Contents lists available atScienceDirect
Thrombosis Research
journal homepage:www.elsevier.com/locate/thromres
Full Length Article
The relationship between heparanase levels, thrombus burden and
thromboembolism in patients receiving unfractionated heparin treatment
for prosthetic valve thrombosis
Emrah Bayam
a, Macit Kalçık
b,⁎, Ahmet Seyfeddin Gürbüz
c, Mahmut Yesin
d, Ahmet Güner
a,
Sabahattin Gündüz
a, Mustafa Ozan Gürsoy
e, Süleyman Karakoyun
f, Sinan Cerşit
a,
Alev Kılıçgedik
a, Özkan Candan
a, Ali Yaman
g, Mehmet Özkan
a,haDepartment of Cardiology, Koşuyolu Kartal Heart Training and Research Hospital, Istanbul, Turkey bDepartment of Cardiology, Hitit University Faculty of Medicine, Çorum, Turkey
cDepartment of Cardiology, Necmeddin Erbakan University Meram Faculty of Medicine, Konya, Turkey dDepartment of Cardiology, Kars Harakani State Hospital, Kars, Turkey
eDepartment of Cardiology, Izmir Katip Çelebi University Atatürk Training and Research Hospital, Izmir, Turkey fDepartment of Cardiology, Kars Kafkas University, Faculty of Medicine, Kars, Turkey
gDepartment of Biochemistry, Marmara University, Faculty of Medicine, Istanbul, Turkey hDivision of Health Sciences, Ardahan University, Ardahan, Turkey
A R T I C L E I N F O
Keywords:
Echocardiography Heparanase
Prosthetic valve thrombosis Thromboembolism Unfractionated heparin
A B S T R A C T
Introduction: Procoagulant activity of heparanase has been recently described in several arterial and venous thrombotic disorders. In this study, we aimed to investigate the role of heparanase with regard to thrombus burden, thromboembolism, and treatment success with unfractionated heparin (UFH) in patients with prosthetic valve thrombosis (PVT).
Methods: This study enrolled 79 PVT patients who received UFH for PVT and 82 controls. Plasma samples which were collected from patients both at baseline and after the UFH treatment and from controls at baseline only, were tested for heparanase levels by heparanase enzyme-linked immunosorbent assay.
Results: The PVT group included 18 obstructive and 61 non-obstructive PVT patients who received UFH infu-sions for a median duration of 15 (7–20) days. The UFH treatment was successful in 37 (46.8%) patients. Baseline heparanase levels were significantly higher in the patient group than in the controls [0.29 (0.21–0.71) vs. 0.25 (0.17–0.33) ng/mL; p = 0.002]. Baseline heparanase levels were significantly higher in obstructive PVT patients. There was a significant increase in heparanase levels after UFH treatment. Post-UFH heparanase levels were higher in patients who experienced treatment failure compared to successfully treated group. Baseline and post-UFH heparanase levels were significantly higher in patients with a thrombus area ≥1 cm2and with a recent history of thromboembolism.
Conclusions: Increased heparanase levels may be one of the esoteric causes for PVT. UFH treatment may trigger an increase in heparanase levels which may affect the treatment success. Increased heparanase levels may be associated with high risk of thromboembolism and increased thrombus burden in PVT patients.
1. Introduction
Heparanase is a β‑glucuronidase that cleaves sugar chains of he-paran sulfate proteoglycans on the cell surface and in the extracellular matrix [1,2]. Heparanase activity is implicated in tumor growth [3], inflammation, tissue remodeling [4], angiogenesis [5] and cell invasion [6]. Heparanase is also directly involved in the activation of the
coagulation system [7], interacting with tissue factor and enhancing the generation of factor Xa [8]. Recent reports have described heparanase procoagulant activity in several arterial and venous thrombotic dis-orders [9,10].
Prosthetic valve thrombosis (PVT) is a serious complication with high mortality and morbidity [11]. Mechanical heart valves are thrombogenic, and anticoagulation is essential to prevent PVT and
https://doi.org/10.1016/j.thromres.2018.09.061
Received 7 July 2018; Received in revised form 8 September 2018; Accepted 25 September 2018 ⁎Corresponding author at: Buharaevler Mah, Buhara 25, Sok. No: 1/A Daire: 22, Çorum, Turkey.
E-mail address:macitkalcik@yahoo.com(M. Kalçık).
Available online 26 September 2018
0049-3848/ © 2018 Elsevier Ltd. All rights reserved.
thromboembolism [12]. Subtherapeutic anticoagulation, surgical tech-nique, endocardial fibrosis, pannus formation, foreign body reaction to the prosthetic valve and suture, atrial fibrillation, left atrial enlarge-ment, ventricular dysfunction, and pregnancy may lead to an increased risk of developing PVT [13].
Treatment modalities for PVT include optimized anticoagulation, thrombolytic therapy (TT) [14–16] and re-do surgery [17]. Guidelines lack definitive class 1a recommendations due to lack of randomized controlled trials; however, recently low-dose slow-infusion TT has been recommended as an effective and confidential treatment as class 1b recommendation in left-sided PVT [18]. On the other hand, optimized anticoagulation is the only treatment choice for patients with contra-indications to both TT and surgery [19]. Continuous infusion of un-fractionated heparin (UFH) for several days to weeks followed by oral vitamin K antagonists may serve as an optimized anticoagulation ap-proach in such patients.
In this study, we aimed to investigate the role of heparanase with regard to thrombus burden, thromboembolic events and treatment success with UFH in patients with PVT.
2. Methods
2.1. Study population
Between January 2014 and January 2017, 79 PVT patients (mean age: 49 ± 13.5 years, male: 28) who had a thrombotic mass > 10 mm in diameter and were eligible for UFH therapy and 82 controls (mean age: 49 ± 14.3 years, male: 31) with normally functioning prosthetic valves were enrolled in this single-center case-control study. Patients with end-stage liver disorders, renal insufficiency, pregnancy, chronic inflammatory diseases, infective endocarditis, coagulopathy and ma-lignancies were excluded from the study. Recent subtherapeutic antic-oagulation was defined as two or more measurements of International Normalized Ratio (INR) with a value of < 2.0 within the last 3 months. Recent thromboembolisms included proved cerebrovascular accidents, coronary and peripheral embolisms within the last 3 months. All pa-tients provided a written informed consent and the study protocol was approved by the local ethics committee of the hospital in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
2.2. Laboratory analyses
In order to detect heparanase levels, venous blood samples were collected after 12-h of fasting by a clean puncture of an antecubital vein from patients at baseline and after the UFH treatment and from the controls at baseline only. Blood samples were taken in 3.8% antic-oagulant tubes with sodium citrate and centrifuged at 2500 rpm for 20 min at room temperature. The plasma samples, obtained after cen-trifugation, were stored at −80 °C, for further analysis. The quantita-tive determination of heparanase concentration in human plasma samples was performed by an enzyme-linked immunosorbent assay (ELISA) using a commercially available Human HPA Elisa kit (ShanghaiSunred Biological Technology Co., Shanghai, China) as pre-viously described [20]. The assay range was within the range 50 pg/ ml–15 ng/mL.
2.3. Echocardiography
The diagnosis of PVT and the efficacy of UFH treatment were evaluated by serial transthoracic echocardiography (TTE), two-dimen-sional (2D) and real-time three-dimentwo-dimen-sional (RT 3D) transesophageal echocardiography (TEE) which were performed by using an X7-2t transducer on an iE33 ultrasound machine (Philips Medical Systems, Andover, Massachusetts). The pressure half-time method and continuity equation were used to assess the prosthetic valve areas. Thrombus was
density to the myocardium located at the valve occluder and/or valve struts and was visualized in all patients with PVT [21]. Patients with prosthetic heart valves who had been proved to be thrombus-free on TEE study and had no previous history of PVT constituted the control group.
During the echocardiographic examination, spontaneous echo con-trast was defined as dynamic smoke-like echoes within the cardiac cavities with a characteristic swirling motion that could not be elimi-nated by changes in gain settings [22]. PVT was classified as obstructive (OT) or nonobstructive (NOT) thrombus. The presence of obstruction was defined on the basis of Doppler echocardiographic measurements (peak velocity, mean gradient, effective orifice area, dimensionless index, and acceleration time as appropriate). The cut-off values for these Doppler parameters were defined based on the recent re-commendations [23].
The largest thrombus area was measured by 2D TEE between 0° and 180° angles where there was less interference with acoustic shadowing. In the presence of a single mass the thrombus was traced, otherwise each thrombi was traced separately and the thrombus areas were finally summed-up [14–16,21].
2.4. Rationale for UFH treatment
Patients with a PVT > 10 mm in diameter who had relative con-traindications to both TT and surgery and/or those who refused both therapy were accepted as suitable for UFH therapy and were included in the study. A 70 units/kg or maximum 5000 unit bolus of UFH was administered, followed by a 16 units/kg or maximum 1000 unit/h as the initial infusion rate. The infusion rate was adjusted with a target aPTT of 60 to 70 s which was assessed every 6 h. When an aPTT of < 50 s was measured, an intermittent mini-bolus (16 units/kg or max-imum 1000 unit) of UFH was administered in addition to dose adjust-ment (2 units/kg/h increase in infusion rate). When an aPTT of > 80 s was measured, UFH was interrupted for 30 to 60 min in addition to dose adjustment (2 units/kg/h decrease in infusion rate). Complete blood count was performed daily to detect any bleeding or heparin induced thrombocytopenia. Weekly TEE was performed for the evaluation of treatment success which was defined as the disappearance of thrombus or ≥75% decrease in thrombus burden by 2D gray scale imaging and resolution of Doppler findings without any fatal and non-fatal major complications as previously described [14–16,21]. Propagation or persistence of thrombus, or a < 50% reduction of thrombus burden, and major embolic or hemorrhagic complications were considered as treatment failure. Patients received UFH infusion until final success for maximum 30 days. Once treatment success was established with TEE, warfarin was reinitiated and UFH was stopped when INR levels reached 2.5. The target INR value was maintained between 2.5 and 4 on follow-up.
2.5. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp. Armonk, NY). Descriptive statistics are reported as mean ± standard deviation for continuous variables with normal distribution or median (25th–75th percentiles) values for continuous variables without normal distribution and as frequency with percentages for the categorical variables. The Shapiro-Wilk test was used to test the normality of the distribution of continuous variables. Student t-test or Mann-Whitney U test was used to compare continuous variables as appropriate. Categorical variables were compared using the chi-square test. The significance level was accepted as p < 0.05 in all statistical analyses. Correlational analyses were performed using Pearson, Kendall's tau or Spearmen's correlation tests as appropriate. A logistic regression analysis was performed in order to identify any in-dependent predictors of PVT and failed UFH treatment. A receiver
op-sensitivity, specificity, area under the curve (AUC) and confidence in-terval (CI) of best heparanase level cut-offs for predicting PVT, thromboembolism and failed UFH treatment.
3. Results
The study population consisted of 79 patients with PVT (28 male, mean age: 49.8 ± 13.5) and 82 control patients (31 male, mean age: 49.9 ± 14.3). The patient group included 12 aortic, 51 mitral, 13 aortic + mitral and 3 tricuspid PVT patients (18 OT and 61 NOT). The control group included 15 aortic, 56 mitral, 10 aortic + mitral and 1 tricuspid valve patients. There was no significant difference in terms of demographic characteristics between PVT patients and controls except for New York Heart Association (NYHA) functional class, recent history of subtherapeutic anticoagulation and recent history of thromboem-bolism. The demographic characteristics of the study population are summarized inTable 1.
Patients received an average of 15 (7–20) days of UFH infusion. UFH treatment was successful in 37 patients (46.8%) and failed in 42 patients. Redo valve surgery was performed in 13 patients and TT was administered to 15 patients in whom UFH therapy was unsuccessful. The remaining 14 patients received oral anticoagulation therapy with close follow-up. Overall 12 (15.2%) patients experienced complications (4 non-fatal major complications, 1 death, 7 minor complications). Non-fatal major complications included 2 thromboembolic events (cerebral), 1 intracranial hemorrhage and 1 gastrointestinal bleeding requiring transfusion. Minor complications included self-limiting bleedings (4 hemoptysis, 1 rectus hematoma, and 2 gastrointestinal bleeding) without need for transfusion.
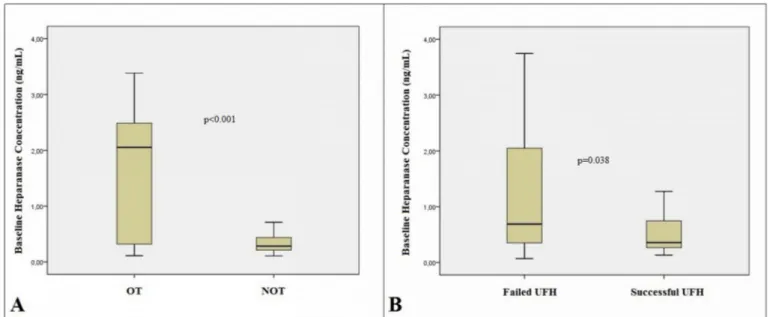
Baseline heparanase levels were higher in the patient group com-pared to control group [0.29 (0.21–0.71) vs. 0.25 (0.17–0.33) ng/ml; p = 0.002] (Fig. 1A) and in patients with OT than those with NOT [2.05 (0.29–2.67) vs. 0.28 (0.15–0.44) ng/mL; p < 0.001] (Fig. 2A). There was a significant difference between baseline and post-UFH heparanase
levels [0.29 (0.21–0.71) vs. 0.48 (0.28–1.27) ng/mL; p < 0.001] (Fig. 1B). Baseline heparanase levels were significantly higher in pa-tients with failed UFH treatment than those who were treated suc-cessfully [0.35 (0.22–1.35) vs. 0.28 (0.19–0.50) ng/mL; p = 0.038] (Fig. 2B). Post-UFH heparanase levels were significantly higher in pa-tients with failed UFH therapy than those with successful outcomes [0.69 (0.34–2.17) vs. 0.36 (0.26–0.79) ng/mL; p = 0.016].
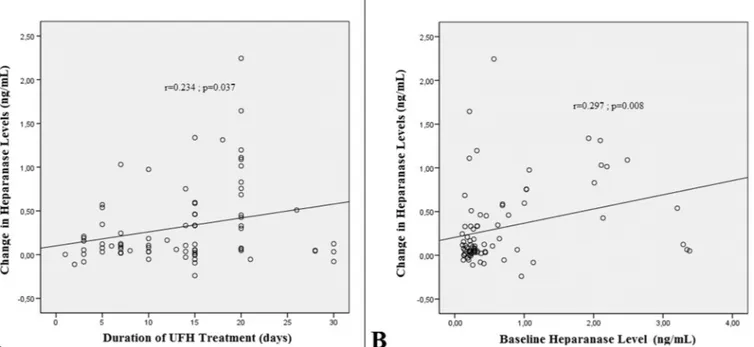
There was a weak but significant positive correlation between the change in heparanase levels and the duration of heparin therapy in patients with PVT (r = 0.234; p = 0.037) (Fig. 3A) and also between the change in heparanase levels and baseline heparanase levels (r = 0.297; p = 0.008) (Fig. 3B).
The parameters which were significantly different between PVT patients and controls in univariate analyses were taken into multiple logistic regression analysis. High NYHA functional class, recent history of subtherapeutic anticoagulation, high baseline heparanase level and recent history of thromboembolism were identified as independent predictors of PVT (Table 2). When analyzed with the ROC curve, a baseline heparanase level above 0.25 ng/mL predicted PVT formation with 60.8% sensitivity and 58.5% specificity (AUC: 0.643; 95% CI: 0.556–0.730; p = 0.002) (Fig. 4A).
Parameters that would affect the outcome of UFH therapy were compared between failed and successful UFH groups. Thrombus type, thrombus area, history of thromboembolism, baseline and post-UFH heparanase levels were significantly different between the subgroups (Table 3). The duration of heparin therapy was higher in the failed group (p = 0.052). Significant and almost significant (0.1 > p > 0.05) variables that could predict failed UFH therapy in univariate analyzes were taken into multiple logistic regression analysis (Table 4). Obstructive thrombus type, high thrombus area and high baseline heparanase levels were identified as independent predictors of failed UFH therapy (OR: 6.296; 95% CI: 1.651–24.012, p = 0.007; OR: 3.181; 95% CI: 0.105–0.456, p = 0.002 and OR: 2.921; 95% CI: 1.224–6.970; p = 0.016 respectively). When analyzed with the ROC curve, it was observed that baseline heparanase levels above 0.31 ng/ mL predicted failed UFH treatment with a sensitivity of 61.9% and a specificity of 64.9% (AUC: 0.636; 95% CI: 0.514–0.758; p = 0.038) (Fig. 4B). Furthermore, thrombus area above 0.87 cm2predicted failed UFH treatment with a sensitivity of %73.2 and a specificity of 61.1% (AUC: 0.696; 95% CI: 0.579–0.814; p = 0.003) (Fig. 5A).
Baseline characteristics and heparanase levels were compared be-tween patients with and without a recent history of thromboembolism. The prevalence of failed UFH therapy, thrombus type, thrombus area, baseline and post-UFH heparanase levels were significantly different between the subgroups (Table 5). Significant variables that could pre-dict thromboembolism in univariate analyzes were taken into multiple logistic regression analysis (Table 6). Only thrombus area was found to be an independent predictors of thromboembolism (OR: 2.285; 95% CI: 0.029–0.426, p = 0.025). By the ROC curve analysis, thrombus area above 1.05 cm2predicted thromboembolism with a sensitivity of %81 and a specificity of 67% (AUC: 0.788; 95% CI: 0.679–0.898; p < 0.001) (Fig. 5B).
PVT patients were divided into 2 groups based on their thrombus area [Group I (n = 42): thrombus area < 1.05 cm2 and Group II (n = 37): thrombus area ≥ 1.05 cm2]. The baseline and post-UFH he-paranase levels were significantly higher in Group II as compared to Group I [0.64 (0.22–1.96) vs. 0.27 (0.21–0.36); p = 0.002 and 0.96 (0.37–2.70) vs. 0.35 (0.26–0.67); p = 0.002 respectively].
The baseline heparanase levels were > 1 ng/mL in 16 patients. In this subgroup, 12 patients had OT and the thrombus area was > 1.5 cm2in the remaining 4 patients with NOT. Furthermore, a recent his-tory of thromboembolism was more common in this subgroup [9/16 (56%) vs. 14/63 (22.2%) patients; p = 0.008].
Table 1
Baseline demographic characteristics of the study population.
PVT
n = 79 Controln = 82 p value Age (years) 49.8 ± 13.5 49.9 ± 14.3 0.423 Gender (male), n (%) 28 (35.4) 31 (38.7) 0.638 Prosthetic valve
position, n (%) Mitral: 51 (64.6)Aortic: 12 (15.1) Aortic + mitral: 13 (16.5) Tricuspid: 3 (3.8) Mitral: 56 (68.3) Aortic: 15 (18.2) Aortic + mitral: 10 (12.3) Tricuspid: 1 (1.2) 0.593
Leaflet status, n (%) Monoleaflet: 12 (15.2) Bileaflet: 67 (84.8) Monoleaflet: 13 (15.8) Bileaflet: 69 (84.2) 0.541 Atrial fibrillation, n (%) 34 (43.1) 37 (45.1) 0.457 SEC, n (%) 22 (27.8) 17 (20.7) 0.192 LAD (mm) 42.5 ± 6.3 43.7 ± 6.1 0.219 NYHA functional class I–II: 62 (78.5)
III–IV: 17 (21.5) I–II: 80 (97.5)III–IV: 2 (2.5) < 0.001 Recent subtherapeutic
INR 57 (72.1) 42 (51.2) 0.006 LV EF (%) 52.0 ± 5.8 53.2 ± 9.1 0.324 Recent history of TE 23 (29.1) 7 (8.5) 0.001 Thrombus type OT: 18 (22.8)
NOT: 61 (77.2) – – Thrombus area, cm2 1.0 (0.6–1.5) – –
Baseline heparanase
levels (pg/dL) 0.29 (0.21–0.71) 0.25 (0.17–0.33) 0.002
INR: International Normalized Ratio; LAD: left atrial diameter; LV EF: left ventricular ejection fraction; NYHA: New York Heart Association; NOT: non-obstructive thrombus; OT: non-obstructive thrombus; PVT: prosthetic valve thrombosis, SEC: spontaneous echo contrast; TE: thromboembolism.
4. Discussion
In this prospective and observational study, we have focused on the role of heparanase in patients with PVT with regard to thrombus burden, thromboembolism, and treatment outcomes of UFH therapy as increased heparanase levels have been shown to be associated with a hypercoagulable state. Baseline heparanase levels were significantly higher in PVT patients than in the controls. There was also an increase in heparanase levels in PVT patients after UFH therapy. Furthermore, high baseline heparanase levels were indicative for increased thrombus burden, longer duration and unsuccessful outcomes of UFH treatment. PVT is a rare complication of valvular replacement surgery that is associated with high mortality and morbidity [14–17]. TTE, 2D and RT-3D TEE and multi-detector cardiac computed tomography play an im-portant role in the diagnosis of PVT [23–26]. Despite technological advancements, prosthetic valves are still thrombogenic because of
foreign body reaction and endothelial damage. Effective antic-oagulation is crucial for preventing PVT and its complications. Al-though subtherapeutic anticoagulation is the most prominent cause of PVT development, it may rarely occur in patients under therapeutic anticoagulation. Several esoteric causes such as genetic mutations [27], elevated fibrinogen [28], anti-cardiolipin antibodies [29], anti-tissue plasminogen activator antibodies [30] and AB0 blood groups [31] may play a role in the aggravation of thrombus formation despite effective anticoagulation. This study revealed increased heparanase levels as a novel esoteric cause of PVT development.
Treatment options for PVT include optimized anticoagulation, TT and re-do valve surgery. Until recently, surgery was recommended as the first-line treatment for left-sided PVT despite high morbidity and mortality rates [12,13,17]. Recently TT has increasingly been per-formed in the treatment of PVT. Complications such as hemorrhage and thromboembolism have been reduced without compromising success
Fig. 1. The box plot graphs revealing the comparison of baseline heparanase concentrations between patients with prosthetic valve thrombosis (PVT) and control
subjects (A) and significant increase in heparanase levels after unfractionated heparin (UFH) therapy (B) (statistical significance of p < 0.05 was calculated using Mann-Whitney U test).
Fig. 2. The box plot graphs revealing the comparison of baseline heparanase concentrations between patients with obstructive (OT) and non-obstructive (NOT)
rates with low dose and slow infusion protocols [14–16]. Following recent studies, the 2017 ACC/AHA valve disease guideline has been revised and TT in PVT therapy has been recommended with an in-dication of class 1b and, alternatively, equivalent to surgical treatment [18]. On the other hand, optimized anticoagulation with UFH is the only treatment choice for patients with contraindications to both TT and surgery [19].
Heparanase has been recently revealed to be an important mod-ulator of blood coagulation and to serve as a cofactor of tissue factor, suggesting that heparanase is directly involved in the activation of the coagulation cascade in an enzymatically independent manner [32]. The
Fig. 3. There was a weak but significant positive correlation between the change in heparanase levels and the duration of heparin therapy in patients with PVT (A)
and also between the change in heparanase levels and baseline heparanase levels (B).
Table 2
The results for multivariate logistic regression analyses of univariate predictors of prosthetic valve thrombosis.
OR 95% CI p value High NYHA functional class 6.411 0.513–0.970 < 0.001 Recent subtherapeutic INR 5.083 0.243–0.552 < 0.001 High baseline heparanase 2.344 0.022–0.257 0.020 Recent history of TE 2.382 0.038–0.405 0.018
CI: confidence interval; INR: International Normalized Ratio; NYHA: New York Heart Association; OR: odds ratio; TE: thromboembolism.
Fig. 4. In ROC curve analyses, baseline heparanase level above 0.25 ng/mL predicted PVT formation with a sensitivity of 60.8% and a specificity of 58.5% (AUC: 643;
95% CI: 0.556–0.730; p = 0.002) (A) and heparanase levels above 0.31 ng/mL predicted failed UFH treatment with a sensitivity of 61.9% and a specificity of 64.9% (AUC: 0.636; 95% CI: 0.514–0.758; p = 0.038).
elevation of heparanase levels in human cancer, together with asso-ciated pro-thrombotic states of most neoplasms suggests possible clin-ical relevance of the procoagulant function of heparanase [5]. In ad-dition, heparanase procoagulant activity has been revealed to be significantly increased in hypercoagulable clinical set-ups, including women at the end of pregnancy [33], women using oral contraceptives [34], patients following orthopedic surgery [35] and patients with stable to vulnerable atherosclerotic plaques [36,37].
Consistent with the literature, increased heparanase levels were
associated with increased PVT risk, high thrombus burden and high thromboembolic complication rates in this study. Baseline heparanase was one of the independent predictors of PVT development and base-line heparanase levels above 1 ng/mL were found to be related with increased thrombus burden. Although the correlation between baseline heparanase levels and absolute change in heparanase level after UFH treatment and the correlation between duration of UFH treatment and absolute change in heparanase level after UFH treatment were very weak correlations, for the sake of the significant p values, we may suggest that patients with higher heparanase levels may need a longer UFH therapy.
In recent studies, in which TT was successfully performed in the management of PVT, anticoagulation with intravenous UFH was with-held during tissue type plasminogen infusion given the safety concerns [14–16]. This strategy may also provide relatively stabilized hepar-anase levels and hence may be in association with high rate of throm-bolytic success. This hypothesis should be considered in prospective trials comparing thrombolysis with or without concomitant UFH treatment in various clinical scenarios including pulmonary embolism, stroke, myocardial infarction and prosthetic valve thrombosis.
Heparanase levels increased significantly after UFH therapy and were found to be associated with the success of the treatment in our study. Like an antigen-antibody reaction, exposure to UFH may trigger an increase in heparanase levels which may influence the success and duration of the treatment. The increase in heparanase levels may be observed due to release from the cell surfaces. The weak but positive correlation between baseline heparanase levels and the change in he-paranase level after UFH treatment supports this hypothesis. Similarly, Peled et al. demonstrated elevated heparanase levels and procoagulant activity in orthopedic surgery patients after receiving a prophylactic dose of enoxaparin [35]. High baseline heparanase levels were one of the independent predictors of failed UFH therapy. Thus, baseline he-paranase levels may have an impact on selection of initial treatment strategy and cessation of UFH therapy during TT in patients with PVT.
4.1. Study limitations
The primary limitation was that our study was a nonrandomized, observational and single center study with a relatively small number of patients. The other limitation of the study was that PVT is a multi-factorial disease, and the genetic and other predisposing esoteric factors were not investigated. In this study, only heparanase concentration was measured. The lack of heparanase activity measurements is another limitation of the present study.
5. Conclusion
This is the first study in the literature that demonstrates the role of heparanase in patients with PVT. Increased heparanase levels may be one of the novel esoteric causes for PVT in patients with prosthetic heart valves. UFH treatment may trigger an increase in heparanase le-vels which may affect the success of the treatment. Patients with high baseline heparanase levels may be prone to develop tromboembolism and increase in thrombus burden. Measurement of plasma heparanase levels in patients with mechanical heart valves may provide useful in-formation regarding PVT risk and outcomes of UFH treatment. Future studies with larger sample sizes are needed to confirm the results of the present study.
Table 3
Comparison of baseline characteristics and heparanase levels between suc-cessful and failed UFH subgroups.
Successful UFH
n = 37 Failed UFHn = 42 p value Age (years) 52.1 ± 11.4 47.9 ± 16.3 0.208 Gender (male), n (%) 12 (32.4) 16 (38.1) 0.600 Prosthetic valve
position, n (%) Mitral: 22 (59.5)Aortic: 5 (13.5) Aortic + mitral: 9 (24.3) Tricuspid: 1 (2.7) Mitral: 29 (69.1) Aortic: 7 (16.6) Aortic + mitral: 4 (9.5) Tricuspid: 2 (4.8) 0.355
Leaflet status, n (%) Monoleaflet: 5 (13.5) Bileaflet: 32 (86.5) Monoleaflet: 7 (16.7) Bileaflet: 35 (83.3) 0.697 Atrial Fibrillation, n (%) 16 (43.2) 18 (42.8) 0.972 SEC, n (%) 10 (27.1) 12 (28.6) 0.879 LAD (mm) 43.3 ± 6.5 41.6 ± 6.0 0.236 LV EF (%) 51.9 ± 6.6 52.1 ± 4.9 0.909 NYHA functional class, n
(%) I–II: 27 (72.9)III–IV: 10 (27.1) I–II: 35 (83.3)III–IV: 7 (16.7) 0.264 Recent subtherapeutic
INR, n (%) 26 (70.2) 31 (73.8) 0.726 Recent history of TE 8 (21.6) 15 (35.7) 0.036 Thrombus type, n (%) OT: 3 (8.1)
NOT: 34 (91.9) OT: 15 (35.7)NOT: 27 (64.3) 0.004 Thrombus area, cm2 0.7 (0.6–1.2) 1.1 (0.7–1.6) 0.003 Baseline heparanase levels (ng/dL) 0.28 (0.19–0.50) 0.35 (0.22–1.35) 0.008 Post-UFH heparanase levels (ng/dL) 0.35 (0.26–0.79) 0.69 (0.34–2.17) 0.016 Duration of UFH treatment (days) 12 (5.5–15) 15 (10−20) 0.052 Change in heparanase levels (ng/dL) 0.09 (0.03–0.41) 0.12 (0.04–0.57) 0.395
INR: International Normalized Ratio; LAD: left atrial diameter; LV EF: left ventricular ejection fraction; NYHA: New York Heart Association; NOT: non-obstructive thrombus; OT: non-obstructive thrombus; SEC: spontaneous echo con-trast; TE: thromboembolism; UFH: unfractionated heparin.
Table 4
The results for multivariate logistic regression analyses of univariate predictors of failed UFH treatment in prosthetic valve thrombosis.
OR 95% CI p value Obstructive thrombus type 6.296 1.651–24.012 0.007 High thrombus area 3.181 0.105–0.456 0.004 High baseline heparanase 2.921 1.224–6.970 0.016 High post-UFH heparanase 1.559 −0.025–0.201 0.079 Long duration of UFH treatment 1.066 0.993–1.145 0.223 Recent history of TE 1.277 −0.095–0.435 0.205
CI: confidence Interval; UFH: unfractionated heparin; OR: odds ratio; TE: thromboembolism.
Addendum
E. Bayam, AS. Gürbüz, M. Kalçık, S. Gündüz and M. Özkan parti-cipated in study design and interpretation, and reviewed the manu-script. AS. Gürbüz, M. Yesin, A. Güner and A. Yaman were responsible for data collection and reviewed the manuscript. E. Bayam, M. Kalçık, S. Gündüz, M.O. Gürsoy, S. Karakoyun analyzed the data, and co-wrote the manuscript. S. Cerşit, A. Kılıçgedik, Ö. Candan revised the manu-script.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Competing interests
All of the authors have no conflict of interest. Funding
The author(s) received no financial support for the research, au-thorship, and/or publication of this article.
Fig. 5. In ROC curve analyses, thrombus area over 0.87 cm2predicted failed UFH treatment with a sensitivity of %73.2 and a specificity of 61.1% (AUC: 0.696; 95% CI: 0.579–0.814; p = 0.003) and thrombus area over 1.05 cm2predicted thromboembolism with a sensitivity of %81 and a specificity of 67% (AUC: 0.788; 95% CI: 0.679–0.898; p < 0.001).
Table 5
Comparison of baseline characteristics and heparanase levels between patients with and without a recent history of thromboembolism.
TE (+)
n = 23 TE (−)n = 56 p value Age (years) 50.3 ± 14.6 49.6 ± 14.2 0.842 Gender (male), n (%) 7 (30.4) 21 (37.5) 0.551 Prosthetic valve
position, n (%) Mitral: 14 (60.8)Aortic: 6 (26.1) Aortic + mitral: 2 (8.7) Tricuspid: 1 (4.4) Mitral: 37 (66.1) Aortic: 6 (10.7) Aortic + mitral: 11 (19.6) Tricuspid: 2 (3.6) 0.282
Leaflet status, n (%) Monoleaflet: 6 (26.1) Bileaflet: 17 (73.9) Monoleaflet: 6 (10.7) Bileaflet: 50 (89.3) 0.084 Atrial Fibrillation, n (%) 13 (56.5) 21 (37.5) 0.121 SEC, n (%) 9 (39.1) 13 (23.2) 0.152 LAD (mm) 43.4 ± 5.5 42.2 ± 6.6 0.454 LV EF (%) 51.6 ± 6.8 52.1 ± 5.4 0.706 NYHA functional class,
n (%) I–II: 20 (86.9)III–IV: 3 (13.1) I–II: 42 (75)III–IV: 14 (25) 0.240 Recent subtherapeutic
INR, n (%) 18 (78.3) 39 (69.6) 0.438 Failed UFH therapy 17 (73.9) 25 (44.6) 0.018 Thrombus type, n (%) OT: 10 (43.5)
NOT: 13 (56.5) OT: 8 (14.3)NOT: 48 (85.7) 0.005 Thrombus area, cm2 1.5 (1.1–1.8) 0.8 (0.6–1.2) < 0.001 Baseline heparanase levels (ng/dL) 0.45 (0.26–2.16) 0.27 (0.21–0.55) 0.003 Post-UFH heparanase levels (ng/dL) 0.90 (0.34–3.33) 0.39 (0.26–0.93) 0.011 Duration of UFH treatment (days) 15 (8.5–20) 14 (7–20) 0.418 Change in heparanase levels (ng/dL) 0.12 (0.04–0.54) 0.10 (0.03–0.40) 0.143
INR: International Normalized Ratio; LAD: left atrial diameter; LV EF: left ventricular ejection fraction; NYHA: New York Heart Association; NOT: non-obstructive thrombus; OT: non-obstructive thrombus; SEC: spontaneous echo con-trast; TE: thromboembolism; UFH: unfractionated heparin.
Table 6
The results for multivariate logistic regression analyses of univariate predictors of thromboembolism.
OR 95% CI p value High thrombus area 2.285 0.029–0.426 0.025 High baseline heparanase 1.845 −0.020–0.529 0.069 High post-UFH heparanase 0.703 −0.281–0.135 0.484 Failed UFH therapy 1.089 −0.088–0.298 0.280 Obstructive thrombus type 0.826 −0.178–0.430 0.412
References
[1] C. Freeman, C.R. Parish, Human platelet heparanase: purification, characterization and catalytic activity, Biochem. J. 330 (1998) 1341–1350.
[2] D.S. Pikas, J.P. Li, I. Vlodavsky, U. Lindahl, Substrate specificity of heparanases from human hepatoma and platelets, J. Biol. Chem. 273 (1998) 18770–18777. [3] Y. Nadir, B. Brenner, Heparanase multiple effects in cancer, Thromb. Res. 133
(2014) 904.
[4] Y. Crispel, S. Ghanem, J. Attias, I. Kogan, B. Brenner, Y. Nadir, Involvement of the heparanese procoagulant domain in bleeding and wound healing, J. Thromb. Haemost. 15 (2017) 1463–1472.
[5] Y. Nadir, B. Brenner, Heparanase-a link between coagulation, angiogenesis and cancer, Rambam Maimonides Med. J. 31 (2012) e0002.
[6] C.R. Parish, C. Freemen, M.D. Hulett, Heparanase: a key enzyme involved in cell invazion, Biochim. Biophys. Acta 1471 (2011) 99–108.
[7] Y. Nadir, B. Brenner, Heparanase procoagulant activity, Thromb. Res. 129 (2012) 76–79.
[8] Y. Nadir, B. Brenner, L. Fux, I. Shafat, J. Attias, I. Vlodavsky, Heparanase enhances the generation of activated factor X in the presence of tissue factor and activated factor VII, Haematologica 95 (2010) 1927–1934.
[9] E. Peled, E. Melamed, T.B. Portal, E. Axelman, D. Norman, B. Brenner, Y. Nadir, Heparanase procoagulant activity as a predictor of wound necrosis following dia-betic foot amputation, Thromb. Res. 139 (2016) 148–153.
[10] Y. Hu, A. Atik, H. Yu, T. Li, B. Zhang, D. Li, H. Cai, Y. Yu, J. Chen, G. Li, L. Yuan, Serum heparanase concentration and heperanse activity in patients with retinal vein occlusion, Acta Ophthalmol. 95 (2017) e62–e66.
[11] L.H. Edmunds Jr., R.E. Clark, L.H. Cohn, G.L. Grunkemeier, D.C. Miller, R.D. Weisel, Guidelines for reporting morbidity and mortality after cardiac valvular operations. Ad hoc liaison committee for standardizing definitions of prosthetic heart valve morbidity of The American Association for Thoracic Surgery and The Society of Thoracic Surgeons, J. Thorac. Cardiovasc. Surg. 112 (1996) 708–711. [12] M. Lengyel, D. Horstkotte, H. Voller, W.P. Mistiaen, Working Group Infection,
Thrombosis, Embolism and Bleeding of the Society for Heart Valve Disease, Recommendations for the management of prosthetic valve thrombosis, J. Heart Valve Dis. 14 (2005) 567–575.
[13] M.O. Gürsoy, M. Kalçık, M. Yesin, S. Karakoyun, E. Bayam, S. Gündüz, M. Özkan, A global perspective on mechanical prosthetic heart valve thrombosis: diagnostic and therapeutic challenges, Anatol. J. Cardiol. 16 (2016) 980–989.
[14] M. Ozkan, S. Gunduz, M. Biteker, M.A. Astarcıoğlu, C. Cevik, E. Kaynak, M. Yıldız, E. Oğuz, A.Ç. Aykan, E. Ertürk, Y. Karavelioğlu, T. Gökdeniz, H. Kaya, O.M. Gürsoy, B. Çakal, S. Karakoyun, N. Duran, N. Özdemir, Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: the TROIA Trial, JACC Cardiovasc. Imaging 6 (2013) 206–216.
[15] M. Ozkan, B. Cakal, S. Karakoyun, O.M. Gursoy, C. Cevik, M. Kalcık, A.E. Oğuz, S. Gündüz, M.A. Astarcioglu, A.Ç. Aykan, Z. Bayram, M. Biteker, E. Kaynak, G. Kahveci, N.E. Duran, M. Yıldız, Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator, Circulation 128 (2013) 532–540. [16] M. Özkan, S. Gündüz, O.M. Gürsoy, S. Karakoyun, M.A. Astarcıoğlu, M. Kalçık,
A.Ç. Aykan, B. Çakal, Z. Bayram, A.E. Oğuz, E. Ertürk, M. Yesin, T. Gökdeniz, N.E. Duran, M. Yıldız, A.M. Esen, A novel strategy in the management of PROsthetic Mechanical valve Thrombosis and the prEdictors of outcomE: the Ultra-slow PROMETEE trial, Am. Heart J. 170 (2015) 409–418.
[17] F.M. Castilho, M.R. De Sousa, A.L. Mendonça, A.L. Ribeiro, F.M. Cáceres-Lóriga, Thrombolytic therapy or surgery for valve prosthesis thrombosis: systematic review and meta-analysis, J. Thromb. Haemost. 12 (2014) 1218–1228.
[18] R.A. Nishimura, C.M. Otto, R.O. Bonow, B.A. Carabello, J.P. Erwin 3rd, L.A. Fleisher, H. Jneid, M.J. Mack, C.J. McLeod, P.T. O'Gara, V.H. Rigolin, T.M. Sundt 3rd, A. Thompson, 2017 AHA/ACC Focused Update of the 2014 AHA/ ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Circulation 135 (2017) e1159–e1195.
[19] F.M. Caceres-Loriga, Heparin in the treatment of prosthetic valve thrombosis, Heart Lung Circ. 24 (2015) 423.
[20] Y. Nadir, G. Sarig, E. Axelman, A. Meir, M. Wollner, I. Shafat, R. Hoffman, B. Brenner, I. Vlodavsky, N. Haim, Heparanase procoagulant activity is elevated and
predicts survival in non-small cell lung cancer patients, Thromb. Res. 134 (2014) 639–642.
[21] M. Ozkan, C. Kaymaz, C. Kırma, K. Sonmez, N. Ozdemir, M. Balkanay, C. Yakut, U. Deligönül, Intravenous thrombolytic treatment of mechanical prosthetic heart valve thrombosis: a study using serial transesophageal echocardiography, J. Am. Coll. Cardiol. 35 (2000) 1881–1889.
[22] I.W. Black, A.P. Hopkins, L.C.L. Lee, W.F. Walsh, Left atrial spontaneous echo contrast: a clinical and echocardiographic analysis, J. Am. Coll. Cardiol. 18 (1991) 398–404.
[23] P. Lancellotti, P. Pibarot, J. Chambers, T. Edvardsen, V. Delgado, R. Dulgheru, M. Pepi, B. Cosyns, M.R. Dweck, M. Garbi, J. Magne, K. Nieman, R. Rosenhek, A. Bernard, J. Lowenstein, M.L. Vieira, A. Rabischoffsky, R.H. Vyhmeister, X. Zhou, Y. Zhang, J.L. Zamorano, G. Habib, Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging, Eur. Heart J. Cardiovasc. Imaging 17 (2016) 589–590.
[24] O.M. Gürsoy, S. Karakoyun, M. Kalçık, M. Özkan, The incremental value of RT three-dimensional TEE in the evaluation of prosthetic mitral valve ring thrombosis complicated with thromboembolism, Echocardiography 30 (2013) E198–E201. [25] M. Ozkan, O.M. Gürsoy, M.A. Astarcıoğlu, S. Gündüz, B. Cakal, S. Karakoyun,
M. Kalçık, G. Kahveci, N.E. Duran, M. Yıldız, C. Cevik, Real-time three-dimensional transesophageal echocardiography in the assessment of mechanical prosthetic mi-tral valve ring thrombosis, Am. J. Cardiol. 112 (2013) 977–983.
[26] S. Gündüz, M. Özkan, M. Kalçik, O.M. Gürsoy, M.A. Astarcioğlu, S. Karakoyun, A.Ç. Aykan, M. Biteker, T. Gökdeniz, H. Kaya, M. Yesin, N.E. Duran, D. Sevinç, T. Güneysu, Sixty-four-section cardiac computed tomography in mechanical pros-thetic heart valve dysfunction: thrombus or pannus, Circ. Cardiovasc. Imaging 8 (2015) e003246.
[27] M. Kalcik, M.O. Gursoy, S. Karakoyun, M. Yesin, M.A. Astarcioglu, M. Ozkan, Potential inherited causes of recurrent prosthetic mitral valve thrombosis in a pregnant patient suffering from recurrent miscarriage, Kor. Circ. J. 44 (2014) 268–270.
[28] A.C. Aykan, T. Gökdeniz, S. Gündüz, M.A. Astarcioğlu, O.M. Gürsoy, E. Ertürk, A.E. Oğuz, Z. Bayram, S. Karakoyun, M. Kalçik, M. Ozkan, Value of serum fi-brinogen levels in the assessment of mechanical prosthetic valve thrombosis, J. Heart Valve Dis. 23 (2014) 222–227.
[29] A.Ç. Aykan, T. Gökdeniz, M. Kalçık, M.A. Astarcıoğlu, S. Gündüz, S. Karakoyun, M.O. Gürsoy, A.E. Oğuz, E. Ertürk, B. Çakal, Z. Bayram, M. Özkan, Role of antic-ardiolipin antibodies in the pathogenesis of prosthetic valve thrombosis: an ob-servational study, Herz 40 (2015) 528–533.
[30] M. Özkan, M. Kalçık, M.O. Gürsoy, L. Öcal, S. Griffini, S. Karakoyun, M. Yesin, S. Gündüz, M.A. Astarcıoğlu, E. Bayam, S. Cerşit, A.Ç. Aykan, M. Cugno, Assessment of anti-tissue type plasminogen activator antibodies in patients with prosthetic heart valve thrombosis: the ATA Trial, J. Cardiovasc. Pharmacol. Ther. 21 (2016) 372–380.
[31] M.A. Astarcıoğlu, M. Kalçık, M. Yesin, M.O. Gürsoy, T. Şen, S. Karakoyun, S. Gündüz, M. Özkan, AB0 blood types: impact on development of prosthetic me-chanical valve thrombosis, Anatol. J. Cardiol. 16 (2016) 820–823.
[32] Y. Nadir, B. Brenner, Heparanase procoagulant activity in cancer progression, Thromb. Res. 140 (Suppl 1) (2016) S44–S48.
[33] Y. Nadir, Y. Kenig, A. Drugan, I. Shafat, B. Brenner, An assay to evaluate heparanase procoagulant activity, Thromb. Res. 128 (2011) e3–e8.
[34] M. Matan, E. Axelman, B. Brenner, Y. Nadir, Heparanase procoagulant activity is elevated in women using oral contraceptives, Hum. Reprod. 28 (2013) 2372–2380. [35] E. Peled, A. Rovitsky, E. Axelman, D. Norman, B. Brenner, Y. Nadir, Increased he-paranase level and procoagulant activity in orthopedic surgery patients receiving prophylactic dose of enoxaparin, Thromb. Res. 130 (2012) 129–134.
[36] C. Osterholm, L. Folkersen, M. Lengquist, F. Ponten, T. Renne, J. Li, U. Hedin, Increased expression of heparanase in symptomatic carotid atherosclerosis, Atherosclerosis 226 (2013) 67–73.
[37] A.B. Baker, W.J. Gibson, V.B. Kolachalama, M. Golomb, L. Indolfi, C. Spruell, E. Zcharia, I. Vlodavsky, E.R. Edelman, Heparanase regulates thrombosis in vas-cular injury and stent-induced flow disturbance, J. Am. Coll. Cardiol. 59 (2012) 1551–1560.