Serum Cardiovascular Risk Factors in
Obstructive Sleep Apnea*
Murat Can, MD; S¸erefden Ac¸ikgo¨z, MD; Go¨rkem Mungan, MD;
Taner Bayraktarog˘lu, MD; Erdem Koc¸ak, MD; Berrak Gu¨ven, MD; and
Selda Demı˙rtas, MD
Background: Obstructive sleep apnea (OSA) patients have increased cardiovascular morbidity
and mortality. The cardiovascular markers associated with OSA are currently not defined.
Objectives: The aims of this study were to determine whether OSA is associated with serum
cardiac risk markers and to investigate the relationship between them.
Methods: Sixty-two male patients were classified into two groups with respect to apnea-hypopnea
index (AHI): group 1, sleep apnea (n
ⴝ 30), with AHI > 5; and group 2 (n ⴝ 32), with AHI < 5. We
compared cardiovascular risk factors in both groups with control subjects (n
ⴝ 30) without OSA (AHI
<
1). Serum cholesterol, triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density
lipoprotein cholesterol (LDL-C), apolipoprotein A-I, apolipoprotein B, lipoprotein (a), C-reactive
protein (CRP), and homocysteine were measured. Statistical significance was assessed with analysis of
variance at p < 0.05. In correlation analysis, Pearson correlation was used.
Results: There was no significant difference between group 1 and group 2 in total cholesterol, LDL-C,
HDL-C, triglyceride, apolipoprotein A-I, apolipoprotein B, and lipoprotein (a). All of the M-mode
echocardiographic parameters were in the normal reference range. Serum homocysteine and CRP
levels were significantly increased in group 1 compared to group 2 (p < 0.05). Serum CRP values
were increased in both group 1 and group 2 when compared with control subjects (p < 0.05). Serum
homocysteine values were higher in group 1 than in control subjects (p < 0.05).
Conclusions: Our results show that OSA syndrome is associated not only with slight
hyperteinemia but also with increased CRP concentrations. Increased plasma concentrations of
homocys-teine and CRP can be useful in clinical practice to be predictor of long-term prognosis for
cardiovascular disease and the treatment of OSA.
(CHEST 2006; 129:233–237)
Key words: C-reactive protein; homocysteine; obstructive sleep apnea
Abbreviations: AHI⫽ apnea-hypopnea index; CPAP ⫽ continuous positive airway pressure; CRP ⫽ C-reactive protein; HDL-C⫽ high-density lipoprotein cholesterol; LDL-C ⫽ low-density lipoprotein cholesterol; OSA ⫽ obstructive sleep apnea
O
bstructive sleep apnea (OSA) is a common
chronic respiratory disorder.
1OSA occurs in
approximately 4% of men and 2% of woman
⬎ 30
years old.
2Increase in the ratio with age may depend
on the role of OSA on the complications of the
disease.
3OSA is well-defined syndrome that includes one or
two of the following symptoms: severe snoring,
nocturnal respiratory arrest, repeated nocturnal
awakening, nonrecuperative sleep, diurnal fatigue,
and altered concentration. These clinical findings are
related to the extent of hypoxemia and hypercapnia
that develop as a result of disordered breathing.
4*From Faculty Of Medicine (Drs. Can, Ac¸ikgo¨z, Mungan, and Gu¨ven), Department Of Biochemistry, and Faculty Of Medicine (Drs. Bayraktarog˘lu and Koc¸ak), Department Of Internal Medi-cine, Karaelmas University, Zonguldak; and Faculty Of Medicine (Dr. Demı˙rtas), Department Of Biochemistry, Ufuk University, Ankara, Turkey.
Manuscript received April 8, 2005; revision accepted July 16, 2005.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal. org/misc/reprints.shtml).
Correspondence to: Murat Can, MD, Karaelmas University, Faculty of Medicine, Department of Biochemistry, Zonguldak, Turkey; e-mail: drcanmurat@yahoo.com
CHEST
Original Research
Although OSA patients have increased
cardiovas-cular morbidity and mortality,
5how much of their
cardiovascular diseases are due to OSA rather than to
other risk factors such as upper-body obesity; insulin
resistance; increased age; alcohol and caffeine
con-sumption; and cigarette smoking is yet unknown.
Therefore, identifying the possible risk factors
in-volved in OSA cardiovascular morbidity and
mortal-ity is of great clinical importance. Several studies
report a strong association between homocysteine,
6C-reactive protein (CRP),
7total cholesterol,
8low-density lipoprotein cholesterol (LDL-C),
9high-den-sity lipoprotein cholesterol (HDL-C),
10triglycer-ide,
11apolipoprotein A-I and B,
12lipoprotein (a)
levels,
13and coronary heart disease.
For editorial comment see page 218
The pathophysiology of the underlying
mecha-nisms and complications of OSA is complex and
multifactorial. The aims of this study were to
deter-mine whether OSA syndrome is associated with
serum cardiac risk markers, and to investigate the
relationship between serum cardiac risk markers.
Materials and Methods
Study DesignPatients were recruited for the study based on medical history and written consent. The study protocol was approved by the university ethics committee and was performed in accordance with the current revision of the guidelines in accordance with the Declaration of Helsinki.
Patients
OSA was diagnosed on the basis of the International Classifi-cation of Sleep Disorders.14Sixty-two male patients who were
referred for suspected sleep apnea underwent an overnight sleep study. Patients were classified into two groups with respect to apnea-hypopnea index (AHI); group 1 (n⫽ 30), AHI ⬎ 5; group 2 (n⫽ 32), AHI ⬍ 5. We compared cardiovascular risk factors in both groups with control subjects (n⫽ 30) without OSA (AHI ⬍ 1). All subjects with OSA snored and reported excessive daytime sleepiness or two or more other features such as impaired concentration, unrefreshing sleep, witnessed apneas, restless sleep, and irritability/personality change. Before enroll-ment, the subjects were asked about their regular medications and medical history regarding diabetes mellitus, renal diseases, and ischemic heart disease. The patients were assessed for coronary artery disease with resting and stress 12-lead precordial ECG. Previous myocardial infarction, unstable angina, prior coronary intervention, arrhythmias, conduction abnormalities, heart failure, digoxin therapy, inability to perform tests, hyper-tension (BPⱖ 140/90 mm Hg or receiving medication), chronic renal disease, and diabetes mellitus were the exclusion criteria. Subjects who smoked or had systemic infections at the time of the study were also excluded. Before polysomnography, baseline demographic data, BP, ECG, and echocardiography were as-sessed throughout the day, and blood samples were collected between 8 pm and 9 pm.
Polysomnography
Polysomnography was started at 9 pm and ended at 6:30 am. Surface electrodes were applied using standard techniques to obtain an EEG, an electromyogram of the chin, an ECG, and an electrooculogram. Sleep was defined according to the criteria of Rechtschaffen and Kales.15 Ventilation was monitored using
inductive plethysmography. Airflow was monitored by ther-mistors placed at the nose and mouth, while arterial oxygen saturation was monitored continuously with a pulse oximeter. A polygraph was run continuously at 10 mm/s to record all of the above physiologic data simultaneously throughout the course of the experiment. All parameters were stored in a data recorder for subsequent analysis. Apnea was defined as the cessation of airflow at the nose and mouth lasting for⬎ 10 s. Hypopnea was defined as a decrease of ⱖ 50% in thoracoabdominal motion associated with a fall in the baseline oxygen saturation ofⱖ 4%. All AHI values were calculated to express the number of episodes of apnea and hypopnea per hour of total sleep time.
Echocardiographic Analyses
We performed echocardiography with M mode (GE-VingMed System 5 Ultrasound System; GE-VingMed Sound AB; Horten, Norway). All studies were performed and interpreted by the same operator and recorded on videotape. Left ventricular end-diastolic dimension, left ventricular end-systolic dimension, and thickness of the interventricular septum and posterior wall were measured. The ejection fraction was calculated from area measurements using the area-length method applied to the average apical area. Echocardiographic data were recorded ac-cording to the guidelines of the American Society of Echocardi-ography.
Blood Collection
All blood samples were obtained in the nonfasting state. The subjects did not perform any specific exercise or apply any specific diet during the study period. For homocysteine, serum samples were centrifuged immediately and placed on ice prior to separation. After centrifugation, the serum aliquots were frozen and stored at⫺ 80°C.
Biochemical Analysis
Serum cholesterol, triglyceride, and HDL-C were measured by enzymatic colorimetric methods with commercially available kits (Cobas Integra 800; Roche Diagnostics GmbH; Mannheim, Germany), and LDL-C was calculated according to the Friede-wald formula: total cholesterol⫺ HDL-C ⫺ (0.45 ⫻ triglycer-ide). Apolipoprotein A-I and apolipoprotein B were measured by immunoturbidimetric method, and lipoprotein (a) and CRP were determined by particle-enhanced immunoturbidimetric method on the Roche Integra 800 analyzer. Serum homocysteine was measured by enzyme-linked immunosorbent assay (Axis Homo-cysteine EIA; Axis-Shield Diagnostics; Dundee, Scotland) on a diagnostic instrument (LP 400; Diagnostics Pasteur; Chaska, MN). Vitamin B12and folate levels were measured by
electro-chemiluminescent immunoassay on a Roche Elecsys 2010 ana-lyzer (Vitamin B12and folate kit; Roche Diagnostics).
Erythro-cyte count, hemoglobin concentration, mean cell volume, and mean cell hemoglobin concentration were measured (MAXM; Beckman Coulter; Fullerton, CA), and stained RBC examinations of the patients were studied.
Statistical Analysis
Results are expressed as mean⫾ SE. We used analysis of variance to analyze any differences in demographic and
hemo-dynamic characteristics between the two groups. In correlation analysis, a Pearson correlation was used. All statistical analysis was performed with a statistical program (SPSS, version 11.0; SPSS; Chicago, IL), defining statistical significance as p⬍ 0.05.
Results
When we compared the patients with the healthy
control subjects, there were no significant
differ-ences between group 1, group 2, and the control
group with respect to age, body mass index, and BP
(Table 1). All of the M-mode echocardiographic
parameters (left ventricular end-diastolic dimension
[LVEDD], 4.46
⫾ 0.45 cm; left ventricular
end-systolic dimension [LVESD], 2.93
⫾ 0.41 cm; EF,
64.6
⫾ 2.66%; IVS, 1.28 ⫾ 0.09 cm; and PW,
1.17
⫾ 0.10 cm) were in normal reference range.
With regard to the conventional parameters (total
cholesterol, LDL-C, HDL-C, triglycerides,
apoli-poprotein A-I, apoliapoli-poprotein B, and liapoli-poprotein (a))
there were no significant differences between group
1 and group 2. Total cholesterol, LDL-C,
triglycer-ide, apolipoprotein B, and lipoprotein (a) values
were increased in both group 1 and group 2 when
compared with control group (p
⬍ 0.05). There were
no significant differences between group 1, group 2,
and the control group in apolipoprotein A-I and
HDL-C results (Table 2). Serum CRP values were
increased in both group 1 and group 2 when
com-pared with control group (p
⬍ 0.05). Serum
homo-cysteine values were higher in group 1 than in
control subjects (p
⬍ 0.05). Comparison of serum
homocysteine and CRP levels revealed a significant
difference (p
⬍ 0.05) between group 1 and group 2.
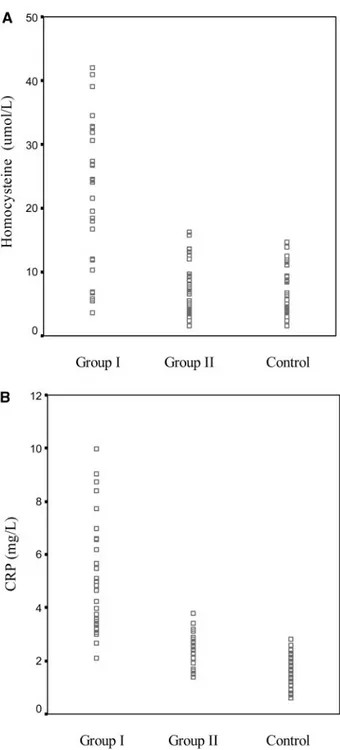
(Table 2). Distribution of plasma homocysteine and
CRP is shown in Figure 1. We did not find a
significant correlation between CRP and
homocys-teine levels (r
⫽ 0.06, p ⬎ 0.05). There was no
rela-tionship between AHI and both CRP and
homocys-teine (r
⫽ 0.12, p ⬎ 0.05; r ⫽ 0.31, p ⬎ 0.05,
respectively). The values of vitamin B
12, folate,
erythrocyte count, hemoglobin, mean cell volume,
and mean cell hemoglobin concentration were in
reference range. Stained RBC examination results
were in the normal range.
Discussion
Homocysteine is a thiol-containing amino acid that
is an intermediate substance produced during
intra-cellular demethylation of methionine. Elevated
lev-els of homocysteine are found in patients with
cardiovascular diseases.
16A clear correlation was
shown between mildly elevated total blood
homocys-teine concentrations and premature coronary artery
diseases,
17stroke,
18peripheral artery diseases, or
venous thrombosis.
19Elevated homocysteine levels in OSA patients
were reported only in patients with associated
isch-emic heart disease and/or hypertension.
20Jordan et
al
21reported 30% decrease in homocysteine level
after long-term continuous positive airway pressure
(CPAP) treatment in a small group of patients mostly
with hypertension or diabetes. However, Svatikova et
Table 1—Clinical Characteristics of OSA Patients and
Control Groups* Characteristics Group 1 (n⫽ 30) Group 2 (n⫽ 32) Control (n⫽ 30) Age, yr 47.14⫾ 1.62 45.31 ⫾ 1.21 42.6 ⫾ 3.2 Body mass index, kg/m² 29.63 ⫾ 0.67 31.12 ⫾ 0.76 20.2 ⫾ 0.82 Systolic BP, mm Hg 124.4⫾ 2.29 125⫾ 2.28 122 ⫾ 4.5 Diastolic BP, mm Hg 76.43⫾ 1.17 76.88 ⫾ 1.03 75.4 ⫾ 2.1
AHI 25.04⫾ 3.85 2.72⫾ 0.28 ⬍1
*Data are presented as mean⫾ SE.
Table 2—Demonstration of the Whole Parameters Detected in Patients and Control Subjects* Parameters Group 1 (n⫽ 30) Group 2 (n⫽ 32) Control (n⫽ 30) Homocysteine,mol/L 21.53⫾ 14.2† 7.4⫾ 5.12 6.8⫾ 4.7 CRP, mg/L 5.08⫾ 3.25†‡ 2.7⫾ 0.60§ 1.8⫾ 0.61 Total cholesterol, mg/dL 212.8⫾ 46.0‡ 197.7⫾ 27.0§ 118.4⫾ 32.2 Triglyceride, mg/dL 153.8⫾ 69.0‡ 132.7⫾ 65.0§ 78.0⫾ 18.8 HDL-C, mg/dL 44.0⫾ 10.0 43.2⫾ 10.9 47.1⫾ 9.4 LDL-C, mg/dL 133.3⫾ 33.3‡ 124.6⫾ 27.1§ 56.4⫾ 20.6 Lipoprotein (a), mg/dL 16.6⫾ 11.0‡ 13.0⫾ 9.3§ 8.7⫾ 3.2 Apolipoprotein A-I, mg/dL 126.4⫾ 20.6 121.7⫾ 39.1 132.1⫾ 27.9 Apolipoprotein B, mg/dL 110.8⫾ 33.7‡ 101.8⫾ 23.3‡ 80.4⫾ 16.7
*Data are presented as mean⫾ SE.
†Significant difference between group 1 and group 2 (p⬍ 0.05). ‡Significant difference between group 1 and control group (p⬍ 0.05). §Significant difference between group 2 and control group (p⬍ 0.05).
al
22have shown that plasma levels of homocysteine
are not elevated in OSA patients, and neither acute
untreated OSA nor treatment with CPAP and
dis-turbed sleep affect plasma homocysteine levels or
obscure its diurnal variation. This result is in contrast
to the findings of our study, in which we found
slightly enhanced homocysteine levels in OSA
pa-tients. The OSA patients in our study did not have
any heart disease and/or hypertension. Thus, we can
say that OSA might be independently associated with
mildly increased blood homocysteine levels.
A recent epidemiologic study
23has shown that
enhanced levels of CRP are a strong independent
predictor of risk of future myocardial infarction,
stroke, peripheral arterial disease, and vascular death
among persons without known cardiovascular
dis-ease. In OSA patients, hypoxia and reoxygenation
episodes can also cause activation of inflammatory
cells, as observed for neutrophils and monocytes,
24and ongoing inflammatory responses play important
roles in atherosclerosis.
25Although CRP is a nonspecific marker of
inflam-mation, epidemiologic studies
23,26suggest that CRP
is an important risk factor in atherosclerosis and
coronary artery disease. CRP that was found at high
concentrations in the atherosclerotic lesion
27has a
direct role on secretion of inflammatory mediators
by vascular endothelium,
28up-regulates the
expres-sion of adheexpres-sion molecules in endothelial cells, and
increases low-density lipoprotein uptake into
macro-phages.
29The development of systemic
inflamma-tion, characterized by elevated levels of certain
potent proinflammatory mediator such as CRP, may
have an important and direct role in the
develop-ment of atherosclerotic lesions and in promoting
cardiovascular morbidity.
30Population-based cross-sectional studies
31have
shown that plasma CRP concentrations are elevated
in obesity.
31In this study, CRP levels of both groups
were found to be significantly higher than those of
the control group. Chronic subclinical inflammation
effects may be one pathophysiologic mechanism
explaining the enhancement of CRP levels in OSA
patients. Shamsuzzaman et al
32reported higher CRP
values in patients with moderate-to-severe OSA than
in control subjects. Yokoe et al
33observed elevated
CRP values in patients with moderate-to-severe OSA
as well, and they noted a decrease in CRP levels by
treatment with nasal CPAP. In agreement with these
studies, we found high levels of CRP in OSA
pa-tients, but this enhancement did not have any
cor-relation with the severity of OSA. Similarly, we did
not find any correlation between CRP and
homocys-teine. Both of the cited authors
32,33reported
signif-icant positive relationship between CRP and AHI;
however, our results disagree with these authors.
The lack of correlation between AHI and CRP levels
is explained by the fact that apnea-related hypoxia
was not sufficient in patients with mild-to-moderate
OSA. This finding shows that CRP may be an
independent risk factor in patients with
mild-to-moderate OSA for future cardiovascular events.
Our results show that OSA syndrome is associated
not only with slight hyperhomocysteinemia but also
with increased CRP concentrations. The lack of
Figure 1. Distribution of plasma homocysteine (top, A) and CRP (bottom, B) in patients and control subjects.
correlation between homocysteine and CRP
sup-ports the possibility that homocysteine and CRP are
independent risk factors for cardiovascular disease in
OSA patients. Increased plasma concentrations of
homocysteine and CRP can be useful in clinical
practice to be predictor of long-term prognosis for
cardiovascular disease and the treatment of OSA,
providing many benefits to the patients and society.
References
1 Olson LG, King MT, Hensley MJ, et al. Community study of snoring and sleep-disordered breathing: prevalence. Am J Respir Crit Care Med 1995; 152:711–716
2 Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing in middle-aged adults. N Engl J Med 1993; 328:1230 –1233
3 Goodday RH. Nasal respiration, nasal airway resistance, and obstructive sleep apnea syndrome. Oral Maxillofac Surg Clin North Am 1997; 9:167–177
4 Peled N, Greenberg A, Pillar G, et al. Contributions of hypoxia and respiratory disturbance index to sympathetic activation and blood pressure in obstructive sleep apnea syndrome. Am J Hypertens 1998; 11:1284 –1289
5 He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea: experience in 385 male patients. Chest 1988; 94:9 –14
6 Mazza A, Bossone E, Mazza F, et al. Homocysteine and cardiovascular risk. Monaldi Arch Chest Dis 2004; 62:29 33 7 Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk
marker or mediator in atherothrombosis? Hypertension 2004; 44:6 –11
8 Morozova S, Suc-Royer I, Auwerx J. Cholesterol metabolism modulators in future drug therapy for atherosclerosis. Med Sci (Paris) 2004; 20:685– 690
9 Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr Atheroscler Rep 2004; 6:381–387
10 Brewer HB Jr. Focus on high-density lipoproteins in reducing cardiovascular risk. Am Heart J 2004; 148(Suppl):S14 –S18 11 Jonkers IJ, Smelt AH, van der Laarse A.
Hypertriglyceride-mia: associated risks and effect of drug treatment. Am J Cardiovasc Drugs 2001; 1:455– 466
12 Walldius G, Jungner I. Apolipoprotein B and apolipoprotein A-I: risk indicators of coronary heart disease and targets for lipid-modifying therapy. J Intern Med 2004; 255:188 –205 13 Rifai N, Ma J, Sacks FM, et al. Apolipoprotein (a) size and
lipoprotein (a) concentration and future risk of angina pecto-ris with evidence of severe coronary atherosclerosis in men: the Physicians’ Health Study. Clin Chem 2004; 50:1364 –1371 14 ASDA Diagnostic Classification Steering Committee. The International Classification of Sleep Disorders: diagnostic and coding manual. 2nd ed. Lawrence, KS: Allen Press, 1997 15 Rechtschaffen A, Kales A. A manual of standardized
termi-nology, techniques and scoring system for sleep stages of human subjects. Washington, DC: National Institute of Health, 1968
16 Graham IM, Daly EL, Refsum HM, et al. Plasma homocys-teine as a risk factor for vascular disease: the European concerted action project. JAMA 1997; 277:1775–1781 17 Nygard O, Nordrehaug JE, Refsum H, et al. Plasma
homo-cysteine levels and mortality in patients with coronary artery disease. N Engl J Med 1997; 337:230 –236
18 Sacco RL, Anand K, Lee HS, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: the Northern Man-hattan Study. Stroke 2004; 35:2263–2269
19 Wuillemin WA, Solenthaler M. Hyperhomocysteinemia: a risk factor for arterial and venous thrombosis. Vasa 1999; 28:151–155
20 Lavie L, Perelman A, Lavie P. Plasma homocysteine levels in obstructive sleep apnea: association with cardiovascular mor-bidity. Chest 2001; 120:900 –908
21 Jordan W, Berger C, Cohrs S, et al. CPAP-therapy effectively lowers serum homocysteine in obstructive sleep apnea syn-drome. J Neural Transm 2004; 111:683– 689
22 Svatikova A, Wolk R, Magera MJ, et al. Plasma homocysteine in obstructive sleep apnea. Eur Heart J 2004; 25:1325–1329 23 Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001; 103:1813–1818 24 Dugovskaya L, Lavie L, et al. Activation of oxidative
metab-olism and CD64 and CD11c expression in peripheral blood monocytes and neutrophils in obstructive sleep apnea syn-drome. Am J Respir Crit Care Med 2002; 165:934 –939 25 Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell
2001; 104:503–516
26 Haverkate F, Thompson SG, Pyke SD, et al. Production of C-reactive protein and risk of coronary events in stable and unstable angina: European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet 1997; 349:462– 466
27 Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation 2001; 103:1194 – 1197
28 Li JJ, Fang CH. C-reactive protein is not only an inflamma-tory marker but also a direct cause of cardiovascular diseases. Med Hypotheses 2004; 62:499 –506
29 Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima; role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterio-scler Thromb Vasc Biol 2000; 20:2094 –2099
30 Hatipoglu U, Rubinstein I. Inflammation and obstructive sleep apnea syndrome pathogenesis: a working hypothesis. Respiration 2003; 70:665– 671
31 Mendall MA, Patel P, Ballam L, Strachan D, et al. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ 1996; 312:1061– 1065
32 Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 2002; 105:2462–2464
33 Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstruc-tive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 2003; 107:1129