https://doi.org/10.1007/s13762-019-02224-7
ORIGINAL PAPER
Metal recovery prediction of elements from anode slime
A. Rüşen1 · S. A. Yildizel1 · M. A. Topçu1Received: 12 June 2018 / Revised: 7 November 2018 / Accepted: 14 January 2019 / Published online: 28 January 2019 © Islamic Azad University (IAU) 2019
Abstract
Metal recovery prediction of elements remains as one of the significant problems in the metal industry due to limited application data. In this research, quasi-Newton training algorithm-based artificial neural network (ANN) was applied for estimating the recovery amount of the elements during the anode slime emerging processes. ANN models were designed with the approximation of the inverse Hessian at each iteration by using gradient information. Temperature, leaching time, solid-to-liquid ratio and ionic liquid concentration were taken as input parameters for the recovery amount estimation. The results showed that the proposed algorithm is highly efficient in predicting metal recovery amount from anode slime. As a precious one, maximum Au recovery amount is predicted by ANN as 82.11% when the solid-to-liquid ratio is 1/25, the temperature is 45 °C, ionic liquid concentration is 40% and the leaching time is 0.5 h.
Keywords Metal recovery · Metal recovery prediction · Anode slime
Introduction
Anode slime emerging during the electro-refining phase in copper production is one of the economically pre-scrap wastes due to having the significant amount of valuable metals (Au, Ag, Cu, Te, Se, etc.) (Chen et al. 2018). Since the composition of the anode slimes can vary from one refinery to another, valuable metals extraction from anode slime as secondary sources differs from each other depend-ing on their chemical and morphological structures (Hait et al. 2004; Aguilera et al. 2016). Some methods based on pyro- or hydrometallurgical extraction have been proposed world over for the processing of copper refinery anode slime (Abd El-Ghaffar et al. 2009; Kılıç et al. 2013). The hydro-metallurgical process is one of the chemical methods used to recover the metal from copper anode slime using leach-ing agents based on acidic (Xleach-ing and Lee 2017: Wang et al.
2017) or necessary solvents (Han et al. 2017; Zhang et al.
2017). However, using these agents can cause several prob-lems such as high solvent consumption, high energy and acid
consumption, re-evaluation or disposal of waste solvents. In order to overcome these problems, scientists are in search of an alternative solvent that is sensitive to the environment and human health.
Ionic liquids with excellent properties and synthesizabil-ity are considered as a convenient/suitable solvent to recover valuable metals from ores and wastes. Ionic liquids used in hydrometallurgical processes generally form an anion (based on imidazolium alkyl chain) and a cation (SO3, Cl, Br, BF4, etc.). The physical and chemical properties of ionic liquids vary depending on the length of the alkyl chain in the ani-ons and catiani-ons (Whitehead et al. 2007). Welton (1999) has reviewed that the ILs, namely green solvents, have some favorable properties such as less vapor pressure, better thermal stability and no harmful effect to the environment and process equipment. Therefore, they are very promising solvents to gain valuable metals from primary or secondary sources and to reduce the environmental issues caused by using organic volatile compounds having a harmful effect on human health and environment. There are various studies on metal recovery from ores and wastes with ionic liquids by solid–liquid extrac-tions or leaching processes (Park et al. 2014).
In recent years, the application areas of ionic liquids have expanded considerably from catalysis, separation technology and lubrication to metal extraction. As a result of getting good results especially in the field of liquid–liquid extraction and leaching processes, the work in this area has become increas-ingly widespread. Initial works in this area were realized by
Editorial responsibility: M. Abbaspour. * S. A. Yildizel
sayildizel@kmu.edu.tr
1 Engineering Faculty, Karamanoglu Mehmetbey University,
Whitehead et al. (2004a, b) performing leach processes for metal (Au, Ag, Cu, etc.) recovery by adding imidazolium-based ionic liquids on the ores. They achieved more than 85% Au and 60% Ag recovery efficiency after the 50-h leaching of ground ores at room temperature with BmimHSO4 (White-head et al. 2004a, b). Whitehead et al. also used ionic liquids (1-alkyl-3-methyl-imidazolium and its derivatives) for the leaching of sulfidic copper, gold and silver ores to examine various variables such as temperature, oxidant and alkali chain length on the metal extraction efficiency. The authors stated that the metal extraction efficiency increases with increasing ionic liquid concentration in the solution, temperature as well as the presence of oxidant (Whitehead et al. 2007, 2009).
With the positive results of the ores, many researchers have recently focused their efforts on the recovery of pre-cious metals (Au, Ag, Cu, Ti, Pd, Ta, Zn, Sc, etc.) from wastes. Huang et al. leached the printed circuit board as a residual material with BmimHSO4 to recover the copper. According to experimental results, 99.92% of copper recov-ery was yielded under the optimum leaching conditions: 80% (v/v) ionic liquid concentration, 1:25 (g/ml) solid-to-liquid ratio, 70 °C leach temperature, 2-h leaching time and 10 ml 30% H2O2 (Huang et al. 2014).
Kılıçarslan et al. studied the copper and zinc recovery from industrial brass residues by using ionic liquid (BmimHSO4) in various ratios (10%, 30%, 50%). In this study, the best recov-ery rate in zinc recovrecov-ery was achieved as 99% after 4 hours without any oxidant additions in 50% ionic liquid added to the solution. Also, the maximum copper recovery rate was yielded as 82% with 50% H2O2 addition (Kilicarslan et al.
2014). In a recent study, Rüsen and Topçu stated that the recovery of the anode slime with high precious metal con-tent could be carried out by using ionic liquids. According to the leaching results, Au recovery efficiency was obtained as 89.07% under the optimum conditions: 80% ionic liquid con-centration, 1/25 g/ml solid-to-liquid ratio, 75 °C temperature and 4-h time (Rüsen and Topçu 2017a).
It is known that early determination of metal recovery efficiencies from primary or secondary sources is an essen-tial factor for any metal producing industry. In copper sector, especially anode slime with the significant amount of valu-able metals is defined as an important secondary source for several elements including Au, Ag, Cu, Te, Se, etc. There are lots of parameters that affect the metal recovery. Instead of performing many experiments to determine the effect of each parameter, it would be very useful to develop an esti-mation method that can predict the metal recovery rates. However, there exists no proved relationship for the predic-tion of metal recovery from anode slime. Therefore, it would be very useful to analyze with experimental data and tests. Data-based ANN models and multiple linear regression sys-tems are commonly used in many engineering applications (Khashman 2012; Yildizel et al. 2017). These models also
provide more detailed and accurate estimations for further understanding of the material properties (Khademi et al.
2016). The quasi-Newton method is commonly used in simi-lar studies due to the facts that it is computationally cheap, it is faster and there are no needs to solve second derivatives and linear systems of the equations (Bogaers et al. 2014; Noack and Funke 2017).
This research is significant due to the fact that the ANN system is proposed for the anode slime in order to reveal quasi-Newton algorithm-based ANN prediction systems potential on the estimation of metal recovery amount for the first time in the literature by this study.
Materials and methods
Material characterization
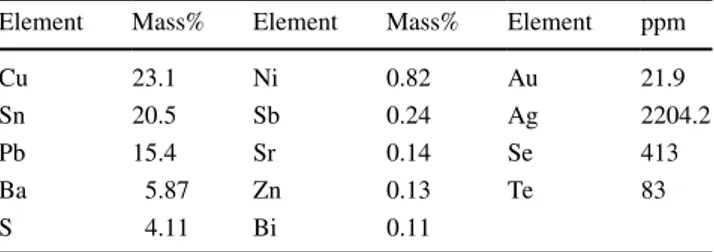
The anode slime used in this study was obtained from Er-Bakır copper company (Denizli Province in Turkey) that treats the secondary sources (bullion copper, scrap copper, etc.) as a charging material in the production process. The composition and morphology of anode slime can vary from refinery to refinery based on the addition to the anode furnace as a starting material, so it is essential to further elaborate on the anode slime provided from a copper refinery plant located in Turkey. Anode slime was obtained as powder form with lower than 100-micron particle size after grinding. In order to specify its significant quantities of precious metal, chemical analysis of anode slime carried out by induced cou-pled plasma-mass spectroscopy (ICP-MS) after applying the appropriate dissolution procedure is given in Table 1.
Anode slime sample was also analyzed by Rigaku NEXCG model XRF in the Department of Metallurgical and Materials Engineering of Necmettin Erbakan Univer-sity (NEU) to check the ICP-MS results. When compared the analyses obtained by ICP-MS and XRF, the results were found to be meaningful with reasonable deviations.
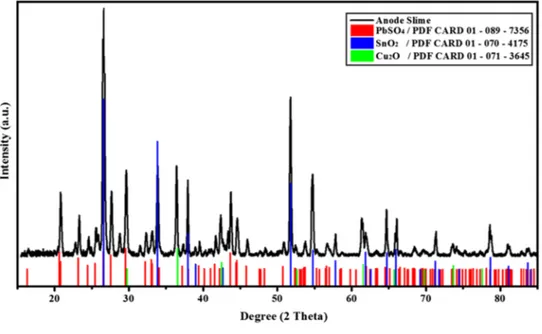
Mineralogical characterization of anode slime was per-formed by X-ray diffractometer (XRD) between 5 and 95° in 2-h range using intervals of 0.0167° with a Cu Kα radiation (40 kV, 40 mA). XRD pattern of original anode slime is shown in Fig. 1.
Table 1 Chemical analysis of original anode slime (Rüsen and Topçu
2017a)
Element Mass% Element Mass% Element ppm
Cu 23.1 Ni 0.82 Au 21.9
Sn 20.5 Sb 0.24 Ag 2204.2
Pb 15.4 Sr 0.14 Se 413
Ba 5.87 Zn 0.13 Te 83
As seen from the XRD pattern of the sample, anode slime is mainly composed of PbSO4, SnO2 and Cu2O. Although the presence of other elements (Ni, Sb, Sr, Bi, Au, Ag, etc.) was detected by the chemical analysis of anode slime, they could not be identified in the XRD pattern. In order to iden-tify other minor components or trace phases in the anode slime, its surface morphology was investigated by scanning electron microscopy (SEM) equipped with EDX. Figure 2
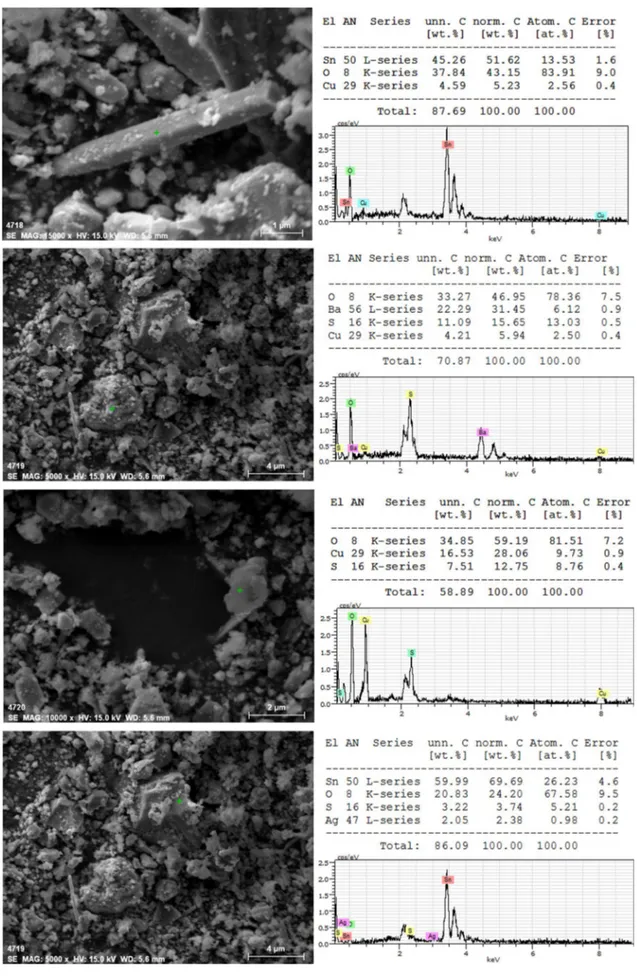
indicates the results of SEM studies of anode slime at differ-ent magnifications and energy-dispersive X-ray spectroscopy (EDX) results for selected points.
According to the EDX results, all of the bar-/rod-type particles in the anode slime belong to the SnO2 compound (Fig. 2a). Also, it is stated that BaSO4 and CaSO4 were spec-ified as minor components in the anode slime (Fig. 2b, c). As shown in Fig. 2d, analysis of the particle by EDX indicates that anode slime includes a small portion of Ag particles which was detected by chemical analysis of anode slime.
In order to determine the effects of leach duration, ionic liquid concentration, solid-to-liquid ratio and temperature parameters (the effect of interfering anions was ignored among these main parameters) on precious metal recovery from anode slime by using 1-ethyl-3-methyl-imidazolium hydrogen sulfate (EmimHSO4) ionic liquid, 16 experiments were designed considering the orthogonal experimental design system as reported in the previous study (Rüsen and Topçu 2017a). In the study, each experiment was performed only one time, but some of them were tested as confirma-tion experiments conducted at the optimum condiconfirma-tions. These leaching experiments were carried out in a 100-ml flask with 25 ml of a constant solution prepared by mixing IL and distilled water. A predefined amount of anode slime according to the solid-to-liquid ratio was mixed with this solution at 600 rpm in a magnetic stirrer and heated to the
desired temperature. The content of the pregnant solution obtained at the end of each experiment was determined by atomic absorption spectroscopy (AAS). Also, the content of the remaining secondary waste was detected by ICP-MS after applying the appropriate dissolution procedure. Metal recovery ratio (R%) was calculated by using Eq. 1 based on the remaining solid (secondary leach residue) analysis result;
where MLR and MSLR are the metal contents of original anode slime and secondary leach residue in mass%, respec-tively. WSLR and WLR are the mass of initial feed and undis-solved portion of the secondary leach residue, respectively.
Artificial neural network development
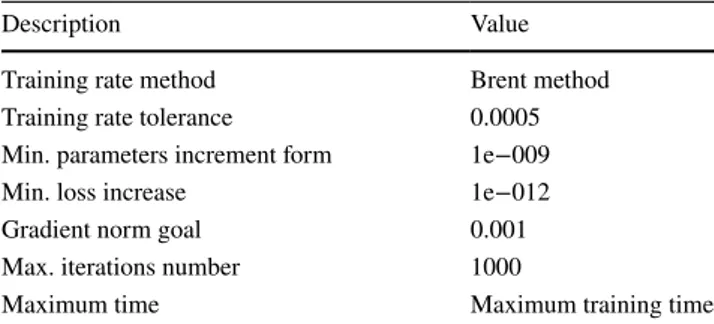
The quasi-Newton algorithm was used within the scope of this study. This method is widely preferred depending on the reason that it does not require the calculation of the second derivatives. The computation was based on the inverse Hes-sian at each iteration of the algorithm with the only usage of gradient information. The training algorithm is given in Table 2. All prediction studies have been proposed with Neural Designer software. Cu, Pb, Ba, Au, Se and Te ele-ments recovery was predicted in this estimation study.
The ANN model was proposed with four inputs for the prediction of the recovery amount, and it is presented in Fig. 3. Temperature, leaching time, solid-to-liquid ratio and ionic liquid concentration were considered as input param-eters based on the experimental studies.
The ANN structure contains a scaling layer, a neural net-work and an unscaling layer. The number of principal com-ponents is 4 and the number of outputs is 1. The complexity
(1)
R(%) =[(MLR ∗ WLR−MSLR∗ WSLR)∕(MLR ∗ WLR)] × 100
Fig. 1 XRD pattern of original
is represented by hidden neurons. Incremental order selec-tion algorithm was applied to the ANN model. This algo-rithm is given in Table 3.
The loss index plays an essential role in the use of a neu-ral network. It defines the task the neuneu-ral network is required to do and provides a measure of the quality of the representa-tion that it is required to learn. The choice of a suitable loss index depends on the particular application.
The root means squared error is used as the error method. It takes the square root of the mean squared error between the outputs from the neural network and the targets in the data set. The neural parameters norm is used as the regu-larization method. It is applied to control the complexity of the neural network by reducing the value of the parameters. Neural parameters norm weight was taken as 0.001.
The norm of the parameters gives a clue about the com-plexity of the predictive model. If the parameters norm is small, the model will be smooth. On the other hand, if the parameters norm is very big, the model might become unsta-ble. It can be also noted that the norm depends on the num-ber of parameters. The parameters norm is 1.32.
Results and discussion
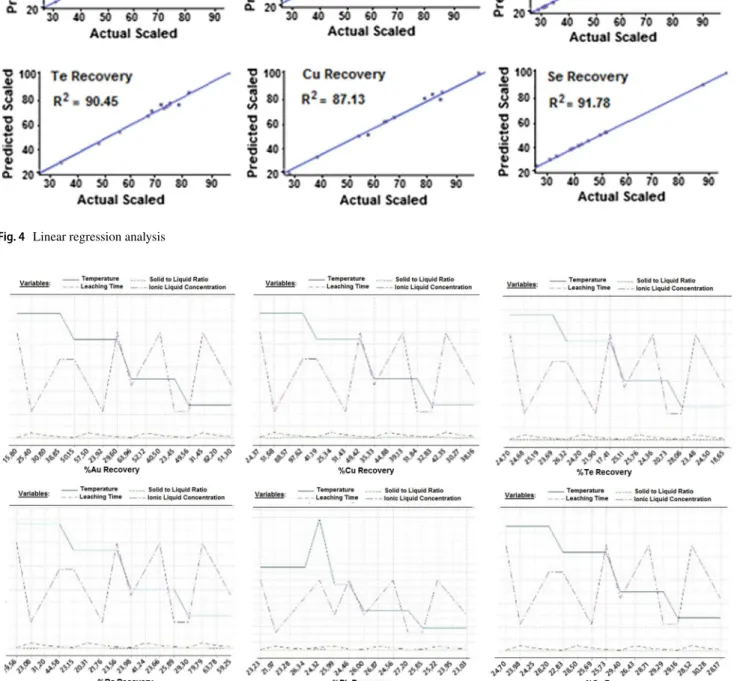
Linear regression analysis of the estimated recovery per-centages is shown in Fig. 4. The estimated values for each metal were plotted versus actual ones as squares. The blue lines show the best linear fits.
R-squared values vary between 91.78 and 87.13, and regression analysis results show that the proposed ANN model exhibits an excellent fitting performance. Ba, Au and Se elements reflect better performance compared to the other metal recovery prediction results.
The output recovery amounts as a function of inputs are presented in Fig. 5. The x- and y-axes are defined by the range of inputs and the output recovery percentages. Generally, the relation between the input and output data is constant and independent; however, it is not valid when evaluated in ANN environment. Each input of the pro-posed ANN model significantly affects the structure.
According to the results of the estimation, the most con-sistency is observed between the temperature, the leaching time and the metal recovery amount. Both inputs have an effect on the recovery amount shown in Fig. 5. Leach-ing time and solid-to-liquid ratio exhibit similar graphi-cal behavior. Ionic liquid concentration and temperature directly affect the recovery amount.
Each metal recovery amount was predicted (Fig. 5), and as a precious one, maximum Au recovery amount was pre-dicted by the proposed neural system as 82.11% when the solid-to-liquid ratio is 1/25, the temperature is 45 °C, the ionic liquid concentration is 40% and the leaching time is 0.5 h. Moreover, the minimum Au recovery is estimated with 95 °C temperature, 60% ionic liquid concentration, 1/10 solid-to-liquid ratio and 1-h leaching time.
Table 2 Training algorithm
Description Value
Training rate method Brent method Training rate tolerance 0.0005 Min. parameters increment form 1e−009 Min. loss increase 1e−012 Gradient norm goal 0.001 Max. iterations number 1000
Maximum time Maximum training time
Fig. 3 Proposed ANN structure
Table 3 Order selection
Description Value
Minimum order 1
Maximum order 10
Trials number 4
Tolerance 0.01
Selection loss goal 0
Maximum selection failures 5
Table 4 shows the errors of the proposed ANN system. The obtained error intervals for all ANN systems are within the acceptable limits. Sum squared error, mean squared error and Minkowski errors were evaluated in this study.
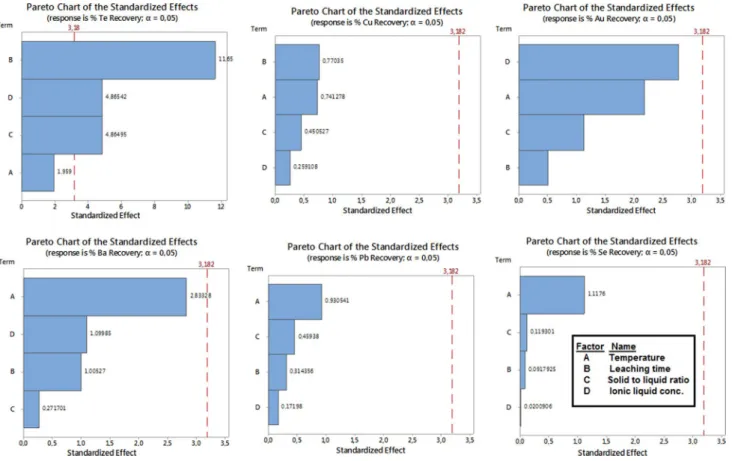
Pareto distribution diagrams are also plotted in order to clearly illustrate which input has the most significant effect on the output, and the diagrams are shown in Fig. 6.
As shown in Fig. 6, ionic liquid concentration is the dominant factor for the Au recovery prediction process. Since the more IL concentration exhibits the more acidic properties, H+ ions in the leach solution increase, which leads to dissolving the gold-bearing mineral in the anode slime (Rüsen and Topçu 2017a).
Leaching time has the most significant effect on the output factor recovery amount for Cu and Te metals compared to the
Fig. 4 Linear regression analysis
other factors. Since copper structures in the anode slime (espe-cially the largest phase; Cu2O) can dissolve even in very low acidic solutions, the effect of the ionic liquid concentration as well as solid-to-liquid ratio on the copper recovery rate is very low. As the ionic liquid concentration increases, the viscosity of the solution also increases, which leads to a reduction in copper recovery in the process (Rüsen and Topçu 2017b). However, leaching efficiency of copper can be enhanced by the addition of oxidants such as H2O2 and FeCl3 (Huang et al. 2014).
According to Fig. 6, temperature significantly affects the recovery amount of the S, Pb, Se and Ba metals. The reason for the parallel dissolution behavior of Pb, Ba and S metals is that they are found in PbSO4 and BaSO4 structure in the anode slime (Figs. 1, 2). Since the dissolution rate of PbSO4 and BaSO4 in water (or low acidic solutions) is very poor, the rate of penetration for Pb and Ba metals into the preg-nant solution is also low. Thus, the solution containing the precious metals will not be further contaminated with the Pb or Ba impurity. Sn found in the anode slime as SnO2 is
also very slowly dissolved in acidic media, so it keeps the original form in the secondary leach waste.
Precious metals in the pregnant solution can be recov-ered by different methods (liquid–liquid extraction, cementation, electrodeposition, etc.). The use of ionic liquids as a solvent will become widespread as costs are reduced due to technological improvements, even though they have higher costs of conventional solvents. Also, it is thought that the application of ionic liquids in the field of metal extraction will increase gradually because of being an environmentally sensitive solvent.
Conclusion
Metal recovery prediction of elements from anode slime has been conducted with the proposed ANN model. Four input parameters and their effect on the recovery
Table 4 Errors table Training Selection Testing
Sum squared error 0.07546–0.08521 0.03231–0.03754 0.01783–0.01846 Mean squared error 0.00777–0.00789 0.01042–0.01049 0.00577–0.00591 Minkowski error 0.98567–1.01234 0.65531–0.66345 0.36601–0.37234
percentages were analyzed in this study. These conclusions can be drawn according to the analysis results:
• The composition and morphology of anode slime can vary from refinery to refinery based on the addition to the anode furnace as a starting material, so it is essen-tial to further elaborate on the anode slime provided from a copper refinery plant. By using the ANN model, precious metal recoveries could be predicted for each copper anode slime.
• The ANN model showed the strong correlation between the metal recovery amount with temperature, leaching time, solid-to-liquid ratio and ionic liquid concentra-tion.
• The analysis results approved that the weighting factor is well calibrated, and they performed a good correla-tion with the previous experimental studies.
• The results of the study can be evaluated with other artificial systems for a better understanding of the effects of the input parameters on the metal recovery amounts.
• Applied ANN algorithm can be used by the other experimental recovery method for detailed investiga-tion of the problem.
Acknowledgements The authors gratefully acknowledge the Karamanoğlu Mehmetbey University Scientific Research Pro-jects (BAP) Coordinating Office for support with Grant Number KMU-BAP-04-YL-16.
Compliance with ethical standards
Conflict of interest The authors certify that they have no affiliations
with or involvement in any organization or entity with any financial in-terest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interests; and expert testimony or patent-licensing ar-rangements) or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this paper.
References
Abd El-Ghaffar MA, Abdel-Wahab ZH, Elwakeel KZ (2009) Extrac-tion and separaExtrac-tion studies of silver(I) and copper(II) from their aqueous solution using chemically modified melamine resins. Hydromet 96(1–2):27–34
Aguilera EM, Vera MCH, Viñals J, Seguel TG (2016) Characteri-zation of raw and decopperized anode slimes from a Chilean refinery. Metall Mater Trans B 47(2):1315–1324
Bogaers AE, Kok S, Reddy BD, Franz T (2014) Quasi-Newton meth-ods for implicit black-box FSI coupling. Comput Methmeth-ods Appl Mech Eng 279:113–132
Chen Y, Liu N, Ye L, Xiong S, Yang S (2018) A cleaning process for the removal and stabilisation of arsenic from arsenic-rich lead anode slime. J Clean Prod 176:26–35
Hait J, Jana RK, Sanyal SK (2004) Mineralogical characteristics of copper electrorefining anode slime and its leached residues. Ind Eng Chem Res 43(9):2079–2087
Han J, Liang C, Liu W, Qin W, Jiao F, Li W (2017) Pretreatment of tin anode slime using alkaline pressure oxidative leaching. Sep Purif Technol 174:389–395
Huang J, Chen M, Chen H, Chen S, Sun Q (2014) Leaching behavior of copper from waste printed circuit boards with Brønsted acidic ionic liquid. Waste Manag 34(2):483–488
Khademi F, Jamal SM, Deshpande N, Londhe S (2016) Predicting strength of recycled aggregate concrete using artificial neural network, adaptive neuro-fuzzy inference system and multiple linear regression. Int J Sustain Built Environ 5(2):355–369 Khashman A (2012) An emotional system with application to
blood cell type identification. Trans Inst Meas Control 34(2–3):125–147
Kılıç Y, Kartal G, Timur S (2013) An investigation of copper and selenium recovery from copper anode slimes. Int J Miner Pro-cess 124:75–82
Kilicarslan A, Saridede MN, Stopic S, Friedrich B (2014) Use of ionic liquid in leaching process of brass wastes for copper and zinc recovery. Int. J Miner Metall Mater 21(2):138–143 Noack MM, Funke SW (2017) Hybrid genetic deflated Newton method
for global optimisation. J Comput Appl Math 325:97–112 Park J, Jung Y, Kusumah P, Lee J, Kwon K, Lee CK (2014)
Appli-cation of ionic liquids in hydrometallurgy. Int J Mol Sci 15(9):15320–15343
Rüşen A, Topçu MA (2017a) Optimization of gold recovery from copper anode slime by acidic ionic liquid. Korean J Chem Eng 34(11):2958–2965
Rüşen A, Topçu MA (2017b) The effect of EmimHSO4
(1-ethyl-3-methyl-imidazolium hydrogen sulfate) on copper recovery from anode slime AKU-J. Sci Eng 17:696–703
Wang S, Cui W, Zhang G, Zhang L, Peng J (2017) Ultra fast ultra-sound-assisted decopperization from copper anode slime. Ultra-son Sonochem 36:20–26
Welton T (1999) Room-temperature ionic liquids. Solvents for syn-thesis and catalysis. Chem Rev 99(8):2071–2084
Whitehead JA, Lawrance GA, McCluskey A (2004a) ‘Green’leaching: recyclable and selective leaching of gold-bearing ore in an ionic liquid. Green Chem 6(7):313–315 Whitehead JA, Lawrance GA, McCluskey A (2004b) Analysis of gold
in solutions containing ionic liquids by inductively coupled plasma atomic emission spectrometry. Aust J Chem 57(2):151–155 Whitehead JA, Zhang J, Pereira N, McCluskey A, Lawrance GA
(2007) Application of 1-alkyl-3-methyl-imidazolium ionic liq-uids in the oxidative leaching of sulphidic copper, gold and silver ores. Hydrometallurgy 88(1–4):109–120
Whitehead JA, Zhang J, McCluskey A, Lawrance GA (2009) Com-parative leaching of a sulfidic gold ore in ionic liquid and aque-ous acid with thiourea and halides using Fe(III) or HSO5−
oxi-dant. Hydrometallurgy 98(3–4):276–280
Xing WD, Lee MS (2017) Leaching of gold and silver from anode slime with a mixture of hydrochloric acid and oxidizing agents. Geosyst Eng 20(4):216–223
Yildizel SA, Tuskan Y, Kaplan G (2017) Prediction of skid resist-ance value of glass fiber-reinforced tiling materials. Adv Civ Eng 2017:1–8
Zhang FY, Zheng YJ, Peng GM (2017) Deselenization and detelluri-zation of precious-metal ore concentrates by swelling oxidizing roasting and successive alkaline leaching. Int J Miner Metall Mater 24(2):147–155