REGULAR ARTICLES
The effects of
Mannheimia haemolytica and albendazole
on marbofloxacin pharmacokinetics in lambs
Feray Altan1&Duygu Neval Sayin Ipek2&Orhan Corum3&Simten Yesilmen Alp4&Polat Ipek5&Kamil Uney6
Received: 4 September 2018 / Accepted: 12 June 2019 / Published online: 23 June 2019 # Springer Nature B.V. 2019
Abstract
The study aimed to define the effects of M. haemolytica and a single oral dose of albendazole on the single-dose pharmacoki-netics of marbofloxacin in lambs. The pharmacokinetic–pharmacodynamic integration of marbofloxacin was applied to describe a 3 mg/kg intramuscular dose in lambs. The 6 healthy and 12 naturally infected with M. haemolytica lambs (Akkaraman, males weighing 10–15 kg and aged 2–3 months) were used in this study. In the marbofloxacin group, 6 healthy lambs received marbofloxacin. In the albendazole group after 2 weeks washout period, the same animals received marbofloxacin on 1 h after albendazole. In the diseased marbofloxacin group, 6 lambs naturally infected with M. haemolytica received marbofloxacin. In the diseased albendazole group, 6 lambs naturally infected with M. haemolytica received marbofloxacin on 1 h after albendazole. The marbofloxacin and albendazole were administered each as a single dose of 3 mg/kg intramuscular and 7.5 mg/kg oral, respectively, in the respective groups. Plasma concentration of marbofloxacin was measured with HPLC-UV and pharmacoki-netic parameters were analyzed by non-compartmental model. Albendazole did not change the pharmacokipharmacoki-netic profiles of marbofloxacin in healthy and diseased lambs. However, M. haemolytica affected the pharmacokinetics of marbofloxacin in diseased lambs, AUC0–24/MIC90ratio was not found to be higher than 125, but Cmax/MIC90ratios was found to be higher than 10 for an MIC value of 0.25μg/mL in all groups. The marbofloxacin dose described in this study may not be effective for the treatment of infections due to M. haemolytica in lambs, with MIC≤ 0.25 μg/mL.
Keywords Albendazole . Lamb . Mannheimia haemolytica . Marbofloxacin . Pharmacokinetics
Introduction
Respiratory system diseases in sheep are common in all ages, particularly in 3- to 12-month-old lambs, and cause
economic loss because of excessive weight loss and in-fertility (Martin 1996; Bell 2008). Small ruminants are susceptible to respiratory infections caused by physio-logical stress and infectious agents such as bacteria, vi-ruses, and parasites (Brogden et al. 1998; Bell 2008). Mannheimia haemolytica (bacterium, M. haemolytica) and Dictyocaulus filaria (lungworm, D. filaria) are the most common agents of respiratory system diseases in sheep and lambs (Brogden et al. 1998; Bell 2008). Marbofloxacin shows excellent antibacterial activity agai nst M. haemolytica ( K r o e m e r e t al . 2 0 12) . Albendazole has low toxicity and high efficacy against D. filaria (Campbell 1990). Co-administration of marbofloxacin and albendazole can be given as an ex-ample of antimicrobial and anthelmintic drug combina-tion used by veterinary clinicians. Although informacombina-tion is available on the pharmacokinetic (PK) profiles of an-thelmintic and antimicrobial drugs in healthy animals (Abo El-Sooud 2003; Atef et al. 2010), there is no sim-ilar information for albendazole and marbofloxacin in diseased animals in the literature.
* Feray Altan
feray.altan@dicle.edu.tr
1
Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Dicle, Diyarbakir, Turkey
2
Department of Parasitology, Faculty of Veterinary Medicine, University of Dicle, Diyarbakir, Turkey
3 Department of Pharmacology and Toxicology, Faculty of Veterinary
Medicine, University of Kastamonu, Kastamonu, Turkey
4
Department of Microbiology, Faculty of Veterinary Medicine, University of Dicle, Diyarbakir, Turkey
5
Department of Physiology, Faculty of Veterinary Medicine, University of Dicle, Diyarbakir, Turkey
6 Department of Pharmacology and Toxicology, Faculty of Veterinary
Marbofloxacin is a third-generation fluoroquinolone anti-microbial drug with rapid bactericidal activity used and devel-oped only for veterinary medicine (Bryskier and Chantot
1995; Thomas et al. 2001). The chemical structure of marbofloxacin is responsible for its alternative PK properties compared with other quinolones (Fitton1992; Brown1996). The tendency of marbofloxacin to accumulate intracellularly in neutrophils facilitates its accumulation in infected tissues (Mahmood et al.2013). Marbofloxacin is a concentration-dependent antibacterial agent and has been used for the treat-ment of respiratory system diseases in cattle because of its broad spectrum and good efficacy (Thomas et al. 2001; Balikci et al.2008). There is information about PK profile of marbofloxacin in healthy lambs (Altan et al.2018), but re-search has been not found about the PK profile of the marbofloxacin in lambs naturally infected with M. haemolytica. However, Skoufos et al. demonstrated the clini-cal effects of marbofloxacin without performing a PK study for respiratory system diseases in lambs (Skoufos et al.2007). Physiopathological changes in disease states and increases in the co-administration of drugs during the treatment of dis-eases lead to the emergence of drug interactions that disrupt or enhance the expected effects of administered drugs (Kumar et al.2009; Palleria et al.2013; Alomar2014). In disease states, it is important to determine the extent to which a co-administered drug changes overall drug concentrations (Elmas et al.2008; Papich2017). Changes in drug concentra-tion may contribute to the emergence of antimicrobial resis-tance and adverse drug effects leading to inadequate treatment of bacterial infections (Toutain et al.2002; Cohen2006). For this reason, clinicians should pay attention to problems that may arise because of the simultaneous use of different drugs in the treatment of diseases.
Effective antimicrobial treatment needed to maximize efficacy and minimize the emergence of resistance can be achieved by integrating PK and pharmacodynamic (PD) parameters of a drug (Toutain et al.2002). The PK/ PD parameters should be considered to design optimal dosage strategies for antimicrobial drugs in veterinary medicine (McKellar et al.2004). The minimum inhibitory concentration (MIC) is the most important PD parameter used to determine the effectiveness of an antibacterial drug against an infectious agent (Craig 1998). The major PK/ PD parameters for concentration-dependent bactericidal agents such as fluoroquinolone are the area under the concentration-time curve/minimum inhibitor concentration ratio (AUC0–24/MIC) and the maximum concentration/ minimum inhibitor concentration ratio (Cm a x/MIC) (Jacobs 2001). Values of AUC0–24/MIC > 125 and Cmax/ MIC > 10 are deemed desirable to achieve effective treat-ment with antibiotics showing concentration-dependent action such as marbofloxacin (Aliabadi and Lees 2002; Sidhu et al.2011).
The aims of this study were the following: (1) determine the effects of M. haemolytica on the PK profile of marbofloxacin in lambs, (2) to assess the effects of albendazole on the PK profile of marbofloxacin in healthy lambs and lambs naturally infected with M. haemolytica, and (3) M. haemolytica.
Materials and methods
Drug
Marbofloxacin (Marbox® CEVA, Turkey) and albendazole (Vilazol® VILSAN, Turkey) were administered as single 3 mg/kg intramuscular and 7.5 mg/kg oral doses, respectively, according to group design. The intramuscular administration of marbofloxacin was injected in the semi-tendinous muscle. The oral dose of albendazole was administered by directly inserting a tablet into the mouth.
Lambs were sedated by administration of xylazine hydro-chloride (Rompun® BAYER, Turkey) and anesthetized with application of ketamine-HCl (Ketasol® INTERHAS, Turkey) prior to collection of bronchoalveolar lavage fluid (BALF).
Animals and husbandry
Six clinically healthy lambs (Akkaraman, weighing 10–15 kg and aged 2–3 months) and twelve lambs naturally infected with M. haemolytica (Akkaraman, weighing 10–15 kg and aged 2–3 months) were used for the study. The animals were obtained from the faculty farm, belonging to the Dicle University, Faculty of Veterinary Medicine. Animals were housed and fed daily with a rye grass and clover hay mix and a supplementary concentrate. Water was given ad libitum. The healthy and infected lambs were housed in separate sta-bles. There was no direct contact between healthy and infected group of lambs. At the end of the study lambs were treated to continue their normal life.
Prior to the start of this study, the lambs were assigned to healthy and diseased groups according to the clinical exami-nation and M. haemolytica isolation. Healthy groups were formed from lambs determined to be clinically healthy. Diseased groups were comprised with these lambs isolated M. haemolytica before marbofloxacin administration. Diseased lambs suffering from pneumonia as a result of clin-ical examination for sings such as coughing, presence of nasal discharge, abnormal sounds at lung auscultation floppy ears were selected from naturally infected flock.
Study design
In the marbofloxacin group (MB), six healthy lambs received marbofloxacin. In the albendazole group (ALMB) after a washout period of at least 2 weeks, the same animals received
marbofloxacin 1 h after albendazole. In the diseases marbofloxacin group (DMB), six lambs naturally infected with M. haemolytica received marbofloxacin. In the diseased albendazole group (DALMB), six lambs naturally infected with M. haemolytica were given marbofloxacin 1 h after albendazole. All research animals were housed in Faculty of Veterinary Medicine (University of Dicle), and all procedures were performed in that same facility.
Blood samples of 2 mL were collected into tubes contain-ing heparin (an anticoagulant) with a catheter placed in the right Vena jugularis. Samples were collected at 0 (prior to treatment), 5, 10, 15, 20, 25, 30, and 45 min and 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, 18, 24, and 36 h post-dosing. After centrifugation (10 min at 2000 g), plasma was stored at− 70 °C until assayed.
The M. haemolytica was isolated from the BALF of lambs with clinical signs of pneumonia in diseased groups for 7 days prior to the start of the study. The BALF samples were col-lected from pneumonic lambs using a previously reported method by Voigt et al. before administration to marbofloxacin (Voigt et al.2007). Lavage was performed 0.9% sodium chlo-ride solution. Samples were stored at− 70 °C until microbio-logical assayed.
M. haemolytica isolation
For determining the diagnostic specificity of M. haemolytica, BALF samples were cultured on sheep blood agar (Oxoid, Aalst, Belgium) and on MacConkey agar (Thermo Fisher Scientific, USA). Plates were incubated at 37 °C in 5% CO2 for 24 h. For the laboratory detection of bacteria, the identifi-cation of suspected bacteria colonies was achieved by obser-vation of colonial morphology under microscopy and the use of some biochemical tests: hemolysis, motility, indole forma-tion, litmus milk, glucose, saccharose, lactose, oxidase, and catalase. Assay for biochemical properties of the bacteria iso-lates were conducted according to MacFaddin’s method (MacFaddin2000). For reliable identification of results, the VITEC II compact (Biomereiux, France) was used.
HPLC assay of marbofloxacin
Plasma concentration of marbofloxacin was measured with high-performance liquid chromatography (HPLC)-ultraviolet (UV) and PK parameters were analyzed by a non-compartmental model.
Plasma marbofloxacin concentrations were determined using a previously reported modified HPLC method (Real et al.2011; Potter et al.2013). The marbofloxacin working solution was prepared in pure water (1 mg/mL) and stored at − 70 °C during the study. The calibration standards of blank plasma spiked with marbofloxacin were linear between 0.04 and 10μg/mL (r2> 0.99). The limits of quantification and
detection of the method were 0.04 and 0.02μg/mL, respec-tively. The interday percent coefficients of variation (CV) for different concentrations of marbofloxacin were as follows: 1.17% (0.1μg/mL), 1.03% (1 μg/mL), and 1.96% (10 μg/ mL). The intraday percent CV for different concentrations of marbofloxacin were as follows: 0.94% (0.1μg/mL), 0.35% (1μg/mL), and 0.48% (10 μg/mL).
Pure marbofloxacin substance was used for quality control. HPLC-grade acetonitrile, trimethylamine, ortho-phosphoric acid, and methanol were purchased from Sigma-Aldrich. The HPLC analysis was controlled using a Shimadzu-LC sys-tem (Shimadzu, Tokyo, Japan) equipped with a CBM-20A controller, LC-20AT pump, DGU-20A degasser, SIL-20A au-to-sampler, SPD-20A UV–VIS detector, and CTO-10A col-umn oven.
Chromatographic separation of marbofloxacin was con-ducted with a Gemini C18 column (250 × 4.6 mm; internal diameter, 5 μm; Phenomenex, Torrance, CA, USA) at 280 nm wave length. The mobile phase was composed of acetonitrile 17% and triethylamine (0.04%) + ortho-phosphoric acid (0.04%) (83%). Flow rate was set at 1 mL/ min. The total analysis time for samples was 25 min. Data were analyzed with a PC-controlled LC solution software pro-gram (Shimadzu, Japan).
Pharmacokinetic analysis
Plasma concentration versus time curves obtained in all ani-mals was fitted using the Phoenix WinNonlin V 6.1.0.173 software (Pharsight, Certara, St. Louis, MO, USA). The PK parameters such as AUC, volume of distribution (Vd), mean residence time (MRT), and total clearance (ClT) were deter-mined using a non-compartmental model analysis using WinNonlin. Elimination rate constant (λz) was estimated by least squares regression of the logarithm of plasma concentra-tion versus time curve over the terminal eliminaconcentra-tion phase. The peak plasma concentration (Cmax) and time to reach Cmax(Tmax) were obtained directly from the concentration-time data. The AUC from concentration-time zero to infinity (AUC0–∞) after a single dose was computed as the sum of AUC0–36and Clast/ λz. The terminal half-life (t1/2λz) was calculated as ln (2)/λz. The trapezoidal rule was used to calculate the area under the plasma concentration-time curve from time zero to the time of the last measurable concentration (AUC0-t).
Pharmacokinetic
–pharmacodynamic integration
The PK/PD integration was achieved by using the AUC0–24 and Cmaxvalues of marbofloxacin obtained in this study and the MIC values acquired from previously published studies for M. haemolytica (Sidhu et al.2010; Kroemer et al.2012).
Statistical analyses
Statistical analyses were carried out with the SPSS 22.0 (IBM Corp, Armonk, NY). Tmax was presented as a median. For time parameters (MRT and t1/2λz), the harmonic mean and the standard deviation (SD) were computed. Other PK param-eters (mean ± SD) were evaluated with one-way variance analysis (ANOVA), and statistical differences between groups were detected with a Duncan test. A p value of < 0.05 was accepted as statistically significant. Results for the main PK parameters are expressed as mean ± SD.
Results
M. haemolytica isolation
Before starting the study, 12 BALF samples collected from lambs which were determined to have pneumonia as a result of clinical examination were examined for bacterial isolation and identification. M. haemolytica were identified in these samples.
Pharmacokinetic analysis
The mean plasma concentration time curves and PK data for marbofloxacin in healthy and naturally infected lambs follow-ing the simultaneous administration of marbofloxacin and albendazole are shown in Fig.1and Table1. There were no statistical differences in the PK parameters of marbofloxacin between the healthy groups (ALMB and MB groups) (Table1). Theλzof marbofloxacin in MB group was signifi-cantly lower than in DALMB group (n:6, Table1). Values of MRT for marbofloxacin after intramuscular administration in healthy lambs (MB, 9.51 h; and ALMB, 9.44 h) were
statistically longer than in naturally infected with M. haemolytica (DMB, 8.36 h; and DALMB: 8.29 h). The ClTof marbofloxacin in DMB group (n:6, 130.19 mL/h/kg) was significantly higher than in MB group (117.55 mL/h/kg).
Pharmacokinetic
–pharmacodynamic integration
P K / P D d a t a ( A U C0– 2 4/ M I C a n d Cm a x/ M I C ) f o r marbofloxacin against M. haemolytica are presented in Table 2. Intramuscular administration of marbofloxacin (3 mg/kg) in MB group provided mean AUC0–24/MIC and Cmax/MIC values of 547.14 and 110.86, respectively, accord-ing to the MIC value of M. haemolytica (0.035μg/mL). For ALMB group, the AUC0–24/MIC and Cmax/MIC were 543.71 and 112.86 respectively, against MIC value of M. haemolytica (0.035μg/mL). The ratios of AUC0–24/MIC and Cmax/MIC were 534 and 130.86, respectively, in DMB group for MIC value of M. haemolytica (0.035μg/mL). In DALMB group, AUC0–24/MIC and Cmax/MIC values of 580 and 126.86, re-spectively, according to the MIC value of M. haemolytica (0.035 μg/mL). According to the MIC90 values of M. haemolytica (0.25 μg/mL), the values of AUC0–24/MIC in all groups were 76.60, 76.12, 74.76, and 81.20, respectively (Table2). The Cmax/MIC ratios of 15.52, 15.80, 18.32, and 17.76 were achieved to 10 in all groups against MIC90values of M. haemolytica (0.25μg/mL).
Discussion
Pharmacokinetic
Pathophysiological changes involving alterations of the alveolocapillary membrane and hemodynamics occurring in respiratory system diseases may lead to changes in the PK of
Fig. 1 Semi-logarithmic plasma concentration-time curves after intramuscular administration of marbofloxacin (3 mg/kg) alone and co-administered with albendazole (7.5 mg/kg) in healthy lambs and naturally infected lambs with M. haemolytica (n = 6) 0.01 0.1 1 10 0 6 12 18 24 30 36 Concentration (µg/mL) Time (hour) MB ALMB DMB DALMB
drugs (Taburet et al.1990). The efficacy of antibiotics used in diseased states can be determined by monitoring clinical re-sponses, but PK and PD parameters should be considered for the development of effective antibiotic treatments that maxi-mize efficacy and minimaxi-mize resistance development (Ambrose et al.2007). Studies based solely on monitoring clinical out-comes may underestimate the effects of highly efficacious drugs and overestimate the effectiveness of weak drugs (McKellar et al.2004).
No significant PK changes were found for marbofloxacin after its co-administration with albendazole in healthy and naturally infected with M. haemolytica. The most common PK interactions occur by inhibition, inactivation, and induc-tion of enzymes metabolizing drugs (Palleria et al.2013). The
inhibition or induction of enzymes responsible for the chem-ical modification of drugs results in changes in the PK profiles of drugs metabolized by these enzymes (Ito et al. 1998; Giacomini et al.2010). Marbofloxacin is metabolized by the liver and is excreted in urine (Schneider et al.1996). Previous studies have shown that albendazole affects cytochrome P450 enzyme activities and that this interaction may lead to changes in the clinical efficacy of other drugs metabolized in the same pathway (Escobar-Garcia et al.2001; Bapiro et al.2002). Abo El-Sooud found that albendazole may cause changes in the metabolism of enrofloxacin by inducing CYP1A (Abo El-Sooud2003). Our study revealed the absence of any effects by albendazole on the PK profiles of marbofloxacin in healthy and naturally infected lambs. Our results may be explained by the fact that albendazole and marbofloxacin are metabolized by different metabolic pathways in both healthy and naturally infected with M. haemolytica lambs.
The ClTsignificantly increased (p < 0.05) in lambs natural-ly infected with M. haemonatural-lytica (DMB) compared with healthy lambs (MB). Although statistically insignificant, we found that the t1/2 zof marbofloxacin was shorter in lambs naturally infected with M. haemolytica (DMB, 14.05 h; DALMB, 12.20 h) compared with healthy lambs (MB, 16.60 h; ALMB, 16.09 h; Table1). The elimination rate con-stants (λz) of marbofloxacin in groups of healthy lambs were significantly lower than in groups of lambs naturally infected with M. haemolytica (Table1). Compared with the healthy lambs groups (MB, 9.51 h; ALMB, 9.44 h), the values of MRT were significantly shorter following intramuscular ad-ministration of marbofloxacin in naturally infected lambs groups (DMB, 8.36 h; DALMB, 8.29 h). Critical infections, such as pneumonia, encourage hyperdynamic state showing that the increase in cardiac output and severe variations in blood flow of vital organs (Blot et al. 2014). The higher
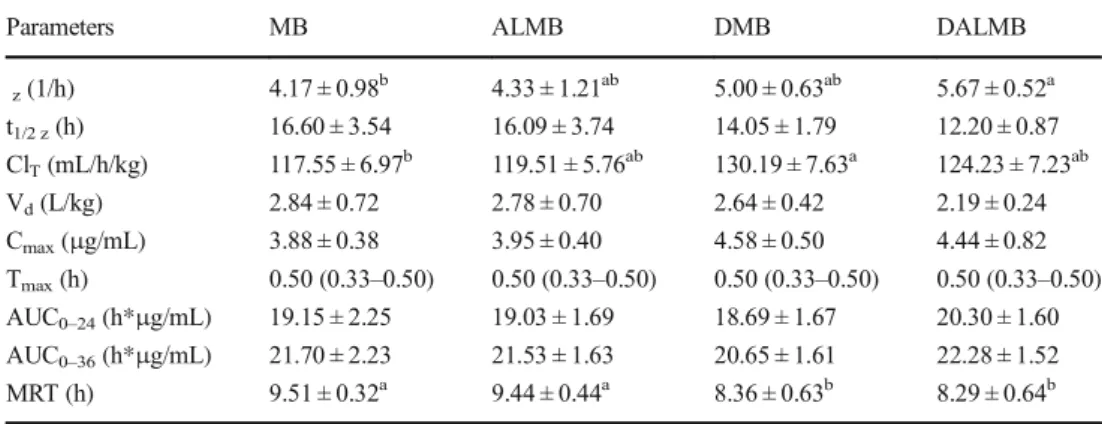
Table 1 Pharmacokinetics parameters (Mean ± SD) obtained after intramuscular administration of marbofloxacin (3 mg/kg) alone and coadministered with oral administration of albendazole (7.5 mg/kg) in healthy and naturally infected with M. haemolytica lambs (n = 6)
Parameters MB ALMB DMB DALMB
z(1/h) 4.17 ± 0.98b 4.33 ± 1.21ab 5.00 ± 0.63ab 5.67 ± 0.52a t1/2 z(h) 16.60 ± 3.54 16.09 ± 3.74 14.05 ± 1.79 12.20 ± 0.87 ClT(mL/h/kg) 117.55 ± 6.97b 119.51 ± 5.76ab 130.19 ± 7.63a 124.23 ± 7.23ab Vd(L/kg) 2.84 ± 0.72 2.78 ± 0.70 2.64 ± 0.42 2.19 ± 0.24 Cmax(μg/mL) 3.88 ± 0.38 3.95 ± 0.40 4.58 ± 0.50 4.44 ± 0.82 Tmax(h) 0.50 (0.33–0.50) 0.50 (0.33–0.50) 0.50 (0.33–0.50) 0.50 (0.33–0.50) AUC0–24(h*μg/mL) 19.15 ± 2.25 19.03 ± 1.69 18.69 ± 1.67 20.30 ± 1.60 AUC0–36(h*μg/mL) 21.70 ± 2.23 21.53 ± 1.63 20.65 ± 1.61 22.28 ± 1.52 MRT (h) 9.51 ± 0.32a 9.44 ± 0.44a 8.36 ± 0.63b 8.29 ± 0.64b
a, bVaried character in the same row are statistically significantly different (P < 0.05).
z, ClTVd,Cmax, AUC0–24,
and AUC0–36values are means ± SD (n = 6). MRT and t1/2 zare means harmonic means (n = 6). Tmaxare median
(range) (n = 6). z; elimination rate constant, t1/2 z; terminal half-life, CLT; total clearance, Vd; volume of
distribu-tion, Cmax; peak plasma concentration, Tmax; time to reach the maximum concentration, AUC; area under the
concentration-versus time curve; MRT, mean residence time; MB, healthy marbofloxacin group; ALMB, healthy albendazole + marbofloxacin; DMB, naturally infected with M. haemolytica marbofloxacin group; DALMB, naturally infected with M. haemolytica albendazole + marbofloxacin
Table 2 Integration of pharmacokinetic/pharmacodynamic data for marbofloxacin against Mannheimia haemolytica (M. haemolytica)
Bacteria Groups AUC0–24/
MIC Cmax/ MIC M. haemolytica MIC 0.035 μg/mL MBALMB 547.14543.71 110.86112.86 DMB 534 130.86 DALMB 580 126.86 MIC900.25 μg/mL MBALMB 76.6076.12 15.5215.80 DMB 74.76 18.32 DALMB 81.20 17.76
For calculations the pharmacokinetic parameters obtained in this study, MIC of 0.035μg/mL (Sidhu et al.2010) and MIC90of 0.25μg/mL
(Kroemer et al.2012) values in previously reports for M. haemolytica. MB, healthy marbofloxacin group; ALMB, healthy albendazole + marbofloxacin; DMB, naturally infected with M. haemolytica marbofloxacin group; DALMB, naturally infected with M. haemolytica albendazole + marbofloxacin group
plasma clearance and shorter terminal half-life obtained in naturally infected lambs indicate that the rate of elimination of marbofloxacin following the intramuscular route in natural-ly infected lambs is faster than in healthy lambs. These effects may be attributed to changes in pH in infected animals and therefore changes in the degree of marbofloxacin ionization. In addition, this alteration in the elimination of marbofloxacin may result from renal and/or hepatic modifications, such as the increase the blood flow in elimination organs, caused by M. haemolytica.
An inflammatory response is known to be triggered by M. haemolytica in sheep (Redondo et al.2011). The balance between pro- and anti-inflammatory agents (cytokines) re-leased by this response determines the outcome of the disease. Secreted agents affect the hepatic P450 enzyme system re-sponsible for drug metabolism, resulting in changes in drug clearance (Morgan2009). In particular, the administration of drugs with effects on pro-inflammatory cytokines may alter the effects of cytokines, resulting in increased drug clearance and rapid removal of the drug from the body (Morgan2009). Many fluoroquinolones inhibit the production of pro-inflammatory cytokines in pneumonia patients (Calbo et al.
2008). In a literature review, we found no studies on the im-munomodulatory activity of marbofloxacin in the case of an-imals with pneumonia. In anan-imals, fluoroquinolones have positive effects on the immune system in some diseases (Rougier et al.2008; Pomorska-Mól and Pejsak2015). In this study, marbofloxacin may have shown an immunomodulatory activity by inhibiting pro-inflammatory cytokines. This effect of marbofloxacin may have caused an increase in its elimina-tion by removing the inhibielimina-tion of the hepatic p450 enzyme system caused by M. haemolytica. Although we did not con-duct any immunological testing in this study, we propose that an immunological effect of marbofloxacin may exist and may regulate the protective immune response.
Pharmacokinetic
–pharmacodynamic
A PK/PD approach is advantageous for determining drug ef-fects and minimizing resistance to antimicrobial drugs (Papich
2014). Parameters such as AUC0–24/MIC and Cmax/MIC are indicative of the antibacterial effects of concentration-dependent antimicrobial drugs in animals with pneumonia (Greko et al.2003; Dorey et al.2017). The two PK/PD indi-ces, AUC0–24/MIC and Cmax/MIC, were determined using the PK parameters provided in the current study and MIC values previously reported for M. haemolytica strains in sheep (Sidhu et al.2010). To achieve high antimicrobial efficacy, AUC0–24/ MIC ratios of 100–125 and Cmax/MIC ratios of 8–10 have been recommended for marbofloxacin (McKellar et al.2004; Papich2014). However, AUC0–24/MIC ratios of 50 are suffi-cient for fluoroquinolones used for the treatment of infections associated with Gram-positive bacteria (Papich 2014).
Additionally, other studies have proposed an AUC0–24/MIC ratio of 125–250 for gram-negative bacteria, respectively (Drusano2007; Dorey et al.2017). The optimal dose should be determined relative to MIC90of the target pathogen in sheep (Sidhu et al.2010).
The MIC value of marbofloxacin against M. haemolytica has not been reported in lambs. For marbofloxacin, Sidhu et al. reported MIC value of 0.035 μg/mL, in sheep strains of M. haemolytica (Sidhu et al.2010). Data in Table2indicate that AUC0–24/MIC and Cmax/MIC ratios required to achieve an appropriate antimicrobial effect were very high for the MIC of M. haemolytica (0.035μg/mL) in all groups. For the MIC90 of M. haemolytica (0.25 μg/mL), the Cmax/MIC ratio was higher than 10, but the AUC0–24/MIC ratio was lower than 125 in all groups (Kroemer et al.2012). The calculated AUC0– 24/MIC and Cmax/MIC ratios for marbofloxacin derived from previously reported MIC values (Sidhu et al.2010), appeared to be higher than the required values to achieve high antimi-crobial efficacy for M. haemolytica in all groups. Nevertheless, for M. haemolytica with a high MIC value, the antimicrobial activity of marbofloxacin is not guaranteed. However, according to the MIC90 of M. haemolytica (0.25μg/mL), a dose of 3 mg/kg intramuscular marbofloxacin administered seems not to be effective in the treatment of pneumonia in lambs.
To the best of our knowledge, this is the first investigation describing the PK profiles of marbofloxacin in healthy lambs and lambs naturally infected with M. haemolytica. Marbofloxacin at 3 mg/kg administered through the intramus-cular route showed the fastest elimination rate in naturally infected lambs. Marbofloxacin may show not excellent anti-bacterial activity with low AUC0–24/MIC and high Cmax/MIC ratios for the MIC of M. haemolytica (0.25μg/mL). For these reasons, a dose of 3 mg/kg intramuscular marbofloxacin should not be considered in pneumonia caused by M. haemolytica. However, for future clinical studies on marbofloxacin, there is a need for more extensive PK and PD studies to determine MIC values for M. haemolytica iso-lated from lambs.
There was no change in the PK profile of marbofloxacin in lambs after administration of albendazole at 7.5 mg/kg dose. Therefore, there is no need to make any changes to the dosage of marbofloxacin co-administered with albendazole. However, further investigations of the pathways of hepatic metabolism and marbofloxacin elimination are required in healthy lambs and lambs naturally infected wi th M. haemolytica.
Acknowledgments This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Thanks are due to Ceva Animal Health Inc., Turkey, for supplying MB pure substance. Abstract presented form at the WVAC 2018 - 34th World Veterinary Association Congress Barcelona, Spain, May 2018.
Compliance with ethical standards
Competing interests The authors declare that they have no conflict of interest.
Ethics approval and consent to participate Animal experiments were carried out under the approval protocol of the Ethics Committee of the Dicle University Animal Experiments (Diyarbakir, Turkey, permit No 28/ 2014), which is in adherence with the Rules for the Working Procedures and Principles of the Ethical Committees of Animal Experiments and Animal Protection Act.
References
Abo El-Sooud, K., 2003. Influence of albendazole on the disposition kinetics and milk antimicrobial equivalent activity of enrofloxacin in lactating goats Pharmacological Research, 48, 389–395 Aliabadi, F.S. and Lees, P., 2002. Pharmacokinetics and pharmacokinetic/
pharmacodynamic integration of marbofloxacin in calf serum, exu-date and transuexu-date. Journal of veterinary pharmacology and thera-peutics, 25, 161–74
Alomar, M.J., 2014. Factors affecting the development of adverse drug reactions (Review article)
Altan, F., Corum, O., Corum, D.D., Atik, O. and Uney, K., 2018. Pharmacokinetics and bioavailability of marbofloxacin in lambs fol-lowing administration of intravenous, intramuscular and subcutane-ous Small Ruminant Research, 159, 5–10
Ambrose, P.G., Bhavnani, S.M., Rubino, C.M., Louie, A., Gumbo, T., Forrest, A. and Drusano, G.L., 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 44, 79–86
Atef, M., El-Gendi, A.Y.I., Amer, A.M. and El-Aty, A.M.A., 2010. Effect of three anthelmentics on disposition kinetics of florfenicol in goats Food and Chemical Toxicology, 48, 3340–3344
Balikci, E., Kizil, O., Karapinar, T., Karahan, M., Ozdemir, H. and Dabak, M., 2008. Efficacy of marbofloxacin for naturally occurring contagious caprine pleuropneumonia Small Ruminant Research, 77, 75–79
Bapiro, T.E., Andersson, T.B., Otter, C., Hasler, J.A. and Masimirembwa, C.M., 2002. Cytochrome P450 1A1/2 induction by antiparasitic drugs: dose-dependent increase in ethoxyresorufin O-deethylase ac-tivity and mRNA caused by quinine, primaquine and albendazole in HepG2 cells. European Journal of Clinical Pharmacology, 58, 537– 542
Bell, S., 2008. Respiratory disease in sheep: 1. Differential diagnosis and epidemiology. In Practice, 30, 200–207
Blot, S.I., Pea, F. and Lipman, J., 2014. The effect of pathophysiology on pharmacokinetics in the critically ill patient— Concepts appraised by the example of antimicrobial agents Advanced Drug Delivery Reviews, 77, 3–11
Brogden, K.A., Lehmkuhl, H.D. and Cutlip, R.C., 1998. Pasteurella haemolytica complicated respiratory infections in sheep and goats Veterinary Research, 29, 233–254
Brown, S.A., 1996. Fluoroquinolones in animal health J Vet Pharmacol Ther, 19, 1–14
Bryskier, A. and Chantot, J.-F., 1995. Classification and Structure-Activity Relationships of Fluoroquinolones Drugs, 49, 16–28 Calbo, E., Alsina, M., Rodríguez-Carballeira, M., Lite, J. and Garau, J.,
2008. Systemic expression of cytokine production in patients with severe pneumococcal pneumonia: Effects of treatment with a β-lactam versus a fluoroquinolone Antimicrobial Agents and Chemotherapy, 52, 2395–2402
Campbell, W.C., 1990. Benzimidazoles: veterinary uses. Parasitology today (Personal ed.), 6, 130–133
Cohen, R., 2006. Approaches to reduce antibiotic resistance in the com-munity. The Pediatric infectious disease journal, 25, 977–980 Craig, W.A., 1998. State-of-the-Art Clinical Article: Pharmacokinetic/
Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men Clinical Infectious Diseases, 26, 1–10
Dorey, L., Pelligand, L. and Lees, P., 2017. Prediction of marbofloxacin dosage for the pig pneumonia pathogens Actinobacillus pleuropneumoniae and Pasteurella multocida by pharmacokinetic/ pharmacodynamic modelling BMC Veterinary Research, 13, 209– 219
Drusano, G.L., 2007. Pharmacokinetics and Pharmacodynamics of Antimicrobials Clinical Infectious Diseases, 45, 89–95
Elmas, M., Yazar, E., Uney, K., Er Karabacak A., and Traş, B., 2008. Pharmacokinetics of enrofloxacin and flunixin meglumine and in-teractions between both drugs after intravenous co-administration in healthy and endotoxaemic rabbits Veterinary Journal, 177, 418–424 Escobar-Garcia, D., Camacho-Carranza, R., Pérez, I., Dorado, V., Arriaga-Alba, M. and Espinosa-Aguirre, J.J., 2001. S9 induction by the combined treatment with cyclohexanol and albendazole Mutagenesis, 16, 523–528
Fitton, A., 1992. The Quinolones: An Overview of their Pharmacology Clinical Pharmacokinetics, 22, 1–11
Giacomini, K.M., Huang, S.-M., Tweedie, D.J., Benet, L.Z., Brouwer, K.L.R., Chu, X., Dahlin, A., Evers, R., Fischer, V., Hillgren, K.M., Hoffmaster, K.A., Ishikawa, T., Keppler, D., Kim, R.B., Lee, C.A., Niemi, M., Polli, J.W., Sugiyama, Y., Swaan, P.W., Ware, J.A., Wright, S.H., Wah Yee, S., Zamek-Gliszczynski, M.J. and Zhang, L., 2010. Membrane transporters in drug development Nature Reviews Drug Discovery, 9, 215–236
Greko, C., Finn, M., Franklin, A. and Bengtsson, B., 2003. Pharmacokinetic/pharmacodynamic relationship of danofloxacin agaisnt Mannheimia haemolytica in a tissue-cage model in calves Journal of Antimicrobial Chemotherapy, 52, 253–257
Ito, K., Iwatsubo, T., Kanamitsu, S., Ueda, K., Suzuki, H. and Sugiyama, Y., 1998. Prediction of pharmacokinetic alterations caused by drug-drug interactions: metabolic interaction in the liver. Pharmacological reviews, 50, 387–412
Jacobs, M.R., 2001. Optimisation of antimicrobial therapy using pharma-cokinetic and pharmacodynamic parameters. Clinical Microbiology and Infection, 7(11), 589–596.
Kroemer, S., Galland, D., Guérin-Faublée, V., Giboin, H. and Woehrlé-Fontaine, F., 2012. Survey of marbofloxacin susceptibility of bacte-ria isolated from cattle with respiratory disease and mastitis in Europe. The Veterinary record, 170(2), 53
Kumar, S., Kumar, S., Kumar, V., Singh, K.K. and Roy, B.K., 2009. Pharmacokinetic studies of levofloxacin after oral administration in healthy and febrile cow calves Veterinary Research Communications, 33, 887–893
MacFaddin, J., 2000. Biochemical Tests for the Identification of Aerobic Bacteria Clinical Microbiology Procedures Handbook, 3rd Edition, 503–642
Mahmood, A., Grice, J.E., Roberts, M.S. and Prow, T.W., 2013. Feasibility of multiphoton microscopy-based quantification of anti-biotic uptake into neutrophil granulocytes. Journal of biomedical optics, 18(7), 076003–1-6
Martin, W.B., 1996. Respiratory infections of sheep. Comparative immu-nology, microbiology and infectious diseases, 19, 171–179 McKellar, Q.A., Sanchez Bruni, S.F. and Jones, D.G., 2004.
Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. Journal of veterinary pharmacol-ogy and therapeutics, 27, 503–514
Morgan, E.T., 2009. Impact of Infectious and Inflammatory Disease on C y t o c h r o m e P 4 5 0 – Mediated Drug Metabolism and Pharmacokinetics Nature, 85, 434–438
Palleria, C., Di Paolo, A., Giofrè, C., Caglioti, C., Leuzzi, G., Siniscalchi, A., De Sarro, G. and Gallelli, L., 2013. Pharmacokinetic drug-drug interaction and their implication in clinical management
Papich, M.G., 2014. Pharmacokinetic–pharmacodynamic (PK–PD) modeling and the rational selection of dosage regimes for the pru-dent use of antimicrobial drugs Veterinary Microbiology, 171, 480– 486
Papich, M.G., 2017. Ciprofloxacin Pharmacokinetics in Clinical Canine Patients Journal of Veterinary Internal Medicine, 31(5), 1508–1513 Pomorska-Mól, M. and Pejsak, Z., 2015. Effect of therapeutic doses of enrofloxacin on circulating lymphocyte subpopulations in pigs Bulletin of the Veterinary Institute in Pulawy, 59(2), 287–293 Potter, T., Illambas, J., Pelligand, L., Rycroft, A. and Lees, P., 2013.
Pharmacokinetic and pharmacodynamic integration and modelling of marbofloxacin in calves for Mannheimia haemolytica and Pasteurella multocida Veterinary Journal, 195, 53–58
Real, R., Egido, E., Pérez, M., González-Lobato, L., Barrera, B., Prieto, J.G., Alvarez, A.I. and Merino, G., 2011. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the secretion of danofloxacin into milk: interaction with ivermectin. Journal of vet-erinary pharmacology and therapeutics, 34, 313–321
Redondo, E., Gázquez, A., García, A., Vadillo, S. and Masot, A.J., 2011. Dominant expression of interleukin-8 vs interleukin-1β and tumour necrosis factor alpha in lungs of lambs experimentally infected with Mannheimia haemolytica New Zealand Veterinary Journal, 59, 225–232
Rougier, S., Vouldoukis, I., Fournel, S., Pérès, S. and Woehrlé, F., 2008. Efficacy of different treatment regimens of marbofloxacin in canine visceral leishmaniosis: A pilot study Veterinary Parasitology, 153, 244–254
Schneider, M., Thomas, V., Boisrame, B. and Deleforge, J., 1996. Pharmacokinetics of marbofloxacin in dogs after oral and parenteral administration Journal of Veterinary Pharmacology and Therapeutics, 19, 56–61
Sidhu, P.K., Landoni, M.F., Aliabadi, F.S. and Lees, P., 2010. PK-PD integration and modeling of marbofloxacin in sheep. Research in veterinary science, 88, 134–141
Sidhu, P.K., Landoni, M.F., Aliabadi, M.H.S., Toutain, P.L. and Lees, P., 2011. Pharmacokinetic and pharmacodynamic modelling of marbofloxacin administered alone and in combination with tolfenamic acid in calves. Journal of veterinary pharmacology and therapeutics, 34, 376–387
Skoufos, J., Christodoulopoulos, G., Fragkou, I.A., Tzora, A., Gougoulis, D.A., Orfanou, D.C., Tsiolaki, K. and Fthenakis, G.C., 2007. Efficacy of marbofloxacin against respiratory infections of lambs Small Ruminant Research, 71, 304–309
Taburet, A.-M., Tollier, C. and Richard, C., 1990. The Effect of Respiratory Disorders on Clinical Pharmacokinetic Variables Clinical Pharmacokinetics, 19, 462–490
Thomas, E., Caldow, G.L., Borell, D. and Davot, J.L., 2001. A field comparison of the efficacy and tolerance of marbofloxacin in the treatment of bovine respiratory disease Journal of Veterinary Pharmacology and Therapeutics, 24, 353–358
Toutain, P.L., Del Castillo, J.R.E. and Bousquet-Mélou, A., 2002. The pharmacokinetic-pharmacodynamic approach to a rational dosage regimen for antibiotics. Research in Veterinary Science, 73(2), 105–114
Voigt, K., Brügmann, M., Huber, K., Dewar, P., Cousens, C., Hall, M., Sharp, J.M. and Ganter, M., 2007. PCR examination of bronchoal-veolar lavage samples is a useful tool in pre-clinical diagnosis of ovine pulmonary adenocarcinoma (Jaagsiekte) Research in Veterinary Science, 83, 419–427
Publisher’s note Springer Nature remains neutral with regard to jurisdic-tional claims in published maps and institujurisdic-tional affiliations.