanol extracts were able to blunt the LPS-induced levels of specific biomar-kers of lipid peroxidation and inflammation. Particularly, we observed that Asphodeline extracts inhibited colon nitrite levels (Figure 2), as an index of free radical production. LPS caused an increase in the nitrite level (102.61 mmoL/g wet tissue) compared to the control group (73.84 mmoL/g wet tissue). Treatment with the plant extracts caused an improvement in nitrite level; the highest reduction of nitrite was exhibited by the A. tenuior extract (69.04 mmoL/g wet tissue (32.7% reduction)) followed by A. cilicica (75.68 mmoL/g wet tissue (26.2% reduction)) and A. damascena (78.95 mmoL/g wet tissue (23.1% reduction)).
Overproduction of reactive oxygen/nitrogen species (ROS/RNS) has long been involved in disruptive peroxidation reactions on cellular substrates such as proteins, lipids, and nucleic acids (Uttara et al.2009). In particular, lipid peroxidation has been recognized in the onset of chronic diseases, including IBDs (Achitei et al.2013). The effects of ROS/RNS, mainly produced by macro-phages and neutrophils, include neutrophils recruitment at the inflamed tissues (Fialkow et al.2007). 8-iso-PGF2α, an isomer of classic prostaglandins deriving from ROS/RNS peroxidation of membrane arachidonic acid, has been long considered a stable marker of lipid peroxidation, in vivo (Pratico
2002). In the present study, LPS treatment induced an increase in the 8-iso-PGF2α level (10 pg/mg
0 5 10 15 20 25 30 35 Vehicle Hydrogen Peroxide ASPHODELINE DAMASCENA SUBP. RUGOSA MEOH
(100µg/mL) ASPHODELINE TENUIOR MEOH (100 µg/mL) ASPHODELINE CICLICA MEOH (100 µg/mL) LDH activ ity-m illiunit/m L
**
**
*
*
Figure 1.Effect of A. tenuior, damascena and cilicica methanol extracts (100 µg/mL) on hydrogen peroxide-induced LDH activity in HCT116 cells. ANOVA, p < 0.001; post hoc, *p < 0.05; **p < 0.01 vs. Hydrogen peroxide group. LDH activity was evaluated through commercial colorimetric assay according to manufacturer’s protocol (Sigma-Aldrich).
wet tissue) compared to the control group (6.4 pg/mg wet tissue). This increase was counteracted by the Asphodeline extracts with the most effective extract being the A. tenuior extract (3.6 pg/mg wet tissue (64% reduction)) followed by A. damascena (3.8 pg/mg wet tissue (62% reduction)) and A. cilicica (7.2 pg/mg wet tissue (28% reduction)). Therefore, the inhibitory effect on nitrite and 8-iso-PGF2α production could account for an antioxidant effect induced by the extracts, ex vivo.
Additionally, in the present study we explored putative anti-inflammatory mechanisms (Lazarova et al. 2016, Zengin et al. 2016b). PGE2 and 5-HT are pro-inflammatory cytokines which have long been involved in colon epithelium inflammation and damage (Nagib et al.2013, Regmi et al.2014). Accordingly with the presentfindings (Figures 3–4), increased PGE2 and 5-HT levels have been previously described in rat colon specimens challenged with LPS (Menghini et al.
2016). Particularly, 5HIIA/5-HT ratio has long been considered as a valuable index of 5-HT turnover, in vivo (Brunetti et al. 2013; Brunetti et al. 2014), possibly mediated by the mono-amineoxidase-a activity (Lee et al. 2001). On the other hand, Asphodeline extracts showing protective effects were able to blunt the increased levels of both biomarkers following LPS challenging. The reduced levels of PGE2 and 5-HT, expressed as pg/mg tissue and 5HIAA/ 5-HT ratio, respectively, could account for anti-inflammatory effects induced by Asphodeline extracts. LPS treatment induced an increase in PGE2 (12.1 pg/mg wet tissue) compared to the control group (9.2 pg/mg wet tissue). The A. tenuior extract was most effective in reducing PGE2 (4 pg/mg wet tissue) followed by A. damascena (6.6 pg/mg wet tissue) and A. cilicica (9.9 pg/mg wet tissue). On the other hand, the decrease in 5HIAA/5-HT ratio induced by LPS (0.23), compared to the control (1.19), was counteracted mostly by the A. damascena extract which resulted in an increase in the 5HIAA/5-HT ratio (0.76) followed by the A. tenuior (0.36) and A. cilicica extract (0.31). We can speculate that the minor activity of A. tenuior and A. cilicica
0 20 40 60 80 100 120 Vehicle LPS ASPHODELINE DAMASCENA SUBP. RUGOSA MEOH
(100µg/mL) ASPHODELINE TENUIOR MEOH (100 µg/mL) ASPHODELINE CICLICA MEOH (100 µg/mL) Nitr ite level - m m o L/g wet tissue
**
**
**
**
Figure 2.Effect of A. tenuior, damascena and cilicica methanol extracts (100 µg/mL) on LPS-induced nitrite level (mmoL/g wet tissue) in rat colon specimens. ANOVA, p < 0.001; post hoc, **p < 0.01 vs. LPS group. Nitrite level was evaluated through Griess assay.
extracts could be partially related to the higher content in emodin which has been reported to exert a stimulatory effect on 5-HT signaling (Mizuno et al.2010). Actually, the protective effects exerted by Asphodeline extracts on rat colon, as revealed by the blunting on tissue levels of all tested biomarkers, could be explained, albeit partially, by the previously reported antiradical and glutathione-S-transferase-stimulating activity (Lazarova et al.2016, Zengin et al.2016b).
The higher efficacy of A. tenuior in reducing H2O2-induced LDH activity and LPS-induced nitrite,
PGE2, and 8-iso-PGF2α, compared to A. damascena and A. cilicica, could be attributed to its phenolic and anthraquinone contents. Indeed, data obtained from the previous studies (Locatelli et al.2017b; Zengin et al. 2016b) found that although A. tenuior did not display the highest total phenolic and flavonoid content among the three species (seeTable 1), it was most abundant in anthraquinones (see
Table 2) especially, chrysophanol and physcione, which could have contributed to its high activity. A previous study by Lin et al. (2015) found that chrysophanol reduced the level of nitric oxide (NO) and PGE2 production by reducing the expression of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2). Chrysophanol was also found to inhibit the production of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and the expression of COX-2 levels induced by LPS (Kim et al.2010). Besides, other compounds present in the extract may interact with each other to contribute to its overall activity. To this regard, we evaluated the effects of the three asphodeline species on IL-6 and TNF-α gene expression, in rat colon. We found that all three species were ineffective in reducing the upregulated IL-6 gene expression induced by LPS (Figure 5), despite there being a reduction trend after treatment with A. tenuior and A. damascena. On the other hand, A. damascena, was able to inhibit LPS-induced TNF-α gene expression, while the other two Asphodelines wereinactive (Figure 5). Considering that A. damascena showed a lower content of chrysophanol compared to A. tenuior, actually (Locatelli et al.
2017b), ourfinding of reduced TNF-α gene expression after A. damascena extract treatment is consistent
0 2 4 6 8 10 12 14
Vehicle LPS ASPHODELINE DAMASCENA
SUBP.RUGOSA MEOH (100 µg/mL) ASPHODELINE TENUIOR MEOH (100µg/mL) ASPHODELINE CICLICA MEOH 100µg/mL) pg /mg we t ti ssue
Prostaglandin E2 8-iso-Prostaglandin F2alpha
**
**
**
**
*
*
*
*
Figure 3.Effect of A. tenuior, damascena and cilicica methanol extracts (100 µg/mL) on LPS-induced prostaglandin E2 (PGE2) and 8-iso-prostaglandin F2α (8-iso-PGF2α) level (pg/mg wet tissue) in rat colon specimens. ANOVA, p < 0.001; post hoc, *p < 0.05; **p < 0.01 vs. LPS group. Prostaglandin levels were evaluated through radioimmunoassay (RIA).
Vehicle LPS ASPHODELINE DAMASCENA SUBP. RUGOSA MEOH (100µg/mL) ASPHODELINE TENUIOR MEOH (100 µg/mL) ASPHODELINE CICLICA MEOH (100 µg/mL) 5-HIIA/5-HT ratio
*
*
**
***
1.40 0.00 1.20 1.00 0.80 0.20 0.40 0.60Figure 4.Effect of A. tenuior, damascena and cilicica methanol extracts (100 µg/mL) on 5HIAA/5-HT ratio in rat colon specimens challenged with LPS. ANOVA, p < 0.001; post hoc, *p < 0.05; **p < 0.01 vs. LPS group. 5HIIA and 5-HT levels were evaluated through liquid chromatography coupled to electrochemical detection.
0 1 2 3 4 5 6 7 Vehicle LPS ASPHODELINE DAMASCENA SUBP. RUGOSA MEOH
(100µg/mL) ASPHODELINE TENUIOR MEOH (100 µg/mL) ASPHODELINE CICLICA MEOH (100 µg/mL) mRNA level (R.Q.) TNF-alpha IL-6
**
** ***
Figure 5.Effect of Asphodeline methanol extracts (100 µg/mL) on TNF-α and IL-6 gene expression in rat colon specimens challenged with LPS. ANOVA, p < 0.01; post-hoc, **p < 0.01; ***p < 0.01 vs. respective LPS group. TNF-α and IL-6 gene expression was evaluated through re.
with its higher content in vanillic acid and gallic acid compared to A. tenuior (Locatelli et al. 2017b; Stanely Mainzen Prince et al.2011; Mohamed and Abd El-Twab2016).
Conclusion
Data obtained from the present study indicates that the three Asphodeline species exerted protective effects in in vitro and ex vivo models of inflammation and oxidative stress. A. tenuior extract was most effective in decreasing most of the tested biomarkers that were elevated by H2O2and LPS. On the
other hand, A. damascene was the most effective in blunting LPS-induced TNF-α gene expression in rat colon. This study adds new insight to the pharmacological actions of Asphodeline species. However, further studies on the isolation and characterization of bioactive compounds responsible for the observed activities are required together with a deeper focus on their molecular pathways.
ORCID
Marcello Locatelli http://orcid.org/0000-0002-0840-825X
References
Achitei D, Ciobica A, Balan G, Gologan E, Stanciu C, Stefanescu G.2013. Different profile of peripheral antioxidant enzymes and lipid peroxidation in active and non-active inflammatory bowel disease patients. Dig Dis Sci. 58:1244–1249.
Altundag E, Ozturk M.2011. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia Soc Behav Sci. 19:756–777.
Brunetti L, Leone S, Orlando G, Ferrante C, Recinella L, Chiavaroli A, Di Nisio C, Shohreh R, Manippa F, Ricciuti A, et al. 2014b. Hypotensive effects of omentin-1 related to increased adiponectin and decreased interleukin-6 in intra-thoracic pericardial adipose tissue. Pharmacol Rep. 66:991–995.
Brunetti L, Orlando G, Ferrante C, Recinella L, Leone S, Chiavaroli A, Di Nisio C, Shohreh R, Manippa F, Ricciuti A, et al.2013. Orexigenic effects of omentin-1 related to decreased CART and CRH gene expression and increased norepinephrine synthesis and release in the hypothalamus. Peptides. 44:66–74.
Brunetti L, Orlando G, Ferrante C, Recinella L, Leone S, Chiavaroli A, Di Nisio C, Shohreh R, Manippa F, Ricciuti A.2014a. Peripheral chemerin administration modulates hypothalamic control of feeding. Peptides. 51:115–121.
Charan J, Kantharia ND. 2013. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 4:303–306.
Chiavaroli A, Brunetti L, Orlando G, Recinella L, Ferrante C, Leone S, Di Michele P, Di Nisio C, Vacca M.2010. Resveratrol inhibits isoprostane production in young and aged rat brain. J Biol Regul Homeost Agents. 24:441.
Table 1.Total phenolic andflavonoid contents of the Asphodeline extracts (Zengin et al.2016b, Locatelli et al.2017b)*. Asphodeline species
Total phenolic content (mgGAE/g extract)
Totalflavonoid content (mgRE/g extract)
A. damascena subsp. rugosa 18.6 ± 0.3 11.8 ± 0.2
A. tenuior subsp. tenuiflora var. tenuiflora
27.5 ± 0.9 27.6 ± 1.3
A. cilicica 49.2 ± 1.5 30.9 ± 1.4
*Values expressed are means ± S.D. of three parallel measurements. GAE: Gallic acid equivalents; RE: Rutin equivalents.
Table 2.Anthraquinones content of the Asphodeline extracts (µg/g) (Zengin et al.2016b, Locatelli et al.2017b)*.
Asphodeline species Aloe-emodine Rheine Emodine Chrysophanol Physcione Total A. damascena subsp. rugosa 9.4 ± 0.8 26.3 ± 2.1 2.3 ± 0.2 171.7 ± 13.7 100.7 ± 8.1 310.3 A. tenuior subsp. tenuiflora var. tenuiflora 5.0 ± 0.4 25.5 ± 2.0 25.6 ± 2.0 249.1 ± 19.9 258.3 ± 20.7 563.5 A. cilicica 7.2 ± 0.9 91.2 ± 10.8 167.2 ± 8.2 - 49.3 ± 0.9 314.9 *Values expressed are means ± S.D. of three parallel measurements.
Ferrante C, Orlando G, Recinella L, Leone S, Chiavaroli A, Di Nisio C, Shohreh R, Manippa F, Ricciuti A, Vacca M. 2016. Central inhibitory effects on feeding induced by the adipo-myokine irisin. Eur J Pharmacol. 791:389–394. Fialkow L, Wang Y, Downey GP.2007. Reactive oxygen and nitrogen species as signaling molecules regulating
neutrophil function. Free Radical Biol Med. 42:153–164.
Gioda A, Fuentes-Mattei E, Jimenez-Velez B.2011. Evaluation of cytokine expression in BEAS cells exposed tofine particulate matter (PM2. 5) from specialized indoor environments. Int J Environ Health Res. 21:106–119. Han X-Z, Ma R, Chen Q, Jin X, Jin Y-Z, An R-B, Piao X-M, Lian M-L, Quan L-H, Jiang J.2018. Anti-inflammatory
action of Athyrium multidentatum extract suppresses the LPS-induced TLR4 signaling pathway. J Ethnopharmacol. 217:220–227.
Ilhan M, Zengin G, Ek AKKOL, Aktümsek A, Süntarİ.2016. The importance of asphodeline species on enzyme inhibition: anti-elastase, anti-hyaluronidase and Anti-collagenase potential. Turkish J Pharm Sci. 13:323–327. Ismail HF, Hashim Z, Soon WT, Ab Rahman NS, Zainudin AN, Majid FAA.2017. Comparative study of herbal
plants on the phenolic andflavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J Tradit Complement Med. 7:452–465.
Kannan N, Guruvayoorappan C.2013. Protective effect of Bauhinia tomentosa on acetic acid induced ulcerative colitis by regulating antioxidant and inflammatory mediators. Int Immunopharmacol. 16:57–66.
Kim HG, Kim M-Y, Cho JY.2018. Alisma canaliculatum ethanol extract suppresses inflammatory responses in LPS-stimulated macrophages, HCl/EtOH-induced gastritis, and DSS-triggered colitis by targeting Src/Syk and TAK1 activities. J Ethnopharmacol. 219:202–212.
Kim S-J, Kim M-C, Lee B-J, Park D-H, Hong S-H, Um J-Y. 2010. Anti-Inflammatory activity of chrysophanol through the suppression of NF-kB/caspase-1 activation in vitro and in vivo. Molecules. 15:6436–6451. Lazarova I, Simeonova R, Vitcheva V, Kondeva-Burdina M, Gevrenova R, Zheleva-Dimitrova D, Zengin G,
Danchev ND.2016. Hepatoprotective and antioxidant potential of Asphodeline lutea (L.) Rchb. roots extract in experimental models in vitro/in vivo. Biomed Pharmacother. 83:70–78.
Lazarova I, Zengin G, Aktumsek A, Gevrenova R, Ceylan R, Uysal S. 2014. HPLC–DAD analysis of phenolic compounds and antioxidant properties of Asphodeline lutea roots from Bulgaria and Turkey. Ind Crops Prod. 61:438–441.
Lee JJ, Chang CK, Liu IM, Chi TC, Yu HJ, Cheng JT.2001. Changes in endogenous monoamines in aged rats. Clin Exp Pharmacol Physiol. 28:285–289.
Lee W, Bae JS. 2018. Inhibitory effects of Kyung-Ok-Ko, traditional herbal prescription, on particulate matter-induced vascular barrier disruptive responses. Int J Environ Health Res. doi:10.1080/ 09603123.2018.1542490
Lin F, Zhang C, Chen X, Song E, Sun S, Chen M, Pan T, Deng X.2015. Chrysophanol affords neuroprotection against microglial activation and free radical-mediated oxidative damage in BV2 murine microglia. Int J Clin Exp Med. 8:3447.
Locatelli M, Ferrante C, Carradori S, Secci D, Leporini L, Chiavaroli A, Leone S, Recinella L, Orlando G, Martinotti S, et al. 2017a. Optimization of aqueous extraction and biological activity of Harpagophytum procumbens root on ex vivo rat colon inflammatory model. Phytother Res. 31:937–944.
Locatelli M, Macchione N, Ferrante C, Chiavaroli A, Recinella L, Carradori S, Zengin G, Cesa S, Leporini L, Leone S, et al.2018. Graminex pollen: phenolic pattern, colorimetric analysis and protective effects in immorta-lized prostate cells (PC3) and rat prostate challenged with LPS. Molecules. 23
Locatelli M, Zengin G, Uysal A, Carradori S, De Luca E, Bellagamba G, Aktumsek A, Lazarova I. 2017b. Multicomponent pattern and biological activities of seven Asphodeline taxa: potential sources of natural-functional ingredients for bioactive formulations. J Enzyme Inhib Med Chem. 32:60–67.
Menghini L, Ferrante C, Leporini L, Recinella L, Chiavaroli A, Leone S, Pintore G, Vacca M, Orlando G, Brunetti L. 2016. An hydroalcoholic chamomile extract modulates inflammatory and immune response in HT29 cells and isolated rat colon. Phytother Res. 30:1513–1518.
Menghini L, Leporini L, Vecchiotti G, Locatelli M, Carradori S, Ferrante C, Zengin G, Recinella L, Chiavaroli A, Leone S, Brunetti L, Orlando G. 2018. Crocus Sativus L. Stigmas and Byproducts: Qualitative Fingerprint, Antioxidant Potentials and Enzyme Inhibitory Activities. Food Res Int. 109:91-98. doi:10.1016/j.foodres.2018.04.028
Mizuno M, Kawamura H, Ishizuka Y, Sotoyama H, Nawa H. 2010. The anthraquinone derivative emodin attenuates methamphetamine-induced hyperlocomotion and startle response in rats. Pharmacol Biochem Behav. 97:392–398.
Mohamed HM, Abd El-Twab SM.2016. Gallic acid attenuates chromium-induced thyroid dysfunction by mod-ulating antioxidant status and inflammatory cytokines. Environ Toxicol Pharmacol. 48:225–236.
Nagarjun S, Dhadde SB, Veerapur VP, Thippeswamy B, Chandakavathe BN. 2017. Ameliorative effect of chromium-d-phenylalanine complex on indomethacin-induced inflammatory bowel disease in rats. Biomed Pharmacother. 89:1061–1066.
Özdemir E, Alpınar K. 2015. An ethnobotanical survey of medicinal plants in western part of central Taurus Mountains: aladaglar (Nigde–Turkey). J Ethnopharmacol. 166:53–65.
Nagib MM, Tadros MG, ELSayed MI, Khalifa AE.2013. Anti-inflammatory and anti-oxidant activities of olme-sartan medoxomil ameliorate experimental colitis in rats. Toxicol Appl Pharmacol. 271:106–113.
Park HH, Park NY, Kim SG, Jeong KT, Lee EJ, Lee E.2015. Potential wound healing activities of Galla Rhois in humanfibroblasts and keratinocytes. Am J Chin Med. 43:1625–1636. Epub 2015/ 12/02.
Pratico D.2002. Alzheimer’s disease and oxygen radicals: new insights. Biochem Pharmacol. Feb 15 63:563–567. Epub 2002/ 05/07.
Regmi SC, Park S-Y, Ku SK, Kim J-A.2014. Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radic Biol Med. Apr;69:377–389. Epub 2014/ 02/15 Sargin SA. 2015. Ethnobotanical survey of medicinal plants in Bozyazı district of Mersin, Turkey.
J Ethnopharmacol. 173:105–126.
Sargin SA, Selvi S, Büyükcengiz M. 2015. Ethnomedicinal plants of Aydıncık district of Mersin, Turkey. J Ethnopharmacol. 174:200–216.
Stanely Mainzen Prince P, Rajakumar S, Dhanasekar K.2011. Protective effects of vanillic acid on electrocardio-gram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur J Pharmacol. 668:233–240.
Tuttolomondo T, Licata M, Leto C, Gargano ML, Venturella G, La Bella S.2014. Plant genetic resources and traditional knowledge on medicinal use of wild shrub and herbaceous plant species in the Etna Regional Park (Eastern Sicily, Italy). J Ethnopharmacol. 155:1362–1381.
Uttara B, Singh AV, Zamboni P, Mahajan R.2009. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 7:65–74.
Verratti V, Brunetti L, Tenaglia R, Chiavaroli A, Ferrante C, Leone S, Orlando G, Berardinelli F, Di Giulio C, Vacca M.2011. Physiological analysis of 8-ISO-PGF2 alpha: a homeostatic agent in superficial bladder cancer. J Biol Regul Homeost Agents. 25:71–76.
Zengin G, Aktumsek A, Girón-Calle J, Vioque J, Megías C.2016a. Nutritional quality of the seed oil in thirteen Asphodeline species (Xanthorrhoeaceae) from Turkey. Grasas Aceites. 67:141.
Zengin G, Aktumsek A, Guler G-O, Cakmak Y-S, Girón-Calle J, Alaiz M, Vioque J.2012. Nutritional quality of protein in the leaves of eleven Asphodeline species (Liliaceae) from Turkey. Food Chem. 135:1360–1364. Zengin G, Aktumsek A, Mocan A, Rengasamy K, Picot C, Mahomoodally M. 2018. Asphodeline cilicica Tuzlaci:
from the plant to its most active part extract and its broad bioactive properties. S Afr J Bot. doi:10.1016/j. sajb.2018.05.014
Zengin G, Locatelli M, Ceylan R, Aktumsek A.2016b. Anthraquinone profile, antioxidant and enzyme inhibitory effect of root extracts of eight Asphodeline taxa from Turkey: can asphodeline roots be considered as a new source of natural compounds? J Enzyme Inhib Med Chem. 31:754–759.
Zengin G, Locatelli M, Stefanucci A, Macedonio G, Novellino E, Mirzaie S, Dvorácskó S, Carradori S, Brunetti L, Orlando G, et al. 2017. Chemical characterization, antioxidant properties, anti-inflammatory activity, and enzyme inhibition of Ipomoea batatas L. leaf extracts. Int J Food Prop. 2017 Dec 29 20;1907–1919
Zhu H, Pu D, Di Q, Zhao X, Ji F, Li H, Zhao Z, Gao J, Xiao W, Chen W.2018. Cirsitakaoside isolated from Premna szemaoensis reduces LPS-induced inflammatory responses in vitro and in vivo. Int Immunopharmacol. 59:384–390.
Zou B, Xiao G, Xu Y, Wu J, Yu Y, Fu M. 2018. Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chem. 255:23–30.