ORIGINAL PAPER
Cu(II) sorption performance
of silane‑modified poly(NaSS‑co‑MA‑co‑AAm)
and poly(NaSS‑co‑MA‑co‑NIPAM) terpolymers
Ahmet Okudan1 · Emre Ozviran1 · Gulsin Arslan2 · Idris Sargin2 Received: 29 August 2019 / Revised: 31 October 2019 / Accepted: 14 November 2019 / Published online: 20 November 2019© Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract
This study reports the adsorption of Cu(II) ion by 3-(2-aminoethylamino) pro-pyldimethoxymethylsilane-modified terpolymers. Water-soluble poly(sodium 4-styrenesulfonate-co-maleic anhydride-co-acrylamide) [poly(NaSS-co-MA-co-AAm)] and poly(sodium 4-styrenesulfonate-co-maleic anhydride-co–N-isopro-pylacrylamide) [poly(NaSS-co-MA-co-NIPAM)] terpolymers were synthesized and then modified with silane to make them water-insoluble. 1H-NMR and FT-IR
spectroscopy was used to study the chemical composition of the terpolymers. Also, acid number and viscosity of the polymers were determined. Cu(II) ion adsorption parameters (adsorbent dosage, contact time, pH and metal ion concentration) were studied, and the equilibrium data were evaluated using adsorption isotherm models; Freundlich isotherm gave the best fit. Cu(II) adsorption performance of poly(NaSS-co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers was found to be 53.42 and 43.10%, respectively. The terpolymers can be used for removal of Cu(II) from aqueous media.
Keywords Adsorption · Silane modification · Water treatment · Terpolymer
Introduction
Heavy metal pollutants are detrimental to living organisms, so their removal from water bodies and industrial effluents is of great importance. So far, many tech-nologies such as chemical precipitation, adsorption, ion exchange and membrane
Electronic supplementary material The online version of this article (https ://doi.org/10.1007/s0028 9-019-03025 -1) contains supplementary material, which is available to authorized users.
* Ahmet Okudan okudan1@gmail.com
1 Department of Chemistry, Faculty of Science, Selcuk University, Konya, Turkey 2 Department of Biochemistry, Faculty of Science, Selcuk University, Konya, Turkey
filtration have been developed and utilized in remediation of wastewaters. Adsorption is one of the widely used methods for removal of heavy metal ions due its low-cost nature and efficiency [1, 2].
Copper ions are released into water bodies through industrial operations like copper mining, electroplating and petroleum refinery [3]. Copper ions in aque-ous media are responsible for developing of many health problems including kid-ney and liver failure and gastrointestinal distress. Although Cu(II) is essential to human health in small amounts, the amount 2.5 mg L−1 is potentially toxic [4].
Despite many efforts, novel adsorbents should be developed for efficient removal of Cu(II) from aqueous solutions [5, 6].
Copolymer systems are employed as efficient systems for removal of heavy metals and dye pollutants from aqueous solutions [7–9]. Sodium 4-styrenesul-fonate (NaSS) is one of the monomers used in copolymer systems, and its anionic exchange group facilitates the removal of heavy metal contaminants by forming salts with heavy metal cations [10–16]. Copolymers containing maleic anhydride/ maleic acid, i.e., ‒COOH groups, also are capable of interacting with metal ions, and thus they are widely used in adsorption of metal cations [17–21]. Compounds containing two adjacent bidentate carboxylic groups can easily interact with metal ions, and therefore they are more effective in metal adsorption. On the other hand, the presence of another cation exchanger together with two carboxylic groups in the structure of a terpolymer can facilitate interactions with metal cations. This is possible through copolymerization of three discrete monomers like 4-styrenesul-fonate, maleic anhydride/maleic acid and acrylamide. When these monomers are copolymerized in the aqueous reaction medium, two different functional groups, ‒COOH and ‒SO3Na, are formed in the structure of the terpolymer. In this case,
readily ionizable groups, ‒COOH groups and ‒SO3Na group, can occur in each
repeating unit of the terpolymer [22, 23]. Many literature studies with NaSS, maleic anhydride (MA), N-isopropylacrylamide (NIPAM) and acrylamide (AAm) different monomers have demonstrated that adsorption of Cu(II) ions could occur through complexation with carboxyl and amide functional groups [24]. However, there are no literature reports on Cu(II) adsorption by terpolymers consisting of these monomers. Polymers consisting of amino, amide, hydroxyl, sulfonate or carboxyl groups in their structure can bind heavy metal through physical adsorp-tion, ion exchange, complexation or chelation [25–28]. Aqueous solutions of such terpolymers can generally exhibit typical polyelectrolyte behavior, and such poly-mers are used in the removal of heavy metal ions from wastewaters.
In the study, it was aimed to synthesize terpolymers containing carboxyl, sul-fonate and amide functional groups for removal of Cu(II) ion from aqueous solu-tions. Terpolymers-based adsorbents consisting of three different functionalities i.e., amide, sulfonate or carboxyl groups were synthesized and used for removal of Cu(II) ions for the first time. Poly(NaSS-co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers were silane-modified to make them water-insoluble. Then, the terpolymers were used as adsorbents to remove Cu(II) ion from aque-ous solutions. The effect of operational conditions, such as adsorbent dosage, contact time, pH and Cu(II) ion concentration, on the adsorption performance of
the silane-modified terpolymers was also studied. Equilibrium data were evalu-ated using Freundlich and Langmuir isotherm models.
Experimental
Materials
Maleic anhydride (MA) (purified by recrystallization from anhydrous benzene), N,
N′-dicyclohexylcarbodiimide (DCC), 1-hydroxybenzotriazole hydrate (HBH),
N-iso-propylacrylamide (NIPAM) (purified by recrystallization from anhydrous benzene), acrylamide (AAm) (recrystallized in hexane), sodium 4-styrene sulfonate (NaSS) (recrystallized in methanol), ammonium persulfate (APS), 3-(2-Aminoethylamino) propyldimethoxymethylsilane (3APS-Si), dimethyl sulfoxide (DMSO), 1,4-dioxane, acetone, diethyl ether, hydrochloric acid, sodium hydroxide, potassium hydroxide and hexane were purchased from Fluka. All the chemicals were used as supplied without any purification treatment except for MA, NIPAM, AAm and NaSS. Deion-ized water was used throughout the experiments.
Synthesis of poly(NaSS‑co‑MA‑co‑AAm) and poly(NaSS‑co‑MA‑co‑NIPAM) terpolymers
The polymerization reactions were carried out in a three-necked flask equipped with a reflux system, a thermometer and nitrogen tank in 1,4-dioxane. For synthe-sis of poly(NaSS-co-MA-co-AAm), 0.01 mol (2.06 × 10−3 mg) NaSS, 0.03 mol
(2.94 × 10−3 mg) MA, 0.01 mol (7.1 × 10−4 mg) AAm and 5 × 10−4 mol APS
were added into the reaction and stirred. Then, the reaction medium was aerated with nitrogen gas for 30 min to ensure deoxygenation and then heated at 70 °C in oil-bath to initiate the polymerization. After 24 h, the separation and purification process was performed and the terpolymer was precipitated out from the reaction media with 1,4-dioxane. The precipitate was recovered by filtration and rinsed with acetone, rested at room temperature for 30 min and then kept at 40 °C in an oven for 24 h. The same procedure was followed for the synthesis of poly(NaSS-co-MA-co-NIPAM) with 0.01 mol (2.06 × 10−3 mg) NIPAM instead of 0.01 mol
(7.1 × 10−4 mg) AAm. In the study, the experiments were also repeated using
dif-ferent monomer ratios to determine the optimum monomer ratios. Table 1 lists the monomer contents that were tested for the synthesis of poly(NaSS-co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers.
Synthesis of silane‑modified poly(NaSS‑co‑MA‑co‑AAm) and poly(NaSS‑co‑MA‑co‑NIPAM) terpolymers
Poly(NaSS-co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers were water-soluble. Therefore, the terpolymers were modified with 3-(2-aminoeth-ylamino) propyldimethoxymethylsilane (3APS-Si) to obtain their water-insoluble
forms. In the modification process, poly(NaSS-co-MA-co-AAm) terpolymer (1.0 g) was dissolved in 30 mL of DMSO. 8.2 × 10−3 mol (2200 mg) DCC and then
8.2 × 10−3 mol (1400 mg) HBH were added into the terpolymer solution. The
solu-tion was first stirred for 12 h at 0 °C and then rested at 25 °C for 24 h. 8.2 × 10−3 mol
(1.9 mL) 3APS-Si was added into the reaction medium dropwise and stirred for 24 h at constant temperature (60 °C). After cooling to room temperature, silane-modified poly(NaSS-co-MA-co-AAm) terpolymer was precipitated with acetone. The precip-itate was recovered by filtration and rinsed with water to get rid of unreacted DCC, HBH and 3APS-Si molecules, rested at room temperature for 30 min and then dried at 25 °C in an oven under vacuum for 24 h. The same procedure was followed for the synthesis of silane-modified poly(NaSS-co-MA-co-NIPAM) but with using dif-ferent amounts of 7.6 × 10−3 mol (1700 mg) DCC, 7.6 × 10−3 mol (1100 mg) HBH
and 7.6 × 10−3 mol (1.8 mL) 3APS-Si. To determine the optimum parameters for
silanization reaction, silane-modified terpolymers were also synthesized with varied amount of DCC, HBH and 3APS-Si at varying temperatures (40, 60 and 70 °C) and reaction time (30, 60 and 65 h) in DMSO.
Characterization of poly(NaSS‑co‑MA‑co‑AAm) and poly(NaSS‑co‑MA‑co‑NIPAM) terpolymers
Fourier transform infrared spectroscopy (FT-IR) spectra of the terpolymers and the silane-modified terpolymers were recorded on PerkinElmer 100 spectrometer in the 4000–600 cm−1 range. The samples for FT-IR analysis were prepared by mixing
the terpolymers with dry KBr powder and pressing into a transparent KBr pellet.
1H- NMR spectra of the terpolymers were obtained on a spectrometer (Varian 400
NMR). For 1H NMR measurements, 10 mg of the sample was dissolved in 1 mL of
deuterated water and tetramethylsilane was used as internal reference. (Results of the characterization studies are given in Supplemental Material.)
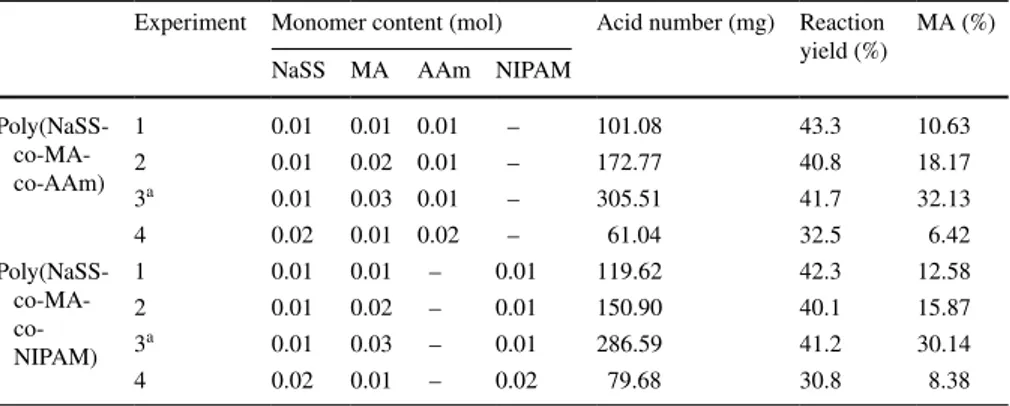
Table 1 The monomer contents, acid number, reaction yield and % maleic anhydride values of poly(NaSS-co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers
a The optimum amount of monomers for the synthesis of co-MA-co-AAm) and Poly(NaSS-co-MA-co-NIPAM) terpolymers
Experiment Monomer content (mol) Acid number (mg) Reaction
yield (%) MA (%) NaSS MA AAm NIPAM
Poly(NaSS- co-MA-co-AAm) 1 0.01 0.01 0.01 – 101.08 43.3 10.63 2 0.01 0.02 0.01 – 172.77 40.8 18.17 3a 0.01 0.03 0.01 – 305.51 41.7 32.13 4 0.02 0.01 0.02 – 61.04 32.5 6.42 Poly(NaSS- MA- co-NIPAM) 1 0.01 0.01 – 0.01 119.62 42.3 12.58 2 0.01 0.02 – 0.01 150.90 40.1 15.87 3a 0.01 0.03 – 0.01 286.59 41.2 30.14 4 0.02 0.01 – 0.02 79.68 30.8 8.38
Intrinsic viscosities of the terpolymers were determined using the Ubbelohde-type viscosimeter placed in a water bath at 25 ± 0.1 °C. Specific viscosity (ηsp) and relative viscosity (ηr) were calculated using Eqs. (1) and (2). Intrinsic viscosity [η] was calculated from Solomon–Ciuta Eq. (3) by using ηr and ηsp values,
where t0 is flow time of solvent, t is flow time of solution:
[η] is the intrinsic viscosity, and C is the molarity of the terpolymer solution [29, 30].
Acid numbers (AN) and % maleic anhydride (MA) of the terpolymers were cal-culated according to the standard titration method using Eqs. (4−7) [31, 32].
Maleic anhydride content of the terpolymers was also calculated from the data of
1H NMR spectrum analysis. Amount of DCC and HBH for silane modification of
the terpolymers were determined according to the amount of carboxylic groups that had been determined by acid number calculations using Eq. 7.
Cu(II) adsorption by silane‑modified poly(NaSS‑co‑MA‑co‑AAm) and poly(NaSS‑co‑MA‑co‑NIPAM) terpolymers
Cu(II) solutions were prepared from copper nitrate salt, and pH of the solutions was adjusted with 0.1 M NaOH and 0.1 M HCl solutions. Adsorption studies were repeated at varying parameters to determine the optimum conditions: amount of the adsorbent, contact time, Cu(II) concentration and pH. The adsorption studies were carried out on a shaker at 200 rpm at 25 °C. At the end of each experiment, the (1) 𝜂r= t t0 (2) 𝜂sp= 𝜂r − 1 (3) [𝜂] = 1.414 C (𝜂sp − ln 𝜂r) 1∕2 (4) COOH(%) =(V1− V2) × N × 10 −3× 45 × 100 m (5) AN( mgKOH g ) = mLKOH× NKOH × 56.11 g polymer (6) MA(%) =AN× 98 2× 561 (7)
Nbaz× Vbaz= mg(APS − Si)
FA
adsorbent was recovered from the adsorption medium by filtration and the amount of Cu(II) ion concentration in the solution or in the filtrate was determined using Contra AA 300 Atomic Absorption Spectrometer. The following formula was used to calculate the amount of Cu(II) adsorbed by the terpolymers (Eq. 8).
where C0 (mmol/L) and C (mmol/L) denote the initial and the equilibrium
concen-trations of Cu(II) ions, V (L) is the volume of aqueous phase, and W (g) is the mass of dry terpolymers [33]. The metal sorption percentage of the terpolymers was cal-culated busing the equation given below.
Determination of the adsorption parameters
To determine the amount of the adsorbent, varying amount of the terpolymer (0.001‒0.100 g) was added into 20 mL of 1.0 × 10−3 M metal solution (pH not
adjusted) and shaken on a shaker for 600 min. Contact time experiments were done for 15‒240 min (amount of the adsorbent: 0.05 g; metal ion solution: 20 mL, 1.0 × 10−3 M; pH: not adjusted; ionic strength: 0.01 M NaCl). The effect of pH of
the metal ion was studied in the following conditions: pH range: 1.0‒5.0 (0.1 M HCl and 0.1 M NaOH solutions were used for pH adjustments); amount of the adsorbent: 0.05 g; metal ion solution: 20 mL, 1.0 × 10−3 M; ionic strength: 0.01 M NaCl. To
study the effect of Cu(II) solution concentration, the adsorption experiments were repeated using different metal ion solutions at varying concentrations; 1.0 × 10−4;
2.0 × 10−4; 4.0 × 10−4; 6.0 × 10−4; 8.0 × 10−4 and 1.0 × 10−3 M. Other parameters
were kept constant; the amount of the adsorbent: 0.05 g; volume of the metal ion solution: 20 mL; pH: not adjusted; ionic strength: 0.01 M NaCl.
Results and discussion
Synthesis and modification of poly(NaSS‑co‑MA‑co‑AAm) and poly(NaSS‑co‑MA‑co‑NIPAM) terpolymers
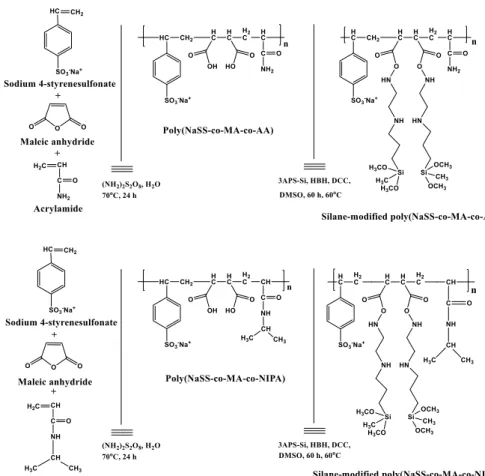
Poly(NaSS-co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers with varying monomer content were synthesized. Figure 1 depicts the polymerization and the silanization reactions of the terpolymers. Table 1 lists the monomer content, the acid number, the reaction yield and the % maleic anhydride values.
In the 1H-NMR spectrum of poly(NaSS-co-MA-co-AAm) (in D
2O at 25 °C)
(Fig-ure S1), the peak at 1.2 ppm was attributed to protons of methylene (–CH2) group
of AAm [34], the peak at 2.5 ppm to protons of CH-CH group of MA terpolymer (8) q= [Co−C V ] W (9) %sorption = amount of Cu(II) ions sorbed
[22] and the peak at 7.8 ppm to aromatic protons of (–CH) of NaSS [35]. Integration of the peaks revealed the contents of the poly(NaSS-co-MA-co-AAm) terpolymer as 23.58, 29.71 and 46.71%, respectively. In the 1H-NMR spectrum of
poly(NaSS-co-MA-co-NIPAM) terpolymer in D2O (Figure S3), protons of methylene (–CH2) group of NIPAM appeared at 0.99 ppm [34]. The peak at 2.5 ppm was attributed to protons of CH-CH group of MA [22]. The peak corresponding to (–CH) aromatic protons of NaSS was observed at 7.8 ppm [35]. The 1H-NMR spectra of the
terpoly-mers were recorded in D2O solvent due to their solubility in water and poor solu-bility in other solvents (DMSO, CDCl3). Therefore, these amide protons (NH and NH2) were not observed in 1H-NMR analysis, indicating the exchanging by D
2O.
Integration of the peaks revealed the contents of the poly(NaSS-co-MA-co-AAm) terpolymer as 25.38, 28.68 and 45.94%, respectively.
Terpolymers that were synthesized through Experiment 3 (refer to Table 1) was used for the adsorption studies. Ionic and hydrophilic groups are present in
Fig. 1 Synthesis of poly(NaSS-co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers and their modification with 3APS-Si (For details of the amount of monomers, reader should refer to Table 1; i.e., Experiment 3)
the structure of the synthesized terpolymers. Therefore, the terpolymers were not suitable for adsorption experiments due to their solubility in water. The maleic anhydride groups go through the ring-opening process in water by form-ing free –COOH groups, and this makes them water-soluble. Their solubility in aqueous medium prevented their use as an adsorbent, and they needed fur-ther modification. To overcome this problem, poly(NaSS-co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers were subjected to silanization treat-ment [36]. In silanization reaction, the polymers were modified with 3APS-Si through their free –COOH groups by giving water-insoluble forms. The –COOH groups, formed as a result of the ring opening occurring during the polymeri-zation of the terpolymers, reacted with the –NH2 groups. The terpolymers that
were synthesized through Experiments 1, 2 and 4 also went through silaniza-tion treatment, but despite the modificasilaniza-tion they were still water-soluble due to their lower acid numbers (refer to Table 1). In other words, low amount of acid groups led to insufficient silylation of the polymers, which in turn made the ter-polymers water-soluble because of low hydrophobic group content of the poly-mer. The FT-IR spectra of the terpolymers and 3APS-Si-modified terpolymers are presented in Figures S2, S4, S5 and S6, and the assignment of the peaks is presented in Tables 2, S1 and S2 [37–40]. The appearance of peaks in the range of 3325–2849 cm−1 confirmed the silane modification of the terpolymers [39].
The reaction yields of the experiments that were conducted to determine the optimum parameters for silanization reaction are listed in Table S3. The opti-mization tests revealed that the reaction yield increased as the DCC, HBH and 3APS-Si contents were increased. Temperatures lower or higher than 60 °C also led to decrease in the reaction yields. Longer reaction times resulted in higher reaction yields, but further increase in reaction time did not contribute to the yields. The optimum amount of reactants and operational parameters for silani-zation of the terpolymers were found as follows: poly(NaSS-co-MA-co-AAm): DCC (2.2 mol), HBH (1.4 mol), 3APS-Si (1.9 mol), temperature 60 °C, reac-tion time 60 h, yield 75.3%; poly(NaSS-co-MA-co-NIPAM): DCC (1.7 mol), HBH (1.1 mol), 3APS-Si (1.8 mol), temperature 60 °C, reaction time 60 h, yield 80.2%. SEM images of the terpolymers are depicted in Fig. 2.
As presented in Table 3, the acid number of the terpolymers that were obtained by volumetric technique was close to one another. Similarly, % MA content of both terpolymers that were determined using volumetric technique or
1H-NMR spectroscopy was close to each other, suggesting that both terpolymers
had a similar number of –COOH groups suitable for modification. Addition of NIPAM monomer to the terpolymer composition led to a lower limit viscosity for MA-co-NIPAM) when compared to that of poly(NaSS-co-MA-co-AAm). This observation could be attributed to the prevention of polymer chain growth in the polymerization by the steric hindrance of isopropyl group of NIPAM. Addition of 3APS-Si to the polymer chains of poly(NaSS-co-MA-co-AAm) or poly(NaSS-co-MA-co-NIPAM) made the terpolymers water-insoluble, which could be attributed to hydrophobic aliphatic composition of 3APS-Si.
Table 2 P eak assignments f or t he FT -IR spectr a of t he ter pol ymers and t he silane-modified ter pol ymers Ter pol ymers Peak s (cm −1) Assignment of t he peak s Pol y(N aSS-co-MA -co-AAm) 1704 Carbo xy l g roups f or med fr om r ing opening of MA 1651 Vibr
ation of amide carbon
yl (–HN–C=O) fr
om AAm
1037 and 836
Symme
tric and asymme
tric vibr ation of ‒SO 2 fr om N aSS Silane-modified pol y(N aSS-co-MA -co-AAm) 3325 Str etc hing of amine (–NH) g roup 2929 and 2849 (C–H) s tre tching of CH 2 and CH 3 g roups 1663 Str etc
hing of amide carbon
yl g
roup (–HN–C=O)
1623
Str
etc
hing of amide carbon
yl (–HN–C=O)
1562
Str
etc
hing of amide II (–N–H bending) fr
om AAm 1037 and 836 Symme tric/asymme tric s tre tching of ‒SO 2 fr om N aSS Pol y(N aSS-co-MA -co-NIP AM) 1714 Carbo xy l g roups f or med fr om r ing opening of MA 1642 Str etc
hing of amide carbon
yl (–HN–C=O) fr om NIP AM 1534 Str etc
hing of amide II (–C=O) fr
om NIP
AM
1042 and 836
Symme
tric and asymme
tric vibr ation of ‒SO 2 fr om N aSS Silane-modified pol y(N aSS-co-MA -co- NIP AM) 3322 Str etc hing of amine (–NH) g roup 2932 and 2849 (C–H) s tre tching of CH 2 and CH 3 g roups 1656 Str etc
hing of amide II (–C=O) fr
om NIP
AM
1623
Str
etc
hing of amide carbon
yl g
roup (-HN-C=O)
1565
Str
etc
hing of amide II (–C=O) fr
om AAm 1037 and 836 Symme tric/asymme tric s tre tching of ‒SO 2 fr om N aSS
Effect of the amount of the terpolymers on Cu(II) adsorption
The effect of the amount of co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers on their Cu(II) adsorption performance is depicted in Fig. 3. Cu(II) adsorption performance of the terpolymers increased to a certain point and then remained unchanged. In the system with
Fig. 2 SEM images of silane-modified co-MA-co-AAm) (a) and silane-modified poly(NaSS-co-MA-co-NIPAM) (b)
Table 3 Viscosity, acid number and % MA content of the terpolymers
Terpolymers [η] dL/g COOH (%) Acid number
(mg KOH/g) MA (%) Volumetric 1H-NMR Poly(NaSS-co-MA-co-AAm) 0.2273 24.51 305.51 32.13 29.71 Poly(NaSS-co-MA-co-NIPAM) 0.1670 23.00 286.59 30.14 28.68 30 40 50 60 70 0.000 0.003 0.006 0.009 0.012 Sorption (% ) Amount of adsorbent, g poly(NaSS-co-MA-co-NIPA) poly(NaSS-co-MA-co-AA)
Fig. 3 The effect of the amount of silane-modified co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers on their Cu(II) adsorption performance
poly(NaSS-co-MA-co-NIPAM) terpolymer, the plateau value was reached at lower adsorbent dosage. In the case of poly(NaSS-co-MA-co-AAm), Cu(II) adsorption was higher and the saturation point was observed at about 0,005 g. It is likely that the steric hindrance of isopropyl group on NIPAM played a role in low Cu(II) adsorption performance of poly(NaSS-co-MA-co-NIPAM).
Effect of the contact time on Cu(II) adsorption by the terpolymers
The effect of the contact time on Cu(II) adsorption performance of the terpol-ymers is presented in Fig. 4. In the first 60 min, relatively fast Cu(II) adsorp-tion by the terpolymers was observed. Cu(II) adsorpadsorp-tion could be related to the presence of cation-exchange group ‒SO3−Na+ and ‒SO
3−H+ on the terpolymer
chains. After 180 min, the adsorption process reached a saturation point for both adsorbents.
Effect of the solution pH on Cu(II) adsorption by the terpolymers
It is possible that the cation-exchange groups ‒SO3−Na+ and ‒SO3−H+ on the
terpolymer structures played a role in Cu(II) ion adsorption and H+ and Na+
ions were exchanged with the metal cations. Also, surface adsorption, chemical adsorption and complex formation could contribute to the adsorption process. As depicted in Fig. 5, Cu(II) adsorption by the polymers was highly dependent on the changes in pH. In acidic medium, desorption of Cu(II) cations occurred, but as pH was increased, the sorption was enhanced. The maximum Cu(II) adsorption was observed at pH 4.5‒5.5. 0 20 40 60 80 0 60 120 180 240 300 Sorption (% ) Time (min) poly(NaSS-co-MA-co-NIPA) poly(NaSS-co-MA-co-AA)
Effect of Cu(II) concentration on adsorption performance of the terpolymers
A rapid increase in Cu(II) adsorption was observed with increasing metal ion concentration, and then the adsorption remained constant after a certain plateau value. The equilibrium data were evaluated according to Freundlich and Lang-muir isotherm models (Figures S7 and S8). Adsorption isotherms were obtained by plotting the amount of Cu(II) per gram of terpolymer against the amount of Cu(II) ion remained in the solution. Linear version of the isotherm models was used.
The Freundlich and Langmuir models were applied to Cu(II) adsorption equi-librium data.
The Freundlich model:
where qe,, amount of Cu(II) adsorbed in mmol g−1; C
e, the equilibrium
concentra-tion of the Cu(II) in mM L−1; and K
F and n, Freundlich constants denoting
adsorp-tion capacity and intensity of the adsorpadsorp-tion. The Langmuir model:
where qe, amount of Cu(II) adsorbed in mmol g−1; Ce, the equilibrium concentration
of the Cu(II) in mmol L−1; and Q0 (in mmol g−1) and b (in L mmol−1) Langmuir
constants related to adsorption capacity and energy of adsorption.
The Cu(II) adsorption performance of NaSS-co-MA-co-AAm terpolymer was relatively higher than that of NaSS-co-MA-co-NIPAM, which could be attrib-uted to amide group of AAm in the structure of NaSS-co-MA-co-AAm. ‒SO3H
(10) log qe= log KF + 1 nlog Ce (11) Ce qe = Ce Q0 + 1 Q0b 0 20 40 60 80 1.5 2.5 3.5 4.5 5.5 6.5 Sorption (% ) pH poly(NaSS-co-MA-co-NIPA) poly(NaSS-co-MA-co-AA)
was effective in Cu(II) adsorption, but since both terpolymers had a nearly same ‒SO3H content, this cation-exchanger group made no significant difference
between the two adsorption systems. Freundlich and Langmuir isotherm param-eters are given in Table 4. Freundlich isotherm gave the best fit. The better fitting to Freundlich isotherm model showed that the terpolymers had heterogeneous surface with adsorption sites having different energy. The values of n were higher than 1, and this indicated that Cu(II) adsorption by the terpolymers was favorable in the specified conditions.
Conclusions
This study reports the synthesis of silane-modified poly(sodium 4-styrenesulfonate-co-maleic anhydride-co-(N-isopropylacrylamide or acrylamide)) terpolymers and their interaction with Cu(II) ion. Silanization procedure with 3APS-Si was used to make poly(NaSS-co-MA-co-AAm) and poly(NaSS-co-MA-co-NIPAM) terpolymers water-insoluble. 1H-NMR and FT-IR spectroscopy revealed the chemical
composi-tion of the terpolymers. Adsorpcomposi-tion parameters (adsorbent dosage, contact time, pH and metal ion concentration) were also studied. The study demonstrated that ter-polymers consisting of three functionalities can be used for adsorption of metal ions from aqueous media after modification by silane compounds. When compared to NIPAM group, the presence of acryl amide group in the structure of the terpolymer enhanced Cu(II) adsorption. In further studies the terpolymers synthesized in this work should be tested in removal of heavy metal contaminants other than Cu(II) ions.
Acknowledgement The authors are thankful to Selcuk University Research Foundation (project number: BAP-14201016) for funding the study.
References
1. Zhao G, Huang X, Tang Z, Huang Q, Niu F, Wang X (2018) Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym Chem Uk 9(26):3562–3582
Table 4 The parameters and constants of Freundlich and Langmuir isotherm plots for the adsorption of Cu(II) by silane-modified poly(NaSS-co-MA-co-NIPAM) terpolymer
Freundlich Langmuir
Terpolymer Slope
Inter-cept n KF R 2 Slope Inter-cept Q 0 b R2 Poly(NaSS-co-MA-co-AAm) 0.408 2.666 2.451 463.447 0.939 0.004 0.001 250 250,000 0.871 Poly(NaSS- co-MA-co-NIPAM) 0.421 2.536 2.375 343.558 0.990 0.005 0.001 200 200,000 0.955
2. Wen J, Fang Y, Zeng G (2018) Progress and prospect of adsorptive removal of heavy metal ions from aqueous solution using metal–organic frameworks: a review of studies from the last decade. Chemosphere 201:627–643
3. Bilal M, Shah JA, Ashfaq T, Gardazi SMH, Tahir AA, Pervez A, Haroon H, Mahmood Q (2013) Waste biomass adsorbents for copper removal from industrial wastewater—a review. J Hazard Mater 263:322–333
4. Prasad M, Freitas H (2000) Removal of toxic metals from solution by leaf, stem and root phytomass of Quercus ilex L. (holly oak). Environ Pollut 110(2):277–283
5. Al-Saydeh SA, El-Naas MH, Zaidi SJ (2017) Copper removal from industrial wastewater: A com-prehensive review. J Ind Eng Chem 56:35–44
6. A. Okudan, B.E. Ataoglu, O. Sengoz, G. Arslan, Cu(II) Sorption Performance of Novel Chitosan/ Ter-(vinyl pivalate-maleic anhydride-N-tert-butylacrylamide) Microcapsules, J Polym Environ (2019) 1–10.
7. Akbari A, Arsalani N, Eftekhari-Sis B, Amini M, Gohari G, Jabbari E (2019) Cube-octameric silsesquioxane (POSS)-capped magnetic iron oxide nanoparticles for the efficient removal of meth-ylene blue. Front Chem Sci Eng 13(3):563–573
8. Eftekhari-Sis B, Akbari A, Motlagh PY, Bahrami Z, Arsalani N (2018) Dye adsorption on cubic polyhedral oligomeric silsesquioxane-Based poly (acrylamide-co-itaconic acid) hybrid nanocom-posites: kinetic, thermodynamic and isotherms studies. J Inorg Organomet Polym 28(5):1728–1738 9. Bahrami Z, Akbari A, Eftekhari-Sis B (2019) Double network hydrogel of sodium
alginate/poly-acrylamide cross-linked with POSS: Swelling, dye removal and mechanical properties. Int J Biol Macromol 129:187–197
10. Brusseau SGN, D’Agosto F, Magnet S, Couvreur L, Chamignon C, Charleux B (2011) Nitroxide-Mediated copolymerization of methacrylic acid and sodium 4-styrenesulfonate in water solu-tion and one-pot synthesis of amphiphilic block copolymer nanoparticles. Macromolecules 44(14):5590–5598
11. Deng H-Y, Xu Y-Y, Zhu B-K, Wei X-Z, Liu F, Cui Z-Y (2008) Polyelectrolyte membranes prepared by dynamic self-assembly of poly (4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA) for nanofiltration (I). J Membr Sci 323(1):125–133
12. Rivas B, Seguel G, Geckeler K (2002) Synthesis, characterization, and properties of polychelates of poly (styrene sulfonic acid-co-maleic acid) with Co(II), Cu(II), Ni(II), and Zn (II). J Appl Polym Sci 85(12):2546–2551
13. Rivas BL, Munoz C (2009) Synthesis and metal ion adsorption properties of poly (4-sodium styrene sulfonate-co-acrylic acid). J Appl Polym Sci 114(3):1587–1592
14. Matsumoto K, Hasegawa H, Matsuoka H (2004) Synthesis of sodium-polystyrenesulfonate-grafted nanoparticles by core-cross-linking of block copolymer micelles. Tetrahedron 60(34):7197–7204 15. Ghosh SK, De P, Khastgir D, De S (2000) Ionic thermoplastic elastomer based on the zinc salt of
sulfonated maleated EPDM rubber. I. Effect of zinc stearate on melt‐flow behavior, and dynamic mechanical, dielectric, and physical properties. J Appl Polym Sci 78(4):743–750
16. Luo X, Goh SH, Lee SY, Huan CHA (1999) Spectroscopic studies of interactions in complexes of poly (1-vinylimidazole) with poly (styrenesulfonic acid) or the zinc salt of poly (styrenesulfonate). Macromol Chem Phys 200(4):874–880
17. Hasanzadeh R, Najafi Moghadam P, Samadi N (2013) Synthesis and application of modified poly (styrene‐alt‐maleic anhydride) networks as a nano chelating resin for uptake of heavy metal ions. Polym Adv Technol 24(1):34–41
18. Hasanzadeh R, Moghadam PN, Samadi N, Asri-Rezaei S (2013) Removal of heavy-metal ions from aqueous solution with nanochelating resins based on poly (styrene-alt-maleic anhydride). J Appl Polym Sci 127(4):2875–2883
19. Abd El-Rehim HA, Hegazy EA, El-Hag Ali A (2000) Selective removal of some heavy metal ions from aqueous solution using treated polyethylene-g-styrene/maleic anhydride membranes. React Funct Polym 43(1):105–116
20. Kawaguchi S, Kitano T, Ito K (1991) Infrared and ultraviolet spectroscopic studies on intramolecu-lar hydrogen bonding in an alternating copolymer of isobutylene and maleic acid. Macromolecules 24(22):6030–6036
21. Samadi N, Ansari R, Khodavirdilo B (2017) Removal of Copper ions from aqueous solutions using polymer derivations of poly (styrene-alt-maleic anhydride). Egypt J Pet 26(2):375–389
22. Rivas BL, Seguel GV, Geckeler KE (2001) Poly(styrene-alt-maleic acid)–metal complexes with divalent metal ions. synthesis, characterization, and physical properties. J Appl Polym Sci 81(6):1310–1315
23. Rivas BL, Seguel GV, Ancatripai C (2000) Polymer-metal complexes: Synthesis, characterization, and properties of poly(maleic acid) metal complexes with Cu(II), Co(II), Ni(II), and Zn(II). Polym Bull 44(5):445–452
24. Chen JJ, Ahmad AL, Ooi BS (2013) Poly(N-isopropylacrylamide-co-acrylic acid) hydrogels for copper ion adsorption: equilibrium isotherms, kinetic and thermodynamic studies. J Environ Chem Eng 1(3):339–348
25. Chauhan GS, Kumar S, Kumari A, Sharma R (2003) Study on the synthesis, characterization, and sorption of some metal ions on gelatin- and acrylamide-based hydrogels. J Appl Polym Sci 90(14):3856–3871
26. Chen JJ, Ahmad AL, Ooi BS (2014) Thermo-responsive properties of poly(N-isopropylacrylamide-co-acrylic acid) hydrogel and its effect on copper ion removal and fouling of polymer-enhanced ultrafiltration. J Membrane Sci 469:73–79
27. Ju X-J, Zhang S-B, Zhou M-Y, Xie R, Yang L, Chu L-Y (2009) Novel heavy-metal adsorption mate-rial: ion-recognition P(NIPAM-co-BCAm) hydrogels for removal of lead(II) ions. J Hazard Mater 167(1):114–118
28. Morales DV, Rivas BL (2014) Poly (Acrylamide-co-Styrene Sodium Sulfonate) and Poly (2-Acryla-mide-2-Methyl-1-Propanesulfonic Acid-co-Acrylic Acid) Resins with Removal Properties for Hg (II), Pb (II), Cd (II), and Zn (II). J Chil Chem Soc 59(2):2420–2426
29. Klumperman B (2010) Mechanistic considerations on styrene–maleic anhydride copolymerization reactions. Polym Chem-Uk 1(5):558–562
30. Tsuchida E, Tomono T (1971) Discussion on the mechanism of alternating copolymerization of sty-rene and maleic anhydride. Die Makromolekulare Chemie Macromol Chem Phys 141(1):265–298 31. Kim BK, Park SY, Park SJ (1991) Morphological, thermal and rheological properties of blends:
Pol-yethylene/nylon-6, polyethylene/nylon-6/(maleic g-polyethylene) and (maleic anhydride-g-polyethylene)/nylon-6. Eur Polymer J 27(4–5):349–354
32. Lucchesi C, Secrets P, Hirn C (1975) Standart method of chemical analysis. Krieger Publishing Company, New York
33. Kocak N, Sahin M, Arslan G, Ucan HI (2012) Synthesis of crosslinked chitosan possessing schiff base and its use in metal removal. J Inorg Organomet Polym 22(1):166–177
34. Travas-Sejdic J, Easteal A (2000) Study of free-radical copolymerization of acrylamide with 2-acrylamido-2-methyl-1-propane sulphonic acid. J Appl Polym Sci 75(5):619–628
35. Genies C, Mercier R, Sillion B, Petiaud R, Cornet N, Gebel G, Pineri M (2001) Stability study of sulfonated phthalic and naphthalenic polyimide structures in aqueous medium. Polymer 42(12):5097–5105
36. Akbari A, Arsalani N (2016) Organic–inorganic incompletely condensed polyhedral oligomeric silsesquioxane-based nanohybrid: synthesis, characterization and dye removal properties. Polym Plastics Technol Eng 55(15):1586–1594
37. Rivas BL, Seguel GV, Geckeler KE (2002) Synthesis, characterization, and properties of polyche-lates of poly(styrene sulfonic acid-co-maleic acid) with Co(II), Cu(II), Ni(II), and Zn(II). J Appl Polym Sci 85(12):2546–2551
38. Deng H, Xu Y, Zhu B, Wei X, Liu F, Cui Z (2008) Polyelectrolyte membranes prepared by dynamic self-assembly of poly (4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA) for nanofiltra-tion (I). J Membr Sci 323(1):125–133
39. Zhu Z, Yang X, He L-N, Li W (2012) Adsorption of Hg 2+ from aqueous solution on functional-ized MCM-41. RSC Adv 2(3):1088–1095
40. Çelik S (2011) Poli (4-vinilpiridin) homopolimeri, maleik anhidrit ve n-izopropil akrilamid ile kopolimer ve terpolimerlerinin sentezi, karakterizasyonu ve özelliklerinin incelenmesi. Fen Bilim-leri Enstitüsü, Gazi Üniversitesi Ankara
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published