Contents lists available atScienceDirect
Industrial Crops & Products
journal homepage:www.elsevier.com/locate/indcropPhytochemical and biological activities of Silene viridi
flora extractives.
Development and validation of a HPTLC method for quanti
fication of
20-hydroxyecdysone
Nilufar Z. Mamadalieva
a,⁎, Stefan Böhmdorfer
b, Gokhan Zengin
c, Markus Bacher
b,
Antje Potthast
b, Davlat Kh. Akramov
a, Abdulaziz Janibekov
a, Thomas Rosenau
baInstitute of the Chemistry of Plant Substances of the Academy Sciences of Uzbekistan, 100170 Tashkent, Uzbekistan
bUniversity of Natural Resources and Life Sciences, Vienna (BOKU University), Department of Chemistry, Division of Chemistry of Renewables, Konrad-Lorenz-Straße 24,
3430, Tulln, Austria
cDepartment of Biology, Selcuk University, Science Faculty, Konya, Turkey
A R T I C L E I N F O Keywords: Silene viridiflora Essential oil Plant extracts 20-Hydroxyecdysone HPTLC
Enzyme inhibitory activity
A B S T R A C T
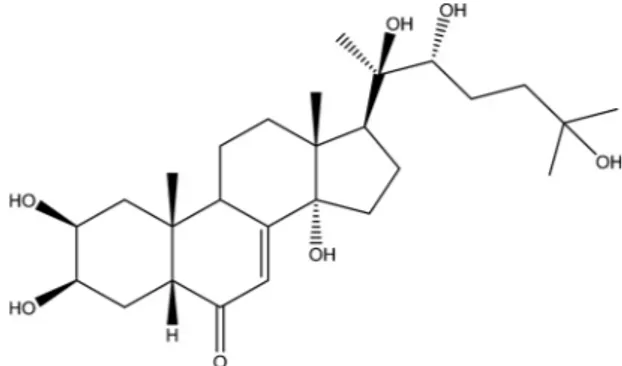
Silene viridiflora (Caryophyllaceae) has adaptogen, tonic, actoprotector and immunomodulator properties that are mainly attributed to ecdysteroids. The aim of the present study is to investigate the chemical constituents of essential oil as well as the 20-hydroxyecdysone content in the methanol extract S. viridiflora and its related biological activity. GC–MS analysis showed the essential oil of S. viridiflora to have a complex composition, which was dominated by methyl palmitate (8.05%), 3-hexen-1-ol (7.54%), 3-hexenyl benzoate (3.86%), β-myrcene (3.80%), 1,1-diethoxy-2-phenylethane (3.65%), hexahydrofarnesyl acetone (3.20%) and 4-terpineol (2.47%). A high performance thin-layer chromatography (HPTLC) method was developed to analyze the me-thanolic extracts of S. viridiflora and to quantify the main ecdysteroid, 20-hydroxyecdysone. Validation showed that the method was accurate over the entire calibration range and resulted in errors of less than 10% in the 100–815 μg/ml concentration range. Antioxidant and enzyme inhibitory potentials of methanol extract and individual compounds of S. viridiflora were evaluated. The results could provide a starting point for designing novel phyto-pharmaceuticals.
1. Introduction
At the present time, there is growing interest all over the world in obtaining bioactive molecules from plants. Thus, there is a need for concerted effort in the investigation of the plants, especially concerning investigation of their biological activities and their chemical composi-tions, isolation of individual biologically-active substances and the development of quantitative and qualitative methods for the analysis of such compounds from those plants.
The genus Silene L. (family Caryophyllaceae) comprises more than 700 species of annual, biennial, and perennial plants, generally dis-tributed in Eurasia, America and Africa (Mamadalieva et al., 2014). Silene viridiflora L. is widespread in Turkey, South, West, and Central Europe, Crimea-Siberia, and East Asia (Yildiz and Çirpici, 2013). It is a perennial herbaceous plant, with a height of 50–120 cm. Previous phytochemical studies of this plant have isolated and identified several ecdysteroids (Mamadalieva et al., 2010b;Simon et al., 2009;Tóth et al.,
2008), lipids (Mamadalieva et al., 2010a), neutral substances, carbo-hydrates, and microelements (Eshmirzaeva et al., 2005). These phyto-chemicals have a range of biological activities. The total ecdysteroid preparation from S. viridiflora acts as an effective immunomodulator in mice leading to secondary immunodeficiency developed under irra-diation as well as acute toxic hepatitis (Shakhmurova et al., 2012). This preparation was also analyzed for actoprotector and adaptogenic ac-tivity in vivo and it increased endurance and reduced the recovery time after a severe physical load. Moreover, the ecdysteroid preparation reduced the stress effects of an extended physical exercises (Shakhmurova et al., 2004; Syrov et al., 2005; Dzakhangirova and Syrov, 2005; Dzakhangirova, 2007). These pharmacological effects have led to the development of ecdysteroid-containing preparations, which are primarily used as legal and non-toxic muscle-promoting substances for bodybuilders. The major active component in such pre-parations is 20-hydroxyecdysone (ecdysterone) (Fig. 1). At this time, S. viridiflora was recognized as a source of 20-hydroxyecdysone (20E) and
https://doi.org/10.1016/j.indcrop.2018.12.041
Received 7 July 2018; Received in revised form 22 November 2018; Accepted 13 December 2018
⁎Corresponding author at: Institute of the Chemistry of Plant Substances, Academy of Sciences of Uzbekistan, Tashkent, 100170, Mirzo Ulugbek Str 77, Uzbekistan. E-mail address:nmamadalieva@yahoo.com(N.Z. Mamadalieva).
Available online 20 December 2018
0926-6690/ © 2018 Elsevier B.V. All rights reserved.
has been cultivated in Uzbekistan since 2004.
We continue to investigate S. viridiflora by studying the chemical composition and biological activities of its essential oil and extracts. We developed and validated a simple and rapid HPTLC quantification method for 20-hydroxyecdysone (20E) in the methanolic extract of S. viridiflora.
2. Materials and methods 2.1. Chemicals
Analytical grade solvents and reagents were used for the study, which were acquired from Merck (Vienna, Austria). 20-Hydroxyecdysone (98.0%) was obtained from the Institute of the Chemistry of Plant Substances (Tashkent, Uzbekistan).
2.2. Plant materials
Aerial parts (flowers, leaves and stems) of S. viridiflora were col-lected from the Botanical Field of the Institute of the Chemistry of Plant Substances (ICPS) (Tashkent, Uzbekistan). The taxonomic authentica-tion was accomplished by the Dr. A. Nigmatullaev at the Department of Herbal Plants of ICPS. The voucher specimen of the plant was deposited in the departmental herbarium under the code 2017/087. Plant mate-rial was air-dried and powdered before use.
2.3. Preparation of the extract
Powdered plant material (200 g) was soaked in 1000 ml of methanol in a round bottomflask at room temperature for 24 h. The mixture was filtered over a Whatman No.1 filter paper. The filtrate was evaporated under vacuum at 40 °C, yielding a crude extract which was freeze-dried to obtain a powder which was stored in airtight containers at 4 °C in the dark for further testing.
2.4. Obtaining of 20-hydroxyecdysone (20E)
20-Hydroxyecdysone was isolated from S. viridiflora as described in our previous studies (Mamadalieva et al., 2003, 2004). 20-Hydro-xyecdysone (20E), C27H44O7: 480.6340; UVλmaxMeOH: 250 nm; HR-MS m/z: for C27H47O7[M + Na]+calcd. 503.29792, found 503.29747; ESI-MS (positive mode): 503 [M + Na]+(100), 481 [M+H]+, 463 [M +H-H2O]+, 445 [M+H-2H2O]+, 427 [M+H-3H2O]+, 409 [M+H-4H2O]+, 385, 301, 157;1H NMR (500 MHz, J/Hz, D2O): 1.38 (Ha-1), 1.88 (He-1), 3.99 (m, Ha-2), 4.07 (m, He-3), 1.75 (Ha-4), 1.75 (He-4), 2.36 (t, H-5), 5.97 (d, J = 2.5, H-7), 3.11 (m, H-9), 1.73 (Ha-11), 1.86 (He-11), 1.96 (Ha-12), 1.90 (Ha-16), 1.75 (Hb-16), 2.34 (m, H-17), 0.87 (s, CH3-18), 1.00 (s, CH3-19), 1.22 (s, CH3-21), 3.43 (d, J = 10.0, H-22), 1.31 (Ha-23), 1.66 (Hb-23), 1.24 (s, CH3-26), 1.24 (s, CH3-27);13C NMR (CD3OD,δ, ppm): 37.46 1), 68.72 2), 68.54 3), 32.81 (C-4), 51.78 (C-5), 206.39 (C-6), 122.12 (C-7), 167.88 (C-8), 35.14 (C-9), 39.28 (C-10), 21.54 (C-11), 32.52 (C-12), 48.65 (C-13), 85.26 (C-14), 31.76 (C-15), 21.54 (C-16), 50.54 (C-17), 18.02 (C-18), 24.38 (C-19), 77.94 (C-20), 21.09 (C-21), 78.41 (C-22), 27.39 (C-23), 42.37 (C-24), 71.29 (C-25), 29.12 (C-26), 29.61 (C-27) (Mamadalieva, 2013). 2.5. Analysis of essential oil composition
2.5.1. Essential oil isolation
The fresh aerial parts of S. viridiflora (200 g) were hydrodistilled for 2 h using a Clevenger-type apparatus. As an only small amount of es-sential oil was present, it was trapped in dichloromethane, which was dried over anhydrous sodium sulfate and stored at -4 °C in the dark until use.
2.5.2. GC–MS analysis
GC–MS characterization of S. viridiflora oils was carried out as previously described (Mamadalieva et al., 2017) using an Agilent 7890B gas chromatograph equipped with a VF-Wax CP 9205 fused si-lica column (100% polyethylene glycol, 30 m × 0.25 mm,film thick-ness 0.25μm, Agilent Technologies, Netherlands), interfaced with an Agilent mass selective detector 5977 A (Agilent Technologies). 2.6. Method development and validation
2.6.1. HPTLC instrumentation and condition
Chromatography was performed on HPTLC plates (20 cm × 10 cm) pre-coated with silica gel 60 F254, 0.20 mm layer thickness (Merck, Germany). Standard solutions and samples were applied as 8 mm bands, starting 20 mm from the left edge and 8 mm from the bottom edge with 11.4 mm of track distance, using an Automatic TLC sampler 4 (Camag, Muttenz, Switzerland) and nitrogen as a nebulizer gas. The development of the plates was carried out in an Automatic Developing Chamber (Camag, Muttenz, Switzerland) with 10 ml of THF / toluene / 1 mM TFA in MeOH / H2O (16:8:2:1, v/v/v/v) to a distance of 7 cm in a twin-through chamber (20 cm × 10 cm) at room temperature and am-bient humidity (43 to 44%) and then dried under a stream of air au-tomatically. For densitometric detection a TLC scanner 3 (Camag, Muttenz, Switzerland) was used in the reflectance absorbance mode at 250 nm. VisionCATS 2.4 software (Camag, Switzerland) was used for instrument control and data evaluation.
2.6.2. Quantification
A stock solution of 20E was prepared by dissolving 2 mg of 20E in 2 ml of methanol (final concentration 1 mg/ml). From this stock solu-tion three independent sets of standard solusolu-tions were prepared with concentrations of 10, 50, 100, 300, 500 and 1000μg/ml. For quanti-tative HPTLC analysis, these calibration standards were applied (2μl) and developed together with the analytes. After detection, a Michaelis-Menten function wasfit through the detected peak heights over con-centration and used to calculate the concon-centration of 20E in the plant samples.
2.6.3. Validation
Validation was performed according to the protocol of SFSTP (Société Française des Sciences et Techniques Pharmaceutiques), which characterizes the performance of an analysis over a concentration range (Hubert et al., 2004, 2007). To this end, triplicates of the standard series were analysed three times on separate plates. The standards were applied to the plates in random order. For each plate separately, the concentration of each standard was calculated from a calibrationfit through the remaining standard levels and related to the expected concentration to determine the bias of the quantification. This in-formation was then used to calculate average bias, expectation toler-ance intervals (set at 0.8) as well as upper and lower limits of quanti-tation using Neolicy software (version 1.8.2.2, Mâcon, France). Recovery was performed by the standard addition method.
2.6.4. Determination of 20-hydroxyecdysone in crude extracts of S. viridiflora
The methanol extract of S. viridiflora was dissolved in chloroform : methanol : water (20:20:5, v/v/v) (20 mg/ml). Afterfiltration through a 0.45μm nylon membrane filter, the solution was applied to an HPTLC plate and analysed according to the conditions described in Section 2.6.1. The concentration of 20E in each sample was calculated using a standard curve constructed from co-developed standards. Each sample was analyzed in triplicate. The content of 20E was expressed in terms of % dry weight of crude extract.
2.7. Total bioactive components
The phenolic content was established according to the Folin-Ciocalteau colorimetric method (Slinkard and Singleton, 1977) and expressed as gallic acid equivalents (GAE/g extract). In addition, the amount of total flavonoid content was determined according to the AlCl3method described byBerk et al. (2011)and was evaluated as rutin equivalents (RE/g extract).
2.8. Antioxidant activity
The antioxidant activity of the MeOH extract of S. viridiflora was evaluated using different assays: free radical scavenging (DPPH), re-ducing power (CUPRAC and FRAP), phosphomolybdenum, and metal chelating. The results of these assays were expressed as trolox equiva-lents (TE/g extract). Metal chelating activity was evaluated as EDTA equivalent (mg EDTA/g extract). The experimental procedures were as previously described (Zengin et al., 2015).
2.9. Enzyme inhibitory activity
Enzyme inhibitory effects were investigated against acet-ylcholinesterase (AChE), butyracet-ylcholinesterase (BChE), tyrosinase, α-amylase, andα-glucosidase. The experimental procedures of these as-says were as previously described (Uysal et al., 2016;Zengin, 2016). The enzyme inhibitory effects were evaluated as standard compound equivalents. Briefly, galanthamine was used for AChE and BChE; kojic acid for tyrosinase and acarbose forα-amylase and α-glucosidase. 3. Results
3.1. Chemical composition of the essential oil
The essential oil from the aerial parts of S. viridiflora were analysed by GC–MS. 75 organic compounds were identified representing 82.18% of the total peak area. The identified chemical compounds are listed in Table 1according to their elution order on a polar VF-Wax capillary column. The oil represents a complex mixture consisting of mainly oxygenated monoterpenes, benzenoids and fatty acid esters. The major organic compounds according to content (with percentages above 1%) were methyl palmitate (8.05%), 3-hexen-1-ol (7.54%), 3-hexenyl benzoate (3.86%), β-myrcene (3.80%), 1,1-diethoxy-2-phenylethane (3.65%), hexahydrofarnesyl acetone (3.20%), 1,1-dimethoxynonane (2.91%), linalool (2.61%), 4-terpineol (2.47%), p-cymene (2.14%), benzyl alcohol (1.72%), 1-hexanol (1.52%), methyl myristate (1.45%), α-campholenal (1.31%), and β-farnesene (1.27%).
3.2. Densitometric quantification of 20E by HPTLC
The objective of method development was to identify and quantify the 20E in methanol extracts by HPTLC. HPTLC offers the advantage of superb robustness and matrix independence (no poisoning of capillaries or columns) while offering striking simplicity and cost-effectiveness as well as similar accuracy levels as HPLC. By a structured solvent screening according to Reich and Schibli (2007), an eluent was
Table 1
Essential oil composition of Silene viridiflora.
RT RIc RIr Compound Peak area, (%)
8.06 1174 1180 Myrcene 3.80 9.76 1218 1216 trans-2-Hexenal 2.87 10.52 1242 1240 γ-Terpinene 0.83 11.25 1266 1268 p-Cymene 2.14 11.63 1278 1280 Terpinolene 0.97 12.76 1316 1316 cis-3-Hexenyl acetate 0.80 13.72 1349 1348 1-Hexanol 1.52 14.16 1364 1366 1,1-Dimethoxyoctane 0.56 14.62 1380 1378 cis-3-Hexenol 7.54 14.80 1386 Bornyl chloride* 0.64 15.20 1400 1400 trans-2-Hexen-1-ol 1.17 16.02 1431 1431 p-Cymenene 0.23 16.14 1435 2,6,10-Trimethyl tridecane* 1.03 16.42 1446 1447 1-Octen-3-ol 0.29 16.94 1465 1465 1,1-Dimethoxynonane 2.91 17.39 1482 1482 α-Campholenal 1.31 17.54 1487 1486 Methyl nonanoate 0.45 18.20 1513 1512 Benzaldehyde 0.52 18.63 1530 Dimethyl acetal benzaldehyde* 0.98 18.97 1544 1543 Linalool 2.61 19.16 1551 1552 1-Octanol 0.90 19.52 1566 1567 Dimethyl acetal decylaldehyde 0.65 20.13 1590 1593 Methyl decanoate 0.35 20.21 1593 1595 4-Terpineol 2.47 20.05 1587 1587 trans-Caryophyllene tr 20.77 1617 1616 Myrtenal 0.32 21.22 1636 1636 trans-2-Decenal 0.71 21.43 1644 1647 Pinocarveol 1.20 21.63 1653 1653 n-Nonanol 0.53 21.82 1661 1660 β-Farnesene 1.27 22.18 1676 1690 1,1-Diethoxy-2-phenylethane* 3.65 22.44 1687 1689 α-Terpineol 0.44 23.47 1733 1732 1,1,6-Trimethyl-1,2-dihydronaphthalene 0.17 23.58 1738 1738 Neryl acetate 0.19 23.70 1743 1743 α-Farnesene 0.70 24.13 1762 1762 Methyl salicylate 1.14 24.87 1795 1793 Methyl dodecanoate 1.09 24.98 1800 1802 2,4-Decadienal 0.31 25.18 1809 1811 β-Damascenone 0.65 25.49 1824 1825 trans-Carveol 0.54 25.78 1838 1838 p-Cymen-8-ol 0.52 25.95 1846 1851 Methyl phenethyl ketone 0.29 26.32 1863 1869 Benzyl alcohol 1.72 27.05 1898 1899 Phenethyl alcohol 0.45 27.49 1918 1915 Neophytadiene 0.44 27.64 1925 1926 β-Ionone 0.37 27.69 1928 1914 cis-Jasmone * 0.29 28.70 1975 1977 trans-β-Ionone-5,6-epoxide 0.41 28.95 1987 1987 Perilla alcohol 0.38 29.18 2003 2000 Methyl myristate 1.45 29.73 2031 2027 Isopropyl myristate 0.46 30.33 2062 2066 Hexyl benzoate 0.36 30.86 2089 2084 10-epi-γ-Eudesmol 0.41 31.19 2106 2108 Methyl pentadecanoate 0.77 31.31 2113 2119 3-Hexenyl benzoate 3.86 31.42 2119 2125 Hexahydrofarnesyl acetone 3.20 32.10 2155 2166 γ-Eudesmol* 0.29 32.19 2160 2166 Methyl 14-methylpentadecanoate 0.14 33.16 2212 2216 Methyl palmitate 8.05 34.07 2263 2245 Methyl palmitoleate* 0.62 34.37 2280 Methyl isoheptadecanoate* 0.44 34.65 2296 2300 n-Tricosane 0.22 34.75 2302 trans-Sobrerol* 0.21 34.92 2312 2309 Methyl heptadecanoate 0.41 36.73 2417 2418 Methyl stearate 0.38 37.06 2437 2445 Methyl elaidate* 0.73 37.84 2484 2482 Methyl linoleate 0.72 38.04 2496 2500 n-Pentacosane 0.17 38.79 2543 2541 Vanillin 0.23 38.92 2551 2550 Methyl linolenate 0.94 39.31 2576 2581 1-Octadecanol 0.17 39.73 2602 2606 Phytol 0.52 39.85 2610 2613 Benzyl benzoate 0.79 40.08 2625 2617 Methyl eicosanoate 0.41
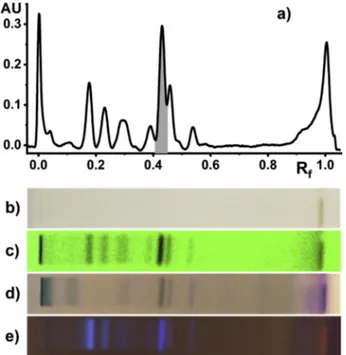
developed with the aim to maximize resolution and number of sepa-rated peaks. WhileReich and Schibli (2007)proposed formic acid as a polar component, this was replaced in our work by a 1 mM solution of TFA in methanol which gave optimum results. It was beneficial in terms of solvent volatility and prevention of irregular solvent fronts while retaining a selectivity similar to formic acid. The peak of 20E at Rf= 0.43 was identified by comparison with an authentic sample iso-lated from S. viridiflora. Densitometric detection of 20E was performed at 250 nm, i.e. the adsorption maximum of 20E (Figs. 2 and 3). The amount of marker compound was calculated using a Michaelis-Menten regression of the calibration curves (Fig. 4).
3.3. Method validation
Validation was based on the protocol of SFSTP, which predicts the performance of an analysis based on a set of validation samples, de-scribing the performance of the analytical method over the entire in-vestigated range (Hubert et al., 2004, 2007). Compared to the more commonly used validation according to ICH (International Council for Harmonisation), it is also applicable to non-linear detectors, such as the scanning densitometer used in this project. Three sets of standard samples covering a concentration range of 10 to 1000μg/ml were analysed in triplicate, and the heights and areas of the detected peaks of 20E were evaluated separately. The lowest standard of 10μg/ml could not be detected reliably and was therefore removed from the data set. The average of the determined value at each concentration was used to describe the bias of the analysis. Quantitation by peak height. It was
found give an almost perfect outcome, while there were considerable deviations at low concentrations when using the peak areas. The ana-lysis by peak height was accurate over the entire calibrated range, that by peak area from 300 to 1000μg/ml. Correlation of the measured concentrations with the known concentrations was 0.9917 according to peak height and 0.9904 according to peak area, the slopes of the cor-relation being 1.0106 and 1.0117 for peak height and peak area, re-spectively. The expectation tolerance interval used in this validation predicts the range of future measurements; the intersections of this interval with the deviation considered acceptable define the Lower and Upper Limits of Quantitation (LLQ and ULQ). Again, quantitation by height yielded better results, with an LLQ of 100μg/ml and a ULQ of 815μg/ml for a deviation of 10%. For an allowed deviation of 20%, the LLQ was 70μg/ml and the ULQ was above the highest investigated concentration of 1000μg/ml. For quantitation by area, the LLQ for 10% was markedly higher at 200μg/ml (85 μg/ml at 20%), while the ULQ was found to be 715μg/ml at 10% and above 1000 μg/ml at 20%. In Table 1 (continued)
RT RIc RIr Compound Peak area, (%)
44.11 2897 2903 n-Hexadecoic acid 0.91
Total identified 82.18
RIc- calculated retention indices relative to C
10-C38n-alkanes on the VF-Wax column, RIr- reported retention induces for Wax column, * - compound iden-tified by MS, tr – trace (less than 0.1% of the peak area).
Fig. 2. HPTLC measurements of a methanol extract of S. viridiflora. The high-lighted peak at Rf = 0.43 corresponds to 20-hydroxyecdysone (20E). a) Densitogram at 250 nm; b) visual detection, transmittance mode, unstained; c) 254 nm, unstained; d) visual detection, transmittance mode, after staining with anisaldehyde; e) 366 nm after staining with anisaldehyde.
Fig. 3. Chromatogram obtained from separation of S. viridiflora methanol ex-tract (leftmost lane) and 20E standards of different concentrations (1000-10 μg/ ml). Visualization under UV light at 254 nm before derivatization.
conclusion, quantitation both by peak area and by height is accurate and sufficiently precise for the analysis of plant extracts; calibration by height being superior in terms of accuracy, expected error and quan-titation range (Table 2andFig. 5).
3.4. Results of antioxidant assays
The antioxidant properties of the methanolic extract of S. viridiflora were examined by different assays, including DPPH free radical scavenging, reducing power (CUPRAC and FRAP), phosphomo-lybdenum and metal chelating tests. Total phenolic and flavonoid content were also measured. The results of the tests are summarized in Table 3. Total amounts of phenolic and flavonoids were found to be 20.91 mgGAE/g and 23.40 mgRE/g, respectively. Based on our results, the methanol extract exhibited moderate antioxidant properties in these assays. The extract was more active on cupric ion (34.37 mgTE/g) than ferric ion (22.19 mgTE/g) in the reducing power assays. Also, the ex-tract was a good metal chelator with the value of 11.23 mgEDTAE/g. 3.5. Results of enzyme inhibitory assays
The enzyme inhibitory properties of methanolic extract and the major compounds (20-hydroxyecdysone, 2-deoxy-20-hydroxyecdysone and 2-deoxyecdysone) of S. viridiflora against cholinesterases (acet-ylcholinesterase (AChE), butyr(acet-ylcholinesterase (BChE)), tyrosinase, amylase and glucosidase were comparatively evaluated. The results are shown inTable 4. Both the studied extract and the isolated compounds exhibited considerable enzyme inhibitory potentials. For example, anti-acetylcholinesterase ability can be ranked in the order 20-hydro-xyecdysone > 2-deoxy-20-hydro20-hydro-xyecdysone > MeOH extract > 2-deoxyecdysone. Noteably, the MeOH extract had the greatest anti-tyr-osinase and anti-amylase effects. 2-Deoxyecdysone showed the weakest inhibitory effects against all tested enzymes.
4. Discussion
Hydrodistillation of S. viridiflora gave dark yellow oils. There are
very few studies on essential oil compositions of Silene species in the literature. It has been reported recently, that the major components of essential oils of Silene species are benzenoids, terpenoids, fatty acid derivatives, phenyl propanoids and nitrogen-containing components (Mamadalieva et al., 2014). According to the report of Azadi and Sohrabi (2015)the main constituents of the essential oils of S. morganae were benzaldehyde (11.6%), (Z)-3-hexenyl acetate (9.6%), (E)- β-oci-mene (8.2%) and linalool (7.4%), whileVivek et al. (2008)observed in the essential oil of S. armeria 1-butene (39.20%), methylcyclopropane (21.48%), 2-butene (17.97%) and caryophyllene oxide (7.20%) as the main constituents. The results of previous studies and ourfindings in-dicate that the oxygenated monoterpenes, benzenoids and esters of fatty acids are major constituents of essential oils of Silene species.
The major active component in Silene species is 20-hydro-xyecdysone (Mamadalieva, 2012). Detection of 20E in plant extracts by TLC is usually performed byfluorescence quenching at 254 nm, and by visualization using the vanillin / sulfuric acid reagent and observing the bluefluorescent reaction product under UV at 366 nm (Chen et al., 2014). A few reports have been published on the determination and quantification of 20E in plants using HPTLC.Jadhav et al. (2007) de-veloped an HPTLC method for quantification of 20-hydroxyecdysone in methanolic extract of Sida rhombifolia; also, HPTLCfingerprints for six different Sida species were obtained. In this study the separation of 20-hydroxyecdysone was achieved in chloroform / methanol (8:2 v/v) as the developing solvent (Rf= 0.37) followed by detection at 250 nm in reflectance/absorbance mode. Yoo et al. (2017) performed simulta-neous HPTLC analysis of 20E in Kochia scoparia extract using chloro-form / methanol / water (7:3:0.5, v/v/v) (Rf= 0.60) as the mobile phase. Quantitative determination of the 20E content in Sesuvium por-tulacastrum was done by HPTLC according to a method by Muchate et al. (2017). They reported that a better separation of 20E was achieved on a TLC plate with ethanol / ethyl acetate / water (8:2:0.5) and developing using a solvent system consisting of chloroform / me-thanol / benzene (12.5:2.5:1.5) (Rf= 0.30) followed by detection by fluorescence quenching at 254 nm. Considering the advantages of Table 2
Figures of merit of the analytical method.
by Height by Area
10% error 20% error 10% error 20% error
LLQ 100μg/ml 70μg/ml 200μg/ml 85μg/ml ULQ 815μg/ml > 1000μg/ ml 715μg/ml > 1000μg/ml Slope of calculated vs. true concentrations 0.9979 1.0106 1.0239 1.0117 R2 0.9960 0.9917 0.9909 0.9904
Fig. 5. Accuracy profiles of the quantitation of 20E at 250 nm (left: based on peak height; right: based on peak area). The black solid line indicates the average relative bias of nine de-terminations at each level; dashed black lines indicate the expectation tolerance interval at a level of 0.8; dashed and dotted grey lines in-dicate the acceptable relative error of 10 and 20%, respectively (For interpretation of the references to colour in thisfigure legend, the reader is referred to the web version of this article).
Table 3
Antioxidant properties for MeOH extract of S. viridiflora.
Parameters Results for MeOH extract of S. viridiflora Total phenolic content (mg GAE/g extract) 20.91 ± .187 Totalflavonoid content (mg RE/g extract) 23.40 ± 1.84 Phosphomolybdenum (mmol TE/g extract) 1.91 ± 0.24 DPPH (mg TE/g extract) 12.83 ± 1.17 CUPRAC (mg TE/g extract) 34.37 ± 2.98 FRAP (mg TE/g extract) 22.19 ± 0.10 Metal chelating activity (mg EDTAE/g extract) 11.23 ± 0.05
*Values expressed are means ± S.D. of three parallel measurements. GAE: Gallic acid equivalent; RE: Rutin equivalent; TE: Trolox equivalent; EDTAE: EDTA equivalent.
HPTLC, i.e. simple method setup, short analysis time and low cost per analysis requiring only a small quantity of sample, quantitative de-termination of the 20E content in S. viridiflora was carried out in our approach according to the validated HPTLC method as presented above (Fig. 2–4). The mobile phase THF / toluene with 1 mM TFA in MeOH / H2O (16:8:2:1, v/v/v/v) gave optimum separation of 20E (Rf= 0.43). The quantities of 20E in the aerial parts of S. viridiflora are reported in Table 2andFig. 4. The developed method might also be useful in fin-gerprinting analysis of other Silene species and herbal preparations.
Because a single method can only provides a limited picture with regard to antioxidant properties, several complementary assays were performed in the present study. DPPH, being a stable radical, is widely using to detect free radical scavenging abilities of plant extracts or pure compounds. Antioxidant compounds can scavenge DPPH by donating electrons or hydrogen atoms, turning the purple DPPH radical into the non-radical reduced species. This transformation is recorded at 515–517 nm. The studied MeOH extract possesses DPPH scavenging ability and this value was found to be 12.83 mg TE/g. This value is comparable to the values reported in the literature for other Silene species such as S. italica (43 mgTE/g) and S. supina (72 mgTE/g) (Zengin et al., 2018a). Reducing power is an important indicator in the antioxidant mechanism, which reflects to electron-donating ability. In the present work, CUPRAC (reduction from Cu2+to Cu+) and FRAP (reduction from Fe3+ to Fe2+) assays were performed to assess the reducing ability of the MeOH extract. The extract exhibited reducing abilities in the assays. However, the reported values were lower than that of some Silene species, for example S. alba (92 mgTE/g for CU-PRAC; 64 mgTE/g for FRAP) and S. italica (87 mgTE/g for CUCU-PRAC; 58 mgTE/g for FRAP) (Zengin et al., 2018a). Similar to the CUPRAC and FRAP assays, the phosphomolybdenum assay is based on the reduction of Mo (VI) to Mo (V) by antioxidants in acidic pH. This assay is also considered as a total antioxidant assays because also non-phenolic an-tioxidants such as ascorbic acid and terpenoids are registered. In the present study, the tested MeOH extract was effective in the phospho-molybdenum assay, giving 1.91 mmol TE/g extract. The obtained value was higher than that of six Silene species (1–1.5 mmol TE/g extract) reported byZengin et al. (2018a). Also, the value for total antioxidant capacity was superior to that of different plant extracts such as Cen-taurea saligna (Zengin et al., 2018b), Iris schachtii (Mocan et al., 2017) and Ballota macrodonta (Uysal et al., 2018). Transition metals are playing destructive role in lipid peroxidation and at this point, the chelating of these metals is considered an important antioxidant me-chanism. Metal chelating ability of the tested MeOH extract was de-tected by ferrozine assay and this extract has good potential as a metal chelator.
Taken together, our findings agree with our earlier observations, which showed that some Silene species exhibited considerable anti-oxidant ability (Boğa, 2017;Foudah, 2017;Golea et al., 2017;Jamali, 2011). The antioxidant abilities observed for Silene species in earlier studies may be linked to phenolic compounds other than ecdysteroids (Golea, et al., 2017;Mamadalieva et al., 2011). The molecular structure of ecdysteroids is not suit for anti-oxidative reactions (Mamadalieva, 2012). In this context, observed antioxidant potential of S. viridiflora MeOH extract may be rather explained by the presence of phenolics.
The prevalence of several diseases have reached alarming rates during the past decades and they are considered as global health pro-blems. For example, about half a million people are affected with dia-betes mellitus in 2014 as globally and this number is dramatically in-creasing day by day (WHO, 2016). In this context, effective strategies are required to manage these global diseases, and amongst probable strategies, key enzyme inhibition is considered to be a very useful, ef-fective and safe approach (Gonçalves and Romano, 2017). These theory targets certain enzymes (for example, cholinesterase for Alzheimer’s disease; amylase and glucosidase for diabetes mellitus; tyrosinase for hyperpigmentation problems) and the inhibition of these enzymes is expected to alleviate the observed symptoms of these diseases (Braidy et al., 2017; Etxeberria et al., 2012). Several inhibitors (tacrine for cholinesterase; acarbose for amylase and glucosidase; kojic acid for tyrosinase) are chemically produced, but most of them exhibited un-pleasant side effects, such as gastrointestinal disturbances, diarrhea and toxicity (Murray et al., 2013;Lee et al., 2016). From this perspective, natural enzyme inhibitors are gaining importance for managing such diseases. To this end, we investigated enzyme inhibitory potentials of MeOH extract and major compounds (20-hydroxyecdysone, 2-deoxy-20-hydroxyecdysone and 2-deoxyecdysone) of S. viridifolia. Apparently, all samples have inhibitory potential against the tested enzymes (except for MeOH extract, which was not active on BChE). 20-Hydroxyecdysone and 2-deoxy-20-hydroxyecdysone exhibited stronger cholinesterase inhibitory effects (both AChE and BChE) as compared other studied samples. Thesefindings were also corroborated by other researchers, who reported the 20-hydroxyecdysone skeleton to have noticeable cholinesterase inhibitory effects (Nejma et al., 2015). The studied MeOH extract had an excellent tyrosinase inhibitory potential, followed by 2-deoxy-20-hydroxyecdysone, 20-hydroxyecdysone and 2-deox-yecdysone. The MeOH extract was also the most active on amylase. This is thefirst scientific report on the enzyme inhibitory potentials of S. viridiflora. We hope that it provides valuable contributions for designing novel phyto-pharmaceuticals.
5. Conclusions
Our phytochemical studies of S. viridiflora have isolated and iden-tified ecdysteroids, lipids, volitile compounds and this plant was re-cognized as a source of major ecdysteroid 20-hydroxyecdysone (20E). For thefirst time we quantified the 20E in S. viridiflora by HPTLC. The developed method was fully validated and successfully used to quan-titate 20E in plants. S. viridiflora and its components exhibited con-siderable biological activity. The present study is thefirst report re-garding enzyme inhibitory potential of the isolated ecdysteroids, which could open new avenues to phytopharmaceutical products from S. vir-idiflora. However, further studies are needed to better understand bio-logical availability and pharmacobio-logical potential of this plant. References
Azadi, B., Sohrabi, Y., 2015. Chemical composition of Silene morganae Freyn volatile oil. Nat. Prod. Res. 29, 791–794.
Berk, S., Tepe, B., Arslan, S., Sarikurkcu, C., 2011. Screening of the antioxidant,
Table 4
Enzyme inhibitory effects of the components from S. viridiflora*.
Sample AChE inhibition (mg GALAE/g sample)
BChE inhibition (mg GALAE/g sample)
Tyrosinase inhibition (mg KAE/g sample)
Amylase inhibition (mmol ACAE/g sample)
Glucosidase inhibition (mmol ACAE/g sample)
MeOH extract 1.43 ± 0.05 na 59.78 ± 0.48 0.63 ± 0.04 0.24 ± 0.01 20-Hydroxyecdysone 1.54 ± 0.01 1.77 ± 0.07 19.65 ± 2.78 0.10 ± 0.03 0.12 ± 0.02 2-Deoxy-20-hydroxyecdysone 1.49 ± 0.01 1.86 ± 0.04 20.34 ± 0.39 0.10 ± 0.03 0.07 ± 0.01 2-Deoxyecdysone 0.55 ± 0.01 0.61 ± 0.01 6.36 ± 0.20 0.05 ± 0.01 0.02 ± 0.01
* Values expressed are means ± S.D. of three parallel measurements. GALAE: Galanthamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; na: not active.
antimicrobial and DNA damage protection potentials of the aqueous extract of Asplenium ceterach DC. Afr. J. Biotech. 10, 8902–8908.
Boğa, M., 2017. Chemical constituents, cytotoxic, antioxidant and cholinesterases in-hibitory activities of Silene compacta (Fischer) extracts. Marmara Pharm. J. 21, 445–454.
Braidy, N., Poljak, A., Jayasena, T., Sachdev, P., 2017. Natural Plant‐Derived Acetylcholinesterase Inhibitors: Relevance for Alzheimer’s Disease. Natural Products Targeting Clinically Relevant Enzymes. Wiley‐VCH Verlag GmbH & Co., KGaA, pp. 297–318.
Chen, S., Marston, A., Stuppner, H., 2014. Handbook of Chemical and Biological Plant Analytical Methods. John Wiley & Sons, USA 1176 pp.
Dzakhangirova, M., 2007. The Pharmacologic Investigation of the Sum Ecdysteroid Preparations Obtained from Silene brahuica, Silene viridiflora and Ajuga turkestanica Plants as Actoprotector Means. PhD thesis. Tashkent Medical Academy, Tashkent, Uzbekistan.
Dzakhangirova, M.A., Syrov, V.N., 2005. Experimental evaluation of effect of stimulation of sum of ecdysteroids from Silene brachuica and S. viridiflora for erythropoiesis in laboratory animals. Pathology 2, 7–9.
Eshmirzaeva, N.E., Khidyrova, N.K., Khodzhaeva, M., Mezhlumyan, L.G., Shakhidoyatov, K.M., 2005. Chemical composition of Silene viridiflora. Chem. Nat. Comp. 41, 451–453.
Etxeberria, U., de la Garza, A.L., Campión, J., Martinez, J.A., Milagro, F.I., 2012. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Therap. Targets 16, 269–297.
Foudah, A.I., 2017. Pharmacognostic standardization, phenolic and in vitro antioxidant activity of Silene villosa (Family: Caryophyllaceae). Asian J. Pharm. Res. Health Care 9, 106–111.
Golea, L., Benkhaled, M., Lavaud, C., Long, C., Haba, H., 2017. Phytochemical compo-nents and biological activities of Silene arenarioides Desf. Nat. Prod. Res. 31, 2801–2805.
Gonçalves, S., Romano, A., 2017. Inhibitory properties of phenolic compounds against enzymes linked with human diseases. In: Soto-Hernández, M. (Ed.), Phenolic Compounds-Biological Activity. InTech., London, pp. 581–770.
Hubert, P., Nguyen-Huu, J.J., Boulanger, B., Chapuzet, E., Chiap, P., Cohen, N., Compagnon, P.A., Dewe, W., Feinberg, M., Lallier, M., Laurentie, M., Mercier, N., Muzard, G., Nivet, C., Valat, L., 2004. Harmonization of strategies for the validation of quantitative analytical procedures, a SFSTP proposal– part I. J. Pharm. Biomed. Anal. 36, 579–586.
Hubert, P., Nguyen-Huu, J.J., Boulanger, B., Chapuzet, E., Cohen, N., Compagnon, P.A., Dewé, W., Feinberg, M., Laurentie, M., Mercier, N., Muzard, G., Valat, L., Rozet, E., 2007. Harmonization of strategies for the validation of quantitative analytical pro-cedures, a SFSTP proposal– part III. J. Pharm. Biomed. Anal. 45, 82–96.
Jadhav, A.N., Rumalla, C.S., Avula, B., Khan, I.A., 2007. HPTLC method for determination of 20-hydroxyecdysone in Sida rhombifolia L. and dietary supplements.
Chromatographia 66, 797–800.
Jamali, R., 2011. Study of saponins and phenolic compounds and their antioxidant ac-tivities in some Silene species. Clin. Biochem. 44, S351–S352.
Lee, S.Y., Baek, N., Nam, T.G., 2016. Natural, semisynthetic and synthetic tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 31, 1–13.
Mamadalieva, N.Z., 2012. Phytoecdysteroids from Silene plants: distribution, diversity and biological (antitumour, antibacterial and antioxidant) activities. BLACPMA 11, 474–497.
Mamadalieva, N.Z., 2013. Phytoecdysteroids: chemistry and occurrence. Natural Compounds: Plant Sources, Structure and Properties 6. Springer, USA, pp. 1–308.
Mamadalieva, N.Z., Zibareva, L.N., Saatov, Z., Lafont, R., 2003. Phytoecdysteroids of Silene viridiflora. Chem. Nat. Comp. 39, 199–203.
Mamadalieva, N.Z., Zibareva, L.N., Evrard-Todeschi, N., Girault, J.-P., Maria, A., Ramazanov, N.Sh., Saatov, Z., Lafont, R., 2004. New minor ecdysteroids from Silene viridiflora. Collection of Czechoslovak. Chem. Commun. 69, 1675–1680.
Mamadalieva, N.Z., Janibekov, A.A., Lafont, R., Girault, J.-P., 2010b. Two minor phy-toecdysteroids of the plant Silene viridiflora. Nat. Prod. Comm. 5, 1579–1582.
Mamadalieva, N.Z., Ul’chenko, N.T., Yuldasheva, N.K., Egamberdieva, D.R., Zhanibekov, A.A., Dzhukharova, M.K., Glushenkova, A.I., 2010a. Fatty-acid composition and an-tibacterial activity of CHCl3extracts of three plants of the genus Silene. Chem. Nat. Comp. 46, 95–96.
Mamadalieva, N.Z., El-Readi, M.Z., Janibekov, A.A., Tahrani, A., Wink, M., 2011.
Phytoecdysteroids of Silene guntensis and their in vitro cytotoxic and antioxidant ac-tivity. Zeitschrift für Naturforschung C 66, 215–224.
Mamadalieva, N.Z., Lafont, R., Wink, M., 2014. Diversity of secondary metabolites of the genus Silene (Caryophyllaceae) - a review. Diversity 6, 415–499.
Mamadalieva, N.Z., Abdullaeva, N.S., Rosenau, T., Fakhrutdinova, M., Azimova, S.S., Böhmdorfer, S., 2017. Composition of essential oils from four Apiaceae and Asteraceae species growing in Uzbekistan. Nat. Prod. Res. 32, 1118–1122.
Mocan, A., Zengin, G., Mollica, A., Uysal, A., Gunes, E., Crişan, G., Aktumsek, A., 2017. Biological effects and chemical characterization of Iris schachtii Markgr. extracts: a new source of bioactive constituents. Food Chem. Toxicol. 112, 448–457.
Muchate, N.S., Kadam, N.S., Rajurkar, N.S., Nikam, T.D., 2017. High-performance thin-layer chromatography and indirect TLC-HRMS-based determination of 20-hydro-xyecdysone in Sesuvium portulacastrum. J. Planar Chromatogr. 30, 193–198.
Murray, A.P., Faraoni, M.B., Castro, M.J., Alza, N.P., Cavallaro, V., 2013. Natural AChE inhibitors from plants and their contribution to Alzheimer’s disease therapy. Curr. Neuropharmacol. 11, 388–413.
Nejma, A.B., Nguir, A., Jannet, H.B., Daïch, A., Othman, M., Lawson, A.M., 2015. New septanoside and 20-hydroxyecdysone septanoside derivative from Atriplex portula-coides roots with preliminary biological activities. Bioorg. Med. Chem. Lett. 25, 1665–1670.
Reich, E., Schibli, A., 2007. High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants. Thieme, New York, pp. 280.
Shakhmurova, G., Djahangirova, M., Batyrbekov, A., Syrov, V.N., 2004. Akademii Nauk RUz. 55–59.
Shakhmurova, G.A., Mamadalieva, N.Z., Zhanibekov, A.A., Khushbaktova, Z.A., Syrov, V.N., 2012. Effect of total ecdysteroid preparation from Silene viridiflora on the im-mune state of experimental animals under normal and secondary immunodeficiency conditions. Pharm. Chem. J. 46, 222–224.
Simon, A., Tóth, N., Tóth, G., Kele, Z., Groska, J., Bathori, M., 2009. Ecdysteroids from Silene viridiflora. Helvetica Chim. Acta 92, 753–761.
Slinkard, K., Singleton, V.L., 1977. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Viticult. 28, 49–55.
Syrov, V., Dzakhangirova, M., Khushbaktova, Z., 2005. Effect of sum of ecdysteroids from Silene brachuica for working capacity on animals in experiments. Pharm. J. 56–59 (In Russian).
Tóth, N., Simon, A., Tóth, G., Kele, Z., Hunyadi, A., Báthori, M., 2008. 26-hydroxylated ecdysteroids from Silene viridiflora. J. Nat. Prod. 71, 1461–1463.
Uysal, A., Zengin, G., Mollica, A., Gunes, E., Locatelli, M., Yilmaz, T., Aktumsek, A., 2016. Chemical and biological insights on Cotoneaster integerrimus: a new (-)- epicatechin source for food and medicinal applications. Phytomedicine 23, 979–988.
Uysal, S., Aumeeruddy-Elalfi, Z., Zengin, G., Aktumsek, A., Mocan, A., Custodio, L., Neng, R.N., Nogueira, J.M.F., Ciric, A., Glamočlija, J., Soković, M., Mahomoodally, M.F., 2018. Insight into the biological properties and phytochemical composition of Ballota macrodonta Boiss. et Balansa, - an endemic medicinal plant from Turkey. Ind. Crop. Prod. 113, 422–428.
Vivek, B., Savita, S., Chul, K.S., 2008. Chemical composition and antifungal activity of essential oil and various extract of Silene armeria L. Bioresource Technol. 99, 8903–8908.
WHO, 2016. Global Report on Diabetes. World Health Organization, Geneva, Switzerland.
Yildiz, K., Çirpici, A.H., 2013. Taxonomic revision of Silene (Caryophyllaceae) sections Siphonomorpha, Lasiostemones, Sclerocalycinae, Chloranthae, Tataricae, and Otites in Turkey. Turk. J. Bot. 37, 191–218.
Yoo, S.R., Jeong, S.J., Lee, N.R., Shin, H.K., Seo, C.S., 2017. Quantification analysis and in vitro anti-inflammatory effects of 20-hydroxyecdysone, momordinic, and oleanolic acid from the fructus of Kochia scoparia. Phcog. Mag. 13, 339–344.
Zengin, G., 2016. A study on in vitro enzyme inhibitory properties of Asphodeline anatolica: new sources of natural inhibitors for public health problems. Ind. Crop. Prod. 83, 39–43.
Zengin, G., Uysal, S., Ceylan, R., Aktumsek, A., 2015. Phenolic constituent, antioxidative and tyrosinase inhibitory activity of Ornithogalum narbonense L. from Turkey: a phytochemical study. Ind. Crop. Prod. 70, 1–6.
Zengin, G., Bulut, G., Mollica, A., Picot-Allain, C.M.N., Mahomoodally, M.F., 2018b. In vitro and in silico evaluation of Centaurea saligna (K. Koch) Wagenitz - an endemic folk medicinal plant. Comput. Biol. Chem. 73, 120–126.
Zengin, G., Mahomoodally, M.F., Aktumsek, A., Ceylan, R., Uysal, S., Mocan, A., Soković, M., 2018a. Functional constituents of six wild edible Silene species: a focus on their phytochemical profiles and bioactive properties. Food Biosci. 23, 75–82.