Contents lists available atScienceDirect
Acta Tropica
journal homepage:www.elsevier.com/locate/actatropica
Genetic diversity of Culicoides species within the Pulicaris complex (Diptera:
Ceratopogonidae) in Turkey inferred from mitochondrial COI gene
sequences
Alparslan Yildirim
a,b,⁎, Bilal Dik
c, Onder Duzlu
a,b, Zuhal Onder
a,b, Arif Ciloglu
a,b,
Gamze Yetismis
a, Abdullah Inci
a,baErciyes University, Faculty of Veterinary Medicine, Parasitology Department, Kayseri, Turkey
bVectors and Vector-Borne Diseases Implementation and Research Center, Erciyes University, Kayseri, Turkey cSelcuk University, Faculty of Veterinary Medicine, Parasitology Department, Konya, Turkey
A R T I C L E I N F O Keywords: Culicoides subgenus COI gene mtDNA Phylogenetics Cryptic species A B S T R A C T
Identification of Culicoides (Diptera: Ceratopogonidae) biting midges to species has become important due to their potential role in the transmission of arboviruses such as bluetongue virus, bovine ephemeral fever virus, Akabane virus, African horse sickness virus, epizootic haemorrhagic disease virus and Schmallenberg virus. In several studies, molecular tools, used for the identification of biting midges, revealed the presence of cryptic and undescribed species especially within Pulicaris complex. The presence of cryptic species within species com-plexes raise questions about their role in viral disease transmission as there are apparent differences in the vectorial capacity between closely related species. In this study, we analyzed the mitochondrial DNA cytochrome oxidase I (COI) gene sequences of species within the Pulicaris complex present in Turkey and determined their phylogenetic relationships. Twenty-one haplotypes within the already described species C. pulicaris P1, C. lu-picaris, C. lupicaris L2, C. newsteadi, C. newsteadi N1, C. punctatus, C. fagineus F2 and C.flavipulicaris were de-termined from the study areas. The molecular analysis revealed further two haplotypes belonging to new non-described cryptic species named as C. lupicaris L3 and Culicoides WBS corresponding to C. lupicaris and Fagineus complex which diverged by 17.9% to 25.7% and 18.7% to 31.8%, respectively from other species in the sub-genus Culicoides. Genetic divergence within species was < 2.0% and phylogenetic analyses of the COI dataset revealed 22 different monophyletic separate clades within two major cluster. The results of this study emphasize the applicability of COI sequences as a diagnostic marker for differentiating Culicoides species and revealing cryptic species.
1. Introduction
Biting midges of the genus Culicoides Latreille, 1809 (Diptera: Ceratopognidae) are known as the smallest blood-sucking flies in the world. More than 38 species groups within approximately 34 subgenera in the single genus Culicoides have been described worldwide (Borkent, 2017). Besides their direct effects on hosts such as biting nuisance and causing dermatitis in equids, Culicoides species are also involved in the transmission of protozoan parasites that include Haemoproteus and Leucocytozoon, the helminth parasite Onchocerca and over 50 arbo-viruses including bluetongue virus (BTV; Orbivirus, Reoviridae), Aka-bane virus (AKAV), bovine ephemeral fever virus (BEFV), epizootic haemorrhagic disease virus (EHDV), Schmallenberg virus (SBV;
Orthobunyavirus, Peribunyaviridae) and African horse sickness virus (AHSV; Orbivirus, Reoviridae) (Mellor et al., 2000;Collins et al., 2018). The unprecedented emergence of SBV and multiple serotypes of BTV in Europe since 2000 has highlighted the veterinary importance of Culi-coides biting midges (Carpenter et al., 2009;Hoffmann et al., 2012; Sailleau et al., 2017).Collins et al. (2018)highlighted the vulnerability of Europe to the introduction of viruses from distant geographical re-gions that may be transmitted by biting midges. Furthermore, the outbreaks of these viruses in Europe over the last two decades have shown that susceptibility to virus infection may indeed be widespread in the genus Culicoides and not linked to one or two species. Therefore, several entomological and surveillance programs as part of contingency plans for the diseases transmitted by Culicoides have been conducted in
https://doi.org/10.1016/j.actatropica.2018.12.005
Received 9 October 2018; Received in revised form 4 December 2018; Accepted 4 December 2018
⁎Corresponding author at: Erciyes University, Faculty of Veterinary Medicine, Parasitology Department, Kayseri, Turkey. E-mail address:yildirima@erciyes.edu.tr(A. Yildirim).
Available online 13 December 2018
0001-706X/ © 2018 Elsevier B.V. All rights reserved.
many European countries (Nielsen et al., 2010; Ander et al., 2012; Ramilo et al., 2012). These surveys resulted in updated species lists within countries and identification of new species in Culicoides genus (Casati et al., 2009;Pages et al., 2009;Nielsen et al., 2010;Lassen et al., 2012a,b;Wenk et al., 2012;Ander et al., 2013;Nielsen and Kristensen, 2015;Sarvasova et al., 2017). A fast-growing attention on arboviruses transmitted by Culicoides and importance of controlling biting midges populations for the protection against the related diseases has also been emphasized by several authors (Benelli et al., 2017; Meloni et al., 2018).
DNA barcoding using a standardized short sequence of COI from mt-DNA is generally considered as a reliable, cost-effective and easy mo-lecular identification tool, with a wide applicability across metazoan taxa (Hebert et al., 2004a,b;Hebert and Gregory, 2005) including tick (Zhang and Zhang, 2014;Zhang et al., 2017), mosquito (Murugan et al., 2016; Vadivalagan et al., 2017;Karthika et al., 2018) and Culicoides species (Sarvasova et al., 2014;Talavera et al., 2017;Liu et al., 2018). Various species belonging to the genus Culicoides may have rather di-verse biology and that knowledge of the biology of a vector species will play a critical in implementing efficient control measures. There are currently about 55 described species in the subgenus Culicoides (Borkent, 2017). Despite the increasing number of studies, the taxo-nomic status of many species in the subgenus Culicoides in the Palearctic region is still unclear (Meiswinkel et al., 2004;Sarvasova et al., 2017). The species within Pulicaris complex (C. pulicaris, C. lupicaris, C. im-punctatus, C. im-punctatus, C. grisescens, C. newsteadi, C. flavipulicaris, C. fagineus, C. subfagineus) have exhibited high variation in molecular studies especially if based on the phylogenetic analyses of mitochon-drial COI sequences (Pages et al., 2009; Lassen et al., 2012b; Wenk et al., 2012;Ander et al., 2013;Sarvasova et al., 2014). These studies revealed the presence of cryptic species and support the notion of the existence of several yet undescribed within in this complex.
The diseases associated with Culicoides, such as BTV, AKAV, EFV, EHDV, and SBV cause problems in ruminant populations through the certain regions of Turkey (Dik et al., 2012,2014;Tonbak et al., 2016). However, the studies on the Culicoides diversity of Turkey are limited and up to date 61 species have been reported (Dik, 2017). These studies mainly used the identification keys based on wing morphology of the specimens collected from particular regions. Thus, thefindings of these studies probably might not reflect the true dispersal of the species be-cause of the sibling and cryptic diversity within the genus and also possible misidentifications due to the unavailability of experienced (Pages et al., 2009;Harrup et al., 2015).
Given the importance of members of Culicoides subgenus due to their role in the transmission of BTV and the features of the suggested presence of sibling species within this subgenus (Pages et al., 2009; Lassen et al., 2012b;Nielsen and Kristensen, 2015), the main objective of this study was to identify and analyze the variations among Culicoides species within the Pulicaris complex using both morphological and molecular techniques for the first time in Turkey. Thus, the present study contributes to knowledge of the diversity of Culicoides subgenus. We analyzed the COI DNA sequences and compared them to published DNA barcodes of targeted species to establish whether the populations in Turkey contained unique and/or cosmopolitan genetic diversity. 2. Materials and methods
2.1. Origin and identification of biting midges
A total of 42 female Culicoides specimens collected from 15 sites in West Black Sea Region and Central Anatolia Region of Turkey were included in the study. The geographical locations of sites are shown in Table 1. Specimens selected for molecular analysis were collected using Onderstepoort-type 220 V blacklight traps with 8 W UV light tubes and stored in 70% ethanol prior to identification. Initial identification of the Culicoides specimens was based on the wing patterns and other
morphological features using the relevant keys (Mathieu et al., 2012).
2.2. Genomic DNA extraction and polymerase chain reaction (PCR) Genomic DNA (gDNA) was extracted from individual Culicoides specimens using the GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific, USA). gDNA concentration of each Culicoides specimen was measured by Qubit Fluorometric Quantitation (Thermo Fisher Scientific, USA) in order to optimize the amount used in the PCR mastermix.
The gDNA from individual biting midges were subjected to PCR analyses with the primers C1J1718/C1N2191 (Dallas et al., 2003) that amplify 472 bp COI gene region. PCR reactions were carried out in a total volume of 25μL, including 12.5 μL of commercial ready to use Master Mix (Maxima Hot StartGreen PCR Master Mix, Thermo Scien-tific, USA), 0.2 μM each primer of each primer and 30 ng of genomic DNA. The reactions were performed in a C1000 Touch™ Thermal Cycler (Bio-Rad, USA), with the described cycling parameters (Pages et al., 2009). The PCR products were separated by 1.5% agarose gel electro-phoresis and visualized under Fusion FX Gel Documentation System (Vilber Lourmat, France).
2.3. Sequence and phylogenetic analysis
The amplification products from COI fragment of the Culicoides specimens were purified from agarose gel with a commercially avail-able kit (High Pure PCR product purification kit, Roche). The amplifi-cation products were sequenced in both directions (Macrogen, Netherlands) using PCR primers. The primer sequences were trimmed from all reads. The resulting forward and reverse sequences were pro-cessed and aligned with Geneious 11.0.2 software (Kearse et al., 2012) to obtain a single consensus sequence. Finalized sequences were sear-ched in the GenBank database by using BLASTn algorithm to compare fragments and constitute the data set for phylogenetic analyses. The COI data set was comprised from a total of 120 sequences including published haplotypes from several countries in the GenBank and the haplotypes obtained in this study. Detailed information about the haplotypes were presented in Supplementary Data Table S1. All se-quences were aligned using MUSCLE (Edgar, 2004) through the plugin available in Geneious 11.0.2. Intra- and inter-specific genetic differ-ences based on the Kimura-2-Parameter (K2P) distance model (Kimura, 1980;Nei et al., 2005) and ts/tv bias (R) in each codon were calculated with MEGA version 7 (Kumar et al., 2016). Haplotype analyses and nucleotide composition, AT bias and genetic diversity indices were calculated using DnaSP v.5.1 (Librado and Rozas, 2009). Neutrality tests (Tajima’s D [Tajima, 1989] and Fu’s F [Fu, 1997]) were carried out in DnaSP v.5.1.
TCS haplotype networks were built to examine the haplotype re-lationship among Culicoides species with default parameters using PopART software (Leigh and Bryant, 2015).
Phylogenetic reconstruction was performed by Maximum Likelihood (ML) and Bayesian inference (BI). The best-fit DNA-sub-stitution model for ML and BI based on the Akaike information criterion (AIC) algorithms was selected as TN93+G + I by using jModeltest v.0.1.1 (Posada, 2008). The ML and BI analyses were run in PhyML (Guindon and Gascuel, 2003) and MrBayes version 3.2.6 (Huelsenbeck and Ronquist, 2001), respectively through the plugins available with Geneious 11.0.2. A bootstrap analysis was performed with 1000 re-plicates in ML. Two Markov Chain Monte Carlo simulations were run simultaneously for 10 million generations, with sampling every 200 generations for the posterior probability calculations in BI. Before constructing a majority consensus tree, 25% of the initial trees in each run were discarded as burn-in.
3. Results
3.1. Morphologic identification of Culicoides
Ten species of the Pulicaris complex were identified morphologi-cally: Culicoides fagineus F1 (Pages et al., 2009), Culicoidesflavipulicaris (Dzhafarov, 1964), Culicoides WBS, Culicoides lupicaris (Dzhafarov, 1964), Culicoides lupicaris L2, Culicoides lupicaris L3, Culicoides newsteadi (Austen, 1921), Culicoides newsteadi N1 (Pages et al., 2009), Culicoides pulicaris (Linnaeus, 1758), and Culicoides punctatus (Meigen, 1804). Wing patterns of all detected species can be seen inFig. 1. The present study provides the first description of C. lupicaris L3 and its wing morphology was more similar to C. lupicaris L2 than C. lupicaris. Culi-coides lupicaris could be differentiated from C. lupicaris L2 and C. lupi-caris L3 by wing pattern, showing bigger elongated dark spot in the proximal part of M2 and wider anterior 3rddark costal spot (d.c.s) in r3. Culicoides lupicaris L3 could also be differentiated from C. lupicaris L2 by wing pattern, showing smaller well-marked dark spots in the cubital wing cell and proximal part of M2. Additionally,five of the specimens with wing morphology similar to C. fagineus F1 (Pages et al., 2009) but with different COI sequences was identified. As this group of specimens were collected from West Black Sea Region of Turkey, they were pro-visionally named Culicoides WBS. The Culicoides WBS specimens dif-fered from C. fagineus in having bigger well-marked and confluent dark spots in wing pattern, more rounded pale spot in anterior cubital cell (cua1) and two pale spots fused with a narrow strait in the distal part of anal cell (an). All the other species determined in the study had similar wing patterns with the described morphological features of the corre-sponding species (Pages et al., 2009;Mathieu et al., 2012)
3.2. COI sequence analyses and divergence
A 472 bp of COI gene sequences were successfully recovered from the 42 specimens sampled from Western Black Sea and Central
Anatolian Regions. No insertions, deletions, or stop codons were de-termined in COI indicating that all sequences constitute functional mitochondrial products. Based on the obtained sequences, together with the corresponding 78 sequences of Culicoides isolates in GenBank totally 89 haplotypes were determined in the COI data set (472 bp length) of the subgenus Culicoides (Supplementary Data Table S1). Among the 23 haplotypes detected in the study, 20 were new for Pu-licaris complex and the remaining three were already reported from other countries. COI fragment of the haplotypes showed significant variations in the base composition indicating more polymorphic sites among the Culicoides species. The AT and GC composition of the entire data set ranged from 60.4 to 66.1% and 33.9– to 39.6% respectively. It was AT biased with 196 variable sites of which 189 were parsimony informative. The transition/transversion bias (R) was observed to be higher in 2nd(7.89) and 1st(3.29) positions, comparative to 3rdcodon position (2.42).
Genetic diversity indices and results of neutrality tests for COI data set were shown inTable 2. Overall haplotype and nucleotide diversities were 0.99 and 0.16 among the Culicoides species. There were low level of haplotype and nucleotide diversities within each species. Tajima’s D and Fu’s F were also not significant (p > 0.05) in any of the Culicoides species.
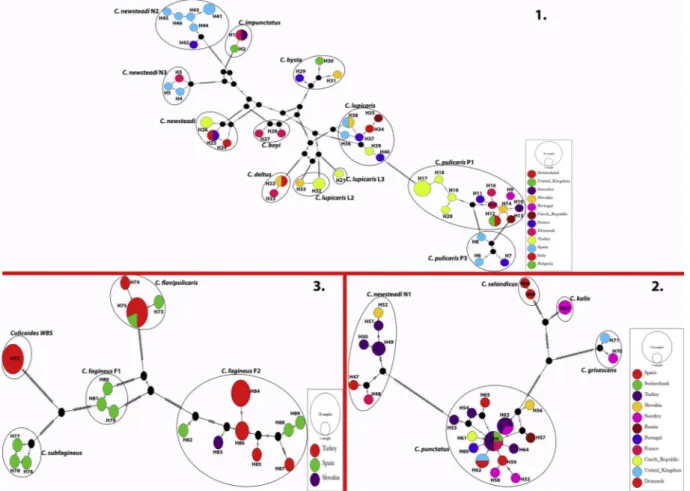
The TCS network of the entire set of the haplotypes from Turkey and from the NCBI was given in Fig. 2. Haplotype networks were con-structed within three phylogenetically related groups. The TCS analyses indicated that COI haplotypes of Culicoides species from several Eur-opean countries resolved in separate networks for each group.
Four haplotypes were determined for C. pulicaris P1 in the study. H17 was the most common haplotype and included 4 sequences (KU754170, KU754173, KU754175, KU754176). The remaining three haplotypes (H18, H19 and H20) were represented by one isolate each (KU754171, KU754172 and KU754174). There were one tofive mu-tational steps among the H1-H4 haplotypes, while 11 to 19 steps were determined between C. pulicaris P1 haplotypes from Turkey and several Table 1
Culicoides species with collection data, number of specimens (n) in the COI data set, and number of haplotypes (nHAP).
Species Location GPS coordinates Date n nHAPa
C. newsteadi Turkey, West Black Sea Region, Duzce, Kaynasli 40.4704 N 31.1901E 14.08.2014 1 1 Turkey, West Black Sea Region, Bartın 41.3641 N 32.1533E 10.07.2014 1
C. newsteadi N1 Turkey, West Black Sea Region, Bolu 40.4557 N 31.5154E 08.07.2014 2 3 Turkey, West Black Sea Region, Bolu, Yenicaga 40.4616 N 32.0147E 18.08.2014 1
Turkey, West Black Sea Region, Kastamonu 41.2719 N 33.4037E 12.06.2014 1
C. pulicaris P1 Turkey, West Black Sea Region, Duzce, Kaynasli 40.4704 N 31.1901E 14.08.2014 1 4 Turkey, West Black Sea Region, Zonguldak, Devrek 41.0622 N 31.5305E 15.08.2014 1
Turkey, West Black Sea Region, Bolu 40.4557 N 31.5154E 08.07.2014 1 Turkey, West Black Sea Region, Bartın, Ulus 41.3256 N 32.3446E 09.06.2014 2 Turkey, West Black Sea Region, Bolu 40.4557 N 31.5154E 14.08.2014 1 Turkey, West Black Sea Region, Bartın, Ulus 41.3256 N 32.3446E 10.06.2014 1
C. lupicaris Turkey, West Black Sea Region, Kastamonu 41.2719 N 33.4037E 12.06.2014 1 1 C. lupicaris L2 Turkey, West Black Sea Region, Zonguldak, Devrek 41.0622 N 31.5305E 15.08.2014 2 1 C. lupicaris L3 Turkey, West Black Sea Region, Duzce 40.4704 N 31.1901E 14.08.2014 1 1 C. punctatus Turkey, West Black Sea Region, Zonguldak, Devrek 41.0622 N 31.5305E 15.08.2014 1 5
Turkey, West Black Sea Region, Zonguldak, Devrek 41.0622 N 31.5305E 15.08.2014 1 Turkey, West Black Sea Region, Bartın, Ulus 41.3256 N 32.3446E 09.06.2014 2 Turkey, West Black Sea Region, Kastamonu 41.2719 N 33.4037E 12.06.2014 1 Turkey, West Black Sea Region, Duzce¸ Kaynasli 40.4704 N 31.1901E 14.08.2014 1 Turkey, West Black Sea Region, Zonguldak, Devrek 41.0622 N 31.5305E 15.08.2014 1
C. fagineus F2 Turkey, West Black Sea Region, Zonguldak, Devrek 41.0622 N 31.5305E 15.08.2014 1 4 Turkey, West Black Sea Region, Bartın 41.3638 N 32.1755E 10.06.2014 3
Turkey, Central Anatolia Region, Konya 37.5120 N 32.3334E 14.07.1997 1 Turkey, Central Anatolia Region, Konya 37.5120 N 32.3334E 16.07.1997 2 Turkey, Central Anatolia Region, Konya 37.5120 N 32.3334E 18.08.1997 1
Culicoides WBS Turkey, West Black Sea Region, Duzce, Kaynasli 40.4704 N 31.1901E 14.08.2014 3 1 Turkey, West Black Sea Region, Zonguldak, Devrek 41.0622 N 31.5305E 15.08.2014 2
C.flavipulicaris Turkey, West Black Sea Region, Kastamonu, Taskopru 41.2800 N 34.1027E 13.07.2014 3 2 Turkey, West Black Sea Region, Zonguldak, Caycuma 41.2931 N 31.5123E 12.07.2014 1
Turkey, Central Anatolia Region, Konya 37.5120 N 32.3334E 16.07.1997 1
European countries (Fig. 2). The mean intraspecific genetic distance for C. pulicaris P1 was determined as 1.84% and our sequences showed 96.2% to 96.9% identity to the isolates reported from several European countries.
We determined three new haplotypes (H39, H32 and H21) within C. lupicaris (KU754161), C. lupicaris L2 (KU754159, KU754160) and C. lupicaris L3 (KU754162), respectively. TCS network analyses (Fig. 2) revealed close relationship between haplotype H39 and haplotype H40 reported from France (KF591629) with only one mutational step. H39 haplotype exhibited two to six mutational steps with the haplotypes from other European countries. H32 haplotype closed to the haplotype H33 from Slovakia (KJ624098) with one mutational step. The third
unique haplotype H21 correspond to C. lupicaris was represented by one isolate (KU754162) and this specimen was provisionally named C. lu-picaris L3. Culicoides lulu-picaris L3 H21 haplotype was closer to H32 and H33 haplotypes of C. lupicaris L2 (with 64 and 65 mutational steps) rather than C. lupicaris H39 haplotype (Fig. 2). The mean intraspecific genetic distances for C. lupicaris and C. lupicaris L2 were designated as 0.49% and 0.20%, respectively. Culicoides lupicaris sequence exhibited 99.3% to 99.8% identity to the isolates reported from several European countries while C. lupicaris L2 sequences were 99.8% identical to the isolate (KJ624098) reported from Slovakia (Fig. 2). Culicoides lupicaris L3 exhibited a mean genetic distance of 18.9% and 17.9% to C. lupicaris and C. lupicaris L2 (Table 3).
Fig. 1. Wing images of the ten Culicoides species identified in Turkey in this study. All the wings belonged to female specimens.
Table 2
Summary of genetic diversity indices and results of neutrality tests (Tajima’s? ? and Fu’s? ???) in the mitochondrial COI gene segment of Culicoides species.
Species n k K h ( ± SD) π ( ± SD) Tajima’sD Fu’s F
C. pulicaris P1 16 26 12 0.942 ± 0.002 0.02080 ± 0.00178 1.04 0.39 C. pulicaris P3 3 6 3 1.000 ± 0.272 0.00989 ± 0.00275 N N C. lupicaris 8 9 7 0.964 ± 0.077 0.00666 ± 0.00127 −0,46 −0.12 C. lupicaris L2 3 1 2 0.667 ± 0.314 0.00141 ± 0.00067 N N C. lupicaris L3 1 – 1 – – N N C. deltus 3 1 2 0.667 ± 0.314 0.00141 ± 0.00067 N N C. newsteadi 5 3 3 0.800 ± 0.164 0.00339 ± 0.00084 0.70 0.70 C. newsteadi N1 7 16 6 0.952 ± 0.096 0.01453 ± 0.00354 0.28 0.72 C. newsteadi N2 7 10 6 0.952 ± 0.096 0.00908 ± 0,00164 0.27 0.55 C. newsteadi N3 3 2 3 1.000 ± 0.272 0.00282 ± 0.00094 N N C. impunctatus 3 1 2 0.667 ± 0.314 0.00141 ± 0.00067 N N C. bysta 3 18 3 1.000 ± 0.272 0.02542 ± 0.00692 N N C. boyi 2 4 2 1.000 ± 0.500 0.00847 ± 0.00424 N N C. punctatus 20 17 14 0.947 ± 0.034 0.00558 ± 0.00090 −1,69 −9.52 C. kalix 2 0 1 0.000 ± 0.000 0.000 ± 0.000 N N C. selandicus 2 1 2 1.000 ± 0.500 0.00212 ± 0.00106 N N C. grisescens 2 3 2 1.000 ± 0.500 0.00636 ± 0.00318 N N C. fagineus F1 3 2 3 1.000 ± 0.272 0.00282 ± 0.00094 N N C. fagineus F2 12 22 8 0.894 ± 0.078 0.01233 ± 0.00292 −0,89 −0.64 C. subfagineus 3 2 3 1.000 ± 0.272 0.00282 ± 0.00094 N N C.flavipulicaris 7 2 3 0.524 ± 0.209 0.00121 ± 0.00054 −1,24 −1.37 Culicoides WBS 5 0 1 0.000 ± 0.000 0.00000 ± 0.00000 – – Overall 120 196 89 0.993 ± 0.002 0.15567 ± 0.00163 0.64 1.63
There were one (H26) and three haplotypes (H49, H50, H51) cor-responding to C. newsteadi and C. newsteadi N1. The mean intraspecific genetic diversities for C. newsteadi and C. newsteadi N1 were 0.33% and 1.45%, respectively. H26 haplotype comprised two isolates (KU754167, KU754164) with 99.4% to 99.6% identity and two to three mutational
steps to C. newsteadi haplotypes from France (H25) and Italy (H24, H25) (Fig. 2). Culicoides newsteadi N1 H49 (KU754165, KU754168), H50 (KU754169) and H51 (KU754166) haplotypes showed 97.3% to 99.6% identity and four to 10 mutational steps to the haplotypes from Slovakia, Spain and France (Fig. 2).
Fig. 2. TCS haplotype network for the COI gene segment of Culicoides species within the Pulicaris complex. Haplotype networks were constituted within three phylogenetically related groups. Each segment on the vertical lines represents a single mutation. Small black circles represent hypothetical intermediates not observed in the data set. Size of the circles is proportional to haplotype frequency. Sampling countries are color-coded.
Table 3
Pairwise genetic distances (%) between species of Culicoides.
No Species 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 1 C. newsteadi 2,9 3,4 2,9 2,9 3,1 2,5 2,8 3,4 3,6 4,2 3,2 3,0 3,2 2,9 3,1 3,3 3,4 3,1 3,2 3,2 3,2 2 C. newsteadi N1 19,6 3,6 3,3 3,2 3,0 3,0 3,1 2,9 3,2 3,3 3,4 3,2 3,3 3,1 3,4 3,1 3,5 3,1 3,2 3,7 3,0 3 C. newsteadi N2 24,0 25,3 3,1 3,0 3,4 3,4 3,4 3,7 3,2 3,4 3,0 3,3 3,7 3,1 3,0 3,2 3,6 2,9 3,6 3,4 3,6 4 C. newsteadi N3 19,9 24,1 22,0 3,0 3,1 2,6 2,9 3,3 3,0 3,5 3,4 2,9 3,2 2,9 2,6 3,6 3,2 3,1 3,2 3,9 3,5 5 C. kalix 18,8 21,6 21,0 20,9 1,6 2,7 2,9 2,9 3,2 3,4 3,0 3,1 3,2 2,7 2,7 2,4 3,3 3,3 3,1 3,7 3,2 6 C. selandicus 20,3 21,5 24,6 22,2 7,8 2,9 3,2 2,9 3,4 3,3 3,1 2,7 3,3 2,9 3,3 2,9 3,1 3,3 3,2 3,4 3,0 7 C. boyi 16,6 20,4 25,0 18,5 18,9 20,8 1,5 3,1 3,0 4,0 2,6 2,9 3,4 2,7 2,6 3,3 2,9 3,1 2,9 3,5 3,1 8 C. bysta 19,6 21,2 25,4 21,7 21,7 24,0 7,1 3,4 3,2 3,9 2,8 3,3 3,4 3,2 3,0 3,3 3,2 3,2 3,3 3,8 3,7 9 C. grisescens 23,6 20,1 27,4 23,7 19,8 20,5 21,5 24,2 3,6 3,4 3,6 3,1 3,5 3,2 3,1 3,1 3,1 2,8 3,0 3,8 2,6 10 C. pulicaris P1 26,4 22,6 23,0 21,3 22,6 24,8 20,9 23,6 25,9 2,2 2,8 3,1 3,2 3,1 3,1 3,6 3,1 3,6 3,6 3,8 3,4 11 C. pulicaris P3 29,8 23,6 24,5 25,7 23,2 24,3 28,7 28,8 25,3 13,0 3,4 3,7 3,4 3,1 3,4 2,9 3,1 3,3 3,7 4,2 3,6 12 C. lupicaris 21,7 24,3 21,1 23,3 21,1 22,1 17,0 20,1 25,0 19,7 23,9 2,6 2,7 2,9 2,9 3,5 3,3 3,6 3,1 3,7 3,5 13 C. lupicaris L2 20,4 22,6 25,0 20,2 18,5 17,7 20,3 23,6 21,0 22,9 24,8 18,3 2,9 3,0 3,0 3,2 3,3 3,5 2,7 3,8 3,2 14 C. lupicaris L3 23,4 24,1 25,7 22,9 22,0 23,5 25,5 25,2 24,2 22,1 23,6 18,9 17,9 3,0 3,1 3,4 3,3 3,0 2,9 3,6 3,1 15 C. deltus 19,3 22,7 20,7 19,2 16,7 19,7 19,0 23,1 22,9 21,3 22,1 20,0 19,7 20,9 3,1 3,2 3,4 3,2 3,4 3,9 3,3 16 C. impunctatus 21,1 24,3 21,2 17,4 18,1 23,1 18,5 21,4 21,0 22,6 24,4 19,4 20,1 20,2 20,6 3,4 3,5 3,4 3,1 3,6 3,5 17 C. punctatus 21,8 20,5 22,9 26,6 15,4 19,1 23,6 25,0 21,3 25,1 20,2 24,4 20,9 23,2 22,7 23,2 3,6 3,7 3,3 3,7 3,2 18 C. fagineus F1 24,1 25,5 26,1 21,7 22,1 23,5 19,6 22,9 21,3 22,8 23,1 23,7 22,8 24,2 23,5 25,6 27,3 2,1 2,0 2,8 2,2 19 C. fagineus F2 22,3 23,3 20,6 22,2 22,9 24,7 21,9 23,5 19,3 26,7 25,2 24,9 25,5 22,0 22,4 24,0 27,4 12,3 2,2 3,1 2,3 20 C. subfagineus 23,3 23,3 27,4 22,4 21,7 23,2 20,6 25,1 20,9 26,7 27,7 21,8 19,3 21,4 24,1 21,4 23,6 11,6 14,1 2,6 2,5 21 Culicoides WBS 23,7 27,0 26,3 29,2 25,5 24,3 24,9 27,6 26,3 28,0 31,8 25,8 27,9 26,7 28,9 26,8 27,1 19,1 22,7 18,7 2,9 22 C.flavipulicaris 22,4 21,1 27,1 25,0 21,6 21,8 22,2 27,0 17,1 24,7 27,0 25,2 23,2 23,6 22,7 25,4 22,2 14,1 15,0 16,4 20,8
A mean intraspecific genetic diversity of 0.56% was found for C. punctatus andfive haplotypes were characterized among the examined specimens. Three haplotypes H54 (KU754177), H55 (KU754182) and H64 (KU754178) exhibited 98.9% to 99.8% identity and one to six mutational steps to the C. punctatus haplotypes reported from several European countries (Fig. 2). While the remaining two haplotypes (H63, H66) comprised two isolates each (KU754179, KU754180 and KU754181, KU754183) were identical to haplotypes reported from Sweden (JQ978450), France (KF591635) and Switzerland (HQ824507), respectively.
We determined four unique haplotypes within C. fagineus F2 and analyzing of COI data set revealed a mean intraspecific genetic diversity of 1.23%. H84 haplotype was the common and included four sequences (KU754148, KU754151, KU754152, KU754154). H86 haplotype was represented by two isolates (KU754147, KU754149) and the remaining H85 and H87 haplotypes were represented by one isolate each (KU754150 and KU754153). The COI sequences of the isolates under the detected haplotypes exhibited 97.7% to 99.6% identity and two to 19 mutational steps to the haplotypes reported from Spain and Slovakia (Fig. 2).
Two haplotypes were detected among the sequences of C. flavipuli-caris in the study and mean intraspecific genetic distance was de-termined as 0.15% for the corresponding species. H75 was the common haplotype and included four isolates (KU754155, KU754156, KU754157, KU754158). COI sequence of the haplotype H75 was identical to the haplotype reported from Spain (GQ338923). H74 haplotype was represented by one isolate (KU754163) that showed 99.5% to 99.8% identity and one to two mutational steps to the Spanish haplotypes (Fig. 2).
In the identification of biting midges from Black Sea Region of Turkey, we detected Culicoides specimens from two collection sites in Duzce and Zonguldak provinces (Table 1) similar in wing morphology to C. fagineus F1. Their COI sequences (KU754184toKU754188) were identical to each other resulting in the presence of one unique haplo-type (H72). TCS network analysis (Fig. 2) revealed the relation with Fagineus complex species among the Culicoides taxa which exhibited 18.7% to 22.7% interspecific genetic distance to the species in corre-sponding complex (Table 2). These specimens were provisionally named as Culicoides WBS. Interspecific pairwise genetic distances among the species detected in our study and the species available in GenBank were also greater than 10.0% (Table 2).
3.3. Phylogenetic analysis
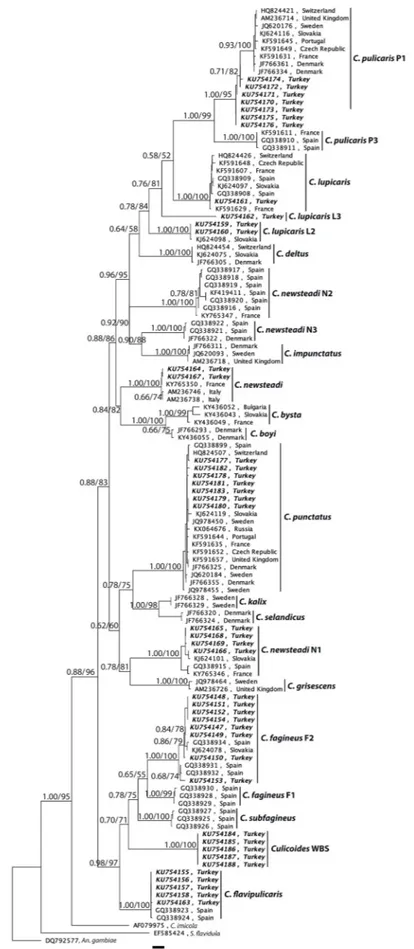
The consensus tree inferred by using maximum likelihood (ML) analyses under the nucleotide substitution model TN93+G + I based on the alignment of COI data set (120 nucleotide sequences) was shown inFig. 3. The tree based on Bayesian inference (BI) also produced si-milar topology with ML tree. Therefore, posterior probabilities were shown with bootstrap values on ML tree. COI sequences from Anopheles gambiae, Stempellinellaflavidula and C. imicola were used as outgroups and the resulting dendrogram showed 22 different separate clades. The Pulicaris complex is recovered as monophyletic with strong supports of bootstrap values (75% to 100%) and posterior probabilities (0.76 to 1.00).
The phylogenetic tree also explored the presence of two major cluster with the support of bootstrap value of 96% and posterior probability of 1.00. Thefirst major cluster divided into two sub clusters. Thefirst included the species C. pulicaris P1, C. pulicaris P3, C. lupicaris, C. lupicaris L2, C. lupicaris L3, C. deltus, C. newsteadi, C. newsteadi N2, C. newsteadi N3, C. impunctatus, C. bysta and C. boyi. Phylogenetic asso-ciation among the species within this sub cluster was strongly sup-ported (bootstrap values ≥ 81%; posterior probabilities ≥0.76). However, inclusion of C. lupicaris and C. deltus in to sub cluster was supported with moderate bootstrap values (52% and 58%) and pos-terior probabilities (0.58 and 0.64). The second sub cluster comprised
the species C. punctatus, C. kalix, C. selandicus, C. newsteadi N1 and C. grisescens. The phylogenetic resolution of this sub cluster also supported by bootstrap values of 75%–100% and posterior probabilities of 0.78–1.00 except the sister relationship between the clade including C. newsteadi N1 and C. grisescens, and the clade including C. punctatus, C. kalix and C. selandicus which exhibited a moderate bootstrap (60%) and posterior probability (0.62) support. The second major cluster included the species C. fagineus F1, C. fagineus F2, C. subfagineus, Culicodes WBS and C.flavipulicaris. The phylogenetic relationships in this cluster also well supported (bootstrap values ≥ 71%; posterior probabilities ≥0.70) except the inclusion of C. fagineus F1 to C. fagineus F2 as a sister group which had moderate bootstrap value of 55% and posterior probability of 0.65.
4. Discussion
DNA barcoding provides rapid, accurate species identification using one or few short, standardized DNA regions and has been widely used effective identification of organisms at the species (Virgilio et al., 2010; Chakraborty et al., 2014). An ideal barcoding marker should be easy to amplify using a universal pair of primers, suitably long (not exceed 800 bp), and sufficiently variable to discriminate among all species (Hebert et al., 2003). Several DNA regions such as mitochondrial genes (COI,16S rRNA) and nuclear genes (18S rRNA; ITS regions) have been utilized as potentially suitable barcodes, (Chen et al., 2012;Xia et al., 2012;Giudicelli et al., 2015). Among these genes, 658 bp region of COI is widely accepted as a universal and standard marker for all animal taxa (Hebert et al., 2004b; Hajibabaei et al., 2006). During the last decade molecular tools, especially DNA barcoding using COI sequences have also provided an efficient solution for identification of Culicoides species in several subgenera and revealed the existence of cryptic spe-cies (Pages et al., 2009;Lassen et al., 2012b;Nielsen and Kristensen, 2015;Sarvasova et al., 2017). The sequence analyses of the 472 bp COI marker successfully confirmed the morphological identification of C. fagineus F2, C.flavipulicaris, C. lupicaris, C. lupicaris L2, C. newsteadi, C. newsteadi N1, C. pulicaris and C. punctatus. Additionally, further speci-mens corresponding to C. lupicaris and C. fagineus complex were iden-tified from Black Sea Region of Turkey for the first time.
We determined two species of C. newsteadi complex from the lo-calities of West Black Sea Region. Specimens from thefirst C. newsteadi species clustered with the Italian isolates reported by Nolan et al. (2007)and a French isolate characterized byPilgrim et al. (2017)with a low genetic diversity (0.3%). Specimens from the second C. newsteadi species were characterized as C. newsteadi N1 which was originally described byPages et al. (2009)and the isolates detected in our study clustered with Spanish, Slovakian and French isolates reported byPages et al. (2009); Sarvasova et al. (2017)and Pilgrim et al. (2017), re-spectively with an overall intraspecific divergence of 1.5%. These re-sults indicated that genetic diversity in C. newsteadi N1 seems more variable than C. newsteadi based on the geographical locations among different countries in Europe. Although the specimens of both species collected from closer regions in West Black Sea Region (Table 1), the interspecific genetic distance between the species was more than 19.0%. Pages et al. (2009) also reported high genetic divergence (21.7% to 22.8%) among the species of C. newsteadi (N1, N2 and N3) from Catalonia, Spain. Further two closely related species C. boyi and C. bysta that were grouped with C. newsteadi in ML three (16.6% to 19.6% mean genetic distance, respectively) has been described from Denmark (Nielsen and Kristensen, 2015) and, Slovakia, Bulgaria and France (Sarvasova et al., 2017), respectively.
We determined specimens belonging to three divergent species from the localities in the West Black Sea Region all exhibited similar wing morphology resembling that of C. lupicaris. The common haplotypes of C. lupicaris have been reported from several European countries in-cluding Switzerland (Wenk et al., 2012), Czech Republic, France (Ramilo et al., 2013), Spain (Pages et al., 2009) and Slovakia
Fig. 3. Phylogenetic analysis of Culicoides species belonging to the subgenus Culicoides. Tree was based on 120 COI sequences belonging to haplotypes available in GenBank and newly characterized haplotypes (in bold italic) from Turkey. Numbers at the nodes represent posterior probability and bootstrap values (1000 re-plicates), respectively. The sequences were given as GenBank accession number and country. Anopheles gambiae, Stempellinellaflavidula and C. imicola sequences were used as the outgroup. The scale bar represents 0.03% divergence.
(Sarvasova et al., 2017) with an overall genetic diversity of 0.7%. An-other unique C. lupicaris haplotype (signed as C. lupicaris L2) was re-corded from Slovakia (Sarvasova et al., 2014) that differed from the above haplotypes over 18% genetic divergence. One of the two hap-lotypes identified in this study clustered with the common haphap-lotypes of C. lupicaris and the other clustered with C. lupicaris L2 haplotype. We also determined a new haplotype that diverged by over 17% from the above haplotypes.Page’s et al. (2009)also reported over 12% diver-gence between two cryptic species within the Pulicaris complex, C. pulicaris P1 and P3 that exhibited similar wing morphology. Similar to thefindings ofPage’s et al. (2009), ourfindings support the presence of cryptic species within C. lupicaris populations and we initially named the corresponding species as C. lupicaris L3.
A group of specimens morphologically and phylogenetically related to Fagineus complex with unknown COI DNA sequences were identified from Culicoides fauna of West Black Sea Region of Turkey. We provi-sionally named this most likely new species as Culicoides WBS. COI sequence of Culicoides WBS diverged by over 18.0% from other mem-bers of Fagineus complex (Table 2). Furthermore, subsequent in-vestigations should be utilized to elucidate the ecological differences between Fagineus complex species exhibiting similar morphology in the region.
A low level of polymorphism was observed within the species of C. pulicaris P1, C. punctatus, C. fagineus F2 and C.flavipulicaris along with the isolates from distant geographical regions. This phylogenetic in-ference corresponds well with thefindings ofNolan et al. (2007);Pages et al. (2009)andRamilo et al. (2013), and might indicate moderately continuous populations and large-scale gene flow within the related species.
In conclusion, our study provides first data on the genetic char-acterization of Culicoides species within the Pulicaris complex in Turkey and adds valuable knowledge to the molecular biology and systematics of the species found in the Palearctic region. COI sequences were suc-cessfully performed as DNA barcoding sequences to identify well known and cryptic species of Culicoides which provide a framework for the assessment of vector potential of these tiny pests in the transmission of BTV and other Culicoides borne diseases. Our results highlight a need for further in-depth studies to investigate and clarify the genetic compo-sition and epidemiology of Culicoides species, and their role in the transmission of pathogens.
Financial support
This research was funded by the Erciyes University Research Fund with the project code TSA-2015-5762.
Authors’ contributions
AY, AI and BD conceived the study; BD conducted thefield work; AY, OD, ZO, AC and GY conducted laboratory works, AY and OD analyzed the data and drafted the manuscript; AI, BD and AC helped with the interpretation of the results. All authors critically revised and approved thefinal manuscript. AY is the guarantor of the paper. Conflict of interest statement
The authors have no conflict of interest concerning the work per-formed in this paper.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.actatropica.2018.12. 005.
References
Ander, M., Meiswinkel, R., Chirico, J., 2012. Seasonal dynamics of biting midges (Diptera: Ceratopogonidae: Culicoides), the potential vectors of bluetongue virus, in Sweden. Vet. Parasitol. 184, 59–67.
Ander, M., Troell, K., Chirico, J., 2013. Barcoding of biting midges in the genus Culicoides: a tool for species determination. Med. Vet. Entomol. 27, 323–331.
Benelli, G., Buttazzoni, L., Canale, A., D’Andrea, A., Del Serrone, P., Delrio, G., Foxi, C., Mariani, S., Savini, G., Vadivalagan, C., Murugan, K., Toniolo, C., Nicoletti, M., Serafini, M., 2017. Bluetongue outbreaks: looking for effective control strategies against Culicoides vectors. Res. Vet. Sci. 115, 263–270.
Borkent, A., 2017. Ceratopogonidae. In: Kirk-Spriggs, A.H., Sinclair, B.J. (Eds.), Manual of Afrotropical Diptera Volume 2. Nematocerous Diptera and Lower Brachycera. Suricata 5. Pretoria: South African National Biodiversity Institute. P. pp. 733–812.
Carpenter, S., Wilson, A., Mellor, P.S., 2009. Culicoides and the emergence of bluetongue virus in northern Europe. Trends Microbiol. 17, 172–178.
Casati, S., Racloz, V., Delecolle, J.C., Kuhn, M., Mathis, A., Griot, C., Stark, K.D., Vanzetti, T., 2009. An investigation on the Culicoides species composition at seven sites in southern Switzerland. Med. Vet. Entomol. 23, 93–98.
Chakraborty, C., Doss, C.G.P., Patra, B.C., Bandyopadhyay, S., 2014. DNA barcoding to map the microbial communities: current advances and future directions. Appl. Microbiol. Biotechnol. 98, 3425–3436.
Chen, R., Jiang, L.Y., Qiao, G.X., 2012. The effectiveness of three regions in mitochondrial genome for aphid DNA barcoding: a case in Lachininae. PLoS One. 7, e46190.
Collins, A.B., Mee, J.F., Doherty, M.L., Barrett, D.J., England, M.E., 2018. Culicoides species composition and abundance on Irish cattle farms: implications for arboviral disease transmission. Parasit. Vectors 11, 472.
Dallas, J.F., Cruickshank, R.H., Linton, Y.M., Nolan, D.V., Patakakis, M., Braverman, Y., Capela, R., Capela, M., Pena, I., Meiswinkel, R., Ortega, M.D., Baylis, M., Mellor, P.S., Mordue Luntz, A.J., 2003. Phylogenetic status and matrilineal structure of the biting midge, Culicoides imicola, in Portugal, Rhodes and Israel. Med. Vet. Entomol. 17, 379–387.
Dik, B., 2017. Vectorial role and Control of Culicoides (Diptera: Ceratopogonidae). In: Ozbel, Y. (Ed.), Control of Vector Artropoda. Turkish Parasitology Society, pp. 74–78 No: 25, Izmir.
Dik, B., Yavru, S., Uslu, U., Yapici, O., Esin, E., 2012. Determination of Culicoides species (Diptera: Ceratopogonidae) and suspect vectors of epizootic haemorrhagic disease virus and bluetongue virus in Culicoides specimens by RT-PCR in southern and wes-tern Anatolia. Rev. Med. Vet. 163, 505–510.
Dik, B., Muz, D., Uslu, U., Muz, M., 2014. The geographical distribution andfirst mole-cular analysis of Culicoides Latreille (Diptera: Ceratopogonidae) species in southern and south-eastern Turkey during 2012 outbreak of bovine ephemeral fever. Parasitol. Res. 113, 4225–4232.
Edgar, R.C., 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797.
Fu, Y., 1997. Statistical tests of neutrality of mutations against popu- lation growth, hitchhiking and background selection. Genetics. 147, 915–925.
Giudicelli, G.C., Mader, G., Brandao de Freitas, L., 2015. Efficiency of ITS sequences for DNA barcoding in Passiflora (Passifloraceae). Int. J Mol. Sci. 16, 7289–7303.
Guindon, S., Gascuel, O., 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704.
Hajibabaei, M., Janzen, D.H., Burns, J.M., Hallwachs, W., Hebert, P.D., 2006. DNA bar-codes distinguish species of tropical Lepidoptera. Proc. Natl. Acad. Sci. U. S. A. 103, 968–971.
Harrup, L.E., Bellis, G.A., Balenghien, T., Garros, C., 2015. Culicoides Latreille (Diptera: Ceratopogonidae) taxonomy: current challenges and future directions. Infect. Genet. Evol. 30, 249–266.
Hebert, P.D., Gregory, T.R., 2005. The promise of DNA barcoding for taxonomy. Syst. Biol. 54, 852–859.
Hebert, P.D., Cywinska, A., Ball, S.L., 2003. Biological identifications through DNA bar-codes. Proc. Biol. Sci. 270, 313–321.
Hebert, P.D., Penton, E.H., Burns, J.M., Janzen, D.H., Hallwachs, W., 2004a. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. U. S. A. 101 (41), 14812–14817.
Hebert, P.D., Stoeckle, M.Y., Zemlak, T.S., Francis, C.M., 2004b. Identification of birds through DNA barcodes. PLoS Biol. 2, e312.
Hoffmann, B., Scheuch, M., Hoper, D., Jungblut, R., Holsteg, M., Schirrmeier, H., Eschbaumer, M., Goller, K.V., Wernike, K., Fischer, M., Breithaupt, A., Mettenleiter, T.C., Beer, M., 2012. Novel orthobunyavirus in cattle, Europe, 2011. Emerg. Infect. Dis. 18, 469–472.
Huelsenbeck, J.P., Ronquist, F., 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755.
Karthika, P., Vadivalagan, C., Thirumurugan, D., Kumar, R.R., Murugan, K., Canale, A., Benelli, G., 2018. DNA barcoding offive Japanese encephalitis mosquito vectors (Culex fuscocephala, Culex gelidus, Culex tritaeniorhynchus, Culex pseudovishnui and Culex vishnui). Acta Trop. 183, 84–91.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P., Drummond, A., 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649.
Kimura, M., 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16, 111–120.
Kumar, S., Stecher, G., Tamura, K., 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874.
hosts of biting midges (Diptera: Ceratopogonidae: Culicoides Latreille) in Denmark. Parasit. Vectors 5, 143.
Lassen, S.B., Nielsen, S.A., Skovgard, H., Kristensen, M., 2012b. Molecular differentiation of Culicoides biting midges (Diptera: Ceratopogonidae) from the subgenus Culicoides Latreille in Denmark. Parasitol. Res. 110, 1765–1771.
Leigh, J.W., Bryant, D., 2015. PopART: full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116.
Librado, P., Rozas, J., 2009. DnaSP v5: a software for comprehen- sive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452.
Liu, Y., Tao, H., Yu, Y., Yue, L., Xia, W., Zheng, W., Ma, H., Liu, X., Chen, H., 2018. Molecular differentiation and species composition of genus Culicoides biting midges (Diptera: Ceratopogonidae) in different habitats in southern China. Vet. Parasitol. 254, 49–57.
Mathieu, B., Cetre-Sossah, C., Garros, C., Chavernac, D., Balenghien, T., Carpenter, S., Setier-Rio, M.L., Vignes-Lebbe, R., Ung, V., Candolfi, E., Delecolle, J.C., 2012. Development and validation of IIKC: an interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the Western Palaearctic region. Parasit. Vectors 5, 137.
Meiswinkel, R., Gomulski, L.M., Delecolle, J.C., Goffredo, M., Gasperi, G., 2004. The taxonomy of Culicoides vector complexes - unfinished business. Vet. Ital. 40, 151–159.
Mellor, P.S., Boorman, J., Baylis, M., 2000. Culicoides biting midges: their role as arbo-virus vectors. Annu. Rev. Entomol. 45, 307–340.
Meloni, G., Cossu, M., Foxi, C., Vento, L., Circosta, S., Burrai, E., Masala, S., Goffredo, M., Satta, G., 2018. Combined larvicidal and adulticidal treatments to control Culicoides biting midges (Diptera: Ceratopogonidae): results of a pilot study. Vet Parasitol. 257, 28–33.
Murugan, K., Vadivalagan, C., Karthika, P., Panneerselvam, C., Paulpandi, M., Subramaniam, J., Wei, H., Alsalhi, M.S., Devanesan, S., Nicoletti, M., Paramasivan, R., Parajulee, M.N., Benelli, G., 2016. DNA barcoding and molecular evolution of mosquito vectors of medical and veterinary importance. Parasitol. Res. 115, 107–121.
Nei, M., Kumar, S., Oxford University, P., 2005. Molecular evolution and phylogenetics. Oxford University Press, Oxford [etc.].
Nielsen, S.A., Kristensen, M., 2015. Delineation of Culicoides species by morphology and barcode exemplified by three new species of the subgenus Culicoides (Diptera: Ceratopogonidae) from Scandinavia. Parasit. Vectors 8, 151.
Nielsen, S.A., Nielsen, B.O., Chirico, J., 2010. Monitoring of biting midges (Diptera: Ceratopogonidae: Culicoides Latreille) on farms in Sweden during the emergence of the 2008 epidemic of bluetongue. Parasitol. Res. 106, 1197–1203.
Nolan, D.V., Carpenter, S., Barber, J., Mellor, P.S., Dallas, J.F., Mordue Luntz, A.J., Piertney, S.B., 2007. Rapid diagnostic PCR assays for members of the Culicoides ob-soletus and Culicoides pulicaris species complexes, implicated vectors of bluetongue virus in Europe. Vet. Microbiol. 124, 82–94.
Pages, N., Munoz-Munoz, F., Talavera, S., Sarto, V., Lorca, C., Nunez, J.I., 2009. Identification of cryptic species of Culicoides (Diptera: Ceratopogonidae) in the sub-genus Culicoides and development of species-specific PCR assays based on barcode regions. Vet. Parasitol. 165, 298–310.
Pilgrim, J., Ander, M., Garros, C., Baylis, M., Hurst, G.D.D., Siozios, S., 2017. Torix group Rickettsia are widespread in Culicoides biting midges (Diptera: Ceratopogonidae),
reach high frequency and carry unique genomic features. Environ. Microbiol. 19, 4238–4255.
Posada, D., 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25, 1253–1256.
Ramilo, D.W., Diaz, S., Pereira da Fonseca, I., Delecolle, J.C., Wilson, A., Meireles, J., Lucientes, J., Ribeiro, R., Boinas, F., 2012. First report of 13 species of Culicoides (Diptera: Ceratopogonidae) in mainland Portugal and Azores by morphological and molecular characterization. PloS ONE 7, e34896.
Ramilo, D., Garros, C., Mathieu, B., Benedet, C., Allene, X., Silva, E., Alexandre-Pires, G., Fonseca, I.P., Carpenter, S., Radrova, J., Delecolle, J.C., 2013. Description of Culicoides paradoxalis sp. nov. From France and Portugal (Diptera: Ceratopogonidae). Zootaxa 3745, 243–256.
Sailleau, C., Breard, E., Viarouge, C., Vitour, D., Romey, A., Garnier, A., Fablet, A., Lowenski, S., Gorna, K., Caignard, G., Pagneux, C., Zientara, S., 2017. Re-emergence of bluetongue virus serotype 8 in France, 2015. Transbound Emerg. Dis. 64, 998–1000.
Sarvasova, A., Kocisova, A., Halan, M., Delecolle, J.C., Mathieu, B., 2014. Morphological and molecular analysis of the genus Culicoides (Diptera: Ceratopogonidae) in Slovakia withfive new records. Zootaxa 3872, 541–560.
Sarvasova, A., Kocisova, A., Candolfi, E., Mathieu, B., 2017. Description of Culicoides (Culicoides) bysta n. sp., a new member of the Pulicaris group (Diptera: Ceratopogonidae) from Slovakia. Parasit. Vectors 10, 279.
Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585–595.
Talavera, S., Munoz-Munoz, F., Verdun, M., Pages, N., 2017. Morphology and DNA bar-coding reveal three species in one: description of Culicoides cryptipulicaris sp. nov. and Culicoides quasipulicaris sp. nov. in the subgenus Culicoides. Med. Vet. Entomol. 31, 178–191.
Tonbak, S., Azkur, A.K., Pestil, Z., Biyikli, E., Abayli, H., Baydar, E., Poel, W.H.M., Bulut, H., 2016. Circulation of Schmallenberg virus in Turkey, 2013. Turk. J. Vet. Anim. Sci. 40, 175–180.
Vadivalagan, C., Pushparaj, K., Murugan, K., Panneerselvam, C., Del Serrone, P., Benelli, G., 2017. Exploring genetic variation in haplotypes of thefilariasis vector Culex quinquefasciatus (Diptera: Culicidae) through DNA barcoding. Acta Trop. 169, 43–50.
Virgilio, M., Backeljau, T., Nevado, B., De Meyer, M., 2010. Comparative performances of DNA barcoding across insect orders. B.M.C. Bioinformatics 11, 206.
Wenk, C.E., Kaufmann, C., Schaffner, F., Mathis, A., 2012. Molecular characterization of Swiss Ceratopogonidae (Diptera) and evaluation of real-time PCR assays for the identification of Culicoides biting midges. Vet. Parasitol. 184, 258–266.
Xia, Y., Gu, H.F., Peng, R., Chen, Q., Zheng, Y.C., Murphy, R.W., Zeng, X.M., 2012. COI is better than 16S rRNA for DNA barcoding Asiatic salamanders (Amphibia: Caudata: Hynobiidae). Mol. Ecol. Resour. 12, 48–56.
Zhang, R., Zhao, A., Wang, X., Zhang, Z., 2017. Diversity of tick species on domestic animals in Shandong Province, China, using DNA barcoding. Exp. Appl. Acarol. 73, 79–89.
Zhang, R.L., Zhang, B., 2014. Prospects of using DNA barcoding for species identification and evaluation of the accuracy of sequence databases for ticks (Acari: Ixodida). Ticks Tick Borne Dis. 5, 352–358.