T. C.
DICLE UNIVERSITY
GRADUATE SCHOOL OF NATUREL AND APPLIED SCIENCE
EFFECT OF SOME BIOLOGICAL PARAMETERS OF ENGLISH GRAIN APHID Sitobion avenae (Hemiptera: Aphididae) ON DIFFERENT WHEAT
CULTIVARS
Omar SAFAR
MASTER THESIS
PLANT PROTECTION DEPARTMENT
DIYARBAKIR
DĠCLE ÜNĠVERSĠTESĠ
FEN BĠLĠMLERĠ ENSTĠTÜSÜ MÜDÜRLÜĞÜ DĠYARBAKIR
Omar SAFAR tarafından yapılan “Effect of some biological parameters of English grain aphid Sitobion avenae (Hemiptera: Aphididae) on different wheat cultivars” konulu bu çalıĢma, jürimiz tarafından Bitki Koruma Anabilim Dalında Yüksek Lisans tezi olarak kabul edilmiĢtir.
Jüri Üyeleri
BaĢkan : Prof. Dr. Erol BAYHAN (DanıĢman) Üye : Doç. Dr. Ramazan ÇETĠNTAġ Üye : Doç. Dr. Ġzzet AKÇA
Tez Savunma Sınavı Tarihi: 25 / 12 / 2015
Yukarıdaki bilgilerin doğruluğunu onaylarım. .../.../2015
Doç. Dr. Mehmet YILDIRIM Enstitü Müdürü
I
Words are not enough to thank God and all the people who gave me strength and support throughout this stage of my life.
I would like to give my sincerest and deepest gratitude to my supervisor, Prof. Dr. Erol BAYHAN for his belief, guidance, assistance and useful feedbacks for carrying out this project.
I also wish to express my thanks to Staff of Agricultural Faculty, Dicle University for giving me laboratory equipment and support I needed during my study. I would also like to give my deepest appreciation to my best friend Mr. Hassan YAHYA AL-MIZORI for giving me always precious advices during my study.
My special thanks go to my friends Ms. Zelal AKGÜL and Necla DAKAK, who helped me during laboratory work.
I am appreciative of my family, especially my parents for their kind support, patience and encouragement, which gave me the energy to continue and to motivate myself towards completing my research study.
II ACKNOWLEDGEMENT……….……..….… I CONTENTS...II OZET……….…III ABSTARCT……….….…IV LIST OF TABLES………...….…V LIST OF FIGURES………...……… VI 1. INTRODUCTION……….…………1 2. REVIEW OF LITERATURE………...5
2.1. Development time, longevity and fecundity of Sitobion avenae ……...…..….…..5
2.2. Survival rate of Sitobion avenae ……….……...….…..…….11
2.3. Mortality rate of Sitobion avenae ………..……...………...13
2.4. Plant resistance to Sitobion avenae ...15
3. MATERIALS AND METHODS……….……….…..19
4. RESULTS AND DISCUSSION …………..……….…..24
5. CONCLUSION………....34
III
Sitobion avenae‘nın geliĢme süresi, canlılık oranı ve doğurğanlığı üzerine Buğday
çeĢitlerinin dayanıklığını etkilemektedir. Bazı buğday çeĢitlerinin S. avenae’nin kanatsız bireylerinin geliĢme süresi, canlılık oranı, üreme ve ömür uzunluğu ve bazı biyolojik parametrelerine etkisi laboratuvar koĢullarında (25 ± 1 °C, % 65 ± 5 nisbi nem ve 16 saat aydınlık: 8 saat karanlık ortamda) beĢ farklı buğday çeĢitleri üzerinde ayrı ayrı üretilmiĢtir. Yaprakbitinin geliĢme süresi Vitorrio, Ceyhan-99, Pehlivan, Stendal ve ġahinbey çeĢitlerinde sırasıyla 9.02, 8.17, 7.88, 6.97 ve 6.62 gün olarak bulunmuĢtur. Ergin öncesi en düĢük canlılık oranı ġahinbey çeĢidinde tespit edilmiĢtir.Ortalama diĢi ömür uzunluğu ġahinbey, Stendal, Pehlivan, Ceyhan-99 ve Vittorio çeĢitlerinde sırasıyla 5.05, 5.63, 7.16, 7.81 ve 8.095 gün olarak saptanmıĢtır.Ortalama doğurganlık en yüksek 12.12 progeny/female ile Pehlivan’da, en düĢük 8.38 progeny/female ile ġahinbey çeĢidinde bulunmuĢtur. ġahinbey çeĢidinde üzerinde beslenen S. avenae’nin biyolojik parametreleri diğer çeĢitlerden elde edilenlerden farklı olduğu saptanmıĢtır.
IV
Resistant varieties of wheat play a vital role in influencing development time, survival rate and fecundity of Sitobion avenae. The impact of certain wheat cultivars on developmental time, survival rate, reproductive rate and longevity and some biological parameters of English grain aphid were studied under laboratory condition (25 ± 1 °C, 65 ± 5 % relative humidity and photoperiod of 16 h light: 8 h dark), and randomly choose apterous aphids and reared on five wheat cultivars. The English grain aphid had a nymphal developmental time of 9.02, 8.17, 7.88, and 6.97 and 6.62 days for Vitorrio, Ceyhan-99, Pehlivan, Stendal and ġahinbey, respectively. The lower survival rates (percentage) of total immature stages were observed on ġahinbey cultivar. Mean longevity of adult female was observed 5.05, 5.63, 7.16, 7.81 and 8.095 days on cultivars ġahinbey, Stendal, Pehlivan, Ceyhan-99 and Vittorio, respectively. Mean fecundity was highest on Pehlivan (12.12 progeny/female) and was lowest on ġahinbey (8.38 progeny/female). The gross reproduction rate (GRR), net reproductive rate (Ro), intrinsic increase rate (rm) and generation time (To) were observed lower (10.55, 4.34, 0.143 and 10.26) on ġahinbey than of the other wheat cultivars. The present study revealed that
Sitobion avenae feeding on ġahinbey cultivar showed significant biological parameters
than those of the other cultivars in the study.
V
Table 1. Development periods (days SE) of immature stages of Sitobion avenae at 25 °C on five varieties of wheat………..………….……..25 Table 2. Survivorship (percentage) of immature stages of Sitobion avenae at 25 °C on five varieties of wheat……….…..……27 Table 3. Mean SE development, reproduction, fecundity per female, longevity of female Sitobion avenaeat 25 °C on five varieties of wheat……….………….29
Table 4. Life table parameters of female Sitobion avenae at 25 °C on five varieties of wheat………..………….…...31
VI
Figure 1. Shows the collection of aphid colonies in the field……….………..…19 Figure 2. Putting aphid colonies in the incubator………...………….…….…….20 Figure 3. Reared aphid colonies in petri dishes………...……...21 Figure 4. Shows the daily observing each petri dish of aphids on five varieties of wheat………..…21
1 1. INTRODUCTION
Wheat is an important cereal crop grown in the world and is regarded as a third grain crop among cereal crops (Anonymous, 2014). Wheat is the most essential food crop between cereal crops. Presently, about 95 % of the wheat crop grown around the world are bread wheat (Triticum aestivum) and it’s also frequently called pasta wheat, and remaining is durum wheat (Triticum durum) (Varga et al., 2002; Bayaner, 2002; Shewry, 2009; Köskel and Cetiner, 2015). Wheat production has been worldly increased from (521 million tons) in 1986 to (732 million tons) in 2014 (Richard and Won, 2015). However, in 2013 the global average wheat yield was about (713 million tons). Generally, the universal wheat yield has augmented day by day in the world (Anonymous, 2015a).
Wheat is a major grain crop in Turkey in the matter of economy, nourishments and as a food sources. Turkey is considered to have a good climatic condition for wheat cultivation. The annual using area for growing wheat in Turkey is approximately (8 million hectares) and annual the yield is between (19-22 million), and wheat crop is almost planted all over Turkish regions especially in the rainfall areas (Altay, 2012).
In Turkey, the average wheat product between 2003-2013 has increased from 19 million tons to 22 million tons, and Turkey has also exported a large amount of wheat flour to many countries (Alpan and Engüzel, 2015). Turkey is known to have best quality, quantity and good varieties of wheat (Gökgöl, 1939; Vavilov, 1950; Harlan, 1971; Özkan et al., 2002). Central Anatolia is the biggest and the best region in Turkey for growing wheat because of favorable conditions for wheat growing. The wheat growing area in central Anatolia in 2015 was about 3,000,000 hectares, and the average wheat yield was about 6.2 million tons (Karabina, 2015).
Wheat can be infested by numerous pests like Aphids, Stink Bugs, Armyworms, cutworms, stalk Borers, Cereal leaf Beetle, Thrips, Hessian fly, Wheat Stem maggot, Sawfly, White Grubs, Wireworms, Grasshoppers, Crickets, Helicoverpa, Mites, Slugs and Snail, depending on environmental conditions and timing of planting. Of these pests,
2
there are about six aphid species attacking small grain crops in the world, including English grain aphid (Sitobion avenae (Fabricius)), Russian wheat aphid (Diuraphis noxis (Mordvilko)), Corn leaf aphid (Rhopalosiphum maidis (Fitch)), rose grass aphid (Metopoliphium dirhodum), Bird cherry-oat aphid (Rhopalosiphum padi (Linnaeus) and Greenbug, (Schizaphis graminum (Rondani)) (Prescott et al., 1986; Anonymous, 2012).
Aphids are considered as one of the most important agricultural pest group in the world and play a significant role in decreasing crop yield by directly causing severe damage (sucking sap) and act as a vector in transmitting viruses (Rochow, 1969; Fereres et al., 1989; Mann et al., 1997; Thackray et al., 2009; Elek, 2013; Herbert et al., 2014). Wheat crop yield can be lost easily as a result of the presence of huge aphid populations (Carver, 1989).
English grain aphid (Sitobion avenae (Fabricius)) and Russian wheat aphid (RWA)(Diuraphis noxis (Mordvilko)) are two major aphid species affecting wheat in Turkey (Özder, 2002;Turanli et al., 2012). The English grain aphid is known prevalent worldwide (Robinson and Hsu, 1963; Dean 1974; Kolbe and Linke, 1974; Kiechefer, 1975; Markkula, 1980; van Emden and Harrington, 2007). Sitobion avenae was recorded as a main insect pest of wheat crop in Turkey (Özder, 2002). The English grain aphid is considered monoecious and has a complex life cycle of a Poacea family (Reimer, 2004). Life cycle of English grain aphid undergoes six stages, including the egg or embryonic stage, four nymphal stages and an adult (Elek, 2013). Eggs are small, ovid shape, green in color and when become nearly to fertilize the color are changing to black color (Dedryver, 1984). Sitobion avenae have four nymphal instars, nymphs color is similarly colored when they become adults, there are five-segmented antennae on the first and second instar stages, but third and fourth instar stages have six-segmented antennae. The presence of bright red-brown color in nymphs is indicated male nymphs, general body color of males are red or reddish brown (Weppler, 2009). Adult body length of S. avenae apterae (wingless aphids) is about 1.3-3.3 mm long and alates (winged aphids) is about 1.6-2.9 mm long spindle shaped aphid. They have a salient black to pale cauda. Sitobion
3
colors are (Yellow, green, purple, brown to red). Alatae have the same color like in aperae, but with the presence of dark inter segmental markings on the upper side of the abdomen. Antennae and siphunculi are blackish, legs are yellow, but femora, tarsi and tibiae are dark color polymorphism (different colors). This is defined in both reactions to environmental conditions and genetic characters, consisting nourishment, with green and brown forms predominating (Jenkins et al., 1999). Sitobion avenae is featured from other aphid species by length of black siphunculi and in part black legs (Weppler, 2009).
In order to control English grain aphid, selecting resistant varieties of wheat is becoming a great deal significant (Schotzko and Bosque-perez, 2000; Özder, 2002). Increasing and decreasing of the developmental nymphal time, longevity of the adult and fecundity of
Sitobion avenae is depending on selecting resistant varieties. Therefore, using resistance
cultivars is good tactics as protection and management of aphid infestations in wheat (Dixon, 1998; Özder, 2002).
Plant resistant varieties and knowledge of the genetic foundation of aphid-plant interaction are very important to decrease reproductive rate, growth rate and inhibiting aphid outbreaks (Xu et al., 2011). Biotypes of plants are needed to generate resistance varieties against aphid (Xo et al., 2011; Elek, 2013; Lowe, 1987; Riazuddin et al., 2004). Temperature is the most powerful factor on reproductive rate and period of developmental nymphal stages survival (Kindlmann et al., 2010). Sitobion avenae has been recorded as a main source of transmissible viral diseases, especially barley yellow
dwarf virus (BYDV) (Johnson and Hershman, 1996; Emden and Harrington, 2007). The
English grain aphid is a vector for transmitting Bean yellow mosaic virus and the Pea
mosaic virus (Basky, 2005). Aphid-infected with viral diseases are producing more
offspring and affecting the duration time of longevity and reproductive rates more than those aphids that are not infected with viral diseases (plant healthy) (Araya and Foster, 1987). When Sitobion avenae afforded with viral disease fecundity rate increased and aphid became enlarged in size was due to the presence of a large quantity of nutrients that affected by the development of aphid life (Ajay and Dewar, 1982).
4
Sitobion avenae was isolated that can overcome some wheat cultivars (Xoet al., 2011).
Aphids produce a large quantity of honeydew that plays an important role as a medium for growing sooty moulds. Large amount of sooty moulds prevent photosynthesis process in plants and consequently leads wheat to become unmarketable (Elek, 2013).
Some biological parameters of Sitobion avenae on wheat varieties have been studied individually (Markkula and Myllymaki, 1963; Markkula and Roukka, 1972; Dixon, 1973; Watt, 1979; Watt and Dixon, 1981; Araya et al., 1987; Di Pietro and Akli, 1987; Leszczynski et al. 1995; Theime and Heimbacha, 1996; De Celis et al., 1997; JunXiang et al., 1999). In a laboratory conditions different wheat varieties were evaluated for their susceptibility and resistance against Sitobion avenae. Also to estimate nymphal developmental period, longevity, fecundity, survival and mortality rates (life table) of aphids.
The development of a sound pest management program depends on a thorough understanding of the biology and ecology of this pest. Basic information on development rate, age-species fecundity, and survival on pomegranate has not been determined for S.
avenae, and thus, effective control of this aphid has been hampered. Consequently, we
report the results of a study in which the effect of different wheat cultivars on the development, longevity, and reproduction of S. avenae was examined.
5 2. REVIEW OF LITERATURE
2.1 Development time, Fecundity and Longevity
Life table is the best strategy for understanding and analyzing the influence of an external factor at development time, survival, reproductive, growth and the average of increasing insect populations (Soleimannejad et al., 2010). Biological or life parameters are very important indicator for duration of developmental stages and growth of insect populations (La rossa and Khan, 2003).
El-Ibrashy et al. (1972) investigated the development time of Rhopalosiphum maidis nymphal instars occurred during constant high temperature of 20 to 30 °C.
Dean (1974) found that the most suitable temperature was 20 °C for highest fecundity and reproductive rate of S. avenae on barley. Additionally, S. avenae sustained the longest reproductive life and life span followed by R. padi (L) and M. dirhodum (Walker). He also determined that onset reproduction of S. avenae was 22.5 °C and the fastest development time to reach adult was 25 °C.
Blackman and Eastop (1984) found that the selection of the host plant is a very important for insects survival and reproduction. They considered that about 95 % of hosts are monophagous and 5 % are polyphagous.
Lykouresis (1985) investigated that the fastest development to adult and to outset of reproduction occurred at (25 °C).
Knight and Bale (1986) reported that the extreme low temperature between -5 °C and -15 °C cause increasing mortality, but above ranges of temperature tested are favorable temperature for surviving aphid colonies as overwintering.
Chongchuan and Jianye (1987) reared S. graminum in the laboratory at 10-30 °C and they found out that the development time of all nymphal instars of aphids reduced with increase of temperature. The study indicated that at 30 °C the development time of all immature stages completed in 4.8 days, but at 10 °C the development period of all
6
immature stages completed in a 16.8 days. It was also recorded that the resistivity of adults was higher than that of nymphs at lower temperatures.
Elliott et al. (1988) pointed out the development time of immature stages apterous of
Rhopalosiphum maidis reared on different host plants was between 10-20 °C.
Tatchell and Parkers (1990) showed that the reproductive rate was high, particularly when the temperature ranges were ideal, plants were favorable to aphid populations to growth, survival, reproduction, fecundity and longevity.
Zhou and Carter (1992) reared aphid populations of rose grain aphid, Metopolophium
dirhodum (Walker), in the laboratory under different temperatures. The survival rate of
an aphid population decreased at high temperature (30 °C) due to the short life span of adults and lack of any nymphs reaching to adult stage.
Plantegenest et al. (1996) recorded that the temperature had affected there productive, survival and fecundity rates of S. avenae. They indicated that the reproductive rate was very sensitive and was affected by temperature. Under favorable conditions, temperature was a major factor for daily fecundity by increasing and reproductive rate.
De Celis et al. (1997) compared the longevity and fecundity of three cereals aphid species named S. avenae, Schizaphis graminum and Rhopalosiphum padi. It was analyzed that the longevity of S. avenae and S. graminum were the same whereas R. padi lived a shorter than two other species. Nevertheless, the longevity and development time were not only depending on food quality but also on temperature. The fecundity ratios were rated as high, intermediate, and low for S. graminum, R. padi and S. avenae, consequently. Schizaphis graminum produced twice number of offspring compared with
S. avenae. It was also observed that the embryonic development of Sitobion avenae
undergoes eight stages. These stages can be called first stage: Egg-shaped, the embryo is colorless, second stage: can be seen much laxness movement and the embryo undergoes cream colored, third stage: caudal end of the body appears, extension and flexure of anterior end, fourth stage: formation of a leg structure occurs, and the proboscis can be observed during early development, and whole body has winding borders that will be
7
basic points for body segmentation and the cauda, fifth stage: during this stage embryo bears eyes, six stage: the proboscis and the eyes clearly occur in the anterior body and appearing of siphunculi in the upper side of abdomen, seventh stage: Internal head structures modification and stylets are more obvious, formation of thoracic segments, The development of eyes and legs can be more visibly, eight stage: the siphunculi can be seen obviously, the legs are very developed and the proboscis is distinctly occur, As well as for each embryonic stage should be observed during twenty four hours.
Abou-Elhagag and Abdel-Hafez (1998) reported that relative–humidity, moderate daily temperature and natural enemies play a central role in decreasing the reproductive rate of aphids and longevity of their populations.
Ciepiela and Sempruch (1999) observed that the rate of fecundity of S. avenae on young leaves was less than old leaves. It means that the rate of fecundity was decreased on young leaves because of the presence of high concentration of defensive compounds against English grain aphid.
Legrand and Barbosa (2000) recorded that the low quality of food had an impact on declining of fecundity rate and increasing of development periods of aphid life span.
Nava-Camberos et al. (2001) recorded that the development time of cereal aphids is depending on the interaction between variation of temperature especially low temperature and host plants.
Asin and Pons (2001) determined the effect of different temperature on growth, reproductive rates and longevity of three cereals aphid species, namely, Sitobion avenae,
Rhopalosiphum padi (L) and Metopolophium dirhodum (Walker). These three species
were exposed to 18, 22, 25, 27.5 and 30 °C temperatures. The most favorable temperature found for development of aphid was 26.5 °C for S. avenae, 28.5 °C for R. padi, and 24.5 °C for M. dirhodum (Walker). When aphid species were exposed to high temperature 30 °C all nymphs were except for nymphs of R. padi. The reproductive rate of R. padi was very low, but its activity was better than other two aphid species. R. padi had also lower nymphal mortality than those two other aphid species, when the temperature increased up
8
to 27 °C the developmental time of S. avenae decreased and when S. avenae exposed to 30 °C no nymphs were reached the adult stage. The maximum temperature for S. avenae survival was around 27.5 °C, and the outset of reproduction rate was between 18-25 °C. The reproductive rate increased when the temperature was 27.5 °C. When the temperature increased up to 27.5 °C the development time of S. avenae decreased, and at 30 °C no nymphs became an adult, however, reproductive rate decreased between 18 °C to 25 °C and increased with increasing temperature 27.5 °C.
Ahmad and Nasir (2001) reported that the population of cereal aphids increased gradually in early growth stage of wheat because producing tillers and aphids cannot reproduce rapidly on the early growth stage of wheat. This is due to the presence of the low quality of food (sap) in early growth stage of wheat. However, during the growing of the wheat stage (life of the plant) changes the quality and quantity of food and influence on longevity, reproduction rate, developmental time (speed), distribution and survival of aphids.
McCornack et al. (2004) described the effect of temperature on aphid populations growth is depending on the net reproductive rate, developmental threshold, intrinsic rate increase and fecundity.
Aslam et al. (2006) reported that aphids reproduce more rapidly and increase their population at earing or heading more than leaves or other parts of wheat crop; this is due to the presence of a large amount of good quality and quantity of food (proteins) in the ear more than other parts.
Ma et al. (2004) showed that above lethal heat temperature and daily fluctuation will result disintegration in aphid populations because of the raised rate of mortality due to accentuated sub-lethal impact on decline of development and reproductive rates. Immature stages of aphids had a longer survival than adult aphids especially when M.
dirhodum exposed to the heat period of more than 8 hours up to four days. The third
instar nymphs survived more than the second and fourth nymphal instars and the third instars sustained the highest survival rates when the exposure was less than four days, 8
9
hours per day. Nymphs of M. dirhodum had a higher tolerance at high temperature than adults, beside survival rate of fourth instar and adult aphids affected by high temperature.
Quareshi and Michaud (2005) reared three cereal aphid species (S. graminum, Diuraphis
noxi and R. padi (L)) on wheat to evaluate survival, development time and fecundity.
Nymphs of all three aphid species were released on wheat plant under laboratory condition at-21.23 °C. Survival rates of aphid species from first nymph instar to onset reproduction reached 90-100 %, growth of Diuraphis noxi was faster than two other aphid species. However, fecundity of these three aphid species reduced during feeding on tandem, and the presence of R. padi were reduced the fecundity of D. noxi and also reduced the survival rate of the second generation of S. graminum.
Kagata et al. (2005) found that aphid predator had very attribute substances depending on genetic characteristics among plants. Toxic substances also showed a direct impact on the development of aphid populations.
Van Emden and Harrington (2007) showed that aphids can choose the plant with good quality and quantity of nutrients, but supply with physical properties like protecting themselves from natural enemies and environmental factors.
Thomson et al. (2010) found that warming environmental conditions are considered as good factors to be a result of the outbreak of aphid populations, for example, increasing number of pest generation every year caused the increasing overwintering survival rate.
Descamps et al. (2011) examined that the nymphal developmental time of
Rhopalosiphum padi on rye had longer development time than those reared on beer
barley, as well as the period of fertility of aphids on beer barley had shorter than those reared on rye. Also the number of offspring produced on beer barley was observed higher than those produced on triticale.
Razmjou et al. (2011) reared S. avenae on five varieties of wheat under laboratory condition (20 ± 1 °C, 60 ± 5 % relative humidity and photoperiod of 14 h light: 10 h dark). During the experiment there were significant differences among varieties of wheat
10
on developmental period of nymphal stages extended from (7.5 days) to (10.8days) on wheat varieties, however, three varieties of wheat showed resistant against S. avenae.
Ricci et al. (2011) studied Russian wheat aphid on wheat and barley, they indicated that the development time and reproductive rate of the pest were varied from year to year.
Tofangsazi et al. (2012) determined immatures development time of S. graminum ranging from 6.60 days at temperature 26 °C to 28.56 days at 10 °C for incorporating nymphs. However, the longest development time appeared at 31 °C and the shortest occurred at 26 °C. The longest nymph instar life was occurring in the first nymph instar of the pest among all immature stages Reproductive rate was found very high between 19 °C - 26 °C.
Gao et al. (2012) studied the effect of cadmium (Cd) on the fecundity, development time and mortality of English grain aphid (Sitobion avenae), by exposing cadmium to soil in different concentrations and measuring its uptake during the growing stages of wheat. It was recorded that concentrations of cadmium affected the development period, reproductive rate, survival rate and mortality of the pest.
Elek (2013) reported that most aphid species can distinguish between good and poor host by sensing the quantity and quality of nutrients that they contain. Eventually the quality of food or host would have an impact on development time, fecundity or mortality.
Jeffs and Leather (2014) found that high temperature (heat stress period) (a total of 16 h at 30 °C) significantly reduced the rate of fecundity and decreased the population growth rate of cereal aphids. Low (cold) temperature (a total of 1.33 h at 15 °C) reduced population growth rate because of the extended development period (in day) but did not have an impact on fecundity. High and low temperature have the extreme impact on mortality of S. avenae, and when S. avenae exposed to low temperature, produced progeny with an abnormal birth weight. Nevertheless, it was observed that heat-treated aphids had significant differences on the fecundity of S. avenae, but no significant differences were observed when the adult of S. avenae exposed to cold. Whereas, the development time of S. avenae is slower when adult of S. avenae exposed to heat
11
indicating that the rate of pre-reproduction development time (in days) of S. avenae increased when exposed to cold treatments. However, the heat and cold treatments didn’t affect the nymphal development, except for nymphal birth weight.
Brabec et al (2014) reported that population growth rate is depending on seasonal variation of aphids dynamic. Usually, after appearance of new generations of cereal aphids in spring, development of stages starts in mid-May on leaves and stems.
Akça et al. (2015) reported that the developmental, survival, and reproduction data for
Aphis fabae Scopoli (Hemiptera: Aphididae) reared Vicia on faba L. ‘Sevilla’ at
laboratory conditions (15, 20, 25, and 30 °C; 70 % relative humidity, and a photoperiod of 16:8 h). For A. fabae, the highest intrinsic rate of increase (rm) and finite rate (λ) were observed at 25 °C.
2.2 Survival rate of Sitobion avenae
Temperature is a vital component of the fluctuation of life history, surviving. Many species of aphids, especially cereal aphids, can survive at low and high extreme temperature (Dixon and Hopkins, 2010).
Leather and Dixon (1981) reported that younger leaves have better defenses against cereal aphids than older leaves. This is due to the availability of a large amount of concentration of defensive compounds than older leaves and aphids can’t grow on young leaves as a result of increased mortality and decreased survival rate on young leaves.
Acreman and Dixon (1989) reported that the life span of an aphid population reduced gradually under rearing-constant temperature above 10 °C-15 °C in S. avenae until the end of producing offspring at 30 °C
Dedryver (1989) reported that nymphs and adults of Rose grain aphid (M. dirhodum) moved to lower parts of plants, particularly lower leaves when the temperature was unfavorable and they could feed and was able to survive with a better chance.
12
Yang (1990) evaluated the effect of temperature and light on the survival rate and populations of S. graminum. They showed that the development time of the pest was delayed at low temperature, and the reproductive rate was also decreased at high temperatures. It was also recorded that the most favorable temperature for growing aphid population was 25 °C in a 12 h light period.
Niehoff (1996) indicated that Rose grain aphid (M. dirhodum) can survive when daily temperature was 30 °C. In this study, the aphid populations delayed when the temperature ranges reached between 22.2 °C - 24.8 °C. When daily temperature goes lower than these ranges, the infestation of aphids in leaves increased from 54 % to 60 %.
Saikia et al. (1998) mentioned that crop varieties play a critical role in decreasing the rates of pre-reproductive, reproductive and post-reproductive. They added that many varieties of wheat significantly differed in reducing fecundity rate and decreased aphid infestations.
Powell and Bale (2004) reared aphid population of S. avenae at temperature 20 °C, survival rates of nymphs and adults augmented from 18 % and 16 % of their tested-ranges temperature (-8 °C and -8.5 °C) to 83 % and 68 %. Many species of cereal aphids maintain a very short time of generation, when the aphid population overwintering winter. Aphid populations in spring are depending on the reproductive rate of winter passed, and it’s considered as possibility fluctuating of aphid populations as a result of being resistant to cold temperature and or some others susceptibilities.
Powell and Bale (2005) evaluated that the survival rate of first nymphs instar and newly reproductive adults of S. avenae. They concluded that the survival rates of first nymphs instar and adults decreased approximately 20 % in a 3 h exposure, when they transferred from 10 °C to sub -11 °C or -12 °C. When they were exposed to low temperature of 0 °C, survival rates of first nymphs instar were increased 26 % in 30 minutes. Temperature survival rates increased to 96 % at 0 °C in a 3 h, but decreased when they were exposed a time interval of 4 to 8 h. Nevertheless, adults of S. avenae increased more rapidly than nymphs and their survival rate increased more rapidly at 0 °C from 22 % to 56 % in a 10
13
min. Survival rates increased more rapidly 70 % in a 30 min at (0 °C), but reduced in an explosion of longer than 30 min. It was also calculated that nymphs surviving of S.
avenae at the discriminating temperature was approximately 86 %, but surviving of adult
aphids decreased gradually at this temperature.
Eleftherianos et al. (2005) reared two cereal aphid species (S. avenae and R. padi) on barley and maize leaves. During his experiment, it was recorded that leaf stages have influences on development time, fecundity and survival rates
Tofangsazi et al. (2012) reported the survival of S. graminum decreased rapidly at the-31 °C, and the lowest rate of life anticipation was reported at this temperature when duration was kept for 7.66 days.
2.3 Mortality of Sitobion avenae
Temperature plays a very significant role in influencing the developmental time, reproductive rate, survival and population growth of the pest (Tofangsazi, 2009). Environmental temperatures have been indicative for the life cycle of aphid species like, fecundity, development time and size (Nealis et al., 1984).
Harrington and Cheng (1984) described that not only low temperature was important for a mortality rate of aphids, but also rainfall and humidity were very important in aphid population dynamics.
Knight and Bale (1987) reported that the temperature was the major factor affecting the development and reproduction of S. avenae in the field in winter and mortality rates increased with decreasing temperature.
Clough et al. (1990) reported that when the temperature increased from 5 °C to 15 °C the mortality rates of population of S. avenae increased from 5 % to 95 %. This suggests that low temperature is a lethal for S. avenae populations.
De Barrow (1992) found that R. paid survived at high temperature of 30 °C, but didn’t survive above that level, causing a mortality rate of 90 %.
14
Bale (1996) observed that the population of S. avenae in the laboratory and field were very susceptible when exposed to low temperature at -8 °C and -12, Mortality at chill (cold) temperatures and had a dramatic effect on their development and survival.
Plantegenest et al. (2001) used an aphid population of S. avenae for knowledge of impact of natural enemies (Entomophthorales fungi and parasitoid) on mortality of aphids.
Entomophthorales fungi were found to be stronger than parasitoid (Hymenoptera) on
mortality and decreasing aphid populations.
Asin and Pons (2001) conducted the mortality of S. avenae nymphs reached 100 % when it exposed to high temperature (30 °C), nymphs of M. dirhodum reached 100 % and 55 % nymphs of R. padi survived at 30 °C.
Powell and Bale (2004) recorded that nymphs of S. avenae induced by sub-zero temperature and when exposed to -7 °C, -8 °C, -9 °C in a 3 h, the rate of mortality increased approximately 80 %. When nymphs of S. avenae exposed to -9 °C for more than 3 h, none of nymphs were survived. This proposes that populations of S. avenae undergo mortality during extensive freeze.
Harmon et al. (2009) reported exposing aphids to an above threshold temperature for even a shorter time can cause modification of proteins resulting physiological problems, consequentially affecting development time and mortality rate.
15 2.4 Plant resistance to cereal aphids
Plant resistance is a way of avoiding aphid infestations by bearing some genetic characteristics as compared to the same host species that lacking those characteristics (Smith, 2005). Using plant resistance against insects mostly influences the development of insect individuals, population growth, survival and fecundity by substances of secondary plants. Host plant resistance can reduce aphid populations and their damage but cannot be the consequence in insect mortality (Cai et al., 2009). Host plant resistance represents a greater role in controlling pests and protection of natural enemies in an agro-ecosystem. The effect of host plant resistance varieties in the declining influence of pests is considered to be prominent (Francis et al., 2001).
Maltais (1951) observed resistance varieties of wheat every consecutive year. Nevertheless a smaller number of susceptible varieties were also his studies.
Wratten and Redhead (1976) reported that incidence levels of aphids were differed in various varieties of wheat due to resistance and susceptible characters of hosts to cereal aphids.
Acreman and Dixon (1985) recorded that alternation of sowing date could be helpful for controlling aphid populations in winter wheat. Aphid population infestations were increased on late sown and causing a reduction in wheat yield as compared to normal sowing. Thus, early sowing was a good method for decreasing aphid populations and reducing aphid infestation, representing a part of biological control (IPM).
Karren (1989) recorded that infestation of aphids at the 1 % level would cause a 0.5 % loss of yield.
Kuroli and Nemeth (1987) found that when the environmental conditions were favorable for growing aphid populations, they could cause a loss of from 50 to 76 % in grain weight per year in spring and winter wheat.
16
Feng et al. (1992) reported that the population of cereal aphids (S. avenae, R. padi, S.
graminum) varied from year to year due to fluctuating of temperature ranges, the
presence of a huge number of predators and parasites.
Kieckhefer and Gellner (1992) also described that about (2.3 % to 2.7 % loss of yield per aphid) means the total number of aphids per plant were 15 aphids and total yield loss reached (35-40 %).
Escobar and Niemeyer (1993) conducted that host plant resistance, wheat germplasm with fractional resistance against aphids and resistance gene were being integrated into breeding programs. Thus, using of biological control (Natural enemies, parasite and entomopathogenic fungi) was being complementary tactics inward integrated pest management (IPM) against cereal aphids.
Gowling and van Emden (1994) reported that using resistance varieties of wheat could effect on reduction of aphid populations and their growth rate without affecting parasitoids.
Chen et al. (1997) found that resistance varieties could reduce growth and survival rates and influence the development period or maturity of S. avenae. However, this situation cannot represent integrated control of all aphid populations.
McCarty et al. (1998) evaluated some germplasm must be assessed before using against aphids as a resistant variety. This could be an extreme influence and supply resistive to future biotypes of aphids during breeding programs.
Brewer et al. (1999) reared Russian wheat aphid (Diuraphis noxia) on barley, and reported that resistant varieties of barley sustain the lower number of aphids than susceptible ones.
McDonald and Linde (2002) classified plant resistance as qualitative and quantitative resistance. Qualitative plant resistance usually has a large phenotypic impact and
17
controlled by single genes called major genes. However, the quantitative plant resistance usually has a small phenotypic impact called minor genes.
Awmack et al. (2002) found that the interaction between insects and their hosts was depending on the quality and quantity of food such as (nitrogen compound, amino acids, carbohydrates, and protein). All these nutrients were very important for growing insect populations and their reproductive life.
Pasalu et al. (2004) reported that biotypes of cereal aphids could overcome on resistant varieties with an extensive planting.
Mornhinweg et al. (2006) studied on resistant and susceptible varieties of spring barley against Russian wheat aphid Diuraphis noxia. It was calculated that some cultivars of wheat were resistance with D. noxia and the average grain yield increased 5 %. In contrast that, some cultivars of spring barley had susceptible with D. noxia and caused a reduction of grain yield and the average reduction reached 56 %.
Sprawka (2007) tested five types of proteins on aphids’ life table, and concluded that there was a high negative impact on feeding behaviors and minimize and increase mortality depending on aphids.
Zaayman et al. (2009) found multiple biotypes of two cereal aphids, D. noxia and S.
graminum, knowledge of these biotypes of cereal aphids was considered by scientists as a
good program for breeding resistance varieties against cereal aphids.
Maneva et al. (2009) investigated the average percent of aphids’ infestation S. avenae in different oat varieties in the field. It was observed that infestation of aphids was 37.53 % when 2.4 aphids found per plant Medium infestion of some oat cultivars was approximately caused a 24.94 % loss of yield at 7.5 aphids per stalk. About 41.67 % loss of grain yield increased (highly infested) when 16.6 aphids were present per stalk.
Akhtar et al. (2010) concluded that various genotypes of wheat had different ranges of aphid infestation. They concluded that aphid cause yield loss from 7.9 % to 34.2 % with increasing infestation.
18
Xu et al. (2011) discovered for the first time the biotypes of S. avenae. English grain aphid biotype is very important since it can reduce the development of aphid-resistant cultivars. Sing resistance varieties of wheat against S. avenae are very useful for understanding genetic characteristics of aphid-resistance in wheat varieties.
Wains et al. (2014) used 464 bread wheat varieties that contain wheat germplasm for rearing three species of cereal aphids, S. avenae, S. graminum and R. padi. The study results indicated that 8 varieties of those were resistant to aphids when 3-4 aphids found per tiller. Overall, 177 of them obtained moderately resistant when 5-6 aphids present per tiller, 141 of them were tolerant when 7-8 aphids found per tiller. Nevertheless, 16 were moderately susceptible, 16 were susceptible and 5 were highly susceptible for 9-10, 11-15 and 16-20 aphids per tiller, respectively. However, approximately 71 wheat varieties were immune when 1-2 aphids found per tiller.
Herrera (2014) described the effects of antibiosis, antixenosis and tolerance on cereal aphids. Antibiosis has an influence on the life history of cereal aphids and can reduce growth rates, cause longer developmental time, lower fecundity and higher mortality. In addition to that, antibiosis contains Alleochemicals (non-nutritional) that can be present in plant resistance genotype that reduces insect damage. On the other hand Antixnosis negatively impacts the host acceptance and host finding activity, thus it can reduce the number of aphids per plant or plant parts (Spike, leaf, tiller, etc.) and also reduces feeding rate. Tolerance, however, is the ability of plant to recover as before when exposed to insect damage and the ability of plants to withstand during insect damaging
19 3. MATERIALS AND METHODS
This research was established in a laboratory at Dicle University, faculty of agriculture in Diyarbakir, Turkey. Aphid colonies were collected from the experimental wheat field of the faculty.
Figure 1. Shows the collection of aphid colonies in the field.
Five seeds of wheat cultivars namely Ceyhan-99, ġahinbey, Vittorio, Stendal and Pehlivan were used to experiment. They were planted individually in plastic pots under laboratory conditions and plants were watered as needed and aphid colonies were reared and maintained on fresh leaves of each wheat cultivar at 25 ± 1 °C and 65 ± 5 % relative humidity and photoperiod of 16 h light: 8 h dark.
20
Figure 2. Putting aphid colonies in the incubator
All populations of aphids were reared separately in every petri dishes about 8.5 cm in diameter 1.3 cm in depth on every varieties of wheat. The fresh leaves of wheat cultivars were washed with distilled water and placed in to petri dishes with labels before the aphid banks were used in the experiment. Two fresh leaves about 4.5 cm of each wheat cultivar were washed with distilled water and placed upside down in each petri dish on filter paper. For immature survival and developmental time, apterous aphids were randomly chosen from aphid banks and relocated separately, placed on a lower side of leaf wheat cultivars into petri dishes about 5.3 cm in diameter by 1.2 cm in depth.
21
Figure 3. Reared aphid colonies in petri dishes
Each petri dish was observed daily under a stereo microscope and all present newborn aphids were removed from petri dishes with a small brush. Filter paper was checked and wetted daily for2-3 days in spite of fresh leaves of each wheat cultivar were supplied to aphid every one or two days.
22
Nymphal stages were depending on appearance of molting (exuviae) time. Adult longevity and reproduction rates were checked daily and after forth nymphal instar and a newborn progeny were pointed out the period of adult preproduction, reproduction, post reproduction and number of new born per day. All data were recorded daily until the death of all aphids. All data obtained was used for a life table of aphids on different wheat cultivars.
Wheat cultivars were planted twice in the experiment. The experiment for biological parameters of (Sitobion avenae) was conducted for 35 replicates aphids at each cultivar.
23 STATISTICAL DATA ANALYSIS
All data of this biological parameters (Nymphal developmental period, daily reproduction, fecundity, survival rate and life span of adults) were analyzed by statistical software (IBM SPSS Statistics version 22). The significant differences were resolved by One-way (ANOVA), Tukey’s, Duncan and Least square difference (LSD) of post hoc differentiation were made with significance at (P≤ 0.05). In addition to that, it was used the computer program TWOSEX-MS Chart to facilitate statistical analysis of raw data and calculation of life table parameters by Chi (2015).
24 4. RESULTS AND DISCUSSION RESULTS
Wheat varieties had significant impacts on total developmental time, total immature stages period, pre-reproduction period and mean newborn offspring of S. avenae.
Developmental time of immature stages
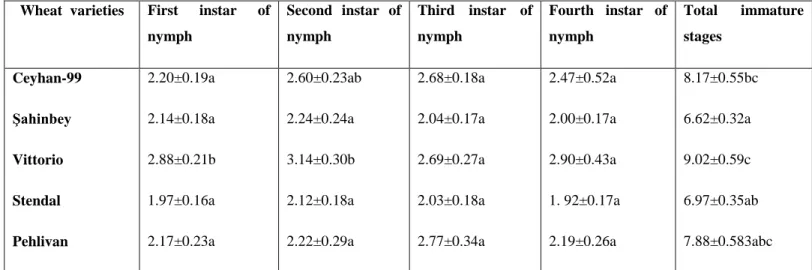
Developmental times of different nymphs of S. avenae on five wheat cultivars are shown in Table 1. There were no significant differences in first, third and fourth nymphal developmental time except for a second nymphal developmental time was the shortest and significantly differed on Stendal. Total developmental period of S. avenae was shortest on ġahinbey (6.62 d), and was the longest on Vittorio (9.02 d) as in Table 1.
25
Table 1. Development periods (days SE) of immature stages of Sitobion avenae at 25 °C on five varieties of wheat
Wheat varieties First instar of nymph Second instar of nymph Third instar of nymph Fourth instar of nymph Total immature stages Ceyhan-99 Şahinbey Vittorio Stendal Pehlivan 2.20±0.19a 2.14±0.18a 2.88±0.21b 1.97±0.16a 2.17±0.23a 2.60±0.23ab 2.24±0.24a 3.14±0.30b 2.12±0.18a 2.22±0.29a 2.68±0.18a 2.04±0.17a 2.69±0.27a 2.03±0.18a 2.77±0.34a 2.47±0.52a 2.00±0.17a 2.90±0.43a 1. 92±0.17a 2.19±0.26a 8.17±0.55bc 6.62±0.32a 9.02±0.59c 6.97±0.35ab 7.88±0.583abc
26 Survivorship of immature stages
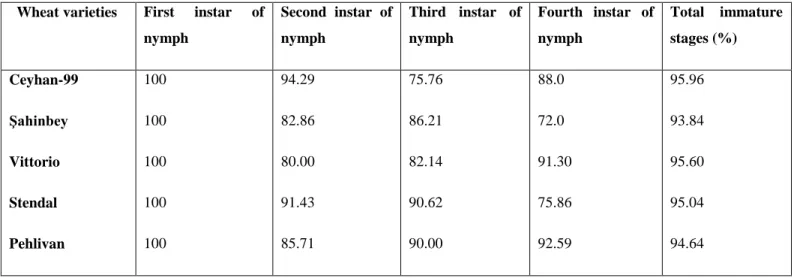
The survival rate (percentage) of second, third and fourth instars were mostly lower than the first instars (Table 2). The survival rate of the total nymphal period was highest with 95.96 % on Ceyhan-99 and was the lowest with 93.84 % on ġahinbey.
27
Table 2. Survivorship (percentage) of immature stages of Sitobion avenae at 25 °C on five varieties of wheat
Wheat varieties First instar of nymph Second instar of nymph Third instar of nymph Fourth instar of nymph Total immature stages (%) Ceyhan-99 Şahinbey Vittorio Stendal Pehlivan 100 100 100 100 100 94.29 82.86 80.00 91.43 85.71 75.76 86.21 82.14 90.62 90.00 88.0 72.0 91.30 75.86 92.59 95.96 93.84 95.60 95.04 94.64
28 Reproduction and Adult longevity
Pre-reproductive, reproductive and post reproductive period, longevity and fecundity of English grain aphid are presented in (Table 3). There were significant differences in pre-reproduction period, the shortest pre-pre-reproduction period was found on ġahinbey (0.83 d) and the longest was found on Vittorio (3.14 d). No significant differences showed in reproduction period (day), post-reproduction and fecundity (total number of new offspring per female) among wheat cultivars. Wheat cultivars had a distinct effect of reproductive period on English grain aphid.
Significant differences were observed in the adult longevity among wheat cultivars. The highest rate of adult longevity was on Vittorio (8.09 d) followed by Ceyhan-99 (7.81 d), Pehlivan (7.16 d), Stendal (5.63) and ġahinbey (5.05).
29
Table 3. Mean SE development, reproduction, fecundity per female, longevity of female Sitobion
avenaeat 25 °C on five varieties of wheat Wheat varieties Pre- reproduction Period Reproduction period Post-reproduction period Longevity of female No. offspring per female Ceyhan-99 Şahinbey Vittorio Stendal Pehlivan 2.31±0.46bc 0.83±0.25a 3.14±0.59c 1.09±0.22a 1.84±0.35ab 4.04±0.72a 3.00±0.49a 3.80±0.80a 3.63±0.83a 4.32±0.54a 1.45±0.22a 1.22±0.17a 1.14±0.22a 0.90±0.11a 0.96±0.14a 7.81±0.67c 5.05±0.39a 8.09±0.76c 5.63±0.75ab 7.16±0.65bc 11.86±2.78a 8.38±1.67a 9.28±2.06a 9.63±2.40a 12.12±1.80a
30 Life table parameters of Sitobion avenae
The gross reproduction rate (GRR), generation time (To), Net reproduction rate (Ro) and intrinsic increase rate (rm) of S. avenae were studied on five wheat cultivars are given in (Table 4). The highest value of GRR was found on Vittorio (37.1), and the lowest value was obtained on ġahinbey (10.55). Generation time (To) was recorded on wheat cultivars and To of Vittorio was found highest (16.59 d) followed by Ceyhan-99 (13.86 d), Pehlivan (11.53 d), Stendal (11.44 d) and ġahinbey (10.26 d). The net reproduction rate (Ro) (Number of progeny per female) had a highest value of Ro (8.7 ) on Pehlivan and the lowest value of Ro was on ġahinbey (4.34).
The intrinsic increase rate (rm) of Pelivan (0.1878) was obtained the highest value of rm because of the higher reproductive rate as in (Table 3), and the lowest value of rmwas found on Vittorio (0.1035).
31
Table 4. Life table parameters of female Sitobion avenae at 25 °C on five wheat varieties.
Wheat varieties GRR To Ro rm Ceyhan-99 Şahinbey Vittorio Stendal Pehlivan 25.83 10.55 37.1 27.38 30.4 13.86 10.26 16.59 11.49 11.53 7.34 4.34 5.57 5.97 8.71 0.143895 0.143154 0.153543 0.155472 0.187821
Means within a row with the same letter are not significantly different ( = 0.05, Duncan multiple range test).
GRR = The gross reproduction rate, To = Generation time, Ro = Net reproduction rate,
rm = Intrinsic rate of increase
The results of this study show the biological parameters of S. avenae on different wheat cultivars and the important impact on developmental time of immature stages, survival rate, fecundity and longevity of adult.
32 DISCUSSION
In the present study, Sitobion avenae reared under laboratory conditions, English grain aphid showed significant differences among wheat cultivars. The average (Percentage) of survival rates of S. avenae was between 93.84 % - 95.96 %, indicating the occurrence of resistance in some wheat cultivars to S. avenae, in total developmental times of nymphal instars, adult longevity and overall number of newborn offspring of S. avenae.
The mean of total nymphal developmental time, pre-reproductive rates and fecundity in a day was very similar to those obtained by (Özder, 2002), but the results on Pehlivan cultivar were different in her study. Total developmental times values of immature stages in this study were similar to that of reported by (Simon et al., 1991). The obtained of total nyphal developmental times of ġahinbey and Pehlivan cultivars were similar to that of reported by (Tofangsazi et al., 2012) but not the same aphid species (S. graminum) on different wheat cultivars. Also, the developmental times of immature stages of ġahinbey and Stendal were nearly the same to that of recorded by Descamps and Chopa (2011) reared R. padi on varied winter cultivars. It has been recorded nearly the same with our results in nymphal developmental time of S. graminum on wheat cultivars by Mojahed et al., 2013. The mean of the total fecundity of this aphid species was lower that investigated by Markkula and Myllymaki, 1963; Dean, 1974.
However, it was obtained occasionally very different rates during many experiments, and aphid clones placed in varied vegetative parts of cereal plants (Dean, 1974). And also the results of life table of this aphid were totally different than that of reported by (Huang et al., 2013). Nevertheless, aphid-host plant and interaction between aphid-host genotypes show the influences on fecundity of S. avenae (Griifths and Wratten, 1979; Gianoli et al., 1997; Lowe, 1980; Dixon, 1987; Radchenko, 1987, Soroka and Mackay, 1991). It was also estimated that cereal aphids reproduced more rapidly on the ears than the leaves of cereal (Vereijken, 1979; Acreman and Dixon, 1989).
33
The rate for English grain aphid was different among all plant cultivars in biological parameters (Table 4). Kennedy and Abou-Ghadir (1979) described the development period from first nymph instar to first reproduction time related to the grade of resistance or susceptibility since high grades of resistance augments reproduction compared with susceptible level.
The intrinsic rate increase (rm) of S. avenae obtained in this study on all wheat cultivars was dissimilar to that reported by (Sempruch et al., 2011) in different winter triticale. The value of (rm) was used to evaluate the level of fitness of many genotypes to aphids’ life span (Birch et al., 1963; Ohba, 1967; Ayaia, 1968). The survival rates and the intrinsic rate of increase (rm) value were lower on ġahinbey than that of the other wheat cultivars.
The results of this investigation showed that cultivar ġahinbey was resistant variety for S.
avenae. Havlikova (1995) found that antibiosis was recorded in Regina cultivar among
34 5. Conclusions
The findings of the present study showed that biological parameters and life history of traits of English grain aphid, Sitobion avenae, were significantly affected by the level of resistance of wheat varieties. During this study ġahinbey occurred resistant Sitobion
avenae because the total nymphal developmetal time, pre reproductive period and
longevity of female of Sitobion avenae were shortest on ġahinbey compared with the others four varieties of wheat. The study revealed an increasing nymphal developmental time and fecundity rate and increasing mortality rate. Analyzing and understanding of investigation of biological parameters of English grain showed a resistant and susceptible response to aphid populations. Biological parameters are considered the best strategy tactic for knowledge, information on population growth and representing a good tactic for integrated pest management (IPM). Knowledge ecology and biology of insects is also regarded as a main part of integrated pest management.
35 6. References
Abou–Elhagag, G. H. and N. A. Abdel–Hafez, 1998. Cereal aphids (Homoptera: Aphididae): Factors affecting their population on wheat in Upper Egypt. Assiut J.
Agric. Sci., 29: 241–52.
Acreman, S. J. and A. F. G. Dixon, 1989. The effects of temperature and host quality on the rate of increase of the grain aphid (Sitobion avenae) on wheat. Annals of Applied Biology 115: 3–9.
Acreman, T. M. and A. F. G. Dixon, 1985. Developmental patterns in the wheat and resistant to cereal aphids. Crop Protect. 4 (3): 322–328.
Aheer, G. M., A. Rashid, M. Afzal and A. Ali, 1993. Varietal resistance/susceptibility of wheat to aphids. Sitobion avenae F. and Rhopalosiphum rufiabdominalis Sesaski. J. Agric. Res. 31(3):307-311.
Aheer, G. M., M. Munir and A. Ali, 2007. Impact of weather factors on population of wheat aphids at Mandi Baha-ud Din District. J. Agric. Res. 45(1): 61-68.
Ahmad, F. and S. Nasir, 2001. Varietal resistance of wheat germplasm against wheat aphid (Sitobion avenae F.).Pakistan Entomol.,23: 5–7.
Ajayi, O. and A. M. Dewar, 1982. The effect of barley yellow dwarf virus on honeydew production by the cereal aphids, Sitobion avenae and Metopolophiumdirhodum. Ann. App\. Bio\. 100(2): 203-212.
Akça, Ġ., T. Ayvaz, E. Yazıcı, C. L. Smith and Hsin Chi, 2015. Demography and Population Projection of Aphis fabae (Hemiptera: Aphididae): with Additional Comments on Life Table Research Criteria. J. Econ. Entomol.,108 (4): 1466-78.
Akhtar, L. H., M. Hussain, R. M. Iqbal, M. Amer and A. H. Tariq, 2010. Losses in grain yield caused by Russian wheat aphid Diuraphis noxia (Mordvilko). Sarhad J.
36
Alkhedir, H., 2009. Molecular characterisation of Sitobion avenae F. clones and their
interaction with different host plants (Doctoral dissertation, PhD Dissertation,
University of Gottingen, Gottingen, Germany).
Alpan, M. and M. Engüzelö, 2015.Turkish flour yeast and ingredients promotion group. Wheat flour report.
Altay, F., 2012. Yield Stability of Some Turkish Winter Wheat (Triticum aestivum L.) Genotypes In The Western Transtional Zone Of Turkey.Turkish Journal of Field
Crops,17(2), 129-134.
Anonymous, 2012. Insect pest management in winter
cereals.https://www.daf.qld.gov.au/plants/field-crops-and-pastures/broadacre- field-crops/integrated-pest-management/ipm-information-by-crop/insect-pest-management-in-winter-cereals#Helicoverpa
Anonymous, 2014. Top 10 wheat production countries of the world.http://worldknowing.com/top-10-wheat-producing-countries-of-the-world/
Anonymous, 2015a. Wheat.https://en.wikipedia.org/wiki/Wheat
Anonymous, 2015b. International wheat production
statistics.https://en.wikipedia.org/wiki/International_wheat_production_statistics
Araya, J. E. and J. E. Foster, 1987. Laboratory study on the effects of barley yellow dwarf virus on the life cycle of Rhopalosiphum padi (L.). Z. Pflanzenkrank. Pflanzenschutz 94(6): 578-583.
Araya, J. E., J. E. Foster, and S. E. Cambron, 1987. A study of the biology of
Rhopalosiphum padi (Homoptera: Aphididae) in winter wheat in northwestern
37
Asin, L. and X. Pons, 2001. Effect of high temperature on the growth and reproduction of corn aphids (Homoptera: Aphididae) and implications for their population dynamics on the northeastern Iberian peninsula. Environmental
Entomology, 30(6), 1127-1134.
Aslam, M. U. H. A. M. M. A. D., M. U. H. A. M. M. A. D. Razaq, F. A. H. E. E. M. Ahmad, M. U. H. A. M. M. A. D. Faheem and W. A. H. E. E. D. Akhter, 2006. Population of aphid (Schizaphis graminum R.) on different varieties/lines of wheat (Triticum aestivum L.). Int J Agric Biol, 6, 974-977.
Awmack, C. S. and S. R. Leather, 2002. Host plant quality and fecundity in herbivorous insects. Annual review of entomology, 47(1), 817-844.
Ayaia, F.J., 1968. Genotype environment and population numbers. Science 162: 1453-1459.
Bale, J. S., 1996. Insect cold hardiness: a matter of life and death.
Basky, Zs., 2005. Aphids’ description, life cycle, damage and control.263 pp.
Bayaner, A., 2002. Wheat Sector in Turkey, Final Report. Ministry of Agriculture and Rural Affairs, Research Planning and Coordination Council.
Birch, L. C., T. Dobzanaky, P.O. Elliott, and R. C. Lewontin, 1963. Relative fitness of geographic races of Drosophila serrata. Evolution 17: 72-83.
Blackman, R.L. and V.F. Eastop, 1984. Aphids on the World’s Crops: An Identification and Information Guide. Wiley, Chichester, 466 pp.
Brabec, M., A. Honěk, S. Pekár and Z. Martinková, 2014. Population dynamics of aphids on cereals: digging in the time-series data to reveal population regulation caused by temperature.
38
Brewer, M., D. Mornhinweg and S. Huzurbazar, 1999. Compatibility of insect management strategies Diuraphis noxia abundance on susceptible and resistant barley in the presence of parasitoids.BioControl., 43(4): 479-491.
Cai, Q. N., X. M. Ma, X. Zhao, Y. Z. Cao and X. Q. Yang, 2009. Effects of host plant resistance on insect pests and its parasitoid: A case study of wheat aphid- parasitoid system. Biol. Control, 49(2): 134-138.
Carver, M., 1989. Biological control of aphids. pp. 141-165. In: A. K. Minks and P. Harrewijn (Editors), Aphids: Their Biology,Natural Enemies and Control. Volume.2C, 322 pp. Amsterdam, Elsevier.
Chen, J. L., J. R. Sun, H. J. Ding, H. X. Ni and X. F. Li, 1997. The resistant patterns and mechanism of biochemical resistance in various wheat cultivars (lines). Acta
Entomologica Sinica, 40:190-195.
Chi, H., 2015. TWOSEX-MSChart: A computer program for the age-stage, two-sex life table analysis. (http://140.120.197.173/Ecology/Download/TWOSEX-MSChart.zip) (accessed 25 June 2015).
Chongchuan, F and X. Jianye, 1987. Influence of temperature on the growth and development of Schizaphis graminum insects knowledge 24: 140-143.
Ciepiela, A. P., C. Sempruch, 1999. Effect of dihydroxyphenylalanine, ornithine and gamma-aminobutyric acid on winter wheat resistance to grain aphid. J. Appl. Ent. 123, 285–288.
Clough, M.S., J. S. Bale and R. Harrington, 1990. Differential cold hardiness in adults and nymphs of the peach–potato aphid Myzus persicae.Annals of Applied Biology 116, 1–9.
De Barro, P. J., 1992. The impact of spiders and high temperatures on cereal aphid (Rhopalosiphum padi) numbers in an irrigated perennial grass pasture in South Australia. Ann. Appl. Biol. 121: 19-26.
39
De Celis, V. R., D. I. R. C. E. U. Gassen, V. L. Valente and A. K. De Oliveira, 1997. Longevity, fecundity and embryogenesis in Brazilian Aphids. Pesquisa
agropecuaria brasileira, 32, 137-146.
Dean, G. J., 1974. Effect of temperature on cereal aphids Metopolophium dirhodum (Wlk.), Rhopalosiphum padi (L.) and Macrosiphum avenae (F.) (Hem., Aphididae). Bull. Entomol. Res. 63: 401-409.
Dedryver, C.A., 1989. A twelve year study of cereal aphids on winter wheat in Brittany. Pest status of Sitobion avenae F., Metopolophium dirhodum Wlk. and Rhopalosiphum padi L. during spring.IOBC/ WPRS Bull.12(1): 7–12.
Dedryver, C. A. and S. Tanguy, 1984. Biology of cereal aphids in western France. V. - Effect of sowing date of winter wheat on infestation of fields by Rhopalosiphum padi (L.), Sitobion avenae (F.) and Metopolophium dirhodum (Wlk.) and on their population development in spring. Agronomie, 4:711-719.
Descamps, L. R. and C. S. Chopa, 2011. Population growth of Rhopalosiphum padi L.(Homoptera: Aphididae) on different cereal crops from the semiarid pampas of Argentina under laboratory conditions. Chilean Journal of Agricultural
Research, 71(3), 390-394.
Di Pietro, J.P. and M. Akli, 1987. Effets des facteurs vari6tal et ph6nologique de diff6rents cultivars de b16 hiver sur le potentiel biotique de Sitobion avenae (E) en conditions c6ntrol6es. Bull, OILB/SROP X/1:166- 169.
Dixon, A. E. G., 1973. Biology of Aphids. The Institute of Biology.Studies in Biology No. 44.Edward Arnold Ltd., London, UK.
Dixon, A. E. G., 1987. Cereal aphids as an applied problem. Agric. Zool, Rev. 2:1-57.
Dixon, A. F. and G. W. Hopkins, 2010. Temperature, seasonal development and distribution of insects with particular reference to aphids.In Aphid Biodiversity
40
Dixon, A. F. G., 1998. Aphid ecology, 2nd ed. Chapman & Hall, London, United Kingdom.
Eleftherianos, I., P. Vamvatsikos, D. Ward and F. Gravanis, 2005. Changes in the levels of plant total phenols and free amino acids induced by two cereal aphids and effects on aphid fecundity. Journal of Applied Entomology, 130(1), 15-19.
Elek, H., P. Werner, L. Smart, R. Gordon-Weeks, M. Nádasy and J. Pickett, 2013. Aphid resistance in wheat varieties. Communications in agricultural and applied
biological sciences, 74(1), 233-241.
El-Ibrashy, M. T., S. El-Ziady and A. A. Riad, 1972. Laboratory studies on the biology of the corn leaf aphid, Rhopalosiphum maidis (Homoptera: Aphididae).
Entomol.Exp. Appl. 15: 166–174.
Elliott, N. C., R. W. Kieckhefer and D. D. Walgenbach, 1988. Effect of constant and fluctuating temperatures on developmental rates and demographic statistics for the corn leaf aphid (Homoptera: Aphididae). J. Econ. Entomol. 81: 1383–1389.
Emden van, F.H. and R. Harrington, 2007. Aphids as crop pests. Cromwell Press, Trowbridge, UK, p12-21.Entomol.93, 369-382.
Escobar, C. A. and H.M. Niemeyer, 1993.Potential of hydroxamic acid in breeding for aphid resistance in wheat. Acta Agriculture Scandinavica, Section B, Soil and Plant Science 43: 163-167.
Feng, M.G., R.M. Nowierski, R.E. Klein, A. L. Scharen and D. C. Sands, 1992. Spherical hyphal bodies of Pandora neoaphidis (Remaudière and Hennebert) Humber (Zygomycetes: Entomophthorales) on Acyrthosiphon pisum Harris (Homoptera: Aphididae): a potential overwintering form. Pan-Pacific Entomologist 68, 100– 104.