See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/327746743
Genetic and biochemical properties of Cicer spp reveal distinction between
wild and cultivated chickpea genotypes
Article in Legume Research · September 2018
DOI: 10.18805/LR-395 CITATIONS 4 READS 129 6 authors, including:
Some of the authors of this publication are also working on these related projects:
Bacillus spp diversity of pistachioView project Ali Ozkan
Faculty of Fine Arts 20PUBLICATIONS 174CITATIONS SEE PROFILE Canan Can Gaziantep University 64PUBLICATIONS 1,309CITATIONS SEE PROFILE Dürdane Mart
Eastern Mediterranean Agricultural Research Institute 20PUBLICATIONS 25CITATIONS SEE PROFILE Ercan Ceyhan Selcuk University 45PUBLICATIONS 440CITATIONS SEE PROFILE
All content following this page was uploaded by Ali Ozkan on 16 February 2019.
www.arccjournals.com/www.legumeresearch.in
*Corresponding author’s e-mail: aozkan27@gmail.com
1Gastronomy and Culinary Arts, University of Gaziantep, Gaziantep 27310, Turkey. 2Department of Biology, University of Gaziantep, Gaziantep 27310, Turkey. 3Eastern Mediterranean Agricultural Research Institute, Yüreğir, Adana, Turkey. 4Department of Field Crops, University of Selcuk, Konya 42030, Turkey. Print ISSN:0250-5371 / Online ISSN:0976-0571
Genetic and biochemical properties of Cicer spp reveal distinction between
wild and cultivated chickpea genotypes
Feyza Nur Kafadar2, Ali Özkan*1, Canan Can2, Yağmur Kar2, Dürdane Mart3 and Ercan Ceyhan4
Department of Biology,
University of Gaziantep, Gaziantep 27310, Turkey.
Received: 27-10-2017 Accepted: 17-03-2018 DOI: 10.18805/LR-395
ABSTRACT
Chickpea (Cicer arietinum L.) is the third most important legumes after beans and peas in the world. Turkey is one of the main chickpea producing countries but the yield is not within the expected quantity considering cultivated area. Breeding chickpea varieties requires well-characterized wild Cicer spp progenitors including C. reticulatum, C. echinospermum, C.
bijugum and C. pinnatifidum that exhibit sympatric distribution in the Southeastern Anatolia region of Turkey. This study
contributes to the identification of similarities and differences among wild and cultivated Cicer spp. ecotypes using DNA marker systems and biochemical parameters. RAPD and ISSR markers revealed four groups and separated C pinnatifidum ecotypes as a single population. Wild Cicer spp and cultivated chickpea species exhibited significant variations for their oil, protein, starch, cellulose, fructose and sucrose content. Furthermore, the cultivated chickpea genotypes had higher content of fat, starch and sucrose than those of the wild progenitors whereas wild Cicer spp ecotypes possessed higher values for cellulose, fructose and glucose than cultivated chickpea genotypes.
Key words: Cicer spp, Genetic diversity, Biochemical parameters, Legume. INTRODUCTION
Legumes have considerable importance for human nutrition in developing countries where many people are not able to reach the animal-based food; therefore, legumes take an important place in food chain (Bressani, 1975). Chickpea (Cicer arietinum L.) is the third legume over the world after bean and pea (Tekeoğlu et al., 2000) and is widely grown in India, West Asia, North Africa, Ethiopia, Turkey, South Europe, Mexico, Australia, North-West of USA and Canada (Singh, 1987; Saxena and Singh, 1984). Chickpea is a traditional crop species in Turkey with 455 thousand tons of production in an area of 359.5 thousand ha (TUIK, 2016). Fertile Crescent including the Southeastern Anatolia region of Turkey and north parts of Syria are considered to be region for domestication and origin regions of chickpea Syria (Ladizinsky and Adler, 1976; Lev-Yadun et al., 2000). The wild C. reticulatum, C. echinospermum, C. bijugum and C.
pinnatifidum species exhibit sympatric growth along with
cultivated chickpea in the Southeastern Anatolia of Turkey (Abbo et al., 2003; Berger et al., 2003; Van der Maesen et
al., 2005).
Chickpea seeds has high nutritional quality and includes considerable amounts of essential amino acids namely lysine, tyrosine, glutamic acid, histidine and lesser
amounts of methionine and cysteine. Starch is main storage carbohydrate and was followed by fiber, oligosaccharides and simple sugars such as glucose and sucrose with low glycemic index (Iqbal et al., 2006; Jukanti et al., 2012). Oil content of chickpea is lower when compared to those of oilseed legume crop plants such as soybean and groundnuts. DNA molecular markers have more advantages than phenotypic markers in order to determine genetic similarity exist inter and intra-specific variations. Herein DNA based molecular markers give more clear results than phenotypic markers which present variability depending on environ-mental factors (Virk et al., 1995; Serret et al., 1997). Likewise, classical and molecular characterisation of agronomic characters in chickpea genotypes concerning biotic, abiotic tolerance/resistance and food items are emphasized by numerous studies (Talebi et al., 2008; Aggarwal et al., 2015). Koinain et al (2016) and Kumar et
al (2017) determined genetic variability among wild and
cultivated Cicer spp. by using DNA markers and reported high polymorphism allowing efficient selection of agronomic characters in chickpea breeding. Sudupak et al. (2002) used RAPD markers to evaluate genetic similarity among perennial and annual wild Cicer spp collected from Turkey and defined two different groups within annual species. They
2 LEGUME RESEARCH-An International Journal
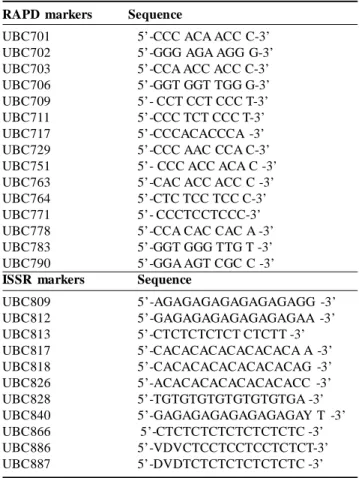
Table 1: Sequences of RAPD and ISSR primers (Talebi et al., 2008; Aggarwal et al, 2015).
RAPD markers Sequence
UBC701 5’-CCC ACA ACC C-3’
UBC702 5’-GGG AGA AGG G-3’
UBC703 5’-CCA ACC ACC C-3’
UBC706 5’-GGT GGT TGG G-3’
UBC709 5’- CCT CCT CCC T-3’
UBC711 5’-CCC TCT CCC T-3’
UBC717 5’-CCCACACCCA -3’
UBC729 5’-CCC AAC CCA C-3’
UBC751 5’- CCC ACC ACA C -3’
UBC763 5’-CAC ACC ACC C -3’
UBC764 5’-CTC TCC TCC C-3’
UBC771 5’- CCCTCCTCCC-3’
UBC778 5’-CCA CAC CAC A -3’
UBC783 5’-GGT GGG TTG T -3’
UBC790 5’-GGA AGT CGC C -3’
ISSR markers Sequence
UBC809 5’-AGAGAGAGAGAGAGAGG -3’ UBC812 5’-GAGAGAGAGAGAGAGAA -3’ UBC813 5’-CTCTCTCTCT CTCTT -3’ UBC817 5’-CACACACACACACACA A -3’ UBC818 5’-CACACACACACACACAG -3’ UBC826 5’-ACACACACACACACACC -3’ UBC828 5’-TGTGTGTGTGTGTGTGA -3’ UBC840 5’-GAGAGAGAGAGAGAGAY T -3’ UBC866 5’-CTCTCTCTCTCTCTCTC -3’ UBC886 5’-VDVCTCCTCCTCCTCTCT-3’ UBC887 5’-DVDTCTCTCTCTCTCTC -3’
reported that C. pinnatifidum, C. judaicum and C. bijugum are genetically more similar to C. montbretii, C. isauricum,
C. anatolicum an d C. incisum th an th ose of C. echinospermum, C. reticulatum and C. arietinum.
Wild annual Cicer spp could be used for transferring valuable agronomic traits such as biotic and abiotic stress tolerance/resistance to cultivated chickpea genotypes (Croser
et al., 2003; Kumar et al., 2017). Among the wild Cicer spp, C. echinospermum, C. pinnatifidum, C. bijugum and C. judaicum exhibit the resistance for Ascochyta blight and
Fusarium wilt (Singh et al., 1998; Collard et al., 2001). C.
reticulatum is considered as progenitor of C. arietinum and
hybridization studies were conducted among wild Cicer spp to enhance biotic and abiotic stress tolerance/resistance in cultigen (Abbo et al 2011; Van oss et al., 2015). Aim of the present study was to determine biochemical content besides genetic diversity of wild Cicer spp which were collected from the Southeastern Anatolia region of Turkey and cultigen
C. arietinum genotypes. Protein, fat, glucose, fructose,
sucrose, cellulose and starch contents were examined and genetic difference among Cicer spp. was analyzed using RAPD and ISSR markers.
MATERIALS AND METHODS
Plant Materials and PCR analyses: Wild chickpea species Cicer pinnatifidium Jaub & Spach, C. reticulatum Ladiz, C. echinospermum P. H. Davis, C. bijugum Rech. Fil and C. anatolicum were collected from Gaziantep, Şanlıurfa,
Diyarbakır, Mardin, Adıyaman and Van provinces of Turkey during 2013-2016. Three chickpea genotypes (Canıtez, ICC 12004, ICC 3979) were included in this study. Seeds of wild
Cicer spp were scarified before planting and 3-4 seeds were
sown to 12x9 cm pots containing soil, peat moss, perlite 1:1:1 (v/v/v) mixture. The pots were maintained in controlled
climate rooms of 22-24 oC, 70% humidity, 16 h light regime
for 3 weeks and plants were irrigated regularly with water. Cicer spp. genotypes were preserved at Biology Department in Gazian tep Un iver sity (Gazian tep) an d Easter n Mediterranean Agricultural Research Institute (Adana). Genomic DNA isolation was made with modified Doyle (1990) method. Isolated total genomic DNA concentrations were measured with nanodrop spectrophotometer (UVS99/ ACT, USA) at 260-280 nm wave length absorbance and adjusted to 15-30 ng/µL. PCR amplifications were conducted in 25 µl containing 0.7 U Taq DNA polymerase (Promega),
2 mM dNTPs (Promega), 25 mM MgCl2, 1X Taq buffer (1
M KCl, 1 M Tris HCl pH 8.3) and 10 mM primer (Operon Technologies, Inc). PCR cycles for RAPD analyses were 1 cycle of 94 ºC for 5 min for denaturation, 35 cycles of 94 ºC for 1 min, 35 ºC for 1 min, 72 ºC for 3 min for extension and fallowed by 1 cycle of 72°C for 10 min. The PCR analyses for ISSR markers were 1 cycle of 94 ºC for 5 min for denaturation, 35 cycles of 94 ºC for 1 min, 50 ºC for 1 min, 72 ºC for 3 min for extension and fallowed by 1 cycle of
72°C for 10 min. The PCR products were separated by 1.5-2 % agarose gel electrophoresis. RAPD and ISSR markers used in this study are given in Table 1.
Determination of protein, fat, glucose, fructose, sucrose, cellulose and starch contents: For cr ude protein determination, 0.5 g of moisture-free samples of the ground chickpea was weighed and the seed protein content was assesed by Kjeldahl method (Kacar, 1972). Protein ratio was calculated from multiplication for 6.25 times by nitrogen content. Oils were extracted from chickpea seeds (each 2 g sample) with n-hexane using a Soxhlet extraction apparatus and gravimetric determination of fat content was conducted. Glucose, fructose, sucrose, cellulose and starch contents were defined according to AOAC-2005 method. Seeds samples were grinded to non-passing form of 1 mm sieve and homogenized. Glucose, fructose and sucrose content were determined by water dissolving of 5 g samples and filled to 50 ml by de-ionized water. Samples were passed from 0,45 mm filter and injected to device.
Statistical analysis: For statistical analysis, results were evaluated as mean of ± Standard Error (Mean ± SE). Comparison of means were made by independent groups t test for two categorized variables while One Way Variance Analyze (ANOVA) was used for more than two variables
and Pearson Correlation Coefficient was used for relations amongst biochemical contents. Hierarchical cluster analyze was made to grouping of the investigated characteristics in the chickpea species while Principal Component Analyze (PCA) was used for grouping of marker data. Results of Duncan Multiple Comparison (post-hoc) in ANOVA were defined as statistically significant (p<0.05).
RESULTS AND DISCUSSION
Phylogenetic relations among Cicer spp: C. pinnatifidium, C. reticulatum, C. echinospermum, C. bijigum, C. anatolicum landraces and C. arietinum genotypes were
examined for determination of genetic similarity in the present study. A total of 15 RAPD and 11 ISSR markers were used in PCR analyses. Among them 6 RAPD (UBC 701, UBC 709, UBC 729, UBC 764, UBC 771, UBC 790) and 6 ISSR markers (UBC 809, UBC 812, UBC 817, UBC 818, UBC 828, UBC 840) exhibited clear polymorphism among Cicer spp (Table 2). Images of the RAPD and ISSR markers are presented in Fig 1 and 2.
Table 2: Polymorphisms of RAPD and ISSR markers.
RAPD ISSR
Primer numbers 15 11
Total number of bands amplified 700 1000
Mean of bands per primer 46.6 90.9
Number of polymorphic bands 528 663
Average of polymorphic bands per primer 35.2 60.2
ISSR markers amplified more bands and created distinctive polymorphisms when compared with those of the RAPD markers (Table 2).
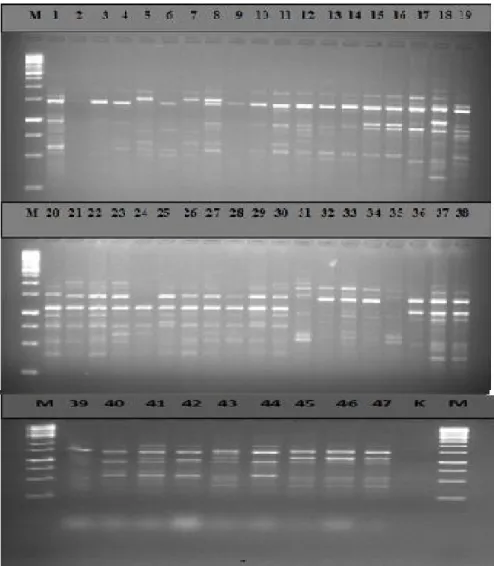
Fig 1: Polymorphism generated among Cicer spp with UBC 701.
M: 1 kb DNA ladder, 1-19 C. pinnatifidum, 20-31 C. echinospermum, 32-34 C. bijigum, 35 C. anatolicum, 36-38 C. arietinum, 39-47 C. reticulatum.
Fig 3 present the clustering of Cicer species by phylogenetic analysis. The ISSR and RAPD markers used in the present study provided clear distinction of the Cicer species and C. pinnatifdum as a separate group. Internal groups and transition points of the species were clearly determined by markers tested in this study (Table 1). C.
anatolicum was placed at the transition point between C. bijugum and C. echinospermum. Furthermore, C. arietinum
4 LEGUME RESEARCH-An International Journal
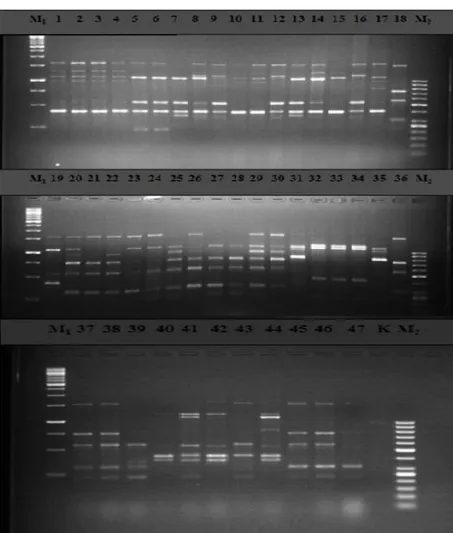
Fig 2: Polymorphism generated among Cicer spp with UBC 812.
M1: 1 kb DNA ladder, M2: 50 bp DNA ladder, 1-19 C. pinnatifidum, 20-31 C. echinospermum, 32-34 C. bijigum, 35 C. anatolicum, 36-38 C. arietinum, 39-47 C. reticulatum
Fig 3: Genetic relations among Cicer spp.
genotypes were grouped together with C. reticulatum and
C. pinnatifidum was located as a separate group.
Annual and perennial Cicer spp were subjected to morphological and DNA based molecular marker analyses for use in breeding programs to enhance biotic and abiotic stress tolerance/resistance of cultigen C. arietinum (Sudupak
et al., 2002; Shan et al., 2005; Kumar et al., 2017) These
studies revealed close genetic diversity between C. arietinum and C. reticulatum but wide genetic polymorphism in annual and perennial wild Cicer spp. Furthermore C. pinnatifidum was reported to possess high DNA polymorphism when compared to other wild annual Cicer spp and placed as a separate branch (Singh et al., 2008).
Biochemical parameters of Cicer spp: Protein, oil and glucose, fructose and sucrose contents was compared on wild and culture chickpea species (Table 3).
Starch, cellulose, fructose and sucrose content exhibited statistically significance (p< .01) groups between wild Cicer spp and C. arietinum genotypes. Chickpea genotypes contained higher starch (t= 3.56; p< .01) and sucrose (t= 1.63; p< .01) content than those of the wild species. Whereas, wild forms had higher values for cellulose
Table 3: Content of oil, protein, starch, cellulose, total sugar, fructose, glucose and sucrose in wild and culture forms of chickpea species.
Wild- Culture N Mean Std. ErrorMean t-test P
Oil ratio Wild 12 2.40 1.23 0.784 0.443
Culture 8 2.9 1.61 Protein Wild 12 19.80 2.42 0.963 0.348 Culture 8 20.77 1.83 Starch Wild 12 32.77 4.65 3.556** 0.002 Culture 8 43.63 5.75 Cellulose Wild 12 18.23 2.82 3.321** 0.004 Culture 8 12.45 4.99
Total sugar Wild 12 2.45 0.56 0.371 0.732
Culture 8 2.36 0.49 Fructose Wild 12 0.70 0.20 0.363** 0.002 Culture 8 0.65 0.37 Glucose Wild 12 0.42 0.37 3.210** 0.001 Culture 8 0.00 0.00 Sucrose Wild 12 1.36 0.28 1.630** 0.004 Culture 8 1.76 0.79
Mean Standard Error. *: p<0,05; **: p<0,01. The same letters mean to non-significance (Duncan Post Hoc,= 0,05).
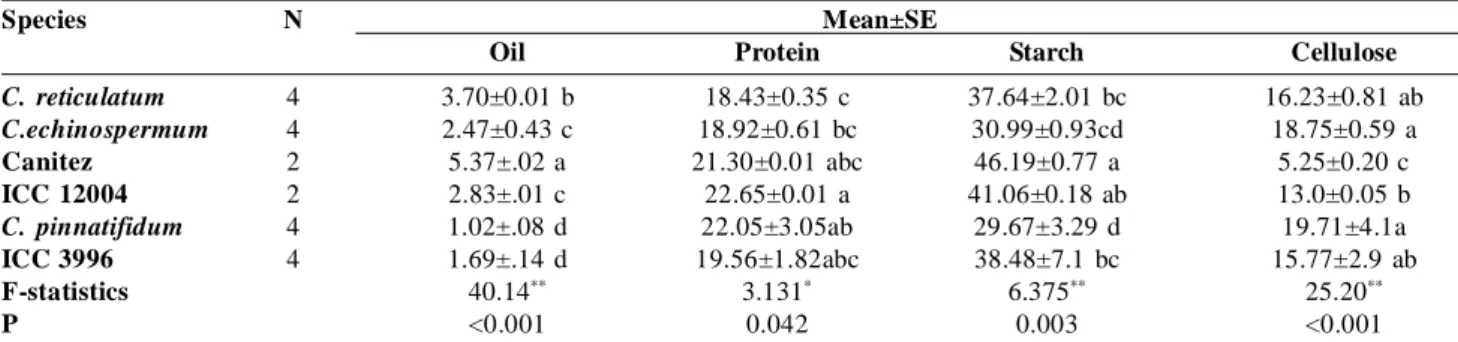
Table 4: Duncan multiple test groups of oil, protein, starch and cellulose in wild Cicer spp and cultigen chickpea genotypes.
Species N Mean±SE
Oil Protein Starch Cellulose
C. reticulatum 4 3.70±0.01 b 18.43±0.35 c 37.64±2.01 bc 16.23±0.81 ab
C.echinospermum 4 2.47±0.43 c 18.92±0.61 bc 30.99±0.93cd 18.75±0.59 a
Canitez 2 5.37±.02 a 21.30±0.01 abc 46.19±0.77 a 5.25±0.20 c
ICC 12004 2 2.83±.01 c 22.65±0.01 a 41.06±0.18 ab 13.0±0.05 b
C. pinnatifidum 4 1.02±.08 d 22.05±3.05ab 29.67±3.29 d 19.71±4.1a
ICC 3996 4 1.69±.14 d 19.56±1.82abc 38.48±7.1 bc 15.77±2.9 ab
F-statistics 40.14** 3.131* 6.375** 25.20**
P <0.001 0.042 0.003 <0.001
Mean Standard Error. *: p< .05; **: p< .01. The same letters mean to non-significance (Duncan Post Hoc, = .05).
(t= 3.32; p< .01), fructose (t= 0.36; p< .01) and glucose (t= 3,21; p< .01) than those of the C. arietinum genotypes. Biochemical content was non-significant by view of oil ratio, protein and total sugar (Table 4, 5).
Among the plant samples analyzed in this study, cv Canitez exhibited the highest oil ratio( = 5,37) and the lowest oil content was obtained from C. pinnatifidum ecotypes ( = 1,022) and ICC 3996 ( = 1,693) genotype. Similar results were reported on oil content of cultigen C.
arietinum (Wood and Grusak, 2007; Shad et al., 2009).
Zia-Ul-Haq et.al., 2007 reported that, the linoleic acid is very important with the nutritional value in dietary for human metabolism.
The data of wild Cicer spp and C. arietinum genotypes were subjected to t-test and one-way variance analyze (ANOVA) to define statistically different groups. T-test was non-significant (t= 0.96; p>.05) for protein ratio among Cicer spp. ANOVA test for protein ratio is given in Table 4 and the difference was significant (F= 3.13; p< .05) for inter-species. cv Canitez, ICC 12004 and C. pinnatifidum had higher protein content than C. reticulatum, C.
echinospermum and ICC 3996 and the difference was
statistically significant. Protein ratio of ICC 12004, cv
x
6 LEGUME RESEARCH-An International Journal
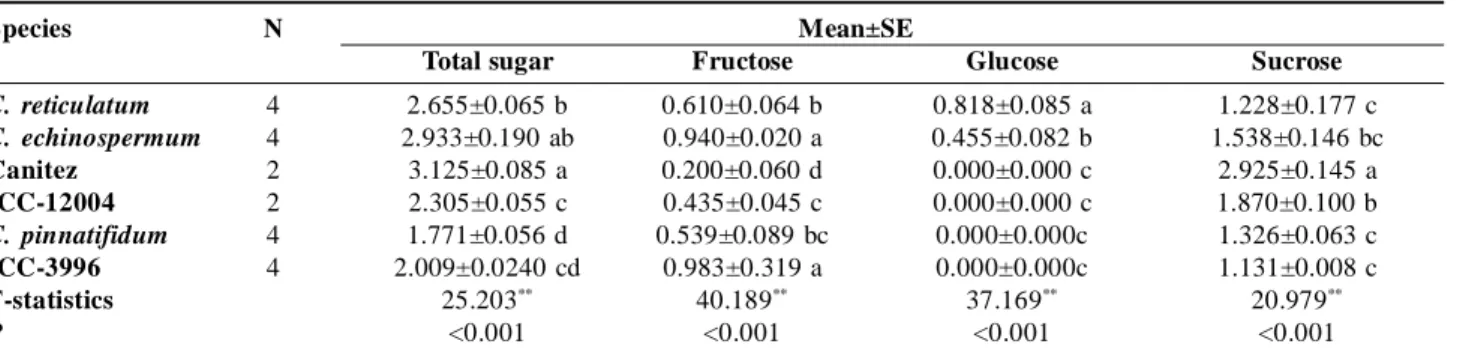
Table 5: Duncan multiple test groups of total sugar, fructose, glucose and sucrose in wild Cicer spp and cultigen chickpea genotypes.
Species N Mean±SE
Total sugar Fructose Glucose Sucrose
C. reticulatum 4 2.655±0.065 b 0.610±0.064 b 0.818±0.085 a 1.228±0.177 c C. echinospermum 4 2.933±0.190 ab 0.940±0.020 a 0.455±0.082 b 1.538±0.146 bc Canitez 2 3.125±0.085 a 0.200±0.060 d 0.000±0.000 c 2.925±0.145 a ICC-12004 2 2.305±0.055 c 0.435±0.045 c 0.000±0.000 c 1.870±0.100 b C. pinnatifidum 4 1.771±0.056 d 0.539±0.089 bc 0.000±0.000c 1.326±0.063 c ICC-3996 4 2.009±0.0240 cd 0.983±0.319 a 0.000±0.000c 1.131±0.008 c F-statistics 25.203** 40.189** 37.169** 20.979** P <0.001 <0.001 <0.001 <0.001
Mean±Standart Error. *: p< .05; **: p< .01. The same letters mean to non-significance (Duncan Post Hoc, = .05).
Canitez and C. pinnatifidum were 22.65%, 21.30% and 22.05, respectively. Protein content of the C. reticulatum,
C. echinospermum and ICC 3996 were 18.43%, 18.92% and
19.56%, respectively, which were lower than the other Cicer spp used in this study. Sibian et al. (2016) studied on raw and germinated chickpea genotypes and found the range as 19.50-21.63% for protein ratio. Nobile et al. (2013) reported protein ratio as 18.46-24.46% in Kabuli type chickpeas. Kaur
et al. (2016) studied on soaked and raw chickpea genotypes
and found the range as 19.40-23.86% for protein ratio. Nevertheless, Dragičevič et al. (2015) found the protein ratio as 11.26-17.63% in 19 chickpea species and Maheri-Sis et
al. (2010) reported the protein ratio as 22.76-24.63% in Kabuli and Desi types. The results obtained through this
study were in accordance with previous studies. The composition and amount of protein found in chickpeas and other legumes may show some differences because of the variety, growing season, environmental factors, as well as geographic location and method of analysis used by the investigators. (Maheri-Sis et al, 2010; Alajaji and El-Adawy, 2006; Zia-Ul-Haq et all., 2007).
Starch ratio of the wild Cicer spp and C. arietinum were statistically significant (Table 4) according to T-test and ANOVA (t= 3.56; p< .01); (F= 6,37; p< .01) Among them, cv Canitez exhibited the highest starch ratio ( =46.19) (F= 15.36; p< .01). Duncan test showed that cv Canitez, ICC 12004 and ICC 3996 contained more starch ratio than
C. reticulatum, C. pinnatifidum and C. echinospermum.
Similar results were reported by Maheri-Sis et al. (2010) that defined the starch ratio as 38.48-39.12% in Kabuli and Desi type chickpeas. Higher starch content of cultivar C.
arietinum could be due to selective domestication process
as indicated by Abbo et al (2011).
Cellulose ratio of wild Cicer spp and C. arietinum genotypes is presented in Table 3-4 and found as statistically significant according to T-test and ANOVA (t= 3.32; p< .01 and F=25.20; p< .01). Cellulose content was defined as C.
pinnatifidum> C. reticulatum> C. echinospermum>
ICC-3996> ICC-12004> cv Canitez. Wild genotypes have more cellulose ratio than culture forms. Vasishtha and Srivastava (2013) studied the cellulose ratio in Desi (4.02-5.91%),
Kabuli (1.2-2.00%) and green type (5.28-5.35%) chickpeas.
Results of the present study showed non-significant values between culture and wild forms of the chickpeas by view of cellulose content (t= 0.37; p > .05)
Total sugar content showed statistically significance on ANOVA test among the Cicer spp (F= 25.20; p< .01) Duncan test for total sugar placed cv Canitez and C.
echinospermum genotypes in the same group while the rest
of them found statistically significant. Sibian et al. (2016) found the total sugar ratio as 8.68-11.34% in Bengal gram chickpea that is lower than the data obtained through present study. Fructose content is presented on Table 5. Differences between wild Cicer spp and C. arietinum were found as statistically significant (F= 40.19; p< .01) The higher fructose content was determined in C. echinospermum and ICC-3996 genotypes (F= 40.19; p< .01) Fructose content in the other genotypes were as C. reticulatum> C. pinnatifidum> ICC-12004> cv Canitez. C. reticulatum had the highest glucose content and the results were statistically significant (F= 21.54; p< .01) Glucose content of cv Canitez and ICC 12004 genotypes were similar. Sucrose content was found as statistically significant among Cicer spp (F= 15.57; p< .01) and cv Canýtez had the highest sucrose content (Table 5). S´anchez-Mata et al. (1998) and Alajaji and El-Adawy (2006) reported sucrose as 1.09%–2.28%. Furthermore, Aman (1979), Wang and Duan (2004) and Aguilera et al. (2009) reported sucrose amount in cultivated chickpea genotypes as 4.3 %, 2.0 % (1.56–2.85) and 15.2 %, respectively.
Glucose and fructose are source of sucrose besides protein. Chickpea seed consist from 80% protein, glucose and sucrose while rest of it consist 0.8-6.4% oil, 2.1-11.7% fiber, 0.2% Ca and 0.3% P (Williams and Singh, 1987). In the developed countries, chickpea is used for animal feed as well due to rich mineral content. Except Sulphur, all of the main amino acids existed substantially. Additionally, chickpea is used for some important human health care status together with other legumes and cereals for CVD, diabetes, digestion diseases and some of the cancer types. In general, chickpea is essential for potential nutrition and health (Jukanti et al., 2012). It does not contain any toxic matters
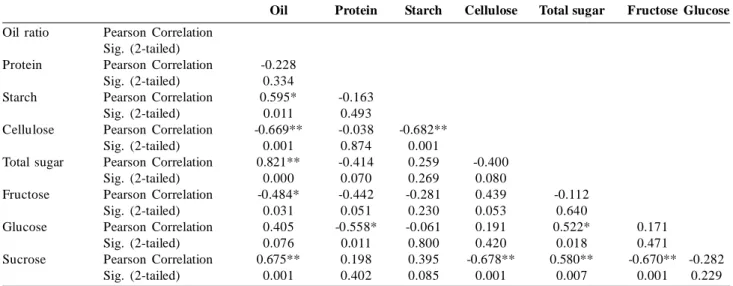
Table 6: Correlation analyze of oil, protein, starch, cellulose, total sugar, fructose, glucose and sucrose content of Cicer spp (N= 20, N= Number of sample).
Oil Protein Starch Cellulose Total sugar Fructose Glucose
Oil ratio Pearson Correlation
Sig. (2-tailed)
Protein Pearson Correlation -0.228
Sig. (2-tailed) 0.334
Starch Pearson Correlation 0.595* -0.163
Sig. (2-tailed) 0.011 0.493
Cellulose Pearson Correlation -0.669** -0.038 -0.682**
Sig. (2-tailed) 0.001 0.874 0.001
Total sugar Pearson Correlation 0.821** -0.414 0.259 -0.400
Sig. (2-tailed) 0.000 0.070 0.269 0.080
Fructose Pearson Correlation -0.484* -0.442 -0.281 0.439 -0.112
Sig. (2-tailed) 0.031 0.051 0.230 0.053 0.640
Glucose Pearson Correlation 0.405 -0.558* -0.061 0.191 0.522* 0.171
Sig. (2-tailed) 0.076 0.011 0.800 0.420 0.018 0.471
Sucrose Pearson Correlation 0.675** 0.198 0.395 -0.678** 0.580** -0.670** -0.282
Sig. (2-tailed) 0.001 0.402 0.085 0.001 0.007 0.001 0.229
*: p< .05; **: p< .01
Fig 4: Dendogram illustrating biochemical properties of Cicer spp. unlike other legumes and, it contains energy, protein, vitamin,
mineral and fiber, substantially (Mukesh et al., 2015). Chickpea that is rich by protein contains higher amounts of Arginine amino acid as well. Therefore, it is used as food source and pharmaceutical purposes (Cortés-Giraldo et al., 2016).
Table 6 presents the correlation analysis of fat, protein, starch, cellulose, total sugar, fructose, glucose and sucrose in wild Cicer spp and C. arietinum genotypes.
Significant and positive relation (r= 0.59; p< .01) was 60% between fat and starch ratio. Additionally, 70% ratio between fat and cellulose was found as significant with negative relation (r= -0.67; p< .01). The relation was 56% between protein and glucose content of the wild and culture forms of chickpeas and the correlation was statistically significant-negative (r= -0.56; p< .05). Relation between protein and fructose was negative and non-significant (r= -0.44; p< .06). Furthermore, the correlation coefficient was 68% between
8 LEGUME RESEARCH-An International Journal starch and cellulose that was significant and negative (r=
-0.68; p< .01).
Two separate groups were determined using biochemical properties of Cicer spp. Wild annual Cicer species were clearly separated from cultivated chickpea genotypes except that of C. reticulatum which is the p r op osi n g p r og en it or of cu l ti g en C . a rie t i nn u m (Ladizinsky and Adler, 1976; Lev-Yadun et al., 2000; Abbo et al., 2003).
Overall, in the present study, 15 RAPD and 11 ISSR markers were used to define phylogenetic relations of Cicer spp. The polymorphic markers provided a clear distinction
of Cicer spp and C. pinnatifidum ecotypes formed a distinct group. Content of the oil, protein, starch, cellulose, total sugar, fructose, glucose and sucrose in wild and cultigen chickpea gen otypes wer e defined. The biochemical parameters defined cultigen C. arietinum and wild Cicer spp. as separate groups. The gene pool could be used in breeding programs for biotic and abiotic stress tolerance/resistance such as drought and diseases.
ACKNOWLEDGEMENT
Present research was supported by Gaziantep University Scientific Research Governing Unit (BAPYB) with project no GSF02.
REFERENCES
Abbo, S., Shtienberg, D., Lichtenzveig, J., lev-Yadun, S., Gopher, A. (2003). The chickpea, summer cropping, and a new model for pulse domestication in the ancient near east. Q Rev Biol 78: 4.
Abbo, S., Lev-Yadun, S., Gopher A. (2011). Origin of Near Eastern plant domestication: homage to Claude Levi-Strauss and ‘‘La Pense´e Sauvage’’, Genet Resour Crop Ev 58:175–179
Aggarwal, H., Rao, A., Kumar, A., Singh, J., Rana, JS., Naik, PK., Chhokar, V. (2015). Assessment of genetic diversity among 125 cultivars of chickpea (Cicer arietinum L.) of Indian origin using ISSR markers. Turk J Bot 39(2): 218-226.
Aguilera, Y., Martı´n-Cabrejas, MA., Benitez, V., Mollá, E., Andréu, FL., Esteban RM. (2009). Changes in carbohydrate fraction during dehydration process of common legumes. J Food Compos Anal 22: 678–683.
Alajaji, SA., El-Adawy, TA. (2006). Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J Food Compos Anal 19: 806–812.
Aman, P. (1979). Carbohydrates in raw and germinated seeds from mung bean and chickpea. J Sci Food Agr 30: 869–875.
AOAC (2005). Official Method of Analysis of the Association of Analytical Chemists International (18th Ed.) Gathersburg, MD USA
Official methods.
Berger, J., Abbo, S., Turner, NC. (2003). Ecogeography of Annual Wild Species. Crop Sci 43(3): 1076-1090.
Bressani R. (1975). Legumes in human diets and how they might be improved. in Nutritional Improvement of Food Legumes by Breeding (Milner M ed). Wiley, New Pages 15-42 York.
Cortés-Giraldo, I., Megías, C., Alaiz, M., Girón-Calle, J., Vioque, J. (2016). Purification of free arginine from chickpea (Cicer arietinum) seeds. Food chem. 192:114-118
Croser, JS., Clarke, HJ., Siddique, KH., Khan, TN. (2003). Low-temperature stress: implications for chickpea (Cicer arietinum L.) improvement. Crit Rev Plant Sci 22(2): 185-219.
Collard, BC., Ades, PK., Pang, EC., Brouwer, JB., Taylor, PW. (2001). Prospecting for sources of resistance to ascochyta blight in wild Cicer species. Ausralas Plant Path 30(3): 271-276.
Doyle J.J. (1990). Isolation of plant DNA from fresh tissue. Focus 12,:13-15.
Dragièević, V., Kratovalieva, S., Dumanović, Z., Dimov, Z, Kravić, N. (2015). Variations in level of oil, protein, and some antioxidants in chickpea and peanut seeds. Chem Biol Technol Agric 2(1): 1-6.
Iqbal, A., Khalil, IA., Ateeq, N., Khan, MS. (2006). Nutritional quality of important food legumes. Food Chem 97: 331–335. Jukanti, AK., Gaur, PM., Gowda, CLL., Chibbar, RN. (2012). Nutritional quality and health beneûts of chickpea (Cicer arietinum L.).
British J Nutr 108: 11-26.
Kacar B (1972). Chemical Analysis of Plant and Soil II: Plant Analysis,. Ankara University, Agriculture Faculty, Issue No: 453-Application Handbook. pp: 155
Kaur, S., Kaur, S., Gupta AK., Kaur, J. (2016) Physiochemical and nutritional attributes of raw and soaked seeds of chickpea (Cicer arietinum L.) genotypes. Legume Res., 39: 359-369
Koinain SA, Hegde VS. and Bharadwaj C. (2016). Genetic diversity analysis among selected short duration chickpea cultivars and breeding lines based on STMS markers, Legume Research, 39: 851-859
Kumar R., Yadav R., Soi S., Srinivasan, Yadav S., Yadav A., Mishra JP., Mittal N., Yadav N., Kumar A., Vaishali, Yadav H., Upadhyaya H.D. (2017). Morpho-molecular characterization of landraces/wild genotypes of Cicer for Biotic/ Abiotic stresses, Legume
Research, 40 : 974-984
Ladizinsky, G., Adler, A. (1976). The origin of chickpea Cicer arietinum L. Euphytica 25: 211-217. Lev-Yadun, S., Gopher, A, Abbo, S. (2000). The cradle agriculture. Science 288: 1602-1603.
Maheri-Sis, N., Chamani, M., Ali-Asghar, S., Mirza-Aghazadeh, A., Aghajanzadeh-Golshani, A. (2010). Nutritional evaluation of kabuli and desi type chickpeas (Cicer arietinum L.) for ruminants using in vitrogas production technique. Afr J Biotechnol 7(16): 2946-2951.
Mukesh, K., Navneet, K., Sunil, M., Arvind, K., Vipin, K. (2015). Molecular characterization of chickpea (Cicer arietinum L) through RAPD and ISSR markırs. Prog Agr 15(2): 277-284.
Nobile, CGM., Carreras, J., Grosso, R., Inga, M., Silva, M., Aguilar, R., Martinez, MJ. (2013). Proximate composition and seed lipid components of “kabuli”-type chickpea (Cicer arietinum L.) from Argentina. Agr Sci 4:729-737.
Sґanchez-Mata, MC., Penuela-Teruel, MJ., Camara-Hurtado, M., Diez Marques, C., Torija-Isasa, ME. (1998). Determination of mono-, di-, and oligosaccharides in legumes by high-performance liquid chromatography using an amino-bonded silica column. J Agr Food Chem 46: 3648–3652.
Saxena, MC., Singh, KB. (1984). Ascochyta blight and Winter Sowing of Chickpeas. Martinus Niijh off, Zoetemeer, the Netherlands. Shad, MA., Pervez, H., Zafar, ZI., Nawaz, H. (2009). Evaluation of biochemical composition and physicochemical parameters of oil
from seeds of desi chickpea varieties cultivated in arid zone of Pakistan. Pak J Bot 41: 655–662
Shan, F., Clarke, HC., Plummer, JA., Yan, G., Siddique, KH. (2005). Geographical patterns of genetic variation in the World collections of wild annual Cicer characterized by ampliûed fragment length polymorphisms. Theor Appl Genet 110(2): 381-91 Serret, MD., Udupa, SM., Weigand, F. (1997). Assessment of genetic diversity of cultivated chickpea using microsatellite derived
RFLP markers: Implications for origin. Plant Breed, 116(6): 573-578.
Sibian, MS., Saxena, DC., Riar, CS. (2016). Effect of pre and post germination parameters on the chemical characteristics of Bengal gram (Cicer arietinum). LWT-Food Sci Technol 65: 783-790
Singh, KB. (1987). Chickpea Breeding. In: Chickpea, [Saxena M.C. and K.B. Singh (Eds)], ICARDA, , Aleppo, Syria. p.127-158. Singh KB., Ocampo B. and Robertson L. D. 1998. Diversity for abiotic and biotic resistance in the wild annual Cicer species. Genet.
Res. Crop Evol., 45: 9-17.
Singh, ARM., Devarumath, RM., Rama Rao, S., Singh, VP., Raina, SN. (2008). Assessment of genetic diversity, and phylogenetic relationships based on ribosomal DNA repeat unit length variation and Internal Transcribed Spacer (ITS) sequences in chickpea (Cicer arietinum) cultivars and its wild species, Genet Resour Crop Ev 55: 65–79
Sudupak, MA., Akkaya, MS., Kence, A. (2002). Analysis of genetic relationships among perennial and annual Cicer species growing in Turkey using RAPD markers, Theor Appl Genet 105: 1220–1228
Talebi, R., Fayaz, F., Mardi, M., Pirsyedi, SM., Naji, AM. (2008). Genetic relationships among chickpea (Cicer arietinum) elite lines based on RAPD and agronomic markers. Int J Agric Biol 8: 1560-8530.
Tekeoğlu, M., Santra, DK., Kaiser, WJ., Muehlbauer, FJ. (2000). Ascochyta blight resistance inheritance in three chickpea recombinant inbred line population. Crop Sci 40: 1251-1256.
Van der Maesen, LJG., Maxted, N., Javadi, F., Coles, S., Davies, AMR. (2005). Taxonomy of the genus Cicer revisited. In Chickpea Breeding and Management[S.S. Yadav, R. Redden, W. Chen, B. Sharma (Eds.)], CAB International, Walling ford, UK (Pp. 14-46).
Vasishtha, H., Srivastava, RP. (2013). Effect of soaking and cooking on dietary fibre components of different type of chickpea genotypes. J Food Sci Tech 50(3): 579-584
Wang, N., Daun, JK. (2004). The chemical composition and nutritive value of Canadian pulses. In Canadian Grain Commission Report, pp. 19–29
Van Oss, R., Abbo, S., Eshed, R., Sherman, A., Coyne, CJ., Vandemark, GJ., Peleg, Z. (2015). Genetic relationship in Cicer sp. expose evidence for gene flow between the cultigen and its wild progenitor. PloS one 10(10): e0139789.
Virk, PS., Newbury, HJ., Jackson, MT., Ford-Lloyd, BV. (1995). The identification of duplicate accessions within a rice germplasm collection using RAPD analysis. Theor Appl Genet 90(7-8): 1049-1055.
Williams, P. C., Singh, U. (1987). Nutritional quality and the evaluation of quality in breeding program. In: Chickpea CAB International, Wallingford Oxon UK, 329–356.
Wood, JA., Grusak, MA. (2007). Nutritional value of chickpea. In Chickpea Breeding and Management, [SS Yadav, R Redden, W Chen and B Sharma, editors]. Wallingford: CAB International. pp. 101–142
Zia-Ul-Haq, M., Ahmad, M., Iqbal, S., Ahmad, S., Ali, H. (2007). Characterization and compositional studies of oil from seeds of desi chickpea (Cicer arietinum L.) cultivars grown in Pakistan. J Am Oil Chem Soc 84(12): 1143-1148.
View publication stats View publication stats