Contents lists available atScienceDirect
Carbohydrate Polymers
journal homepage:www.elsevier.com/locate/carbpol
Production of magnetic chitinous microcages from ephippia of zooplankton
Daphnia longispina and heavy metal removal studies
Idris Sargin
a,⁎, Gulsin Arslan
a, Murat Kaya
baSelcuk University, Faculty of Science, Department of Biochemistry, 42075, Konya, Turkey
bAksaray University, Faculty of Science and Letters, Department of Biotechnology and Molecular Biology, 68100, Aksaray, Turkey
A R T I C L E I N F O Keywords: Ephippia Chitin scaffold Biopolymer A B S T R A C T
This is thefirst study on production of three dimensional chitinous microcages from ephippial eggs of a mi-crocrustacean, Daphnia longispina (waterflea) by keeping the original shape of its chitinous structure. Iron-based magnetic particles were successfully loaded into the chitinous microcages to enhance its heavy metal sorption capacity. The FT-IR, SEM-EDX and TGA analysis proved the purity of chitin and demonstrated that the loading of magnetic particles into the chitinous microcages was achieved. These newly obtained three-dimensional chitin microcages and magnetic particles loaded microcages were tested in Cd(II), Cr(III), Cu(II), Ni(II) and Zn(II) removal from aqueous solutions. Magnetic particles loaded microcages exhibited a better performance in re-moval of Cd(II), Cu(II) and Ni(II) ions; while unloaded microcages showed a higher affinity for Cr(III) and Zn(II). This study demonstrated that the chitin microcages are suitable carriers for iron-based magnetic particles. Here these new materials were studied only for removal offive heavy metal ions but these promising materials have a potential to be used in variousfields.
1. Introduction
Polymeric matrixes of biological origins have attracted much at-tention over the past decade due to their unique nature, biocompat-ibility, biodegradability, abundance and high affinity for charged spe-cies (Sankar, Chennazhi, Nair, & Jayakumar, 2012). Among the biopolymers, chitin, a water-insoluble polysaccharide, and particularly its deacetylated water-soluble form, chitosan, have been extensively exploited to design carriers or matrixes for biomolecules and particles. Thanks to pendant amino groups in its structure, chitosan dissolves in acidic aqueous solutions, and therefore can be cross-linked easily, making chitosan much more advantageous in polymeric matrix pro-duction over chitin. In the literature related to chitin, there have al-ready been numerous studies on chitosan matrixes (Chen, Wang, Wei, Mo, & Cui, 2010; Guo, Xia, Wang, Song, & Zhang, 2005). However, there is relatively less literature available on the use of chitin as matrix for composite production, apparently stemming from the insolubility of chitin in many solvents because of its rigid crystalline structure re-sulting from the hydrogen bonds (Tamura, Furuike, Nair, & Jayakumar, 2011). Additionally, obtaining chitin with intact three dimensional shape from exoskeleton of organisms is another problem. Therefore, many researchers have had to use chitin powder for production of chitin composites. In those studies, chitin powder orflakes were used
after dissolution in dimethylacetamide-lithium chloride solution (Mi, Lin, Wu, Shyu, & Tsai, 2002), 1,1,1,3,3,3-hexafluoro-2-propanol (Min & Lee et al., 2004), phosphoric acid (Min, You, Kim, Lee, & Park, 2004) or calcium chloride saturated methanol (Sankar et al., 2012). Even in more recent studies, chitin slurry was prepared in water from chitin powder (Deng, Li, Yang, & Li, 2014).
However, there have been attempts to extract chitin scaffolds from large organisms such as pens of the common squid (Loligo sp.) (Falini, Fermani, & Ripamonti, 2002), Verongida sponges (Ehrlich et al., 2010), freshwater sponge Spongilla lacustris (Ehrlich, Kaluzhnaya et al., 2013), marine sponge Aplysinafistularis (Wysokowski, Bazhenov et al., 2013) and crab shell (Chen et al., 2010). On the other hand, although it has been long known that chitin occurs in the exoskeleton of freshwater microcrustaceans Daphnia (waterflea) (Seidman & Larsen Jr, 1979) and chitin is the main structural part of the robust shell of their resting eggs, called ephippia (Kawasaki et al., 2004), there are few studies on chitin isolation from Daphnia (Cauchie, Jaspar-Versali, Hoffmann, & Thomé, 2002) itself or from its ephippial eggs (Kaya et al., 2014,2013).
Chitin is found as mineralized polysaccharide in many organisms (Falini & Fermani, 2004). In living organisms, chitin coexists with mi-nerals like calcium phosphate, calcium sulphate, calcium carbonate and silica. This urged many researchers to synthesize biologically inspired synthesis of mineral–chitin/chitosan composites for applications tissue
https://doi.org/10.1016/j.carbpol.2018.11.072
Received 26 July 2018; Received in revised form 22 November 2018; Accepted 22 November 2018
⁎Corresponding author at: Department of Biochemistry, Faculty of Science, Selcuk University, 42075 Konya, Turkey. E-mail address:idris.sargin@selcuk.edu.tr(I. Sargin).
Available online 28 November 2018
0144-8617/ © 2018 Elsevier Ltd. All rights reserved.
engineering or scaffold production (Ehrlich, Simon et al., 2013;Ehrlich, 2010; Muzzarelli & Muzzarelli, 2002; Ogasawara, Shenton, Davis, & Mann, 2000;Rusu et al., 2005;Wysokowski, Motylenko et al., 2013). In the lights of current attempts in production of chitin matrixes, we hy-pothesize that chitinous scaffold of a microcladoceran ephippia can be obtained intact, and subsequently the chitin microcages can be used as a carrier forfine particulates. Using the ephippia of Daphnia longispina as a model, we demonstrated here for thefirst time that ephippial eggs of a microcladoceran can be used as a source for production micro sized chitin scaffolds, and fine particles of magnetic material can be in-corporated into the polymeric matrix. In the study, the characterisation of the microcages was performed by FT-IR, SEM-EDX, TGA and light microscopy. This study also reports heavy metal ion sorption perfor-mance of the chitinous microcages and the iron-based magnetic parti-cles loaded microcages under different conditions.
2. Materials and method
2.1. Materials
NH3, HCl, FeCl3.6H2O and FeSO4.7H2O were purchased from Merck. NaClO solution was obtained from a local supplier and was diluted to 3% prior to its use.
2.2. Collection of D. longispina ephippial resting eggs
The resting eggs used in this study were from the stock that was used in earlier studies (Kaya et al., 2014,2013). Briefly, the floating cladoceran ephippia were collected from the shores of Mamasın Dam reservoir in Central Anatolia (Aksaray, Turkey) following a peak ob-served in cladoceran (Daphnia sp.) population (November 2012). Col-lection of cladoceran ephippia was accomplished with the use of plankton net. The samples were subsequently shipped to the laboratory for identification by a light microscopy. The samples were washed with water and then ephippial eggs belonging to Daphnia species were identified. Ephippia of D. longispina were picked and used in the ex-periments. It should be noted that Daphnia species can be easily cul-tured and resting egg production in Daphnia can be also be induced by manipulating the environmental conditions and the produced ephippial resting eggs can be collected (Abrusán, Fink, & Lampert, 2007;Alekseev & Lampert, 2001).
2.3. Preparation of chitinous microcages from ephippia of D. longispina
Resting eggs of cladoceran D. longispina are encased in chitin shell layers with randomly distributed pitsfilled with minerals such as cal-cium phosphate or sulphate (Kawasaki et al., 2004). Under the exterior layer occurs a layer of protein-chitinfibres (Cáceres, 1998;Seidman & Larsen Jr, 1979). A treatment should be done to remove the chitinous structure from the matrix. In this study, chitinous microcages were isolated by removing the content of the eggs through a line of simple treatments at ambient conditions; demineralization with dilute HCl solution, deproteinization with NaOH solution and bleaching with NaClO solution. It was previously reported that sodium hypochlorite solution had an effect on the ephippial eggs of Daphnia (Gray, Duggan, & MacIsaac, 2006; Pancella & Stross, 1963); therefore, sodium hypo-chlorite solution was preferred as a bleaching agent.
Briefly, the ephippia (10 g) were treated with 500 mL of 0.5 M HCl solution for 1 h at 50 °C. Then, after rinsing with water until neutrality, the samples were stirred in 500 mL of 0.5 M NaOH solution for 1 h at 50 °C. The samples were separated from the alkali medium and washed with water. In thefinal step, the samples were subject to the bleaching treatment in 400 mL of NaClO solution (3%) at 50 °C for 6 min. The bleached samples were removed byfiltration and washed with 8 L of water to wash away hypochlorite ions and to reach neutral pH. Then, half of the wet microcages were left to dry at room temperature for
48 h. The other half in wet form was allotted for magnetic particle loading.
2.4. Loading of iron-based magnetic particles into the chitinous microcages
Synthesis of iron-based magnetic particles was conducted following the method reported elsewhere with some modifications (Ozmen et al., 2010). The incorporation of iron-based magnetic particles into the microcages was achieved as summarised below. The microcages were transferred into 200 mL of water, and subsequently 0.445 g of FeSO4.7H2O was added; the resulting mixture was stirred at 70 °C for 1 h. Then, 0.756 g of FeCl3.6H2O was added and the mixture was stirred for 1 h at the same temperature. This was followed by addition of 4.0 mL of NH3solution. The hot reaction medium was stirred for 1 h. Finally, the microcages with magnetic particles werefiltrated out and washed with water. The magnetic particles loaded microcages were dried at room temperature for 48 h.
2.5. Characterisation of the chitinous microcages and the instrumentation
Chemical structure of the chitinous microcages and magnetic par-ticles loaded chitinous microcages was examined by Fourier transform infrared spectroscopy (Bruker Vertex 70 FTIR spectrometer in range of 4000 and 500 cm−1). Thermogravimetric analysis of the microcages were performed by an EXSTAR S11 7300. Surface examination of the ephippial resting egg of D. longispina, the microcages and the magnetic particles loaded microcages were carried out with a scanning electron microscope (SEM) (EVO LS 10 ZEISS). SEM/Energy Dispersive X-Ray (EDX) analysis was performed to detect the presence of iron atoms in the microcages upon loading of magnetic particles. Metal ion sorption experiments were conducted on a shaker (Heidolph Promax 2020) at 200 rpm. A flame atomic absorption spectrometer (ContrAA 300, Analytikjena) was used to determine the metal ion concentration of the solutions from heavy metal ion sorption experiments.
2.6. Heavy metal ion sorption studies
Affinity of the chitinous microcages and iron-based magnetic par-ticles loaded chitinous microcages for heavy metal cations was tested in batchwise sorption experiments at room temperature. Chitinous mi-crocages (0.150 g) or iron-based magnetic particles loaded chitinous microcages (0.150 g) was added into 25 mL of solution with initial metal ion concentration of 10 mg L−1and then agitated on the shaker for 4 h. Aqueous phase was separated from the medium byfiltration (Whatmanfilter paper, No: 42) or with a magnet. To make comparison between the adsorptive performances of both adsorbents (loaded or unloaded) the samefiltration procedure was followed. Then, metal ion concentration in thefiltrate was determined using atomic absorption spectrometry (on a flame atomic absorption spectrophotometer; ContrAA 300, Analytikjena). Prior to the measurements, a calibration curve was prepared using standard solutions with the known con-centration of the metal ions.
In preliminary sorption studies, optimum contact time was found to be 240 min. The optimum amount of the chitinous microcages (empty or magnetic particles loaded) was determined in a range of 0.050–0.250 g. The effect of metal solution pH was investigated. The initial pH of the metal solutions were Cd(II): 5.69; Cr(III): 4.02; Cu(II): 5.40; Ni(II): 5.80 and Zn(II): 5.62. Then, pH of the metal solutions was adjusted to lower pHs by adding a few drops of 0.1 M HCl solution into the metal solutions: Cd(II), Cu(II), Ni(II) and Zn(II): 4.50 and 3.50, Cr (III): 3.50 and 3.00. Equilibrium (in range of 2–12 mg L−1metal solu-tion), contact time (60–480 min) and thermodynamic (25, 35 and 45 °C) studies were done for each metal ion.
Metal ion sorption capacities of the microcages (empty or magnetic particles loaded) (qein mg g−1) were calculated by using the following equation.
qe= [(Ci–Ce)V]/W (1) where Ci and Ce are the initial and equilibrium liquid-phase con-centrations of metal ions (mg L−1), respectively; V is the volume of metal solution (L), and W (g) is the mass of the chitinous microcages (empty or magnetic particles loaded).
Sorption yield for each of the metal ions was calculated from fol-lowing equation;
Sorption (%) = [(Ci– Ce)/ Ci] × 100 (2) where Ciand Ceare the initial and equilibrium concentration of metal ion (mg L−1).
3. Results and discussion
3.1. Characterisation of chitinous microcages
3.1.1. Fourier transform infrared spectroscopy (FT-IR) of chitinous microcages
Based on its physiological role in the tissues, chitin occurs in the organisms in three different polymorphic forms: α, β and γ (Jang, Kong, Jeong, Lee, & Nah, 2004). Compared to the other crystalline forms, α–chitin which has piles of anti-parallel alignment occurs widely in many organisms. In this form, chitin chains are organised tightly thanks to the hydrogen bonds between carbonyl groups and amide groups or side groups (−CH2OH). FT-IR spectra analysis revealed that the mi-crocages consisted of chitin and its polymorphic form isα. As presented in Fig. 1, the characteristics absorption bands of α–chitin were ob-served: 1652 cm−1(amide I), 1620 cm−1(amide II) and 1553 cm−1 (CeN stretching). The splitting of the amide bond is the indication of α chitin allomorph. The shoulder next to the peak at 1620 cm−1is as-cribed to amide I and is the indication ofα allomorph of chitin. Ad-ditionally, the bands observed at 1069 and 1010 cm−1correspond to asymmetric stretching of CeOeC and CeO of the polysaccharide ring. All these observations are consistent with FT-IR spectra of commercial α–chitin (Liu et al., 2012). After magnetic particles loading, the ab-sorption bands corresponding to α–chitin were observed with very small shifting, confirming that chitin did not hydrolyse during the magnetic particles loading procedure. The absorption bands at low wave numbers (≤700 cm−1) can be ascribed to vibrations of Fe–O bonds of iron oxide (Ozmen et al., 2010). The bands at lower wave numbers such as the ones at 694 cm−1 and 528 cm−1 disappeared while a band corresponding to the vibrations of Fe–O bonds was re-corded at 556 cm−1, demonstrating the presence of iron-based mag-netic particles.
3.1.2. Light microscopy images
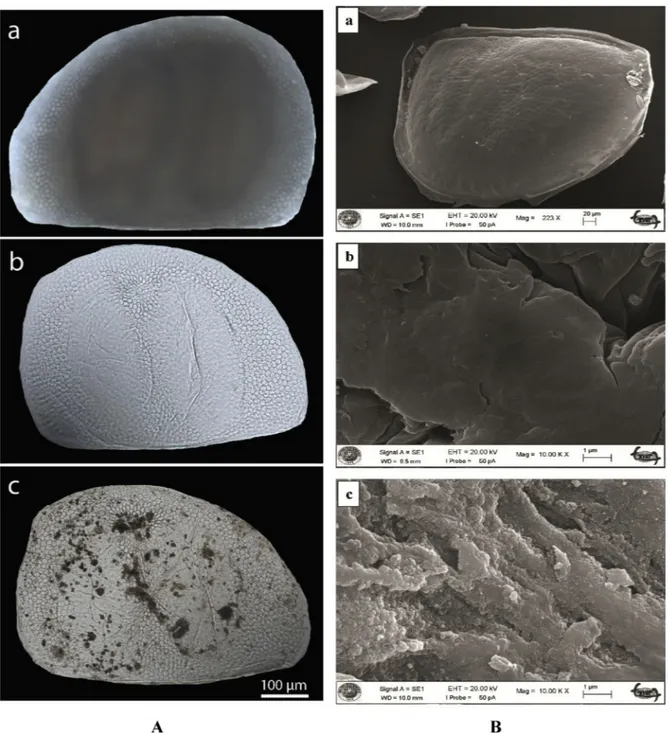
The light microscopy images demonstrated that the method fol-lowed here was effective at isolating the chitinous microcages from ephippia of D. longispina by keeping the original shape of the chitin matrix while emptying the resting eggs contents successfully. The mi-crocages retained their original structure after the emptying treatment (Fig. 2A). Upon the treatment, two resting eggs encased in the ephip-pium (darker bean shaped regions inFig. 2Aa) vanished completely. On the other hand, honeycombed structure of the ephippium remained unchanged (Fig. 2Ab). In iron-based magnetic particles loaded micro-cages, magnetic particles were dispersed randomly throughout the structure, but denser magnetic particles accumulation was observed particularly near the sites where the resting eggs had rested (Fig. 2Ac).
3.1.3. SEM images of the microcages
SEM images of the ephippium of D. longispina and the chitin speci-mens from the microcages and iron-based magnetic particles loaded microcages are presented in Fig. 2B. The chitin specimen from the unloaded microcages had a smooth surface morphology. Upon
magnetic particles loading, the surface feature of the chitin was com-pletely changed into a rough surface with particulates of magnetic material.
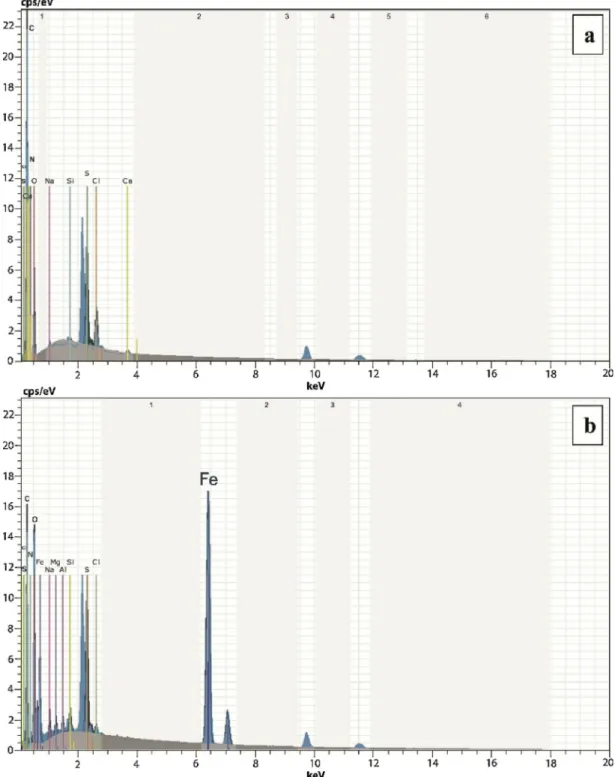
3.1.4. SEM-EDX analysis of the microcages
SEM-EDX analysis of the microcages and the iron-based magnetic particles loaded microcages revealed the elemental identification and surface characteristics of the samples (Fig.3). The Fe peak in the SEM-EDX spectrum of the magnetic particles loaded microcages was the indication of magnetic particles deposition in the microcages (Fig. 3b).
3.1.5. Thermal gravimetric analysis of magnetic particles loaded chitinous microcages
The thermogram demonstrated that iron-based magnetic particles loaded chitinous microcages had two main decomposition steps (Fig. 4). Thefirst decomposition step observed at temperatures lower than 150 °C can be attributed to the evaporation of the absorbed water molecules. In the second decomposition step, about 53% mass loss was observed, indicating the degradation of the polysaccharide structure and decomposition of the acetylated and deacetylated units of chitin polymer (Jayakumar, Egawa, Furuike, Nair, & Tamura, 2009). All these observations are comparable to the thermogravimetric analysis of ephippial pure chitin extracted from ephippia of another micro-cladoceran, Daphnia magna (Kaya et al., 2013) and to that of com-mercial α–chitin from crab shell (Juárez-de la Rosa, Quintana, Ardisson, Yáñez-Limón, & Alvarado-Gil, 2012). However, at elevated temperatures (higher than 400 °C) lower mass loss was recorded. Higher amount of residual content can be corresponded to the inorganic content of the magnetic particles loaded chitinous microcages. Ad-ditionally, main decomposition temperature was recorded at 331 °C, which was lower than pure ephippial chitin from D. magna (351.6 °C) (Kaya et al., 2013) and commercialα–chitin (350 °C) (Juárez-de la Rosa et al., 2012). The catalytic effect of magnetite particles on decomposi-tion of organic matrixes have been reported in the literature ( Ziegler-Borowska, Chełminiak, & Kaczmarek, 2015). It appears that the iron-based magnetic particles catalysed the thermal degradation of chitinous matrix.
3.2. Metal ion sorption studies
It has already been acknowledged that magnetite particles enhance the interaction with metal cations (Gong et al., 2012; Reddy & Lee, 2013;Zhang, Sui, Li, Tang, & Cai, 2012). Affinity of the chitinous crocages and the iron-based magnetic particles loaded chitinous mi-crocages for metal cations was tested to reveal the contribution of magnetic particles. Metal sorption studies were conducted under dif-ferent conditions and the equilibrium experimental data was also evaluated thermodynamically.
3.2.1. Effect of sorbent dosage
The optimum amount of chitinous microcages and iron-based magnetic particles loaded chitinous microcages for each metal ion was determined in a range of 0.05-0.25 g (Fig.5a, b). The sorption system for each of sorbents reached a saturation point at about 0.15 g; and further increments in the amount of the sorbent made a less contribu-tion to the sorpcontribu-tion. Among the metal ions, chitinous microcages ex-hibited the highest sorption capacity for Cr(III) followed by Zn(II), Cu (II), Cd(II) and Ni(II) ions. Magnetic particles loading changed this order; magnetic particles loaded chitinous microcages showed the highest affinity for Cu(II) ions, followed by Cr(III), Cd(II), Zn(II) and Ni (II) ions. When the contribution of magnetic particles was evaluated, it was observed that magnetic particles loading significantly enhanced Ni (II) (about 4.56 times), Cd(II) (3.16 times) and Cu(II) (2.91 times) ions sorption capacity of chitinous microcages. Lowest enhancement in sorption was recorded for Cr(III) and Zn(II) ions; 1.19 and 1.04 times, respectively.
3.2.2. Effect of contact time
The effect of contact time on the metal ion sorption systems with chitinous microcages and iron-based magnetic particles loaded chit-inous microcages was recorded as presented in Fig. 6. After thefirst 240 min, sorption systems of the metal ions reached to the equilibrium except Zn(II) ions. Sorption of Zn(II) ions took longer time to reach the equilibrium. Sorption of Cu(II) ions by chitinous microcages was fast and the equilibrium was attained within thefirst 120 min.
3.2.3. Effect of solution pH
The effect of metal solution pH on the sorption of metal ions by chitinous microcages and iron-based magnetic particles loaded chit-inous microcages was studied in range of pH 3.0–6.0 (Fig. 7). Metal sorption studies were not conducted for higher pH because of the possible precipitation of metal ions as hydroxides. Lower metal ion sorption occurred at more acidic solutions for both sorbents. In sorption system with chitinous microcages, Cd(II) ion sorption was least affected Fig. 1. FT-IR spectra of chitinous microcages isolated from the ephippium of D. longispina (waterflea) (The arrow points the shoulder that appeared at 1652 cm−1 and this is the indication ofα allomorph of chitin) and FT-IR spectra of the magnetic particles loaded chitinous microcages from the ephippium of D. longispina. The arrow points the band at 556 cm−1and it was ascribed to vibrations of Fe–O bonds of iron oxide).
by the changes in pH. At pH 3.5 and 4.5, Zn(II) sorption was higher than in the sorption system with magnetic particles loaded chitinous microcages.
In metal ion sorption systems, the changes in pH affect metal ion speciation and the charge density on the sorbent through protonation and ionisation of functional groups (Crini, Peindy, Gimbert, & Robert, 2007). Enhancement in sorption percentage observed at higher pH can be attributed to the free lone pair of electrons on oxygen atoms of hy-droxyl groups and nitrogen atoms of acetamide groups. These electron donor groups provided suitable coordination sites for metal cations through the electron pair sharing (Kalmykova, Strömvall, & Steenari, 2008). Additionally, at lower pH, the presence of the competitor H+ ions for the sorption sites hindered the coordination of the metal cations with the electron donor groups. In other words, protonation of the
metal interacting groups on the sorbents created electrostatic repulsion between the positively charged surface and the metal cations. A pos-sible interaction of metal ions with magnetic particles and chitin is presented inFig. 8. The images of chitinous microcages and iron-based magnetic particles loaded chitinous microcages in water are also pre-sented inFig. 8.
3.2.4. Adsorption isotherm analysis
Freundlich (Freundlich, 1906), Langmuir (Langmuir, 1918), Scatchard (Crist et al., 1994) and Dubinin–Radushkevich (D–R) (Dubinin & Radushkevich, 1947) isotherm models were employed tofit the experimental data obtained from metal concentration effect studies. The isotherm constants, parameters and correlation coefficients are tabulated inTable 1.
Fig. 2. A. Images of pristine ephippium of D. longispina (waterflea), produced chitinous microcages and the iron-based magnetic particles loaded microcages. Light microscopy images the ephippium of D. longispina (waterflea) (a), chitinous microcages isolated from the ephippium of D. longispina (b) and the magnetic particles loaded chitinous microcages from the ephippium of D. longispina (c).B. Surface morphology. SEM images of the ephippium of D. longispina (a) and the chitin specimens from the chitinous microcages (b) and the magnetic particles loaded chitinous microcages isolated from the ephippium of D. longispina (c).
The linearized form of Freundlich isotherm model is represented by the following equation. log qewas plotted against log Ceto determine the constants and the parameters.
log qe= log KF+ (1/n) log Ce (3)
where qeis the amount on metal ions sorbed by the sorbent (in mmol g−1); Ceis the equilibrium metal ion concentration (mmol L−1); and KF (mmol g−1) is the indicator of the adsorption capacity and n is the Freundlich constant denoting the adsorption intensity.
The linear form of Langmuir model is expressed by the equation given below. The slope and intercept of the plot (Ce/qevs. Ce) gave the maximum adsorption capacity Cm (mmol g−1) and the energy of
adsorption KL(L mmol−1).
Ce/qe= Ce/Cm+1/(CmKL) (4)
where qeis the amount on metal ions sorbed by the sorbent (in mmol g−1); Ceis the equilibrium metal ion concentration (in mmol L−1).
The Scatchard plot analysis is expressed by the following equation;
qe/Ce= Ks(Qs–qe) (5)
where Ceis the equilibrium concentration of metal ions (mmol L−1); qe is the equilibrium metal ion sorption capacity (mmol L−1); Ks (L mmol−1) and Qs(mmol g−1) are the Scatchard isotherm constants.
The D–R model is expressed by the following equation:
Fig. 3. SEM-EDX spectra of the chitin specimens from the chitinous microcages (a) and the iron-based magnetic particles loaded chitinous microcages isolated from the ephippium of D. longispina (b).
Fig. 4. Thermal analysis of the iron-based magnetic particles loaded chitinous microcages. TG-DTG curves of the magnetic particles loaded chitinous microcages isolated from the ephippium of D. longispina.
Fig. 5. Effect of sorbent dosage on the sorption of metal ions by chitinous microcages (a) and iron-based magnetic particles loaded chitinous microcages (b) isolated from the ephippium of D. longispina (25 mL of solution with initial metal ion concentration of 10 mg L−1, temperature 25 °C, contact time 4 h).
Fig. 6. Effect of contact time on the sorption of metal ions by chitinous mi-crocages (a) and iron-based magnetic particles loaded chitinous mimi-crocages (b) isolated from the ephippium of D. longispina (25 mL of solution with initial metal ion concentration of 10 mg L−1, temperature 25 °C).
lnqe= lnXm−Kε2 (6) where qeis the amount of metal ions adsorbed by chitinous microcages or iron-based magnetic particles loaded chitinous microcages (mmol g−1); Xmis the adsorption capacity (mmol g−1) and K (mol2kJ−2) is a constant related to the adsorption energy. Polanyi Potential (ε) was determined using the equation below;
ε = RT ln(1 + (1/Ce)); (7)
The intercept and slope of the lnqeversusε2plot gave Xm and K. The mean free energy of adsorption (E) was calculated by using the equa-tion:
E = (–2K)− 0.5 (8)
In the metal ion sorption system with magnetic particles loaded chitinous microcages, Langmuir model gave betterfits to the experi-mental data, demonstrating the homogeneous distribution of binding sites on the sorbent. On the other hand, experimental data of the sorption system with chitinous microcages exhibited relatively betterfit to Freundlich model.
In the Scatchard plot analysis of Cd(II), Cr(II) and Zn(II) ions sorption by the chitinous microcages and Cr(III), Cu(II), Ni(II) and Zn (II) ions by magnetic particles loaded chitinous microcages, the plots of qe/Ceagainst qedid not yield the straight line (low R2); demonstrating the presence of more than one type binding site on the sorbents. On the other hand, Cu(II) and Ni(II) sorption by chitinous microcages and Cd (II) sorption by magnetic particles loaded chitinous microcages gave relatively higher R2, indicating that ion sorption occurred through one type binding site (Akar, Ozcan, Tunali, & Ozcan, 2008). E values ob-tained in the D–R adsorption model analysis of sorption of metal ions (except Zn(II)) by magnetic particles loaded chitinous microcages were
recorded higher than those by chitinous microcages. E values lower than 8 kJ mol−1can be attributed to physisorption of the metal ions, and higher E values of Cd(II), Cr(III), Cu(II) and Zn(II) ions possibly indicated that the sorption of these ions by magnetic particles loaded chitinous microcages proceeded through chemical ion-exchange (Tuzen & Sarı, 2010).
In this study according to the data of adsorbent dosage experiments we recorded these sorption capacities; by chitinous microcages Cd(II): 16.2 mg g−1, Cr(III): 57.5 mg g−1, Cu(II): 22.1 mg g−1, Ni(II): 6.7 mg g−1, Zn(II): 39.9 mg g−1and by iron-based magnetic particles loaded chitinous microcages Cd(II): 51.1 mg g−1, Cr(III): 68.4 mg g−1, Cu(II): 64.6 mg g−1, Ni(II): 26.8 mg g−1, Zn(II): 48.5 mg g−1. In a pre-vious study on adhesive stalks of diatom Didymosphenia geminata a high sorption capacity for Cd(II) (145.86 mg g−1) and Ni(II) (130.27 mg g−1) was reported (Wysokowski et al., 2017). Another publication reported a high adsorption capacity for Zn(II); 107.21 mg g−1(Mousavi, Parvini, & Ghorbani, 2018). In a recent study the sorption capacity of the chitin/lignin material was reported as fol-lows; Ni(II): 70.41 mg g−1, Cu(II): 75.70 mg g−1, Zn(II): 82.41 mg g−1 (Bartczak et al., 2017). Considering these reports and finding of the present study, it appears that sorption capacity of magnetic particles loaded chitinous microcages is moderate.
3.2.5. Evaluation of thermodynamic parameters
In temperature effect studies, metal sorption experiments were conducted at 25, 35 and 45 °C. By employing the equations given below, changes in the enthalpy (ΔH°), the entropy (ΔSº) and Gibbs free energy (ΔGº) during the sorption were calculated. The slope and the intercept of the plot of logarithm of the distribution coefficient (KD) values versus 1/T gave the parameters.
Log KD= (ΔS°/2.303R) – (ΔH°/2.303RT) (9)
ΔG°= ΔH°–TΔS° (10)
where R is universal gas constant, (8.314 J mol−1K−1) and T is the temperature (K).Table 2lists the thermodynamic parameters for the sorption of Cd(II), Cr(III), Cu(II), Ni(II) and Zn(II) by chitinous micro-cages and magnetic particles loaded chitinous micromicro-cages. The positive values ofΔH° indicated that the sorption of the metal ions by chitinous microcages or by magnetic particles loaded chitinous microcages was of endothermic nature; whereas the negative values exhibited that metal ion sorption proceeded through an exothermic reaction. Positive values ofΔSº were recorded for all the metal ions (except Cu(II) ions) sorption by magnetic particles loaded chitinous microcages; indicating that the randomness of the sorption system increased during the sorption. On the other hand, among thefive metal ions examined, only for sorption of Zn(II) ions by chitinous microcages, positive values ofΔS° were re-corded, demonstrating that the randomness of the sorption system de-creased during the sorption. With exception of Ni(II) ions, metal ion sorption by magnetic particles loaded chitinous microcages was spon-taneous and thermodynamically feasible under the specified conditions. Also, an increase in Gibbs free energy values was observed at higher temperatures, suggesting that the Cd(II), Cr(III) and Zn(II) sorption by magnetic particles loaded chitinous microcages was more efficient at higher temperatures.
4. Conclusions
Three dimensional chitinous microcages and its iron-based mag-netic particles loaded counterparts were produced from ephippial resting eggs of a cladoceran species (D. longispina) for thefirst time. FT-IR spectra analysis revealed that the microcages produced from ephippial resting eggs consisted of α-chitin. SEM-EDX analysis con-firmed the incorporation of the magnetic particles into the chitinous microcages. Thefindings of heavy metal sorption experiments demon-strated that magnetic particles enhanced the affinity of the chitinous Fig. 7. Effect of pH on the sorption of metal ions by chitinous microcages (a)
and iron-based magnetic particles loaded chitinous microcages (b) isolated from the ephippium of D. longispina (25 mL of solution with initial metal ion concentration of 10 mg L−1, temperature 25 °C, sorbent dosage 0.15 g).
microcages for Cd(II), Cu(II) and Ni(II) ions. In conclusion, it was re-vealed that these biological materials are effective sorbents for heavy metal ions. These newly produced materials also can find biotechno-logical applications in various areas in further studies.
Conflict of Interests
The authors declare no conflict of interests.
Fig. 8. The images of chitinous microcages and iron-based magnetic particles loaded chitinous microcages in water and the scheme showing the possible interaction of metal ions with iron-based magnetic particles and chitin matrix.
Acknowledgements
This study was funded by Selcuk University Research Foundation, Turkey" (project number: BAP-14201082).
References
Abrusán, G., Fink, P., & Lampert, W. (2007). Biochemical limitation of resting egg pro-duction in Daphnia. Limnology and Oceanography, 52(4), 1724–1728.
Akar, T., Ozcan, A. S., Tunali, S., & Ozcan, A. (2008). Biosorption of a textile dye (Acid Blue 40) by cone biomass of Thuja orientalis: Estimation of equilibrium, thermo-dynamic and kinetic parameters. Bioresource Technology, 99(8), 3057–3065.
Alekseev, V., & Lampert, W. (2001). Maternal control of resting-egg production in Daphnia. Nature, 414(6866), 899.
Bartczak, P., Klapiszewski,Ł., Wysokowski, M., Majchrzak, I., Czernicka, W., Piasecki, A., et al. (2017). Treatment of model solutions and wastewater containing selected ha-zardous metal ions using a chitin/lignin hybrid material as an effective sorbent. Journal of Environmental Management, 204, 300–310.
Cáceres, C. E. (1998). Interspecific variation in the abundance, production, and emer-gence of Daphnia diapausing eggs. Ecology, 79(5), 1699–1710.
Cauchie, H.-M., Jaspar-Versali, M.-F., Hoffmann, L., & Thomé, J.-P. (2002). Potential of using Daphnia magna (Crustacea) developing in an aerated waste stabilisation pond as a commercial source of chitin. Aquaculture, 205(1), 103–117.
Chen, Z. G., Wang, P. W., Wei, B., Mo, X. M., & Cui, F. Z. (2010). Electrospun collagen–Chitosan nanofiber: A biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomaterialia, 6(2), 372–382.
Crini, G., Peindy, H. N., Gimbert, F., & Robert, C. (2007). Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Separation and Purification Technology, 53(1), 97–110.
Crist, R. H., Martin, J. R., Carr, D., Watson, J. R., Clarke, H. J., & Crist, D. L. R. (1994). Interaction of metals and protons with algae .4. Ion-exchange vs adsorption models and a reassessment of Scatchard plots - ion-exchange rates and equilibria compared with calcium alginate. Environmental Science & Technology, 28(11), 1859–1866.
Deng, Q., Li, J., Yang, J., & Li, D. (2014). Optical andflexible α-chitin nanofibers re-inforced poly(vinyl alcohol) (PVA) compositefilm: Fabrication and property. Composites Part A, Applied Science and Manufacturing, 67(0), 55–60.
Dubinin, M. M., & Radushkevich, L. V. (1947). Equation of the characteristic curve of activated charcoal. Chem. Zentr. 1, 875–889.
Ehrlich, H. (2010). Chitin and collagen as universal and alternative templates in biomi-neralization. International Geology Review, 52(7-8), 661–699.
Ehrlich, H., Ilan, M., Maldonado, M., Muricy, G., Bavestrello, G., Kljajic, Z., et al. (2010). Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. International Journal of Biological Macromolecules, 47(2), 132–140.
Ehrlich, H., Kaluzhnaya, O. V., Brunner, E., Tsurkan, M. V., Ereskovsky, A., Ilan, M., et al. (2013). Identification and first insights into the structure and biosynthesis of chitin from the freshwater sponge Spongilla lacustris. Journal of Structural Biology, 183(3), 474–483.
Ehrlich, H., Simon, P., Motylenko, M., Wysokowski, M., Bazhenov, V. V., Galli, R., et al. (2013). Extreme biomimetics: Formation of zirconium dioxide nanophase using chitinous scaffolds under hydrothermal conditions. Journal of Materials Chemistry B, 1(38), 5092–5099.
Falini, G., & Fermani, S. (2004). Chitin mineralization. Tissue Engineering, 10(1-2), 1–6.
Falini, G., Fermani, S., & Ripamonti, A. (2002). Crystallization of calcium carbonate salts into beta-chitin scaffold. Journal of Inorganic Biochemistry, 91(3), 475–480.
Freundlich, H. M. F. (1906). Over the adsorption in solution. Zeitschrift für Physikalische Chemie, 57, 385–471.
Gong, J., Wang, X., Shao, X., Yuan, S., Yang, C., & Hu, X. (2012). Adsorption of heavy metal ions by hierarchically structured magnetite-carbonaceous spheres. Talanta, 101(0), 45–52.
Gray, D. K., Duggan, I. C., & MacIsaac, H. J. (2006). Can sodium hypochlorite reduce the risk of species introductions from diapausing invertebrate eggs in non-ballasted ships? Marine Pollution Bulletin, 52(6), 689–695.
Guo, T.-Y., Xia, Y.-Q., Wang, J., Song, M.-D., & Zhang, B.-H. (2005). Chitosan beads as molecularly imprinted polymer matrix for selective separation of proteins. Biomaterials, 26(28), 5737–5745.
Jang, M.-K., Kong, B.-G., Jeong, Y.-I., Lee, C. H., & Nah, J.-W. (2004). Physicochemical characterization ofα-chitin, β-chitin, and γ-chitin separated from natural resources. Journal of Polymer Science Part A: Polymer Chemistry, 42(14), 3423–3432.
Jayakumar, R., Egawa, T., Furuike, T., Nair, S. V., & Tamura, H. (2009). Synthesis, characterization, and thermal properties of phosphorylated chitin for biomedical applications. Polymer Engineering and Science, 49(5), 844–849.
Juárez-de la Rosa, B. A., Quintana, P., Ardisson, P. L., Yáñez-Limón, J. M., & Alvarado-Gil, J. J. (2012). Effects of thermal treatments on the structure of two black coral species chitinous exoskeleton. Journal of Materials Science, 47(2), 990–998.
Kalmykova, Y., Strömvall, A.-M., & Steenari, B.-M. (2008). Adsorption of Cd, Cu, Ni, Pb and Zn on Sphagnum peat from solutions with low metal concentrations. Journal of Hazardous Materials, 152(2), 885–891.
Kawasaki, T., Yoshimura, H., Shibue, T., Ikeuchi, Y., Sakata, M., Igarashi, K., et al. (2004).
Table 1
The parameters and constants of Freundlich, Langmuir, Scatchard and D–R isotherm models for sorption of Cu(II), Cd(II), Cr(III), Ni(II) and Zn(II) by chitinous microcages and iron-based magnetic particles loaded chitinous microcages isolated from the ephippium of D. longispina (volume of metal salt solution 25 mL, sorbent dosage 0.15 g, temperature 25 °C).
Isotherms Freundlich Langmuir Scatchard D-R
Metal ions Sorbents KF n R2 Cm KL R2 Ks Qs R2 Xm K E R2
Cd(II) Chitinous microcages 0.950 1.508 0.960 0.318 1.482 0.819 14.15 0.328 0.658 0.320 0.015 5.773 0.953 Magnetic chitinous microcages 1.595 3.401 0.926 0.562 112.486 0.998 325.2 0.560 0.965 0.785 0.005 10.00 0.995 Cr(III) Chitinous microcages 224.905 0.551 0.942 −0.634 −3.583 0.736 8.90 0.656 0.881 9.563 0.048 3.227 0.934 Magnetic chitinous microcages 5.847 2.923 0.949 1.980 495.05 0.938 134.0 1.915 0.804 2.437 0.006 9.128 0.944 Cu(II) Chitinous microcages 2.172 1.672 0.946 0.412 29.479 0.981 241.6 0.397 0.971 0.567 0.007 8.451 0.992 Magnetic chitinous microcages 13.001 3.297 0.743 0.980 22706.63 0.969 9056 1.514 0.407 6.896 0.004 11.180 0.879 Ni(II) Chitinous microcages 5.662 0.499 0.995 −0.059 −0.015 0.882 4.676 0.043 0.943 0.540 0.065 2.773 0.988 Magnetic chitinous microcages 0.588 4.291 0.720 0.374 17.855 0.926 16.82 0.630 0.891 0.812 0.02 5.00 0.989 Zn(II) Chitinous microcages 1.032 8.928 0.263 0.750 750.188 0.980 100.1 1.457 0.051 0.858 0.002 15.811 0.300 Magnetic chitinous microcages 2.884 2.958 0.647 0.804 8045.052 0.984 232.2 1.179 0.413 1.381 0.005 10.00 0.796
Table 2
Thermodynamic parameters for the sorption of Cu(II), Cd(II), Cr(III), Ni(II) and Zn(II) by chitinous microcages and iron-based magnetic particles loaded chitinous microcages isolated from the ephippium of D. longispina (25 mL of solution with initial metal ion concentration of 10 mg L−1, sorbent dosage 0.15 g).
Metals Sorbents ΔG° (J mol−1)
ΔH° (J mol−1) ΔS° (J K−1mol−1) T = 298.15 (K) T = 308.15 (K) T = 318.15 (K)
Cd(II) Chitinous microcages −14598.511 −58.325 2791.121 3374.372 3957.623 Magnetic chitinous microcages −196.651 10.014 −3182.461 −3282.461 −3382.750 Cr(III) Chitinous microcages −6054.629 −12.273 −2395.156 −2272.417 −2149.678 Magnetic chitinous microcages 4411.7222 30.311 −4625.630 −4928.75 −5231.86 Cu(II) Chitinous microcages -26635.007 −94.802 1630.282 2578.304 3526.327
Magnetic chitinous microcages −49574.431 −115.061 −15264.014 −14118.404 −12967.795 Ni(II) Chitinous microcages −5794.215 −37.262 5315.511 5688.133 6060.755
Magnetic chitinous microcages 1634.673 4.710 230.257 183.153 136.049 Zn(II) Chitinous microcages 2818.600 11.565 −629.639 −745.294 −860.948
Crystalline calcium phosphate and magnetic mineral content of Daphnia resting eggs. Zoological Science, 21(1), 63–67.
Kaya, M., Cakmak, Y. S., Baran, T., Asan-Ozusaglam, M., Mentes, A., & Tozak, K. O. (2014). New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnology and Bioprocess Engineering, 19(1), 58–69.
Kaya, M., Sargin, I., Tozak, K.Ö., Baran, T., Erdogan, S., & Sezen, G. (2013). Chitin ex-traction and characterization from Daphnia magna resting eggs. International Journal of Biological Macromolecules, 61, 459–464.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and pla-tinum. Journal of the American Chemical Society, 40, 1361–1403.
Liu, S., Sun, J., Yu, L., Zhang, C., Bi, J., Zhu, F., et al. (2012). Extraction and character-ization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules, 17(4), 4604.
Mi, F.-L., Lin, Y.-M., Wu, Y.-B., Shyu, S.-S., & Tsai, Y.-H. (2002). Chitin/PLGA blend microspheres as a biodegradable drug-delivery system: Phase-separation, degrada-tion and release behavior. Biomaterials, 23(15), 3257–3267.
Min, B.-M., Lee, S. W., Lim, J. N., You, Y., Lee, T. S., Kang, P. H., et al. (2004). Chitin and chitosan nanofibers: Electrospinning of chitin and deacetylation of chitin nanofibers. Polymer, 45(21), 7137–7142.
Min, B.-M., You, Y., Kim, J.-M., Lee, S. J., & Park, W. H. (2004). Formation of nanos-tructured poly(lactic-co-glycolic acid)/chitin matrix and its cellular response to normal human keratinocytes andfibroblasts. Carbohydrate Polymers, 57(3), 285–292.
Mousavi, S. J., Parvini, M., & Ghorbani, M. (2018). Experimental design data for the zinc ions adsorption based on mesoporous modified chitosan using central composite design method. Carbohydrate Polymers, 188, 197–212.
Muzzarelli, C., & Muzzarelli, R. A. (2002). Natural and artificial chitosan–inorganic composites. Journal of Inorganic Biochemistry, 92(2), 89–94.
Ogasawara, W., Shenton, W., Davis, S. A., & Mann, S. (2000). Template mineralization of ordered macroporous chitin−silica composites using a cuttlebone-derived organic matrix. Chemistry of Materials, 12(10), 2835–2837.
Ozmen, M., Can, K., Arslan, G., Tor, A., Cengeloglu, Y., & Ersoz, M. (2010). Adsorption of Cu(II) from aqueous solution by using modified Fe3O4magnetic nanoparticles. Desalination, 254(1–3), 162–169.
Pancella, J. R., & Stross, R. G. (1963). Light induced hatching of Daphnia resting eggs.
Chesapeake Science, 4(3), 135–140.
Reddy, D. H. K., & Lee, S.-M. (2013). Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Advances in Colloid and Interface Science, 201–202(0), 68–93.
Rusu, V. M., Ng, C.-H., Wilke, M., Tiersch, B., Fratzl, P., & Peter, M. G. (2005). Size-controlled hydroxyapatite nanoparticles as self-organized organic–inorganic compo-site materials. Biomaterials, 26(26), 5414–5426.
Sankar, D., Chennazhi, K. P., Nair, S. V., & Jayakumar, R. (2012). Fabrication of chitin/ poly(3-hydroxybutyrate-co-3-hydroxyvalerate) hydrogel scaffold. Carbohydrate Polymers, 90(1), 725–729.
Seidman, L. A., & Larsen Jr, J. H. (1979). Ultrastructure of the envelopes of resistant and nonresistant Daphnia eggs. Canadian Journal of Zoology, 57(9), 1773–1777.
Tamura, H., Furuike, T., Nair, S. V., & Jayakumar, R. (2011). Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydrate Polymers, 84(2), 820–824.
Tuzen, M., & Sarı, A. (2010). Biosorption of selenium from aqueous solution by green algae (Cladophora hutchinsiae) biomass: Equilibrium, thermodynamic and kinetic studies. Chemical Engineering Journal, 158(2), 200–206.
Wysokowski, M., Bartczak, P.,Żółtowska‐Aksamitowska, S., Chudzińska, A., Piasecki, A., Langer, E., et al. (2017). Adhesive stalks of diatom Didymosphenia geminata as a novel biological adsorbent for hazardous metals removal. CLEAN–Soil, Air, Water, 45(11), 1600678.
Wysokowski, M., Bazhenov, V. V., Tsurkan, M. V., Galli, R., Stelling, A. L., Stöcker, H., et al. (2013). Isolation and identification of chitin in three-dimensional skeleton of Aplysinafistularis marine sponge. International Journal of Biological Macromolecules, 62(0), 94–100.
Wysokowski, M., Motylenko, M., Stöcker, H., Bazhenov, V. V., Langer, E., Dobrowolska, A., et al. (2013). An extreme biomimetic approach: Hydrothermal synthesis of β-chitin/ZnO nanostructured composites. Journal of Materials Chemistry B, 1(46), 6469–6476.
Zhang, C., Sui, J., Li, J., Tang, Y., & Cai, W. (2012). Efficient removal of heavy metal ions by thiol-functionalized superparamagnetic carbon nanotubes. Chemical Engineering Journal, 210(0), 45–52.
Ziegler-Borowska, M., Chełminiak, D., & Kaczmarek, H. (2015). Thermal stability of magnetic nanoparticles coated by blends of modified chitosan and poly(quaternary ammonium) salt. Journal of Thermal Analysis and Calorimetry, 119(1), 499–506.