DOES MEAN PLATELET VOLUME SHOW DISEASE ACTIVITY IN BEHÇET’S DISEASE?
OZGEGUNDUZ1, BURCUT. AYANOGLU2, AYSELGURLER3, FATMAG. ERDOGAN4, ASLIHANALHAN5
1Assistant Professor of Dermatology, Ufuk University Faculty of Medicine, Department of Dermatology, Ankara - 2Dermatologist, Aksaray State Hospital, Department of Dermatology, Aksaray - 3Professor of Dermatology, Ufuk University Faculty of Medicine, Department of Dermatology, Ankara - 4Associated Professor of Dermatology, Ufuk University Faculty of Medicine, Department of Dermatology, Ankara - 5Assistant Professor of Statistics, Ufuk University Faculty of Arts and Sciences, Department of Statistics, Ankara, Turkey
Introduction
Behçet’s disease (BD) is a multisystemic inflammatory disease characterized by exacerbation and periods of remission. The pathogenesis of the disease appears to be complex and multifactorial; hence, it remains unknown, but vascular endothelial pathologies, hypercoagulability and clotting disor-ders have been proposed as possible causes(1).
Distinctively in BD, the occlusion can affect both veins and arteries, and in histopathological exami-nation a nonspecific inflammation is seen(2).
It was observed that episodes of thrombosis tend to occur in younger patients with BD as well as during periods of increased disease activity(3).
Recurrent aphthous stomatitis (RAS) is a chronic painful ulceration of the oral mucosa with an unknown etiology, but possible initiating factors include trauma, infections, immune mechanisms, or genetic predisposition(4). Furthermore, RAS that
recurs more than three times a year is a major crite-rion for the diagnosis of BD and is usually the ini-tial sign of this disease.
Received Febrary 13, 2016; Accepted April 02, 2016
ABSTRACT
Introduction: The mean platelet volume (MPV) shows the average size of platelets in blood and indicates platelet function and
activation, which are correlated with thrombosis and inflammation. Platelet activation might be related with the pathophysiology of Behçet’s disease (BD) since this disease is also prone to inflammation and thrombosis.
We aimed to determine whether MPV can be used as a marker for BD in disease activation and severity.
Materials and methods: The data of 33 BD patients during the active and the inactive periods of the disease were collected
retrospectively, and the results were then compared with 35 recurrent aphthous stomatitis (RAS) patients and 60 healthy controls who were age- and gender- matched. If at least two clinical findings were present, BD was considered to be active.
Results: In the active period of BD, the MPV levels (8,6 fL) were slightly higher than those in RAS (8,4 fL) and control groups
(8,4 fL) (p=0,148), however, the MPV levels of active period (8.66 fL) were significantly higher than the inactive period (8.3 fL), (p=0.007). Moreover, 8 BD patients with vascular event history had significantly higher levels of MPV during active (9,3 fL) than inactive period (7,3 fL) of the disease (p=0,012).
Conclusion: In conclusion, elevation of MPV levels should be considered for each patient individually, and during follow-ups
MPV measurements can be valuable to determine disease activity. Additionally, an elevation in MPV could alarm clinician as BD is getting activated and that there might be an increased risk of vascular involvement with BD.
Key words: Behçet’s disease, Mean platelet volume, disease activity.
The mean platelet volume (MPV) shows the average size of platelets in the blood(5). Thus, an
increase in platelet volume and size indicates platelet function and activation, which is also related to thrombosis and inflammation(6).
Correlations between increased MPV values and some thrombotic diseases, such as deep vein thrombosis, acute myocardial infarction, or acute ischemic cerebrovascular events, have previously been reported(7). In other inflammatory processes
such as rheumatoid arthritis (RA) and ankylosing spondylitis (AS), it was reported that the MPV was significantly higher, reflecting, in addition, disease activity(8).
Until now, in previous studies MPV levels were reported to be higher in BD patients than con-trols, but in different patients with active and inac-tive phase of BD, and no correlation has been found regarding the disease activity(9, 10). However, the
dif-ferences in MPV levels during the active and inac-tive phase of BD have not been compared in the same individuals. Here, we aimed to determine whether the MPV can be used as a marker for BD in disease activation and severity, therefore, we per-formed this study to compare the same patients’ MPV levels during the active and inactive periods of BD with RAS and control group.
Materials and methods
The diagnosis of BD was based on the clinical criteria established by the International Study Group of Behçet’s Disease in 1990(11). If at least two
clinical findings were present at the time of diag-nose or during follow up, the BD was considered to be active. Patients with typical painful oral ulcers that recurred at least 3 times a year without any organ involvement of BD were diagnosed as RAS.
The files of BD and RAS patients who were followed up in our Behçet’s Disease Center between February 2006 and February 2014 were reviewed, and data of the 33 BD patients who had routine complete blood cell (CBC) count, erythro-cyte sedimentation rate (ESR) and C-reactive pro-tein (CRP) results during the active and the inactive periods of the disease were included in this study. They were compared with age- and gender matched 35 RAS patients with active ulcers at the time of testing, and 60 patients who were diagnosed with non-inflammatory skin disorders (e.g. telogen efflu-vium) served as controls. The median age of BD patients, RAS patients and controls were 29 (18-63)
years, 36 (18-65) years and 30 (18-65) years, respectively (p=0.405). The male to female ratio was 13:20 in BD group, 13:22 in RAS group and 23:37 in control group (p=0.982). The baseline demographic findings of the BD patients are shown in Table 1.
Patients with BD, RAS or controls who were using aspirin or any other drugs that could have had an influence on platelet functions were excluded from the study. In addition, patients or controls with diabetes mellitus, peripheral vascular disease, car-diovascular disease, cerebrovascular events, hema-tological disorders, inflammatory bowel disease, or malignancies were also not included. This study was approved by the Ufuk University Local Ethics Committee (Approval number: 280520142).
According to our laboratory protocol, all of the blood samples were taken by venipuncture between 8 and 11 a.m. after a fasting period of 12 hours, and these were then analyzed within one hour of receipt. The CBC parameters from routine tubes containing ethylene diamine tetra acetic acid (EDTA) were studied, and the analyses were per-formed using the Abbott CELL DYN 3700 auto-mated hematology analyzer (Abbott Laboratories, Abbott, IL, USA). In our laboratory, the reference range for the MPV, ESR and CRP levels are ranged from 6.9 to 10.4 fL, 0,01 to 20 mm/h and 0.01 to 5 mg/L, respectively.
Statistical analysis
The Predictive Analytics Software (PASW) version 18.0 for Windows (SPSS Inc., Chicago, IL,
Males/Females 13/20 Age (years) 32.50 (± 12.88) Duration of the disease (years) 9.85 (± 6.44)
Family history 9 Mucocutaneous involvement 33
Positive pathergy test 14 Vascular involvement* 8
Table 1: Demographic properties of the Behçet’s Disease patients (n=33).
* vena cava superior syndrome (n=1), Pulmonary thromboem-bolism (n=1), Transverse sinus thrombosis (n=1), Venous thrombosis of Sigmoid sinus (n=1), Deep venous thrombosis (n=1), superficial thrombophlebitis (n=3).
USA) was used for all analyses. To determine data distribution, a single-sample Kolmogorov-Smirnov test was performed, and one-way analysis of vari-ance (ANOVA), Wilcoxon and Mann-Whitney U tests were used to compare the groups. In addition, p values of less than 0.05 were considered to be sta-tistically different.
Results
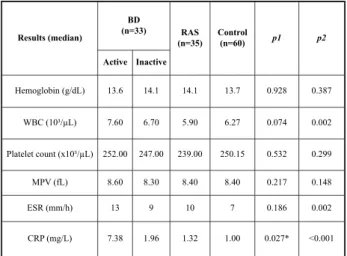
A comparison of the groups with regard to their laboratory findings is presented in Table 2. In the active period of BD, the MPV levels were slightly higher than those in RAS and control groups (ANOVA, p=0,148); however, MPV levels were significantly higher during the active period than inactive period of the BD (Wilcoxon test, p= 0,007). Moreover, comparison of MPV levels in 8 BD patients with vascular event history yielded that, during the active period of the disease (9,3 fL; 7,7-10,5) MPV levels were statistically significant-ly higher than inactive period (7,3 fL; 6-8,5) (Wilcoxon test, p=0,012). Also, in the active period, the MPV levels of the same 8 BD patients with vas-cular event history (9,3 fL; 7,7-10,5) were higher than 25 patients without vascular involvement (8,6 fL; 5,93-13,10), however, no statistically significant differences were found between the groups (Mann-Whitney U test, p=0,165).
Discussion
The elevation in MPV is accepted as a sign of inflammation and is also associated with platelet activation. Larger platelets are more reactive and contain more dense granules filled with higher amounts of active substances (e.g. thromboxane A2)(12). The exact pathogenic mechanism associated
with the formation of vascular lesions in BD remains unclear, but endothelial dysfunction is thought to have a major role in the pathogenesis of thrombosis(13). Akar et al. reported that the platelets
in the BD patients were more reactive than in the controls, which contributed to the thrombotic ten-dency(14). Additionally, IL-6 levels were shown to be
elevated in BD patients with an active disease, which can also induce megakaryopoiesis and releasing of bigger platelets from the bone mar-row(15, 16). Therefore, we hypothesized that platelet
activation might be related with the pathophysiolo-gy of BD since this disease is prone to inflamma-tion and thrombosis. We also thought that because the MPV is routinely measured in CBC counts, it could have served as a practical biomarker of dis-ease activation.
To the best of our knowledge, no studies exist that have compared the MPV levels in the same individuals during the active and inactive periods of BD. In their study, Acikgoz et al. concluded that there were no significant differences in the MPV levels between the active (8.20 fL) and inactive (8.08 fl) groups of BD, and that it was significantly higher in the BD patients (8.14 fl) than the controls (7.48 fL)(9). Similarly, Ekiz et al. also determined
that the MPV levels in the active phase (8.21 fL) of BD were not significantly higher than the inactive phase (8.44 fL), although, they reported significant-ly higher MPV levels in patients with BD and RAS than controls(10). Balta et al. found significantly
higher MPV levels in the BD patients (8.86 fl) in their study compared with the controls (8.39 fl), and MPV was also significantly increased in BD patients in the active phase (9.07 fL) than those in inactive phase (8.40 fL)(17).
In our study, the MPV levels in the active peri-od of BD were found to be high but not statistically higher than the RAS and control groups. Therefore, we concluded that it was not possible to use our MPV results to discriminate BD from RAS and controls. However, this difference might be related to the relatively small sample size in our study.
Results (median) BD (n=33) RAS (n=35) Control (n=60) p1 p2 Active Inactive Hemoglobin (g/dL) 13.6 14.1 14.1 13.7 0.928 0.387 WBC (10³/µL) 7.60 6.70 5.90 6.27 0.074 0.002 Platelet count (x10³/µL) 252.00 247.00 239.00 250.15 0.532 0.299 MPV (fL) 8.60 8.30 8.40 8.40 0.217 0.148 ESR (mm/h) 13 9 10 7 0.186 0.002 CRP (mg/L) 7.38 1.96 1.32 1.00 0.027* <0.001
Table 2: Comparison of laboratory findings between Behçet’s disease, RAS and controls.
BD: Behçet’s disease; RAS: Recurrent aphthous stomatitis; WBC: White blood cell; MPV: Mean platelet volume; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; p1: Comparison of inactive phase of BD with RAS and control groups p2:Comparison of active phase of BD with RAS and control groups
Also, we showed that, the MPV levels were significantly increased during the active period (8.6 fL) of the disease compared to the inactive period (8.30 fL), probably because of the fact that we had studied MPV levels in the same individuals during the active and inactive periods of the disease. On the contrary, Lee et al. found lower MPV levels in patients with BD (9.97 fL) than in the control group (10.5 fL)(18). We also found that MPV levels in the
inactive phase were even lower than RAS and con-trol group. Our finding might help us to understand the discrepancy of Lee et al.’s study, because dis-ease activity was not taken into consideration in their study. Also, Turkcu et al. reported lower MPV levels in the active uveitis phase of BD than in the controls(19). These results might differ from our
study because non-ocular involvement of the dis-ease was not compared in their study, and ocular involvement of the disease might also have addi-tional etiopathogenetic factors than non-ocular involvement.
Our patients with vascular involvement had significantly higher levels of MPV during the active period of the disease than the inactive period, but the MPV levels in these patients with vascular involvement during the active period was not statis-tically higher than patients without vascular involvement. Here, we cannot show statistically significant results due to relatively little number of patients with vascular involvement (n=8), but Acikgoz et al. found that the MPV was statistically higher in BD patients with thrombosis (8.45 fL) than in those without thrombosis (7.96 fL)(9). These
results also showed that increased platelet reactivity was to blame for the development of vascular lesions associated with BD. In contrast, Lee et al. and Ekiz et al. determined that there were no signif-icant differences with regard to thrombosis(10, 18).
Their results might differ from our study due to the fact that MPV levels were not compared in the same individuals during the active and inactive periods of the disease.
The discrepancies between previous studies could also stem from the fact that anticoagulants (EDTA or citrate) might potentially affect platelet size, and that measurements from various types of automated cell count analyzers have shown the dis-parity in MPV levels(20). The main limitation of this
study was its retrospective design. Hence, a non-standardized delay after venipuncture could affect the MPV results(21). Another limitation was the
rela-tively small number of patients included in our
study.
In conclusion, our results showed that MPV levels of active BD patients did not differ from RAS and controls, and also MPV levels in patients with vascular involvement during the active period was not statistically higher than patients without vascular involvement. However, MPV was signifi-cantly increased in BD patients during active phase compared to inactive phase, and also in BD patients with vascular event history MPV levels during the active period of the disease were statistically signif-icantly higher than inactive period. These results show us that, MPV levels are not useful to discrimi-nate BD from RAS, and the elevation of MPV lev-els should be considered for each patient individu-ally. In conclusion, our results suggest that during follow-ups the MPV measurements can be valuable while predicting to determine disease activity. In addition, we might say that an elevation in the MPV could alarm clinician as the patient’s BD is getting activated and that there might be an increased risk of vascular involvement with BD.
References
1) Emmi G, Silvestri E, Squatrito D, D’Elios MM, Ciucciarelli L, Prisco D, et al. Behçet’s syndrome
pathophysiology and potential therapeutic targets.
Intern Emerg Med 2014; 9: 257-265.
2) Mat C, Yurdakul S, Sevim A, Özyazgan Y, Tüzün Y.
Behçet’s syndrome: Facts and controversies. Clinics in
Dermatology 2013; 31: 352-361.
3) Gaffo AL. Thrombosis in vasculitis. Best Pract and Res Clin Rheum 2013; 27: 57-67.
4) Chavan M, Jain H, Diwan N, Khedkar S, Shete A, Durkar S. Recurrent aphtous stomatitis: a review. J Oral Pathol Med 2012; 41: 577-583.
5) Buttarello M, Plebani M. Automated blood cell counts. Am J Clin Pathol 2008; 130: 104-116.
6) Colkesen Y, Muderrisoglu H. The role of mean platelet
volume in predicting thrombotic events. Clin Chem Lab
Med 2012; 50: 631-634.
7) Van der Loo, Martin JF. Megakaryocytes and platelets
in vascular disease. Bailliere’s Clinical Haematology
1997; 10: 109-123.
8) Yazici S, Yazici M, Erer B, Erer B, Calik Y, Ozhan H, et al. The platelet indices in patients with rheumatoid
arthritis: mean platelet volume reflects disease activity.
Platelets 2010; 21(2): 122-125.
9) Acikgoz N, Karincaoglu Y, Ermis N, Yagmur J, Atas H, Kurtoglu E, et al. Increased mean platelet volume in
Behçet’s disease with thrombotic tendency. Thoku J
Exp Med 2010; 221: 119-123.
10) Ekiz O, Balta I, Sen BB, Rifaioglu EN, Ergin C, Balta S, et al. Mean platelet volume in recurrent aphthous
stomatitis and Behçet disease. Angiology 2014; 65: 161-165.
11) International Study Group of Behcet’s disease. Criteria
for diagnosis of Behcet’s disease. Lancet 1990; 335: 1078-1080.
12) Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD.
Mean platelet volume: a link between thrombosis and inflammation. Current Pharmaceutical Design 2011;
17: 47-58.
13) Kiraz S, Ertenli I, Oztürk MA, Haznedaroğlu IC, Celik I, Calgüneri M. Pathological haemostasis and
‘pro-thrombotic state’ in Behçet’s disease. Thrombosis
Research 2002; 105: 125-133.
14) Akar S, Ozcan MA, Ateş H, Gürler O, Alacacioglu I, Ozsan GH, et al. Circulated activated platelets and
increased platelet reactivity in patients with Behçet’s disease. Clinical and Applied Thrombosis/hemostasis
2006; 12: 451-457.
15) Adam B, Calikoglu E. Serum interleukin-6,
procalci-tonin and C-reactive protein levels in subjects with active Behçet’s disease. J Eur Acad Dermatol Venereol
2004; 18: 318-20.
16) Kurzrock R. Thrombopoietic factors in chronic bone
marrow failure states: the platelet problem revisited.
Clin Cancer Res 2005; 11: 1361-1367.
17) Balta I, Balta S, Koryurek OM, Demirkol S, Celik T, Akbay G, et al. Mean platelet volume is associated with
aortic arterial stiffness in patients with Behcet’s disease without significant cardiovascular involvement. J Eur
Acad Dermatol Venereol 2014; 28(10): 1388-93. 18) Lee WS, Kim TY. Is mean platelet volume increased in
Behcet’s disease with thrombosis? Tohoku J Exp Med
2010; 222: 225-226.
19) Türkcü FM, Cingü AK, Yüksel H, Cınar Y, Akkurt M, Sahin M, et al. Mean platelet volume in ocular Behçet’s
disease. The Scientific World Journal 2013; ID215912,
4 pages.
20) Beyan C. Is mean platelet volume a predictive marker
in patients with venous thrombosis? Clinical and
Applied Thrombosis/Hemostasis 2012; 18(6): 670-671. 21) Lancé MD, van Oerle R, Henskens YM, Marcus MA.
Do we need time adjusted mean platelet volume mea-surements? Lab Hematol 2010; 16(3): 28-31.
_______
Corresponding author
OZGEGUNDUZ, MD.
Ufuk University Faculty of Medicine, Department of Dermatology
06520 Balgat Ankara