Contents lists available atScienceDirect
Aquaculture
journal homepage:www.elsevier.com/locate/aquaculture
Pharmacokinetics and bioavailability of ceftriaxone in brown trout (Salmo
trutta fario) after intravenous and intramuscular administration
Orhan Corum
a,⁎, Ayse Er
b, Duygu Durna Corum
a, Orkun Atik
c, Kamil Uney
b aDepartment of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Kastamonu, 37200 Kastamonu, Turkey bDepartment of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Selcuk, 42031 Konya, TurkeycDepartment of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Afyon Kocatepe, 03200 Afyonkarahisar, Turkey
A R T I C L E I N F O Keywords: Ceftriaxone Brown trout Pharmacokinetics Bioavailability A B S T R A C T
Ceftriaxone (CTX) is a third-generation cephalosporin that has proven to be effective in the treatment of in-fections caused by a wide range of gram-positive and gram-negative microorganisms. This study aimed to de-termine the plasma and muscle pharmacokinetics of CTX after its administration via the intravenous (IV) and intramuscular (IM) routes to brown trout (Salmo trutta fario) at temperatures of 10 °C–13 °C. In total, 140 healthy brown trout (body weight, 245 ± 38 g) were used. The brown trout received single IV and IM injections of CTX at 25 mg/kg. The IV doses were injected into the caudal vein, whereas the IM doses were injected into the right epaxial muscles. The plasma and muscle tissue concentrations of CTX were measured using high-performance liquid chromatography. Pharmacokinetic parameters were calculated using noncompartmental methods. Following the IV administration of CTX, the elimination half-life (t1/2ʎz), volume of distribution at steady state,
total body clearance, and area under the concentration–time curve (AUC0–72) in plasma were 5.83 h, 0.09 L/kg,
0.02 L/h/kg, and 1079.46 h*μg/mL, respectively. After the IM administration of CTX, plasma t1/2ʎz, peak plasma concentration (Cmax), time to reach Cmax, and bioavailability were 22.78 h, 87.92μg/mL, 0.5 h, and 27.19%,
respectively. The AUCMuscle/AUCPlasmaratio following the IV administration was 0.02 and that following the IM
administration was 0.04. CTX exhibited low bioavailability and prolonged t1/2ʎzafter the IM administration. The prolonged t1/2ʎzof CTX could thus be beneficial in brown trout. Nevertheless, future studies that aim to
de-termine the clinical efficacy and pharmacokinetics after repeated administration of CTX are warranted.
1. Introduction
There has been rapid growth in the aquaculture industry, and it has become a major sector in both developed and developing countries. Subsequently, growth in the cultivation of different fish populations in confined areas for the achievement of more products has been ob-served. This rise infish populations has led to the spread of bacterial infections and mortality infish. In addition, manipulation in fish in-duces stress, weakens the immune system, and increases vulnerability to bacterial infections. Thus, the use of antibiotics infish for prophy-lactic and therapeutic purposes has been recommended (Alderman and Hastings, 1998; Cabello, 2006; Romero et al., 2012; Serrano, 2005). Specific antibiotics are not available for fish; therefore, antibiotics used tofight bacterial infections in other species have generally been used forfish (Smith, 2008). However, the use of unknown dose regimens of
antibiotics in fish may cause bacterial resistance and occurrence of antibiotic residues. The presence of antibiotic residues infish and fish products is a potential threat to public health and is responsible for adverse effects, such as allergies, toxicities, gut flora dysbiosis, and bacterial resistance in humans (Heuer et al., 2009). Therefore, the dose regimens and pharmacokinetics of antibiotics administered to fish should be known (Serrano, 2005; World Health Organization, 2006; Yanong, 2016).
Trout is one of the most extensively producedfish species world-wide. Similar to that in other animals and humans, bacterial diseases are frequently observed in trout (Noble and Summerfelt, 1996). Gram-negative and gram-positive bacteria, such as Vibrio spp., Yersinia ruckeri, Streptococcus spp., Lactococcus garvieae, Aeromonas hydrophila, and Ed-wardsiella tarda (Gauthier and Rhodes, 2009;Nakai and Park, 2002; Rowe et al., 2014;Stock and Wiedemann, 2001), are the most common
https://doi.org/10.1016/j.aquaculture.2018.10.026
Received 6 April 2018; Received in revised form 6 August 2018; Accepted 14 October 2018
Abbreviations: AUC, Area under the plasma concentration versus time curve; ClT, Total body clearance; Cmax, Maximum plasma drug concentration; CTX,
Ceftriaxone; F, Bioavailability; HPLC, High-performance liquid chromatography; LOD, Limit of detection; LOQ, Limit of quantification; t1/2ʎz, Elimination half-life;
Tmax, Time to maximum drug concentration; Vdss, Apparent volume of distribution at steady state ⁎Corresponding author.
E-mail address:orhancorum@kastamonu.edu.tr(O. Corum).
Available online 15 October 2018
0044-8486/ © 2018 Elsevier B.V. All rights reserved.
microorganisms that have been isolated from trout with hemorrhagic septicemia, enteric redmouth disease, exophthalmia, and me-ningoencephalitis (Dalsgaard and Madsen, 2000; Toranzo, 2004; Toranzo et al., 2005;Uhland et al., 2000).
Ceftriaxone (CTX) is a third-generation cephalosporin antibiotic with bactericidal activity, acting via the inhibition of cell wall synthesis in gram-negative and gram-positive bacteria. It possesses a broad spectrum of activity against bacteria, including Staphylococcus spp., Streptococcus spp., Vibrio spp., Lactococcus spp., Aeromonas spp., Salmonella spp., Escherichia coli, and Yersinia spp. (Cormier et al., 2007; Fernando et al., 2011;Perry and Schentag, 2001;Ryzhko et al., 2006; Stock and Wiedemann, 2001;van Alphen et al., 2014). The use of CTX in veterinary medicine has not yet been approved by the EMEA and FDA. However, its off-label use has been reported in foals for the treatment of meningitis and septicemia at an intravenous (IV) dose of 25 mg/kg every 12 h (EMEA, 2017). In some countries, commercial preparations of CTX are available for use in cattle, buffalo, goat, sheep, horse, zoo animals, dogs, and cats at a dose of 5–50 mg/kg to treat infections caused by susceptible pathogens (Anonymous, 2018a, 2018b, 2018c).Plumb (2008)has reported the increasing use of CTX in ve-terinary medicine with some advantages, including excellent gram-ne-gative coverage and significantly less toxicity potential. The pharma-cokinetics of CTX have been determined in calves (Maradiya et al., 2010), cows (Kumar et al., 2010), buffalo calves (Dardi et al., 2004), horses (Ringger et al., 1996), neonatal foals (Ringger et al., 1998), camels (Goudah, 2008), lactating goats (Ismail, 2005), lactating ewes (Goudah et al., 2006), cats (Albarellos et al., 2007), and dogs (Rebuelto et al., 2002). CTX can also be used in brown trout because of its valu-able pharmacokinetic properties, such as long elimination half-life (t1/ 2ʎz) and high bioavailability noted in mammalian species. However, the obvious differences in the pharmacokinetics of CTX of the mammalian species make it crucial to investigate its pharmacokinetics in brown trout. Therefore, in the present study, we aimed to determine the plasma and muscle pharmacokinetics of IV and intramuscular (IM) administrations of CTX at a dose of 25 mg/kg in brown trout. 2. Materials and methods
2.1. Chemicals
CTX standard was obtained from Sigma-Aldrich (St. Louis, Mo., USA). Methanol (MeOH) and trifluoroacetic acid (TFA) were obtained from Merck (Darmstadt, Germany) and were used at the proper purity for performing high-pressure liquid chromatography (HPLC). For the injection, a CTX formulation (Unacefin 1.0 g powder for IV injection; Yavuz Drug Comp., Turkey) was used. An aqueous solution of CTX was prepared by dissolving the powder of the CTX formulation in sterile water at 50 mg/mL. Ultra-distilled water was obtained through a dis-tilled water system (aqua Maxi-Ultra System; Youngl Instrument Co., Ltd., Korea) and was used for preparing the solutions.
2.2. Animals
In total, 140 healthy brown trout (Salmo trutta fario) with an average body weight of 245 ± 38 g were used; these trout were not administered any drugs 2 months before the testing. The brown trout were procured from afish farm (Bozkir, Konya, Turkey). Two ponds (20 m3) belonging to thefish farm were accessed. The brown trout were housed under normal daily lighting conditions (daylight and dark) in running water with a pH of 7.1–7.5 and temperature of 10 °C–13 °C. The brown trout were maintained in the ponds for 2 weeks for acclimati-zation. They were given commercial pellet feed (Sibal, Sinop, Turkey) every day. The ethics committee of the Faculty of Veterinary Medicine (University of Selcuk, Konya, Turkey) approved all the study protocols for thefish.
2.3. Experimental groups and drug administrations
We randomly and equally divided the brown trout into two study groups. Thereafter, 14 equal subgroups were prepared from each of the two study groups. CTX (single dose at 25 mg/kg) was administered intravenously to the brown trout in the subgroups of thefirst study group (n = 70) and intramuscularly to those in the subgroups of the second study group (n = 70). The IV dose of CTX was injected into the caudal vein, whereas the IM dose of CTX was injected into the right epaxial muscles.
Blood samples (2 mL) were drawn fromfive brown trout at each sampling point under MS-222 (tricaine methanesulfonate, 200 mg/L) anesthesia at 0 (pre-dose sample), 0.25, 0.5, 1, 2, 4, 6, 8, 12, 18, 24, 36, 48, and 72 h. The collected samples were added to anticoagulant tubes that contained heparin, using 26-gauge needles, and were centrifuged (10 min, 4000 ×g) to obtain the plasma samples. After the blood samples were collected, the brown trout were euthanized under high-dose MS-222 (300 mg/L) anesthesia, and samples of the left epaxial muscles (1 g) were taken. Plasma and muscle samples were stored at −70 °C until analyses.
2.4. HPLC analysis of CTX
The plasma and muscle tissue concentrations of CTX were de-termined using an HPLC system (Shimadzu, Tokyo, Japan), as per a slightly modified version of the method previously described by Maradiya et al. (2010). The muscle tissues that were collected during sampling were homogenized at 10,000 rpm for 1 min with a tissue homogenizer (Heidolph Silent Crusher M, Germany). The samples were then vortexed by adding 200μL of MeOH to 100 μL of plasma and 400μL of MeOH to 100 μg of muscle tissue; they were then centrifuged at 10,000 rpm for 10 min. The resultant clean supernatant was trans-ferred to HPLC vials, and 10μL of the clear supernatant was injected. CTX was detected using a SPD-10A UV–VIS detector at a wavelength of 274 nm and a Gemini™ C18 column (250 mm × 4.6 mm, id 5 μm; Phenomenex, Torrance, CA, USA). While the column temperature was maintained at 35 °C using a column oven, the temperature of the au-tosampler (SIL 20A) was maintained at room temperature. Before the introduction into the system, all the solutions were passed through a 0.45-μm filter under vacuum and subjected to sonication (T840DH; Elma, Germany) for 20 min. The mobile phase that contained MeOH and 0.1% TFA (34:66 v/v) was degassed (DGU-14A) and transferred to the HPLC system through a pump with a low-pressure gradient (LC-20AT with CBM-20A system controller). Theflow rate was set to 1 mL/ min, and the injection volume was 10μL.
The method was found to be linear in the range from 0.1 to 1000μg/mL for plasma and in the range from 0.1 to 40 μg/g for muscle tissue. The limit of detection (LOD) and limit of quantitation (LOQ) values for the plasma and muscle tissue were 0.1μg/mL (g) and 0.04μg/mL (g), respectively. To determine the precision and accuracy of the assay,five replicates at 0.4 μg/mL, 4 μg/mL, and 40 μg/mL for plasma and at 0.1μg/g, 1 μg/g, and 10 μg/g for muscle tissue were tested forfive consecutive days to determine the coefficients of varia-tion and recoveries, respectively. The recoveries for CTX were de-termined to be > 90 for plasma and muscle tissue. Both the intraday and interday coefficients of variation were < 6.55%.
2.5. Pharmacokinetic calculations
The plasma and muscle tissue concentration–time curves of CTX were analyzed with WinNonlin 6.1.0.173 (Pharsight Corporation, Scientific Consulting Inc., North Carolina, USA) software. The phar-macokinetic variables over the average CTX concentrations at each time point were assessed using noncompartmental analysis. Total clearance (ClT) and volume of distribution at steady state (Vdss) for plasma were determined after the IV administration, whereas area under the
concentration versus time curve (AUC0–72) and terminal t1/2ʎz for plasma and muscle following both the IV and IM administrations were calculated. The elimination rate constant (λz) was calculated from the linear portion of the terminal phase with linear regression analysis. The t1/2ʎzwas estimated with ln2/λz. The area under the curve was cal-culated with the linear-log method. The absolute bioavailability (F) of CTX was calculated by dividing the AUC0–72value following the IM administration by the AUC0–72value following the IV administration.
The peak plasma concentration (Cmax) and time to reach Cmax(Tmax) after the IM administration were determined directly from the mean plasma and muscle tissue concentration–time curves. The penetration of CTX into the muscle tissue was evaluated using the ratio of AUC(0–72) for muscle tissue and AUC(0–72)for plasma (AUCMuscle/AUCPlasma). 2.6. Statistical analyses
Plasma and muscle tissue concentrations are presented as mean ± standard deviation (SD) values. The mean pharmacokinetic parameters that were calculated after the IV and IM administrations of CTX were evaluated by using the percentage difference calculated using the fol-lowing formula: (100*[value obtained after IM administration− value obtained after IV administration] / value obtained after IV adminis-tration). The values≤(−)25% and ≥ (+)25% were considered to be significant (Hudachek and Gustafson, 2013).
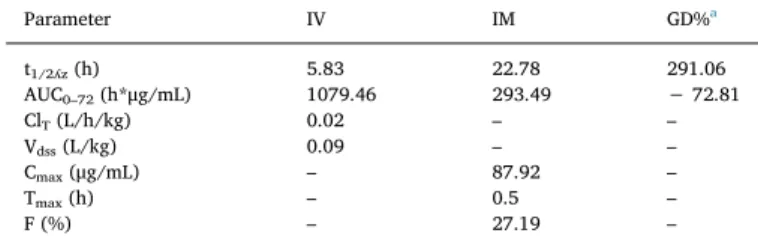
3. Results
Semilogarithmic plasma concentration–time curves and pharmaco-kinetic parameters after the IV and IM administrations of CTX (single dose at 25 mg/kg) in brown trout are presented inFig. 1andTable 1, respectively. The plasma t1/2ʎzand AUC0–72after the IM administration were significantly longer and lower, respectively, than those after the IV administration. After the IV administration of CTX, ClTwas 0.02 L/ h/kg and Vdss was 0.09 L/kg. However, after the IM administration, CTX reached a Cmaxof 87.92μg/mL, a Tmaxof 0.5 h, and F of 27.19%. Semilogarithmic muscle tissue concentration–time curves and pharmacokinetic parameters after the IV and IM administrations of CTX (single dose at 25 mg/kg) in brown trout are presented inFig. 2and Table 2, respectively. After the IM administration of CTX, t1/2ʎzand Tmaxof CTX in the muscle tissue were significantly longer and the AUC0–72and Cmaxwere significantly lower than those after the IV ad-ministration. AUCMuscle/AUCPlasmaratios following the IV and IM ad-ministrations were 0.02 and 0.04, respectively.
4. Discussion
We did not observe any local or systemic adverse drug reaction after either IV or IM administration of a single dose of CTX at 25 mg/kg in
brown trout. In addition, different doses of CTX (10–50 mg/kg) have been shown to be well tolerated by goats, sheep, and cats (Albarellos et al., 2007;Goudah et al., 2006;Sar et al., 2008, 2013).
The IV administration of CTX at a single dose of 25 mg/kg infish elicited a longer t1/2ʎz (5.83 h) than that in cats (1.87 h;Albarellos et al., 2007), dogs (1.73 h;Rebuelto et al., 2002), sheep (1.75 h;Goudah et al., 2006), goats (1.44 h;Ismail, 2005), cattle (1.02 h;Kumar et al., 2010), calves (1.58 h;Maradiya et al., 2010), buffalo calves (3.79 h; Dardi et al., 2004), foals (3.25 h;Ringger et al., 1998), and camels (2.57 h;Goudah, 2008). In the present study, ClTinfish (0.02 L/h/kg) was found to be lower than that in cats (0.37 L/h/kg;Albarellos et al., 2007), cows (0.30 L/h/kg; Kumar et al., 2010), sheep (0.14 L/h/kg; Goudah et al., 2006), goats (1.41 L/h/kg;Sar et al., 2008), and buffalo calves (0.26 L/h/kg;Dardi et al., 2004). Vdsswas found to be lower in 0.01 0.1 1 10 100 1000 0 12 24 36 48 60 72 Concentration (µg/mL) Time (hour) IV IM
Fig. 1. Mean ± standard deviation (SD) of plasma concentration–time curves (semilogarithmic plot) after intravenous (IV) and intramuscular (IM) adminis-trations of a single dose of ceftriaxone at 25 mg/kg in brown trout (n = 5). (For interpretation of the references to colour in thisfigure legend, the reader is referred to the web version of this article.)
Table 1
Plasma pharmacokinetic parameters after intravenous (IV) and intramuscular (IM) administrations of a single dose of ceftriaxone at 25 mg/kg in brown trout.
Parameter IV IM GD%a t1/2ʎz(h) 5.83 22.78 291.06 AUC0–72(h*μg/mL) 1079.46 293.49 − 72.81 ClT(L/h/kg) 0.02 – – Vdss(L/kg) 0.09 – – Cmax(μg/mL) – 87.92 – Tmax(h) – 0.5 – F (%) – 27.19 –
t1/2ʎz, elimination half-life; AUC, area under the concentration versus time
curve; ClT, total clearance; Vdss, volume of distribution at steady state; Cmax,
peak plasma concentration; Tmax, time to reach the maximum concentration; F,
absolute bioavailability.
a Refers to percentage (%) difference of IM administration compared with
that of IV administration ([IM− IV]/IV*100).
0.2 2 20 0 12 24 36 48 60 72 Concentration (µg/g) Time (hour) IV IM
Fig. 2. Mean ± standard deviation (SD) of muscle tissue concentration–time curves (semilogarithmic plot) after intravenous (IV) and intramuscular (IM) administrations of a single dose of ceftriaxone at 25 mg/kg in brown trout (n = 5). (For interpretation of the references to colour in thisfigure legend, the reader is referred to the web version of this article.)
Table 2
Muscle tissue pharmacokinetic parameters after intravenous (IV) and in-tramuscular (IM) administrations of a single dose of ceftriaxone at 25 mg/kg in brown trout. Parameter IV IM GD%a t1/2ʎz(h) 18.22 30.31 69.11 AUC0–72(h*μg/g) 118.39 79.79 −32.61 Cmax(μg/g) 8.75 3.14 −64.07 Tmax(h) 0.5 2 300
t1/2ʎz, elimination half-life; AUC, area under the concentration versus time
curve; Cmax, peak plasma concentration; Tmax, time to reach the maximum
concentration.
a Refers to percentage (%) difference of IM administration compared with IV
fish (0.09 L/kg) than in dogs (0.28 L/kg; Rebuelto et al., 2002), cats (0.57 L/kg;Albarellos et al., 2007), calves (0.2 L/kg;Maradiya et al., 2010), sheep (0.27–0.28 L/kg;Goudah et al., 2006), horses (1.6 L/kg; Ringger et al., 1998), and goats (0.37 L/kg;Ismail, 2005). CTX binds to plasma proteins at the rate of 87%–96% in humans (Stoeckel et al., 1981), 39%–45% in goats (Ismail, 2005), and 29%–37% in sheep (Goudah et al., 2006). CTX is converted to the active metabolite cefti-zoxime via the liver CYP450enzymes. This metabolite is only detected in the urine and can reportedly be detected in the plasma in case of liver injury in goats (Sar et al., 2006, 2008, 2013). CTX is eliminated through both biliary and urinary excretion (Stoeckel et al., 1988). The urinary excretion of CTX differs among species. Although a significant amount of CTX was detected in the urine of healthy sheep (Goudah et al., 2006), calves (Soback and Ziv, 1988), and buffalo calves (Dardi et al., 2004), no urinary excretion has been reported in healthy goats. Cephalosporins are weakly acidic drugs that are present in an ionized form at blood pH (Goudah et al., 2006). CYP450enzymes are also known to be present in fish with some differences from those in mammals (Goldstone et al., 2010). The kidneys and bile are also important in drug excretion infish. Fish have a kidney structure similar to that in mammals, with some differences, such as in the renal portal system. In addition, the kidneys are reportedly more important in drug metabolism in fish (Toutain et al., 2010). Such differences in t1/2ʎz, ClT, and Vdssbetweenfish and other species may be due to the differences in the plasma protein binding profile as well as differences in the metabolism and elimination pathways of CTX in various species. In addition,fish are heterothermic animals, and the water temperature has a significant influence on their drug pharmacokinetics (Lees and Shojaee Aliabadi, 2002). In the pre-sent study, the low water temperature (10 °C–13 °C) may have sig-nificantly contributed to the longer t1/2ʎzinfish than that observed in other species.
In the present study, the plasma concentration at thefirst sampling point (15 min) after the IV administration of CTX was 430.19μg/mL, whereas Cmaxafter the IM administration was 87.92μg/mL. Cmax ob-tained with a Tmaxof 0.5 h following the IM administration was similar to the concentration at 4 h following the IV administration. Following the IM administration of CTX (25 mg/kg), the Cmaxwas found to be higher in fish than in cats (54.40 ± 12.92 μg/mL; Albarellos et al., 2007) and the Tmaxwas found to be longer infish than in cats (0.33 h; Albarellos et al., 2007) and calves (0.25 h;Maradiya et al., 2010). The absolute bioavailability of CTX after the IM administration in brown trout was 27.19%, which was slightly lower than that reported pre-viously for calves (47%; Maradiya et al., 2010) and lower than that reported previously for cats (85.72%; Albarellos et al., 2007), dogs (102%;Rebuelto et al., 2002), and sheep (83.6%;Goudah et al., 2006). CTX is present in an ionized form at blood pH; therefore, it can be found in an ionized form in muscle tissue that has a pH value similar to that of blood (Gatica et al., 2010). The ionized form of drugs is associated with lower absorption (Riviere, 2009). Moreover, the skin and gills play a role in osmoregulation in freshwaterfish. Therefore, after IM admin-istration, the drug can be diffused via the injection channel or via the skin into the surrounding water (Wilkie et al., 2007). The low bioa-vailability that we observed in the present study could also be attrib-uted to the above phenomenon.
After the IM administration of CTX in brown trout, the t1/2ʎzwas significantly longer and the AUC0–72was significantly lower than those after the IV administration. The cardiovascular system offish is phy-siologically adapted to changes in the environmental temperature, and cardiac output and tissue perfusion depend on the environmental temperature (Barron et al., 1987;Lees and Shojaee Aliabadi, 2002). Fish have two types of muscles, white and red, and 90% of the muscles in trout are white muscles (Johnston, 1981;White et al., 1988). Red muscles have higher vascularization than white muscles (White et al., 1988). In the present study, decreased vascularization, owing to lower water temperature (10 °C–13 °C), in the white muscle tissues that form most of the muscles in trout may have limited drug excretion because of
the slow absorption of the CTX administered via the IM route. In the current study, the muscle t1/2ʎzand Tmaxafter the IM ad-ministration of CTX increased by 69.11% and 300%, respectively, but the muscle AUC0–72and Cmaxfell by 32.61% and 64.07%, respectively, compared with those after the IV administration of CTX. The AUCMuscle/ AUCPlasmaratios after the IV and IM administrations of CTX were 0.02 and 0.04, respectively. The muscle concentration at 6 h after the IV administration of CTX (dose: 100 mg/kg) in rats was found to be 1μg/g (Kwon and David, 1990). In contrast, in the present study, the muscle concentration at 24 h after the IV and IM administrations of CTX was 1μg/g. The tissue/plasma ratio of CTX varies across tissues. It was re-portedly 0.29 in the epididymis (Geny et al., 1993), 0.04 in the vitreous humor (Sharir et al., 1989), 7.82 in the cortical bone (Garazzino et al., 2011), and 0.5 in the brain (Granero et al., 1995).
In the present study, the muscle tissue concentration of CTX at the last sampling point was above the LOQ after both the IV (0.26μg/g) and IM (0.43μg/g) administrations. The maximum residue limit (MRL) and withdrawal time for CTX in veterinary use have not been reported. Because CTX is used at repeated doses in the treatment of infections caused by susceptible pathogens, the determination of the MRL and withdrawal time for CTX following a single-dose administration in the present study was not significant. In terms of its off-label use, the standard withdrawal time forfish has been set at 500 degree-days in the European Union (FAO, 2002). When CTX is used at repeated doses in fish, its withdrawal time may be determined using the rule of 500 de-gree-days. However, regulatory authorities for the protection of human health must establish the MRL and actual withdrawal time for CTX. In contrast, regulations including the MRL and withdrawal time in the specific use of CTX, such as the prevention and treatment of diseases in brood stock trout, are not necessary.
Resistance development against commonly used antibiotics infish, such as tetracycline, streptomycin, and ampicillin, has been reported (Dias et al., 2012;Done et al., 2015). Therefore, the use of alternative antibiotics is necessary particularly in valuable fishes. CTX suscept-ibility in Aeromonas spp. isolated from fishes has been determined (Rajpakshe et al., 2012; Yucel et al., 2005). CTX can alternatively be used infishes for the treatment of infections caused by pathogens that are resistant to other antibiotics. The oral route and bath treatment with antibacterials are the most common administration routes in fish. However, parenteral administration is preferred particularly in valuable fishes, such as brood stock and aquarium fishes, for disease prevention and treatment (Kumar and Roy, 2017). Because CTX is poorly absorbed following oral administration, it must be administered parenterally (Lee et al., 2006). Therefore, the parenteral use of CTX infish farming may not be practical. However, CTX will have a greater potential for use in brood stock trout.
5. Conclusion
CTX exhibited a prolonged t1/2ʎzwith a low absolute bioavailability; further, CTX was well tolerated when administered via the IM route in brown trout. The penetration of CTX into the muscle tissue was weak. The prolonged t1/2ʎzof CTX could be advantageous in brown trout. However, further studies are warranted to determine the clinical effi-cacy and pharmacokinetics of CTX following repeated administration.
Funding
This work was supported by the Coordination of Scientific Research Projects, University of Selcuk, Turkey (project No. 16401134).
Declarations of interest
Acknowledgments
This study was presented in abstract form as oral presentation to the 1. International Congress on Engineering and Life Science “ICELIS 2018,” Kastamonu, Turkey, 26–29 April 2018.
References
Albarellos, G.A., Kreil, V.E., Landoni, M.F., 2007. Pharmacokinetics of ceftriaxone after intravenous, intramuscular and subcutaneous administration to domestic cats. J. Vet. Pharmacol. Ther. 30, 345–352.
Alderman, D.J., Hastings, T.S., 1998. Antibiotic use in aquaculture: development of an-tibiotic resistance–potential for consumer health risks. IJFST 33, 139–155. Anonymous, 2018a.https://www.squarepharma.com.bd/downloads/Ceftron-Vet%20.
Injection.pdf, Accessed date: 20 February 2018.
Anonymous, 2018b.https://renata-ltd.com/wp-content/uploads/2015/04/Renacef_Inj. pdf, Accessed date: 10 July 2018.
Anonymous, 2018c.http://www.morvellab.com/products/veterinary-products/ antibiotics/ceftriaxone-inj-morcef-vet/, Accessed date: 10 July 2018.
Barron, M.G., Tarr, B.D., Hayton, W.L., 1987. Temperature-dependence of cardiac output and regional bloodflow in rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 31, 735–744.
Cabello, F.C., 2006. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ. Microbiol. 8, 1137–1144.
Cormier, G., Lucas, V., Varin, S., Hamelin, J.P., Tanguy, G., 2007. Yersinia pseudotu-berculosis infection of a lumbar facet joint. Joint Bone Spine 74, 110–111.
Dalsgaard, I., Madsen, L., 2000. Bacterial pathogens in rainbow trout, Oncorhynchus mykiss (Walbaum), reared at Danish freshwater farms. J. Fish Dis. 23, 199–209.
Dardi, M.S., Sharma, S.K., Srivastava, A.K., 2004. Pharmacokinetics and dosage regimen of ceftriaxone in buffalo calves. Vet. Res. Commun. 28, 331–338.
Dias, C., Mota, V., Martinez-Murcia, A., Saavedra, M.J., 2012. Antimicrobial resistance patterns of Aeromonas spp. isolated from ornamentalfish. J. Aqua. Res. Dev. 3, 1000131.
Done, H.Y., Venkatesan, A.K., Halden, R.U., 2015. Does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? AAPS J. 17, 513–524.
EMEA, 2017. Reflection Paper on Off-label Use of Antimicrobials in Veterinary Medicine in the European Union. EMA/CVMP/AWP/237294/2017.http://www.ema.europa. eu/docs/en_GB/document_library/Scientific_guideline/2017/07/WC500232226.pdf, Accessed date: 28 June 2018.
FAO, 2002. Basic overview of the regulatory procedures for authorisation of veterinary medicines with emphasis on residues in food animal species. In: Antibiotic Report–Final-17.05.02, . http://www.fao.org/ docrep/004/AC343E/AC343E00. HTM, Accessed date: 28 June 2018.
Fernando, R.R., Krishnan, S., Fairweather, M.G., Ericsson, C.D., 2011. Vibrio para-hemolyticus septicaemia in a liver transplant patient: a case report. J. Med. Case Rep. 5, 171.
Garazzino, S., Aprato, A., Baietto, L., D'Avolio, A., Maiello, A., De Rosa, F.G., Aloj, D., Siccardi, M., Biasibetti, A., Massè, A., Di Perri, G., 2011. Ceftriaxone bone penetration in patients with septic non-union of the tibia. Int. J. Infect. Dis. 15, e415–e421.
Gatica, M.C., Monti, G.E., Knowles, T.G., Gallo, C.B., 2010. Muscle pH, rigor mortis and blood variables in Atlantic salmon transported in two types of well-boat. Vet. Rec. 9, 45–50.
Gauthier, D.T., Rhodes, M.W., 2009. Mycobacteriosis infishes: a review. Vet. J. 180, 33–47.
Geny, F., Costa, P., Bressolle, F., Galtier, M., 1993. Ceftriaxone pharmacokinetics in el-derly subjects and penetration into epididymis. Biopharm. Drug Dispos. 14, 161–169.
Goldstone, J.V., McArthur, A.G., Kubota, A., Zanette, J., Parente, T., Jönsson, M.E., Nelson, D.R., Stegeman, J.J., 2010. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics 11, 643.
Goudah, A., 2008. Pharmacokinetic parameters of ceftriaxone after single intravenous and intramuscular administration in camels (Camelus Dromedarius). Res. Vet. Sci. 84, 483–489.
Goudah, A., Shin, H.C., Shim, J.H., Abd El-Aty, A.M., 2006. Characterization of the re-lationship between serum and milk residue disposition of ceftriaxone in lactating ewes. J. Vet. Pharmacol. Ther. 29, 307–312.
Granero, L., Santiago, M., Cano, J., Machado, A., Peris, J.E., 1995. Analysis of ceftriaxone and ceftazidime distribution in cerebrospinalfluid of and cerebral extracellular space in awake rats by in vivo microdialysis. Antimicrob. Agents Chemother. 39, 2728–2731.
Heuer, O.E., Kruse, H., Grave, K., Collignon, P., Karunasagar, I., Angulo, F.J., 2009. Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 49, 1248–1253.
Hudachek, S.F., Gustafson, D.L., 2013. Coadministration of lapatinib increases exposure to docetaxel but not doxorubicin in the small intestine of mice. Anti-Cancer Drugs 24, 958–968.
Ismail, M.M., 2005. Pharmacokinetics, urinary and mammary excretion of ceftriaxone in lactating goats. J. Vet. Med. A Physiol. Pathol. Clin. Med. 52, 354–358.
Johnston, I.A., 1981. Structure and function offish muscles. Symp. Zool. Soc. Lond. 48, 71–113.
Kumar, V., Roy, S., 2017. J. Aquac. Res. Develop. 8, 1000510.
Kumar, S., Srivastava, A.K., Dumka, V.K., Kumar, N., Raina, R.K., 2010. Plasma
pharmacokinetics and milk levels of ceftriaxone following single intravenous ad-ministration in healthy and endometritic cows. Vet. Res. Commun. 34, 503–510.
Kwon, K.I., David, W.A., 1990. Bourne physiological pharmacokinetic model of cef-triaxone disposition in the rat and the effect of caffeine on the model. Ach. Pharm. Res. 13, 227–232.
Lee, S., Kim, S.K., Lee, D.Y., Chae, S.Y., Byun, Y., 2006. Pharmacokinetics of a new, orally available ceftriaxone formulation in physical complexation with a cationic analogue of bile acid in rats. Antimicrob. Agents Chemother. 50, 1869–1871.
Lees, P., Shojaee Aliabadi, F., 2002. Rational dosing of antimicrobial drugs: animals versus humans. Int. J. Antimicrob. Agents 19, 269–284.
Maradiya, J.J., Goriya, H.V., Bhavsar, S.K., Patel, U.D., Thaker, A.M., 2010. Pharmacokinetics of Ceftriaxone in Calves. Veterinarski Arhiv. Vol. 80. pp. 1–9.
Nakai, T., Park, S.C., 2002. Bacteriophage therapy of infectious diseases in aquaculture. Res. Microbiol. 153, 13–18.
Noble, A.C., Summerfelt, S.T., 1996. Diseases encountered in rainbow trout cultured in recirculating systems. Annu. Rev. Fish Dis. 6, 65–92.
Perry, T.R., Schentag, J.J., 2001. Clinical use of ceftriaxone: a pharmacokinetic-phar-macodynamic perspective on the impact of minimum inhibitory concentration and serum protein binding. Clin. Pharmacokinet. 40, 685–694.
Plumb, D.C., 2008. Plumb's Veterinary Drug Handbook, Sixth Edition. Blackwell Publishing Professional, 2121 South State Avenue Ames, Lowa, pp. 50014–80300.
Rajpakshe, A.D.W.R., Prasad, K.P., Mukherjee, S.C., Kundan, K., Brahmachari, R.K., Meena, C.T., Kumar, H., 2012. In vitro sensitivity of three bacterial pathogens of koi carp (Cyprynus carpio l.) to certain antibiotics. J. Agric. Sci. Technol. 2, 93–98.
Rebuelto, M., Albarellos, G., Ambros, L., Kreil, V., Montoya, L., Bonafine, R., Otero, P., Hallu, R., 2002. Pharmacokinetics of ceftriaxone administered by the intravenous, intramuscular or subcutaneous routes to dogs. J. Vet. Pharmacol. Ther. 25, 73–76.
Ringger, N.C., Pearson, E.G., Gronwall, R., Kohlepp, S.J., 1996. Pharmacokinetics of ceftriaxone in healthy horses. Equine Vet. J. 28, 416–419.
Ringger, N.C., Brown, M.P., Kohlepp, S.J., Gronwall, R.R., Merritt, K., 1998. Pharmacokinetics of ceftriaxone in neonatal foals. Equine Vet. J. 30, 163–165.
Riviere, J.E., 2009. Absorption, distribution, metabolism and elimination. In: Riviere, J.E., Papich, M.G., Adams, H.R. (Eds.), Veterinary Pharmacology and Therapeutics. Wiley-Blackwell, pp. 11–47.
Romero, J., Feijooo, C.G., Navarrete, P., 2012. Antibiotics in aquaculture–use, abuse and alternatives, health and environment in aquaculture. In: Carvalho, E.D., David, G.S., Silva, R.J. (Eds.), Health and Environment in Aquaculture. InTech.https://doi.org/ 10.5772/28157.Available from: https://mts.intechopen.com/books/health-and-environment-in.aquaculture/antibiotics-in-aquaculture-use-abuse-and-alternatives.
Rowe, H.M., Withey, J.H., Neely, M.N., 2014. Zebrafish as a model for zoonotic aquatic pathogens. Dev. Comp. Immunol. 46, 96–107.
Ryzhko, I.V., Moldavan, I.A., Tsuraeva, R.I., Shcherbaniuk, A.I., 2006. Prophylactic use of ceftriaxone in combination with F I antigen immunization in studies on uninbred albino mice infected by Yersinia pestis. Antiplague immunity development. Antibiot. Khimioter. 51, 8–12.
Sar, T.K., Mandal, T.K., Das, S.K., Chakraborty, A.K., Bhattacharyya, A., 2006. Pharmacokinetics of ceftriaxone in healthy and mastitic goats with special reference to its interaction with polyherbal drug (Fibrosin). Int. J. Appl. Res. Vet. Med. 4, 142–154.
Sar, T.K., Mandal, T.K., Das, S.K., Chakraborty, A.K., 2008. Pharmacokinetics of cef-triaxone in carbontetrachloride-induced hepatopathic and uranyl nitrate-induced nephropathic goats following single dose intravenous administration. Drug Metab. Lett. 2, 23–28.
Sar, T.K., Mandal, T.K., Patra, P.H., Samanta, I., 2013. Disposition of ceftriaxone in he-patopathic goats following single-intramuscular dosing. Eur. J. Drug Metab. Pharmacokinet. 38, 269–273.
Serrano, P.H., 2005. Responsible Use of Antibiotics in Aquaculture. FAO Fisheries Technical Paper. No. 469. FAO, Rome, pp. 97.
Sharir, M., Triester, G., Kneer, J., Rubinstein, E., 1989. The intravitreal penetration of ceftriaxone in man following systemic administration. Invest. Ophthalmol. Vis. Sci. 30, 2179–2183.
Smith, P., 2008. Antimicrobial resistance in aquaculture. Rev. Sci. Tech. 27, 243–264.
Soback, S., Ziv, G., 1988. Pharmacokinetics and bioavailability of ceftriaxone adminis-tered intravenously and intramuscularly to calves. Am. J. Vet. Res. 49, 535–538.
Stock, I., Wiedemann, B., 2001. Natural antibiotic susceptibilities of Edwardsiella tarda, E. ictaluri, and E. hoshinae. Antimicrob. Agents Chemother. 45, 2245–2255.
Stoeckel, K., McNamara, P.J., Brandt, R., Plozza-Nottebrock, H., Ziegler, W.H., 1981. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin. Pharmacol. Ther. 29, 650–657.
Stoeckel, K., Trueb, V., Dubach, U.C., McNamara, P.J., 1988. Effect of probenecid on the elimination and protein binding of ceftriaxone. Eur. J. Clin. Pharmacol. 34, 151–156.
Toranzo, A.E., 2004. Report aboutfish bacterial diseases. In: Alvarez-Pellitero, P., Barja, J.L., Basurco, B., Berthe, F., Toranzo, A.E. (Eds.), Mediterranean Aquaculture Diagnostic Laboratories. Zaragoza: CIHEAM, pp. 49–89.
Toranzo, A.E., Magarinos, B., Romalde, J.L., 2005. A review of the main bacterialfish diseases in mariculture systems. Aquaculture 246, 37–61.
Toutain, P.L., Ferran, A., Bousquet-Mélou, A., 2010. Species differences in pharmacoki-netics and pharmacodynamics. Handb. Exp. Pharmacol. 199, 19–48.
Uhland, F.C., Hélie, P., Higgins, R., 2000. Infections of Edwardsiella tarda among Brook Trout in Quebec. J. Aquat. Anim. Health 12, 74–77.
van Alphen, N.A., Gonzalez, A., McKenna, M.C., McKenna, T.K., Carlsen, B.T., Moran, S.L., 2014. Ciprofloxacin-resistant aeromonas infection following leech therapy for digit replantation: report of 2 cases. J. Hand Surg. 39, 499–502.
White, F.C., Kelly, R., Kemper, S., Schumacker, P.T., Gallagher, K.R., Laurs, R.M., 1988. Organ bloodflow haemodynamics and metabolism of the albacore tuna Thunnus alalunga (Bonnaterre). Exp. Biol. 47, 161–169.
WHO, 2006. Antimicrobial Use in Aquaculture and Antimicro- bial Resistance. Report of a Joint FAO/OIE/WHO Expert Consultation on Antimicrobial Use in Aquaculture and Antimicrobial Resistance. World Health Organization, Seoul, Korea.
Wilkie, M.P., Morgan, T.P., Galvez, F., Smith, R.W., Kajimura, M., Ip, Y.K., Wood, C.M., 2007. Division of comparative physiology and biochemistry, society for integrative
and comparative biology. Physiol. Biochem. Zool. 80, 99–112.
Yanong, R.P.E., 2016. Use of antibiotics in ornamentalfish aquaculture 1. Circular 84.
Yucel, N., Aslim, B., Beyatlı, Y., 2005. Prevalence and resistance to antibiotics for Aeromonas species isolated from retailfish in Turkey. J. Food Quality. 28, 313–324.