www.revportpneumol.org
ORIGINAL
ARTICLE
Evaluation
of
vascular
endothelial
growth
factor-A
and
Endostatin
levels
in
induced
sputum
and
relationship
to
bronchial
hyperreactivity
in
patients
with
persistent
allergic
rhinitis
monosensitized
to
house
dust
夽
˙I.
Yılmaz
a,∗,
N.
Bayraktar
b,
K.
Ceyhan
c,
D.
Sec
¸il
a,
S.
Yüksel
d,
Z.
Mısırlıgil
a,
S.
Bavbek
aaAnkaraUniversity,SchoolofMedicine,DepartmentofChestDiseases,DivisionofImmunologyandAllergicDiseases,Ankara, Turkey
bBaskentUniversity,DepartmentofBiochemistry,Ankara,Turkey
cAnkaraUniversity,SchoolofMedicine,DepartmentofPathology,DivisionofClinicalCytology,Ankara,Turkey dAnkaraUniversity,DepartmentofBiostatistics,Ankara,Turkey
Received25December2014;accepted30April2015 Availableonline4June2015
KEYWORDS Bronchial hyperreactivity; Endostatin; Eosinophil; Inducedsputum; Persistentallergic rhinitis; Vascularendothelial growthfactor; Vascularremodeling Summary
Background: Studiesaboutthepathogenesisofbronchialhyperreactivity(BHR)inpatientswith persistentallergicrhinitis(PAR)anditsrelationshipwithlowerairwayremodelingareextremely limited.
Objective: Thisstudyevaluatedbronchialvascularremodelingviathemeasurementof angio-genic factor, vascular endothelial growth factor-A (VEGF-A), and anti-angiogenic factor, Endostatin,andevaluatedtheirrelationshipwithBHRinpatientswithPAR.
Methods:The study group consistedof 30 patients with PARmonosensitized to housedust mitesand14non-allergichealthycontrols.Allsubjectsunderwentinducedsputumand metha-choline(M)bronchialprovocationtests.VEGF-AandEndostatinlevelsweremeasuredbyELISA ininducedsputumsupernatants.
Results:The percentages of eosinophils in induced sputum were significantly increased in patients with PAR compared with healthy controls. There were no significant differences between patients with PAR and healthy controls in terms of levels of VEGF (37.9pg/ml, min---max: 5---373pg/ml vs. 24.9, min---max: 8---67pg/ml, p=0.8 respectively), Endostatin
夽 Thestudywasapprovedbythelocalethicscommittee,andwritteninformedconsentwasobtained.ThestudywassupportedbyAnkara
UniversityResearchFund(projectnumber:09B3330019).
∗Correspondingauthor.
E-mailaddress:insu2004@yahoo.com(˙I.Yılmaz). http://dx.doi.org/10.1016/j.rppnen.2015.04.006
(532.5pg/ml,min---max: 150---2125pg/ml vs.644,min---max:223---1123pg/ml, p=0.2 respec-tively) and VEGF/Endostatinratio (0.057 vs. 0.045,p=0.8 respectively). In addition,there werenosignificantdifferencesbetweenpatientswhoareBHRpositive(n=8),ornegativeto M(n=22)intermsoflevelsofVEGF,EndostatinandVEGF/Endostatinratioandnocorrelations amongvalueofPD20toMandlevelsofVEGF,EndostatinandVEGF/Endostatinratio.
Conclusion:WeconcludethatVEGF-AandEndostatindidnotdifferbetweenpatientswithPAR andhealthycontrolsregardlessofBHRtoM.
©2014SociedadePortuguesadePneumologia.PublishedbyElsevierEspaña,S.L.U.Allrights reserved.
Introduction
The presence of inflammation and airwayremodeling are cornerstonesinthepathogenesisofasthma.1,2Angiogenesis
has recently attracted considerable attention as a com-ponentof airwayremodeling in bronchial asthma. Oneof the key molecules for angiogenesis is VEGF; it is widely expressedwithinmanyhighlyvascularizedorgansincluding thelungsandisapotentinducerofendothelialcellgrowth.3
Vascularremodelingandincreasedexpressionofassociated growthfactorssuch asVEGF arewell-recognizedfeatures of asthma.4,5 Endostatin is a strong endogenous inhibitor
ofangiogenesisandisproduced byvarioustypesof cells.6
Endostatinspecificallyinhibitsendothelial cellgrowthand migrationanddirectlyantagonizesthebiologicaleffectsof VEGF.7The vascularcomponentofremodelingisregulated
byabalancebetweenangiogenicandanti-angiogenic fac-tors.However,therearenodataregardingthebalanceof majorangiogenic andanti-angiogenic factorsinthelower airwaysofpatientswithallergicrhinitis(AR)without con-comitantasthma.
AR,whichisparticularlyassociatedwithbronchial hyper-reactivity(BHR),is considered asarisk factor for asthma development.8,9 The mechanism of BHR in AR is notfully
understoodanditisnotknownwhethertheBHRinasthma and AR have the same pathophysiologies. Studies on the pathogenesisofBHRinpatientswithARanditsrelationship withlower airway remodeling areextremely limited.10---13
In ourfirst trial, we evaluatedbronchial vascular remod-eling and its relationship with BHR via measurement of VEGF-A and Endostatin levels in allergic rhinitis patients monosensitizedtopollen.10 Inthepresentstudy,bronchial
vascularremodelingparametersandtheirrelationshipwith BHRwereevaluatedbymeasuringthesame angiogenic/anti-angiogenicfactorsinpatientswithpersistentallergicrhinitis (PAR).
Methods
Subjects
Inclusioncriteriaforpatientswithrhinitiswereasfollows: (1)ahistoryofpersistentrhinitiswithoutcough,wheezing, orshortnessofbreathduringnaturalexposure,(2)positive
skin test to house dust mites only, (3) baseline forced expiratory volumein 1second (FEV1)greater than 80% of
predictedvalue.Pulmonaryfunctiontests,Bronchial Provo-cationTest(BPT)tomethacholine(M)andinducedsputum were performed. All subjects denied any past or present symptomssuggestiveofasthmaincludingintermittent dys-pnea,wheezing,orarecurrentcough,andanyrespiratory infectionduringthemonthprecedingthisstudy.Control sub-jectshadnormalspirometryandairwayresponsivenesstoM (PC20>16mg/ml),hadnegativeskinprick testtocommon
inhalantallergens,nohistoryofrhinitis,nocurrentorpast symptomssuggesting asthma, andnorespiratoryinfection duringthemonth beforeenrollment.Patientsandcontrols wereallnonsmokersandwerefreeofallsystemicdiseases andmalignancies.Nonehadeczemaorhistoryofnasal poly-posis. None of the patients had previously been treated withimmunotherapy.Allpatientsdiscontinuedtheir medi-cations(nasalsteroidandoralantihistamine)atleast1week beforeMBPT,but theywereallowed touse nasal antihis-taminesprayifnecessary.Patientswereclassifiedaccording to the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines.14 ThestudywasapprovedbyAnkara University
MedicalSchool’sEthicsCommittee(DecisionNo:152-4759).
Evaluationofatopy
Skinpricktestswereperformedbyusingacommonpanel, includingD.pteronyssinus,D.farinae,grass,tree,andweed pollens,cat,dog,Alternaria,Cladosporium,andcockroach allergen extracts (Allergopharma, Stockholm, Sweden). The positive and negative controls used were histamine (10mg/mL)andphenolatedglycerolsaline,respectively.A meanwhealdiameterof3mmorgreaterthanthatobtained withthecontrolsolutionwasconsideredpositive.
Pulmonaryfunctiontestsandnonspecificbronchial
provocationtest
Pulmonary function tests (Flowhandy Zan 100 USB, Germany) were performed before sputum induction to determine baseline FEV1. BPT using M was performed
between 8:30 and 10:30 AM according to the method described byCockcroft etal.15 After inhalationof
from 0.25 to 16mg/mL diluted in physiologic saline. The challengewasstopped whenthe FEV1 decreased by more
than 20% from the post saline level or when the highest concentrationofMhadbeenadministered.The resultwas expressedasPC20 M. The PC20 Mwascalculated fromthe
logconcentrationresponsecurvebylinearinterpolationof thetwolastpoints.APC20 Mofmorethan 16mg/mLwas
acceptedasthecutoffpoint.16
Sputuminductionandprocessing
Sputum induction was performed between 9 and 10 AM accordingtothemethodproposedbyPizzichini etal.and slightlymodifiedandadaptedaccordingtoPavordetal.17,18
Sputuminductionwasappliedatleastthreedaysafterthe MBPT.Beforeinhalation of hypertonic salinesolution, all patients inhaled 2puffs of salbutamol200g(VentolinTM,
GSK, UK) administered through a metered dose inhaler andunderwentspirometry10minlater.Thenanaerosolof sterile3%salinesolutionwasgeneratedbyanultrasonic neb-ulizer(outputsetat1ml/min,OmronNE-U17,Japan) and inhaledfor7minthroughamouthpiecewithoutavalveor noseclip.Ifthepatientcouldnotexpectorateatthisstage, theconcentrationofsalinewasincreasedgradually accord-ingtothedecreaseinFEV1measurementsfrombaseline.In caseofalessthan10%decreaseinFEV1,theconcentration
ofsalinewasincreasedfrom3%to4%andthento5%. Spu-tuminductionwasperformedthreetimes.Aftereachperiod ofinhalation,patientswereaskedtorinsetheirmouthsand throatscarefully,swallowthewater,andblowingthenose beforeexpectorationtominimizecontaminationwithsaliva andpostnasaldrip.Theywereencouragedtocoughdeeply at 3min intervals thereafter. The sputum was collected into a container. The collected sputum was pooled and immediately processed.The volume of theentire sputum samplewasdetermined,andanequalvolumeof0.1% dithio-threitol (Sputolysin R; Calbiochem, San Diego, CA, USA) was added. The sputum samples were mixed gently with avortexmixerandincubatedfor15minatroom tempera-turetoensurecompletehomogenization. Sputumviability wasdeterminedwiththetrypanblueexclusionmethodto ensurethatviabilitywasadequateandthenfilteredsputum wascentrifugedat 450×gfor10min(Rotina38RHettich, Germany).The resultingcell pellets wereresuspended in phosphatebuffersaline.Atotalcellcountwascarriedout usingahemocytometerandcellconcentrationswerethen adjustedto1.0×106cells/ml.Slideswerestainedwith
May-Grünwald---Giemsa stain for differential cell counts which wereperformedbythecountingof400nonsquamouscellsby acytologistinamannerblindtoclinicaldetails.The super-natant was stored at −80◦C (Sanyo freezer MDF-U3086S, Japan)forsubsequentassaysforVEGFandEndostatin.
Inflammatorycellcountsandangiogenicmediators
ininducedsputum
Fourhundredcellswerecountedineachslide,and inflam-matorycells(macrophages,neutrophils,lymphocytes,and eosinophils) were determined aspercentages of the total cellsusinglightmicroscopy(400×).
Vascularendothelialgrowthfactor(HumanVEGF-AELISA, BenderMedSystemsGmBH,Vienna,Austria)andEndostatin (Quantikine®,HumanEndostatinImmunoassay,R&Dsystem
Inc.,Minneapolis,USA)weremeasuredbyELISAusinga spe-cificELISAkitaccordingtothemanufacturer’sinstructions ininducedsputumsupernatant.VEGF-AandEndostatin con-centrationswerequantitatedbycomparisonwithastandard curve generated using recombinant. The detection limits were as follows: VEGF-A 7.9pg/ml, Endostatin 23pg/ml. Theintra- andinter-assayvariabilitieswere, respectively, VEGF-A6.8and8.3%,Endostatin6.9and7.9%.
Statistics
Dataare expressed as median and min---max. Differences amonggroups were examined by means of Kruskal---Wallis andMann---WhitneyU-tests.Thesignificanceofcorrelations wasevaluatedbydeterminingtheSpearman’srho correla-tioncoefficients.Asignificancelevelwastakenas0.05while testingthehypothesis.DatawereanalyzedusingSPSSv.11.5 (SPSSInc.,Chicago,IL,USA).
Results
A total of 42 patients with PAR and 22 healthy con-trols were included in this study. All patients had severe persistent allergic rhinitis according to the current ARIA classification.14Sufficientsputumsampleswereprovidedin
30ofthe42PARpatients(F/M:21/9,meanage:31.9±11.4 years) and 14 of the 22 controls (F/M: 5/9, mean age: 30.6±6.3 years). There were no significant differences between patients and healthy controls in terms of age, FEF25---75,andFEV1value, weightof theentiresputumand
cellviability,butfemalegenderwassignificantlyhigherin thePARgroup(p=0.049)thaninthecontrols.Cellviability was>50%intheallsubjects.Eight PARpatients,butnone ofthecontrols,werepositiveforPC20M(PC20M<16mg/ml).
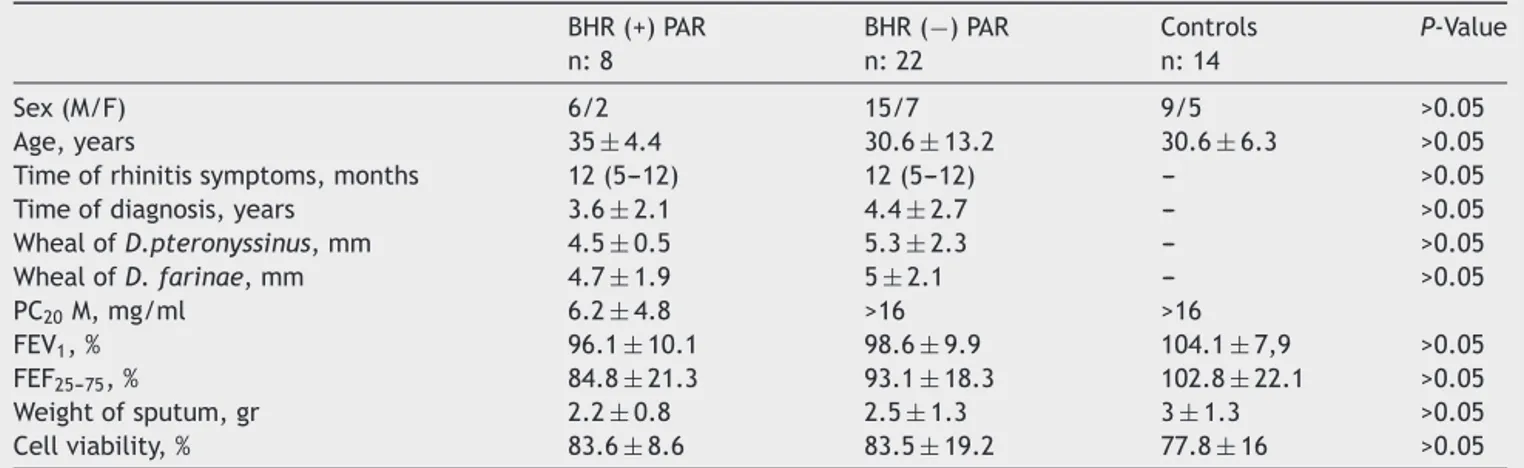
Thepatientsweredividedintotwogroupsaccordingtothe presenceorabsenceofBHR.Therewerenosignificant dif-ferencesbetweenpatientswithorwithoutBHRandcontrols intermsofsex,age,timeofrhinitissymptomsand diagno-sis,FEF25---75,andFEV1 value, weightoftheentire sputum
andcellviability(Table1).
Asignificantlygreaternumberofeosinophilswerefound in the sputum of PAR patients comparedto the nonaller-gic controls, their median (min---max) percentage counts being0.5(0---7)and 0(0---0.2)(p<0.001), respectively.No significant differences wereobserved for other cell types betweencontrols andpatients withPAR.The percentages of eosinophils in the induced sputum were significantly increasedinPARpatientswithBHRcomparedtoPARpatients without BHR and controls (p<0.001). No significant dif-ferences were observed for other cell types among the threegroups(Table2).Therewasnosignificantcorrelation betweenthenumberofeosinophilsandPC20Mvalue.
ThemedianlevelsofVEGFwerenotstatisticallyhigher in PAR patients than in healthy controls (37.9pg/ml, min---max: 5---373pg/ml vs. 24.9, min---max: 8---67pg/ml,
p=0.8respectively).Similarly, themedian levelsof Endo-statinwerenotsignificantlyhigherinpatientswithPARthan inhealthycontrols(532.5pg/ml,min---max:150---2125pg/ml
Table1 Patients’demographic,clinical,functionalrespiratory,andinducedsputumdata. BHR(+)PAR n:8 BHR(−)PAR n:22 Controls n:14 P-Value Sex(M/F) 6/2 15/7 9/5 >0.05 Age,years 35±4.4 30.6±13.2 30.6±6.3 >0.05
Timeofrhinitissymptoms,months 12(5---12) 12(5---12) --- >0.05
Timeofdiagnosis,years 3.6±2.1 4.4±2.7 --- >0.05
WhealofD.pteronyssinus,mm 4.5±0.5 5.3±2.3 --- >0.05 WhealofD.farinae,mm 4.7±1.9 5±2.1 --- >0.05 PC20M,mg/ml 6.2±4.8 >16 >16 FEV1,% 96.1±10.1 98.6±9.9 104.1±7,9 >0.05 FEF25---75,% 84.8±21.3 93.1±18.3 102.8±22.1 >0.05 Weightofsputum,gr 2.2±0.8 2.5±1.3 3±1.3 >0.05 Cellviability,% 83.6±8.6 83.5±19.2 77.8±16 >0.05
Resultsareexpressedasmeans±SDforage,timeofdiagnosis,whealofDerp,whealofDerf,FEV1,FEF25---75,weightofsputum,cell
viabilityandPC20 Mvaluesare expressedasgeometricmeans±SD.Results areexpressedasmedian(min---max)fortimeofrhinitis
symptoms.BHR(−)PAR,pesistentallergicrhinitispatientswithoutBHR;BHR(+)PAR,persistentallergicrhinitispatientswithBHR;M, methacholine.
Table2 CellcountsintheinducedsputumofcontrolandrhinitissubjectswithorwithoutBHR.
BHR(+)patientswithPAR BHR(−)patientswithPAR Controls Totalcellnumber/mLofsputum 1.1(0.4---3.8) 1.0(0.4---2.8) 1.2(0.4---3.6)
Eosinophils,%(min---max) 2.9(0.3---7)* 0.4(0---1.6)** 0(0---0.2)
Macrophages%(min---max) 62.5(52---86) 78(52---92) 70.9(42.5---92)
Neutrophils%(min---max) 30.5(10---42) 20(5.5---46) 26.7(7.5---54.8)
Lymphocytes%(min---max) 1(0---8) 1.2(0---3.8) 1(0.2---6)
* SignificantlydifferentfromthevaluesforcontrolsubjectsandPARpatientswithoutBHR(p<0.001).
**Significantlydifferentfromthevaluesforcontrolsubjects(p<0.001).Dataarepresentedasmedians,withinterquartilerangesin parentheses.
vs. 644, min---max: 223---1123pg/ml, p=0.2 respectively). The VEGF/Endostatin ratio wasnot statistically higher in patientswithPARthaninhealthycontrols(0.057vs.0.045,
p=0.8respectively).Theseresultsshowthattherewasno statisticaldifferencebetweengroups.Inaddition,thereno significantdifferencesbetweenpatientswhowereBHR pos-itive(n=8)ornegativetoM(n=22)andcontrolsintermsof levelsofVEGF,EndostatinandVEGF/Endostatinratio.There werenocorrelationsamongvalueofPD20toMandlevelsof VEGF,EndostatinandVEGF/Endostatinratio.
Discussion
This is the first study about the levels of VEGF-A, Endo-statin,andtheVEGF-A/Endostatinratioininducedsputum inpatientswithPAR.The levelsofVEGF-A,Endostatinand theratioofVEGF-A/Endostatinwerenotsignificantly differ-entbetweenpatientswithPARandhealthycontrols.There were no significant differences between patients with or withoutBHR toMandcontrols interms oflevelsofVEGF, EndostatinandVEGF/Endostatinratio.Theonlysignificant differencebetweenpatientsandcontrolswastheincreased numberofsputumeosinophilsinpatientswithPAR.
AlthoughtheinflammatorypathogenesisofBHRis under-stood in asthmatic patients, light has not been shed on the precise mechanism of BHR in patients with AR.11,19,20
Inourtrial,wedemonstratedhighereosinophilnumbersin patients with PAR compared tohealthy controls indepen-dentofBHR,asinpreviousstudiesincludingourfirststudy inallergicrhinitispatientsmonosensitizedtopollen.10,21---25
Also,thepercentageofeosinophils ininduced sputumwas found tobe significantly higher in PAR patients with BHR when compared withthose without BHR The presence of sputumeosinophils inourpatientsseemedtobelinkedto thepresenceofbothARandBHR.However,nocorrelation betweenPC20 Mvaluesandeosinophillevelswasobserved.
Thismayberelatedtoaprobablecorrelationbetween air-wayinflammationandotherindirectagentsofBPTsuchas adenosine butnotM. thelimitednumberof patientswith PARwithBHRmaybeotherfactoraffectingthelackof cor-relationbetweenPC20M.Ontheotherhand,eosinophilsmay
notbetheonlyinflammatorycellsresponsibleforthe devel-opmentofBHRwhenweconsiderthefactthattheanti-IL-5 antibodydecreasedperipheralbloodandsputumeosinophil levelsbutneverthelesshadnoeffectonBHRin asthmatic patients.26Thesefindingsimplythatotherpathologiesmay
underliethemainmechanismofBHRapartfromeosinophilic inflammationofthelowerairwaysinARpatients,asalready showninasthma.
Previousstudiesexaminingbronchialbiopsieshaveruled out inflammation as the single factor responsible for the developmentofBHRandhavesupportedstructuralchanges playaroleinthisprocess.27---29Eventhoughtherehavebeen
numerous studies reporting airway remodelingin asthma, onlya fewof themhave researchedairway remodelingin patientswithAR.Inthesestudiescollagendepositionwas demonstratedinbronchialbiopsiesandthiswasthoughtto beresponsiblefor RBMthicknessinARpatients.12,13 Some
ofthedrawbacksencounteredinthesestudiesincluded dif-ficultiesintechnicalapplication,lackofsufficientsamples ortheinabilityofsamplestomirrorthewholelowerairway whichinturncouldmaketheevaluationofinflammationand lower airway remodelingusing bronchial biopsies imprac-tical.Inthisrespect,current researchhasfocused onthe useofnon-invasivetechniquessuchasinducedsputumthat couldpossiblyreflectinflammationremodelingofthelower airway. A recent study carried out with induced sputum showed that AR patients have increased VEGF mRNA lev-elscomparedtohealthycontrols.11Inanotherrecentstudy
conductedwithinducedsputum,ARpatientswerefoundto have significantlyhigherVEGF levelscomparedtohealthy controls.25 Inthesestudies,thepossibilityofangiogenesis
inthelowerairwaytractofnonasthmaticpatientswithAR wasindicated.
Althoughangiogenesisisregulatedbyabalanceof angio-genicandanti-angiogenicfactors.3,30---32 therelativelevels
ofantiangiogenicfactors(Endostatin)inthelowerairways of patientswithARhave onlyrecently been evaluatedby ourgroup.10 Thisstudycompareddataobtainedduringthe
pollenseasonfrompatientsmonosensitizedtopollenwithor withoutBHRtoMandhealthycontrols;itwasfoundthatthe levelsofVEGF-AandtheratioofVEGF-A/Endostatinwere significantlyhigherandthelevelof endostatinwas signifi-cantlylowerinallergicrhinitispatientsmonosensitizedto pollenwithBHR.10However,contrarytoourexpectations,in
thepresenttrial,neithertheparametersofvascular remod-elingnortheirassociationwithBHRcouldbedemonstrated inpatientswithPARmonosensitizedtohousedust.We spec-ulatethatthereasonforthismaybemultifactorial,suchas thedurationandintensityofallergenexposure,severityand durationofsymptoms.Severityoftherhinitisdidnotseem tobe afactor sinceallsubjects in the current study and almostallsubjectsinourprevioustrialhadsevererhinitis. VEGFis notonly aremodelingmediatorbutalsoa media-torofinflammation becauseithasspecificallybeenshown toincreaseTh2-mediatedinflammation.33Therefore,inour
firsttrialwespeculatedthatthehighlevelsofVEGFinthe induced sputumofpatients withmonosensitizedtopollen duringthepollenseasonmightbeassociatedwithincreased allergicinflammationinthisseason.However,inthepresent study,wedidnotcheckthedurationand/orintensityofdust miteallergenexposureinthehomesand/or workplacesof patientswithPAR.Evaluation ofthe relationshipbetween theselevelsandtheresultsofinduced sputumcouldhave yieldedmorereliableresults.
Themainlimitationofthecurrentstudywastheindirect evaluationofvascularremodelingbasedononlytwogrowth factorsandtheabsenceofdirecthistopathologicalstudies fromthelower airway.However,previous studies found a correlationbetweenmeasuredvascularremodeling param-eters(VEGF,MMP-9)usinginduced sputumandparameters measuredwithbronchialbiopsy.34,35 Althoughethicalissue
andtechnicalconcernsseemtobeobstaclesinwidelyuse ofbronchoscopyinroutinepracticeandresearchsettingsin patientswithallergicrhinitis,futurestudieswithbronchial
biopsyandBALincludingotherpotentialfactors contribut-ingangiogenesiscouldreinforceourdata.Anotherlimitation wasthe limitednumberof patientswithPAR,particularly caseswithBHRtoM.AlthoughthemeanlevelofVEGF-Aand ratioofVEGF-A/EndostatinwerehigherinPARpatientsthan in healthy controls, the differences werenot statistically significant.This wasthecase forthe meanlevelof Endo-statinaswell.Inabilitytoobtainstatisticallysignificantdata andthecorrelationamongPC20Mvalueandlevelsof
VEGF-A,Endostatin,andVEGF-A/Endostatinratiomayberelated tothisnumericalrestrictionandlargerpatientnumbersmay leadtomoremeaningfulresults.
In conclusion, based on in induced sputum samples, therewaseosinophilicinflammationinthelowerairwayof patientswithPARwithmoreremarkableinflammationinPAR patientswithBHRbutitscorrelationwithPC20Mvaluecould
notbedemonstrated.However,levelsofVEGF-Aand endo-statindidnotdifferbetweenpatientswithPARandhealthy controls regardless of BHR toM. These non-invasive find-ingsshouldbeconfirmedinalargerandwelldefinedstudy populationalongwithsystemicbiomarkers
Ethical
disclosures
Protection of human and animal subjects.The authors declarethatnoexperimentswereperformedonhumansor animalsforthisstudy.
Confidentialityofdata.Theauthorsdeclarethatnopatient dataappearinthisarticle.
Right to privacy and informed consent.The authors declarethatnopatientdataappearinthisarticle.
Author
contributions
˙InsuYılmazselectedpatientsforthestudy,performed spu-tuminductionandwrotethemanuscript,NilüferBayraktar performedELISAmeasurements.DeryaSec¸ilperformedthe sputuminductionprocess,SelcenYükselperformedthe sta-tisticalanalysisand,ZeynepMısırlıgilwasinvolvedinpatient selection,SevimBavbekdesignedthestudyandrevisedthe manuscript.All authors have read andapproved the final manuscript.
Conflicts
of
interest
Theauthorshavenoconflictsofinteresttodeclare.
References
1.Vignola AM,Mirabella F,CostanzoG, DiGiorgi R, Gjomarkaj M, Bellia V, et al. Airway remodeling in asthma. Chest. 2003;123:417---22.
2.Elias JA, ZhuZ, Chupp G, Homer RJ. Airway remodeling in asthma.JClinInvest.2003;111:291---7.
3.FolkmanJ. Angiogenesisin cancer, vascular,rheumatoidand otherdiseases.NatMed.1995;1:27---30.
4.Chetta A, Zanini A, Foresi A, D’Ippolito R, Tipa A, Castag-naroA,etal.Vascularendothelialgrowthfactorup-regulation
and bronchial wall remodeling in asthma. Clin Exp Allergy. 2005;35(11):1437---42.
5.Simcock DE, Kanabar V, Clarke GW, O’Connor BJ, Lee TH, HirstSJ.Proangiogenicactivityinbronchoalveolarlavagefluid from patients with asthma. Am J Respir Crit Care Med. 2007;176:146---53.
6.O’Reilly MS,Boehm T, Shing Y, FukaiN, Vasios G, Lane WS. Endostatin:anendogenousinhibitorofangiogenesisandtumor growth.Cell.1997;88:277---85.
7.YamaguchiN,Anand-ApteB,LeeM,SasakiT,FukaiN,ShapiroR, etal.EndostatininhibitsVEGF-inducedendothelialcell migra-tionandtumorgrowthindependentlyofzincbinding.EMBOJ. 1999;18:441---523.
8.Greisner WA, Settipane RJ, Settipane GA. Co-existence of asthmaandallergicrhinitis:a23yearfollow-upstudyofcollege students.AllergyAsthmaProc.1998;19:185---8.
9.DanielssonJ,JessenM.Thenaturalcourseofallergicrhinitis during12yearsoffollow-up.Allergy.1997;52:331---4.
10.YılmazI,BayraktarN, CeyhanK, Sec¸ilD,Yüksel S, Mısırlıgil Z, et al. Evaluation of vascularendothelial growth factor A and endostatinlevels ininduced sputum and relationship to bronchial hyperreactivity in patients with seasonal allergic rhinitis.AmJRhinolAllergy.2013;27:181---6.
11.SohnSW,LeeHS,ParkHW,ChangYS,KimYK,ChoSH,etal. EvaluationofcytokinemRNAininducedsputumfrompatients withallergicrhinitis:relationship toairway hyperresponsive-ness.Allergy.2008;63:268---73.
12.BraunstahlGJ, FokkensWJ, OverbeekSE,KleinJan A, Hoog-steden HC, Prins JB. Mucosal and systemic inflammatory changesinallergicrhinitisandasthma:acomparisonbetween upper and lower airways. Clin Exp Allergy. 2003;33(5): 579---87.
13.BavbekS,SencerH,MısırlıgilZ,BederS,GurbuzL.Lightand electronmicroscopestudyinallergicrhinitispatients(ARP)with orwithoutbronchialhyperreactivity(BHR).JInvestAllergolClin Immunol.1996;6:172---82.
14.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, TogiasA,etal.Allergicrhinitisanditsimpactonasthma(ARIA) 2008 update (in collaboration with the World Health Orga-nization, GA(2)LENand AllerGen).Allergy. 2008;63Suppl86: 8---160.
15.CockroftDW,HargreaveFE.Airwayhyperresponsiveness: def-inition, measurementand clinicalrelevance.In: KalinerMA, BarnesP,PerssonCGA,editors.Asthma:itspathologyand treat-ment.NewYork,NY:MarcelDekkerInc.;1991.p.51---64. 16.PowerC,ScreenanS,HursonB,BurkeC,PoulterLW.Distribution
of immunocompetent cells in the bronchial wall of clini-callyhealthysubjectsshowingbronchialhyperresponsiveness. Thorax.1993;48:1125---38.
17.PizzichiniE,PizzichiniMM,EfthimiadisA,EvansS,MorrisMM, SquillaceD,etal. Indicesofairwayinflammationininduced sputum:reproducibilityandvalidityofcellandfluidphase mea-surements.AmJRespirCritCareMed.1996;154:308---17. 18.PavordID,PizzichiniMM,PizzichiniE,HargreaveFE.Theuse
ofinducedsputumtoinvestigateairwayinflammation.Thorax. 1997;52:498---501.
19.CanbazP,Uskudar-TekeH,AksuK,KerenM,GulbasZ,KurtE. Nasaleosinophiliacanpredictbronchialhyperresponsivenessin persistentrhinitis:evidenceforunitedairwaysdiseaseconcept. AmJRhinolAllergy.2011;25(2):120---4.
20.SuhDI,LeeJK,KimJT,MinYG,KohYY.Bronchial hyperrespon-sivenessinpreschoolchildrenwithallergicrhinitis.AmJRhinol Allergy.2011;25(5):186---90.
21.Foresi A, Leone C, Pelucchi A, Mastropasqua B, Chetta A, D’IppolitoR, et al. Eosinophils, mast cells,and basophils in inducedsputumfrompatientswithseasonalallergicrhinitisand perennialasthma:relationshiptomethacholineresponsiveness. JAllergyClinImmunol.1997;100:58---64.
22.Polasa R, CiamarraI, ManganoG, Prosperini G, Pistorio MP, VancheriC, et al. Bronchialhyperresponsiveness and airway inflammationmarkersinnonasthmaticswithallergicrhinitis. EurRespirJ.2000;15:30---5.
23.Semik-Orzech A, Barczyk A, Wiaderkiewicz R, Pierzchała W. Eotaxin:butnotIL-8,isincreasedinupperandlowerairwaysof allergicrhinitissubjectsafternasalallergenchallenge.Allergy AsthmaProc.2011;32(3):230---8.
24.AlvarezMJ,Olaguibel JM,GarcíaBE,RodríquezA, Tabar AI, UrbiolaE.Airwayinflammationinasthmaandperennialallergic rhinitis.Relationshipwithnonspecificbronchialresponsiveness andmaximalairwaynarrowing.Allergy.2000;55(4):355---62. 25.KristanS,MalovrhM,SilarM,KernI,FlezarM,KosnikM,etal.
Airway angiogenesis in patients with rhinitis and controlled asthma.ClinExpAllergy.2009;39:354---60.
26.LeckieMJ,tenBrinkeA,KhanJ,DiamantZ,O’ConnorBJ,Walls CM,etal.Effectsofaninterleukin-5blockingmonoclonal anti-bodyoneosinophils,airwayhyper-responsiveness,andthelate asthmaticresponse.Lancet.2000;356:2144---8.
27.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107:295---301.
28.WardC,PaisM,BishR,ReidD,FeltisB,JohnsD,etal.Airway inflammation,basement membrane thickeningand bronchial hyperresponsivenessinasthma.Thorax.2002;57:309---16. 29.Boulet L-P, Laviolette M, Turcotte H, Cartier A, Dugas M.
Bronchialsubepithelialfibrosiscorrelateswithairway respon-sivenesstomethacholine.Chest.1997;112:45---52.
30.AsaiK,KanazawaH,OtaniK,ShiraishiS,HirataK,Yoshikawa J.Imbalancebetweenvascularendothelialgrowthfactorand endostatinlevelsininducedsputumfromasthmaticsubjects.J AllergyClinImmunol.2002;110:571---5.
31.Hanahan D, Folkman J. Patterns and emerging mecha-nisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353---64.
32.Goodwin AM. In vitro assays of angiogenesis for assessment of angiogenic and anti-angiogenic agents. Microvasc Res. 2007;74:172---83.
33.Lee CG, Link H, Baluk P. Vascular endothelial growth fac-tor(VEGF) induces remodelling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095---103.
34.SiddiquiS,SutcliffeA,ShikotraA,WoodmanL,DoeC,McKenna S, et al. Vascular remodeling is a feature of asthma and nonasthmaticeosinophilicbronchitis. JAllergy ClinImmunol. 2007;120(4):813---9.
35.Lee KS, Min KH, Kim SR, Park SJ, Park HS, Jin GY, et al. Vascular endothelial growth factor modulates matrix metalloproteinase-9expressioninasthma.AmJRespirCritCare Med.2006;174(2):161---70.