RESEARCH
Usage of ZnO containing wastes in the sanitaryware bodies
Fazilet Gungor1&Iskender Isik2&Ersin Gungor3&Elif Eren Gültekin4Received: 4 June 2018 / Revised: 19 October 2018 / Accepted: 13 December 2018 / Published online: 2 January 2019 # Australian Ceramic Society 2019
Abstract
Galvanized waste is characterized from a mineralogical point of view in order to investigate the effects of its utilization in sanitary ware bodies. Galvanized waste was added to a sanitary ware body recipe at 1–10% weight in place of albite and kaolin content. In an attempt to investigate the effects of its utilization on the firing temperature of the body, the bodies were sintered at the temperatures 1200 °C and 1250 °C. The physical properties, such as deformation, water absorption, and coloring parameters were investigated. The phase and microstructural development of the selected samples were characterized using XRD and SEM, respectively. Experimental studies have shown that the utilization of galvanized waste at an optimum level, in place of albite and kaolin in sanitary ware bodies sintered at 1200 °C, has similar water absorption (%), lower deformation, and higher whiteness values compared with standard sanitary ware bodies sintered at 1250 °C. It was foreseen that a 4.75% energy saving would be achieved by decreasing the firing temperature from 1250 to 1200 °C.
Keywords Galvanized waste . Energy saving . ZnO . Gahnite . Sanitary ware . Sintering
Introduction
The implementation of energy saving methods are of great importance, not only because of increased production costs as a result of the worldwide energy crisis, but also for being able to meet increasing demand with rising expectations. Within the framework of increasing global commercial com-petition in the field of white ware, the main effort in process-ing is currently directed towards the reduction of production costs, without changing productivity rates or product quality. Therefore, advanced technologies, offering better energy management and reduced pollution, are welcome. Fuels with energy sources from coal, oil, and natural gas have increased the amount of greenhouse gases in the atmosphere and
influenced global warming. Correspondingly, with an increase in energy use, CO2emissions have increased.
Zinc has been widely-used over the past century in numer-ous industrial areas, from agriculture to electronics, from chemicals to die casting, and from iron and steel production to production of ZnO for ceramics. Due to its electrochemical features, the predominant application of zinc is a galvanic pro-tective coating for steel. Nearly 50% of annual zinc production is consumed in galvanizing processes. The origins of this use can be traced to the coating of iron sheets with zinc in Swansea, Wales and, beginning in 1837, the making of so-called corru-gated iron. Considerable amounts of galvanized waste are likely to occur as a result of galvanization. Galvanized products, which are no longer in use, are another source for galvanized waste. All metals which are galvanized are 100% recyclable. The presence of a zinc coating on steel does not restrict its recyclability. Galvanized steel is recycled with other steel scrap in the steel production process; it volatiles early in the process and is collected for reprocessing [1,2].
In traditional ceramics, zinc oxide (ZnO) is used in the manufacture of glass, glazes, frits, porcelain enamels, and magnetic ferrites [3]. It is known to be a strong flux and one of the major components in commercial glazes for sanitary ware and tiles. It controls the thermal expansion coefficient, reduces glaze viscosity, and enhances surface glossiness. A combination of zinc oxide with coloring constituents has a * Fazilet Gungor
faziletgungor@kutahyaporselen.com.tr 1
Kütahya Porselen, R&D Center, Atatürk Bulv. 8. Km., Kutahya, Turkey
2 Department of Ceramic Engineering, Dumlupınar University, Kutahya, Turkey
3
Hisarlar San. A.Ş., Eskişehir, Turkey
4 Department of Airframe and Powerplant Maintenance, Selçuk University, Konya, Turkey
capacity for different crystal formations and distribution. ZnO has been utilized in the production of opaque, transparent, and matte glazes [4–10].
Even though there has been much research conducted into the effects of ZnO on crystallization in glazes, studies concerning its effects on bodies are very rare. For glaze rec-ipes, the initial raw materials melt, and crystallization occurs according to the chemical composition of the glaze and the cooling cycle of the kiln. However, traditional ceramic bodies are partial melt and their microstructures are complex. Raw materials partially dissolve in the glassy phase and new crys-talline phases develop. Therefore, the crystallization behavior of ZnO in ceramic bodies must be different from glazes. In some studies, the incorporation of ZnO containing glazes into porcelain bodies is investigated [11,12]. The addition of ZnO containing glazes favors the early formation and distribution of a glassy phase throughout the entire porcelain body, resulting in highly homogeneous and dense structures. An appropriate addition of ZnO containing glazes into a porcelain batch formulation can result in faster densification and a de-creased firing temperature, wider maturing range, improved mechanical properties, lower sintering shrinkage values, higher whiteness, and higher thermal shock resistance [11].
Capoglu investigated the effect of ZnO in bone china. According to the results, the whiteness of the bodies increased because of the development of Zn-spinel phases [13]. Lee and et al. investigated the effect of ZnO in porcelain bodies. ZnO is added to the body recipe, from between 1 and 5%. The gahnite phase increased, water absorption (%) decreased, and flexural strength and densification improved with an increased amount of ZnO [14]. In another study, the addition of zinc oxide was also investigated in a standard porcelain formulation. The in-vestigated formulations in the experiment were determined by general factorial design. Zinc oxide was found to be the most effective main factor on theL* value. When added, gahnite crystals occurred, and an increased amount of ZnO increased the gahnite and cristobalite peaks’ intensities forming X-ray diffraction (XRD) pattern [15].
The objective of this study is therefore to investigate ZnO containing galvanized waste on deformation, water absorp-tion, and coloring parameters of sanitary ware. It was found that by waste addition sanitary ware bodies could be produced at 1200 °C with low deformation, high whiteness, and low water absorption.
Experimental procedure
Materials and sample preparation
A standard sanitary ware recipe (20% quartz, 30% albite, 25% kaolin, and 25% ball clay) was selected. Instead of albite and kaolin, galvanized waste was used for the selected sanitary ware composition. Galvanized wastes contain Zn greater than Table 1 Chemical analysis of raw materials utilized in the body recipe
(by % weight) Oxide K A BC Q SiO2 52.60 69.70 48.73 98.90 Al2O3 34.24 18.30 30.51 0.96 Fe2O3 0.12 0.10 1.16 0.05 TiO2 0.11 0.16 0.04 – CaO – 0.81 0.22 – MgO – – 0.21 – Na2O – 10.05 0.23 – K2O 1.40 0.26 2.22 0.02 L.O.I. 11.20 0.27 16.83 0.30
K kaolin, A albite, BC ball clay, Q quartz, L.O.I., loss on ignition
Table 2 Sanitary ware compositions for the experimental studies (by % weight)
Code GW-0 GW-1 GW-2 GW-3 GW-4 GW-5 GW-6 GW-7 GW-8 GW-9 GW -10 raw material (wt.%) Quartz 20 20 20 20 20 20 20 20 20 20 20 Albite 30 29.5 29 28.5 28 27.5 27 26.5 26 25.5 25 Kaolin 25 24.5 24 23.5 23 22.5 22 21.5 21 20.5 20 Ball clay 25 25 25 25 25 25 25 25 25 25 25 Galvanized waste 0 1 2 3 4 5 6 7 8 9 10
Table 3 Particle size distribution of raw materials
Raw materials d (0.1) (μm) d (0.5) (μm) d (0.9) (μm)
Quartz 2.09 22.22 207.80
Albite 2.23 17.27 64.08
65% [16]. The galvanized waste is waste material obtained from the Toscelik Galvanization Plant, Iskenderun, Turkey. The chemical compositions of the raw materials, determined by X-ray fluorescence (Rigaku ZSX Primus) and used in the present study, are listed in Table1. Eleven compositions, des-ignated as GW-0, GW-1 GW-2, GW-3, GW-4, GW-5, GW-6, GW-7, GW-8, GW-9, and GW-10 [17]. The ratios of the raw materials for each recipe are given in Table2.
The particle size distribution of quartz, albite, and galva-nized waste was measured by laser diffraction method (master sizer, Malvern 2000) and the results are shown in Table3. The sieve residuals (+ 125μm) of ball clay and kaolin are 0.1 and 0.4%, respectively. Synthesized new slurry batches were put into the mixer and stirred with distilled water for 2 h. After 24 h of slip aging, the prepared slurries were sieved through a 250-μm sieve. Na-silicate added as a defloculant in order to obtain a stable quick casting slip. The slip was poured into bar-shaped plaster molds. The dimensions of the bars are 35 mm × 200 mm × 10 mm. Specimens were dried at 110 °C and sintered in a laboratory scale natural gas furnace Forno Ceramica kiln by two different heat treatment parameters for observing the phases developed in the microstructure. Experimental heat treatment parameters and amount of con-sumed gases were given in Table4. The HT01 firing cycle was the cycle that used for standard sanitaryware sintering. The HT02 firing cycle was chosen according to achieve stan-dard properties of GW-0 sintered at HT01 firing cycle. The economic effect of lowering the firing temperature was calcu-lated based on the amount of gas consumed during the 10 kg product sintered in the experiment. Consumed gas was mea-sured from the counter of the kiln and the difference between consumed gas amounts used for determination of energy
sav-ing. Amount of gas was consumed can be reduced 4.75% by the decreasing of the temperature from standard sanitaryware firing cycle (HT01) to HT02.
Sample characterization
Crystal phase identification was performed by X-ray diffrac-tion using a Rigaku diffractometer (Rigaku Rint 2000 Series). Whereas waste was scanned from 2θ, 20 to 70°, sintered sam-ples were scanned from 2θ, 5 to 70°, at a scanning speed of 1°/ min. The fracture surfaces of the samples were used for scan-ning electron microscope investigation. Samples were etched in a 10% HF solution for 1 min. For SEM observations, a Zeiss Supra TM 50 VP scanning electron microscope (SEM) equipped with an FEG electron source was used in secondary electron (SE) imaging modes. Water absorption was deter-mined by water saturation and Archimedes principle (ISO 10545-3). The coloring parameters of all the samples were determined using a Minolta CR-30C model spectrophotome-ter and the results were expressed inL*, a*, b* values.
The pyroplastic index (PI) of each sample was determined by three-point test method after firing: whereh is the thickness of the body,S is the maximum deflection and L is the distance between the refractory supports as shown in Fig.1[18].
PI¼4h
2
3L4S ð1Þ
Table 4 Heating parameters and amount of consumed gases
Heating rate Dwell temperature Dwell time Consumed gas (K min−1) (°C) (minutes) (m3)
HT01 10 1250 60 4.21
HT02 10 1200 35 4.01
Fig. 1 Positioning of the sample before and after firing for pyroplastic index assay [16]
Results and discussion
Figure2shows the XRD pattern of the galvanized waste. The major phases of the waste are zincite and hematite. Zincite is the mineral form of ZnO. Figure3shows the XRD patterns of specimens sintered at 1250 °C. The addition of ZnO resulted in the development of ZnAl2O4(gahnite). Gahnite formation
is assumed to be related to ZnO dissolution and its consequent crystallization inside the glass as a gahnite phase, instead of a direct solid-state reaction between ZnO and Al2O3containing
raw materials [14]. Crystallization of gahnite depends on the amount of flux in the chemical composition, since gahnite crystals start to develop when the flux melts and the viscosity of the glass is low to allow for crystallization [7,19].
Figure 4 shows the diffraction patterns of specimens sintered at 1200 °C. The amount of gahnite phase increased by increasing the amounts of galvanized waste, and a willem-ite phase was not detected. It has been reported that willemwillem-ite (Zn2SiO2) was not detected in the porcelain body, possibly
due to a greater amount of alumina bearing raw materials [14]. The crystallization of gahnite started to increase when the content of ZnO was greater than 2% in the composition. Peak intensity of mullite was not changed as the amount of gahnite was increased. Mullite formation and the sizes of the crystals strongly depend on the viscosity of the glassy phase. Güngör indicated that as the amount of CaO in the composi-tion was increased, anorthite was observed and the amount of mullite was decreased [20]. But in this study, gahnite forma-tion did not negatively affect the mullite formaforma-tion due to the highly viscosity reducing effect of ZnO.
The water absorption results of the samples are shown in Fig. 5. According to the results, water absorption (%) decreased with an increased sintering temperature and ZnO content. Feldspar is a relatively low melting mineral and acts as a flux during firing, causing an increment at the liquid phase, which facilitates densification of the ce-ramic body by filling interconnected pores. The sintering temperature has a proportional relationship with the amount of the liquid phase. ZnO behaves in the same way as alkaline earth oxides within glazes. However, the dissolution rate of ZnO is somewhat faster and occurs at slightly lower temperatures than alkaline earth oxides [21]. Sintering occurs most readily when the liquid thor-Fig. 3 XRD patterns of the specimens sintered at 1250 °C,✩: gahnite, ◊:
quartz,●: mullite
Fig. 4 XRD patterns of specimens sintered at 1200 °C,✩: gahnite, ◊: quartz,●: mullite
Fig. 5 Water absorption results of samples including or not including ZnO sintered at 1200 and 1250 °C
oughly wets the solid particles at the sintering tempera-ture. The liquid in the narrow channels among the parti-cles results in substantial capillary pressure, which aids densification [22]. The difference in water absorption be-tween two GW-0 sintered at 1250 and 1200 °C is 1.4%. On the other hand, the difference in water absorption be-tween GW-10 (sintered at 1200 °C) and GW-0 (sintered at 1250 °C) is 0.18%, indicating that the addition of an in-creased amount of ZnO content, dissolved and crystallized as gahnite, consequently decreased water absorption and increased densification.
The deformation results of the samples are shown in Fig.6. The experimental results show that the addition of galvanized
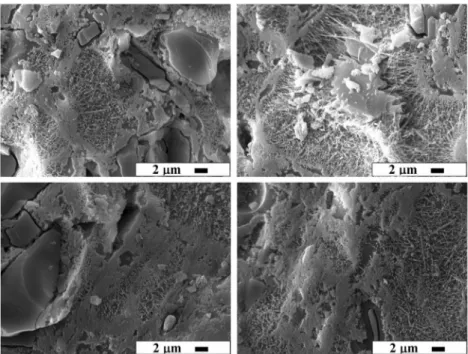
waste, in place of albite and kaolin, results in an increase in deformation. However, the deformation values for the samples sintered at 1200 °C are lower than for the samples sintered at 1250 °C. The deformation value for GW-10 sintered at 1200 °C is almost the same as for GW-0 sintered at 1200 °C. The mean values of color for 1200 °C and 1250 °C are shown respectively in Table 5. CIE 1976 L*, a*, b* (CIELAB) is a color scale based on the opponent color theory. TheL* value indicates the level of light and dark, the a* value indicates redness or greenness, and the b* value indicates yellowness or blueness. All three values are required to completely describe an object’s color. A three dimensional representation ofL*, a*, b* color space is shown in Fig.7[23]. There is a proportional linear rela-tionship between the galvanized waste and the whiteness of the body for samples sintered at 1200 °C. An increased amount of ZnO containing waste increased the L* value. The whiteness of the samples sintered at 1200 °C increased because of the whitening effect of ZnO. There is little whiteness change detected due to the high amount of the glassy phase with an increased waste addition for samples sintered at 1250 °C. SEM analysis results for the samples of GW-0 and GW-5 sintered at 1200 and 1250 °C respec-tively were given in Fig.8. The sizes of mullite crystals in the samples sintered at 1250 °C were greater than in the samples sintered at 1200 °C. The mullite crystals in the samples sintered at 1200 °C were both round and needle--shaped but the crystals in the samples sintered at 1250 °C were almost needle-shaped. Amount of needle-shaped mullite crystals in the sample of GW-5 sintered at 1200 °C was more than the samples of GW-0 sintered at 1200 °C. Crystals derived from pure clay agglomeration are cuboidal and referred as primary mullite. Elongated needle shaped mullite crystallizing from the feldspar rich melt is termed as secondary mullite. The size and shape of mullite crystals are controlled by the fluidity of the local matrix from which they grow, depending on the composi-tion and temperature [24,25]. It is thought that galvanized waste due to high amount of ZnO in it acted as a flux, lowered the viscosity, and increased the dissolution capac-ity of the melt at the early stages of densification.
Conclusions
It is concluded that increasing the amount of galvanized waste addition, in place of kaolin and albite, in a sanitary ware body recipe can result in decreased water absorption, greater white-ness, faster densification, and a lower firing temperature. Gahnite crystals were developed and increased in densification, Table 5 Mean values of color of samples sintered at 1200 °C and
1250 °C 1200 °C 1250 °C Sample code L* a* b* L* a* b* GW-0 87.08 0.51 7.71 81.40 − 0.06 6.61 GW-1 87.14 0.36 7.70 81.20 − 0.78 6.06 GW-2 87.29 0.33 7.94 81.20 − 0.80 5.96 GW-3 87.45 0.30 8.00 81.20 − 0.84 5.74 GW-4 87.63 0.27 8.11 81.20 − 0.90 5.52 GW-5 87.74 0.25 8.15 81.10 − 0.95 5.31 GW-6 87.85 0.22 8.22 81.14 − 0.99 5.22 GW-7 87.93 0.21 8.40 81.11 − 1.02 4.90 GW-8 88.02 0.19 8.61 81.20 − 1.08 4.75 GW-9 88.11 0.19 8.70 81.10 − 1.12 4.49 GW-10 88.21 0.17 8.90 81.15 − 1.14 4.35
with an increase of ZnO containing galvanized waste. At 1200 °C, using 10% waste, sanitary ware bodies were produced with less deformation, greater whiteness, and almost the same water absorption compared with a standard body sintered at 1250 °C. Achieving a reduction of sintering temperature from 1250 to 1200 °C with galvanized waste offers a 4.75% energy saving. Using waste material and saving energy are beneficial from both a commercial and an environmental aspect.
References
1. Bright, M.A., Deem, N.J., Fryatt, J.: The advantages of recycling metallic zinc from the processing wastes of industrial molten zinc applications. In: The Minerals, Metals and Materials Society (ed.) 2007 TMS Annual Meeting & Exhibition, pp. 101–109, Orlando (2007)
2. Gordon, R.B., Graedel, T.E., Bertram, M., Fuse, K., Lifset, R., Rechberger, H., Spatari, S.: The characterization of technological zinc cycles. Resour Conserv Recycl. 39(2), 107–135 (2003) 3. Perl, A.S.: Zinc oxide. Am Ceram Soc Bull. 80(8), 106–107 (2001) 4. Göncü, Y.: Investigation of the usage of wastes containing ZnO in crystal glazes. Master of Science Thesis, Anadolu University, Eskişehir (2006)
5. Karasu, B.: Macrostructure harmonie of various coloring oxides in soft porcelain zinc oxide crystal glazes. J Ceram Art Sci Tech Turkish Ceram Soc. 14, 33–35 (2001)
6. Karasu, B., Çakı, M., Turan, S.: The development and characteri-sation of zinc crystal glazes used for Amakusa-like soft porcelains. J Eur Ceram Soc. 20(12), 2225–2231 (2000)
7. Karasu, B., Çakı, M., Yeşilbaş, Y.G.: The effect of albite wastes on glaze properties and microstructure of soft porcelain zinc crystal glazes. J Eur Ceram Soc. 21(8), 1131–1138 (2001)
8. Karasu, B., Turan, S.: Effects of cobalt, copper, manganese and tita-nium oxide additions on the microstructures of zinc containing soft porcelain glazes. J Eur Ceram Soc. 22(9–10), 1447–1455 (2002)
9. Öztürk, Z.B., Atay, B., Çakı, M., Ay, N.: An investigation of color development by means of the factorial design in wall tile glazes with ferrochromium fly ash. Indian J Eng Mater S. 22, 215–224 (2015)
10. Yıldız, B., Ozturk, Z.B., Kara, A.: Effects of alumina and white fused alumina addition on transparent wall and floor tile glazes. Acta Phys Pol A. 127, 1000–1004 (2015)
11. Tulyaganov, D.U., Agathopoulos, S., Fernandes, H.R., Ferreira, J.M.F.: The influence of incorporation of ZnO-containing glazes on the properties of hard porcelains. J Eur Ceram Soc. 27(2–3), 1665–1670 (2007)
12. Mondal, A.: Effect of incorporation of ZnO containing glaze on the densification of porcelain. Indoceram. 48(2), 42–45 (2012) 13. Capoglu, A.: Elimination of discoloration in reformulated bone
China bodies. J Eur Ceram Soc. 25(13), 3157–3164 (2005) 14. Lee, S.-M., Kim, S.-K., Yoo, J.M., Kim, H.-T.: Crystallization
be-havior and mechanical properties of porcelain bodies containing zinc oxide additions. J Eur Ceram Soc. 25(11), 1829–1834 (2005) 15. Eren, E.: Investigation of zinc oxide addition into a porcelain body. Master of Science Thesis, Anadolu University, Eskişehir (2006) 16. http://www.mebmetal.com/hammaddeler/galvaniz-elek-alti-kulu/.
Accessed 12 Feb 2017
17. Güngör, E., Işık, İ., Güngör, F.: A preliminary study for utilization of the galvanized wastes in the vitrified bodies. In: Karasu, B. (ed.) Proceedings of the 1st International Ceramic, Glass, Porcelain, Enamel, Glaze and Pigment Congress (SERES’09), pp. 977–984. Ongar Electronic Print and Photocopy Center, Eskişehir (2009) 18. Conserva, L.R.D.S., Melchiades, F.G., Nastri, S., Boschi, A.O.,
Dondi, M., Guarini, G., Raimondo, M., Zanelli, C.: Pyroplastic deformation of porcelain stoneware tiles: wet vs. dry processing. J Eur Ceram Soc. 37(1), 333–342 (2017)
19. Yekta, B.E., Marghussian, V.K.: Sintering ofβ. qss and gahnite glass ceramics. J Eur Ceram Soc. 19(16), 2963–2968 (1999) 20. Güngör, F.: Investigation of pyroplastic deformation of whitewares:
effect of crystal phases in theBCaO^ based glassy matrix. Ceram Int. 44(11), 13360–13366 (2018)
21. Tkalcec, E., Kurajica, S., Ivankovic, H.: Crystallization behavior and microstructure of powdered and bulk ZnO-Al2O3-SiO2 glass-ceramics. J Non-Cryst Solids. 351(2), 149–157 (2005)
Fig. 8 SEM images for specimens of 0 (a) and GW-5 (b), sintered at 12GW-50 °C; GW-0 (c) and GW-5 (d), sintered at 1200 °C
22. Richerson, D.W.: Modern Ceramic Engineering, Properties, Processing, and Use in Design. Taylor and Francis Group, Boca Raton, FL (2006)
23. Sharifzade, S., Clemmensen, L.H., Borggaard, C., Støier, S., Ersbøll, B.K.: Supervised feature selection for linear and
non-linear regression of L*a*b* color from multispectral images of meat. Eng Appl Artif Intell. 27, 211–227 (2014)
24. Tuncel, D.Y., Özel, E.: Evaluation of pyroplastic deformation in sanitaryware porcelain bodies. Ceram Int. 38(2), 1399–1407 (2012) 25. Lee, W.E., Iqbal, Y.: Influence of mixing on mullite formation in
![Fig. 1 Positioning of the sample before and after firing for pyroplastic index assay [16]](https://thumb-eu.123doks.com/thumbv2/9libnet/4974199.100740/3.892.76.433.109.200/fig-positioning-sample-firing-pyroplastic-index-assay.webp)
![Fig. 7 L*, a*, b* color space [ 23]](https://thumb-eu.123doks.com/thumbv2/9libnet/4974199.100740/5.892.75.435.119.351/fig-b-color-space.webp)