Contents lists available atScienceDirect
Organic Electronics
journal homepage:www.elsevier.com/locate/orgel
An electrochemically and optically stable electrochromic polymer film based
on EDOT and 1,2,3,4-tetrahydrophenazine
Emine Gül Cansu Ergun
a,*, Deniz Eroglu
baDepartment of Electrical and Electronics Engineering, Baskent University, TR-06810, Ankara, Turkey bDepartment of Chemistry, Middle East Technical University, TR-06800, Ankara, Turkey
A R T I C L E I N F O Keywords: Ethylenedioxythiophene Tetrahydrophenazine Quinoxaline Electrochromic polymers Electrochromic device A B S T R A C T
In this work, 3,4-ethylenedioxythiophene (EDOT) and 1,2,3,4-tetrahydrophenazine based donor-acceptor-donor type monomer (EBE) was synthesized, electropolymerized and the resulting polymer film (P(EBE)) was in-vestigated in terms of its electrochemical and spectroelectrochemical properties. The polymer film showed ex-cellent electrochemical stability upon 1000 switchings by saving almost full of its electroactivity. Moreover, 95% of the optical contrast was maintained after 500 switchings. The optical band gap value was calculated using Tauc Plot method and found as 1.5 eV. P(EBE) film revealed multichromic behaviour between its fully neutral and oxidized states; dark cyanish-green at −0.8 V, more green at −0.5 V and transmissive green beyond 0.6 V potentials. Finally, a dual type electrochromic device (ECD) was constructed with P(EBE) and poly(3,4-ethy-lenedioxythiophene) (PEDOT) films and the spectroelectrochemical behaviour of ECD was depicted.
1. Introduction
Electrochromism is a phenomenon that switching the optical prop-erties of a material between its oxidized and reduced states, under an applied potential [1]. Moreover, reversibility of this electrochromic behaviour perfectly meets with the expectations of the electrocromic device applications such as displays, smart windows, car rear-view mirrors, camuflage materials, sensors, supercapacitors and many other applications [2–9]. Since molecular modifications are possible and each change on the molecule exhibits different optical properties, organic conjugated molecules, especially electron donor-acceptor-donor (DAD) type materials are in full of attention in these applications [10].
Obtaining different colors are the main topic of research in the electrochromic fields. The primary colors, red-green-blue (RGB) are the basic colors and any other color can be obtained by mixing these pri-mary colors. The synthesis of electrochromic polymers, generally pre-senting red or blue colors in their neutral state, have been achieved and reported many times. On the other hand, green color is also necessary to complete the RGB color spectrum. Neutral state green color polymers are the special type of electrochromic polymers exhibiting dual optical absorption band in the visible region. Because of the difficulty to obtain these two absorptions centered at the blue and red regions of the visible spectrum, the neutral state green colored electrochromic polymers took more time to be synthesized and could not have been achieved up to
2000's. After the first green electrochromic polymer reported in the literature [11], various type of molecules have designed and reported, containing different electron donor and acceptor units in the same polymer backbone (DAD), presenting different shades of green or cyan. 3,4 ethylenedioxythiophene (EDOT) is a widely used donor unit in green colored polymer synthesis, since EDOT has excellent donor property and electrochemical/optical stability [12]. When electron withdrawing units combined with EDOT, resulting polymer possesses lower optical band gap, easier oxidation, and better stability. Molecules with quinoxaline rings, on the other hand, are used as the electron acceptor due to their electron-deficient nature, with having an ad-vantage of functionalization of quinoxaline ring by bis-substitution or direct aromatic or cyclic substitution. Several studies have been pub-lished about DAD polymers containing EDOT and different quionoxa-line derivatives as electrochromic materials in the literature [13–23], most of them are green in their neutral state. In the light of these reports in the literature, exhibiting neutral state green color can be attributed to strong electron acceptor combined with a strong electron donor and hues of green can be altered by modifying the acceptor or donor group by different substitutions.
Here, we are chasing another neutral state green polymer by com-bining EDOT with a novel acceptor unit, cyclohexyl substituted qui-noxaline. This polymer, P(EBE), gave a very attractive green color in the neutral state, while showed a high transparency in the oxidized
https://doi.org/10.1016/j.orgel.2019.105398
Received 21 June 2019; Received in revised form 3 August 2019; Accepted 5 August 2019 *Corresponding author.
E-mail address:egulcansu@baskent.edu.tr(E.G. Cansu Ergun).
Available online 06 August 2019
1566-1199/ © 2019 Elsevier B.V. All rights reserved.
state. All electrochemical and spectroelectrochemical properties of the monomer and its electrochemically synthesized polymer were shown and discussed. Meanwhile, basic properties of the corresponding polymer were compared with that of other similar polymers in the lit-erature. Last part of the study includes a dual type electrochemical device (ECD) application of P(EBE) with the homopolymer film of EDOT.
2. Experimental
The chemicals used in this study (acetonitrile (ACN), toluene, di-chloromethane (DCM), ethyl alcohol (EtOH), diethylether (Et2O), acetic
acid (AcOH), hexane, 4,7-dibromobenco[c] [1,2,5]thiadiazole, cyclo-hexane-1,2-dione, Palladium(II), magnesium sulphate, 3,4-ethylene-dioxythiophene (EDOT), sodium borohydride (NaBH4),
tetra-butylammonium tetrafluoroborate (TBABF4)) were purchased from
Sigma Aldrich. Gamry PCI4/300 potentiostat–galvanostat was used for the electrochemical studies. Fluorescence emission measurement of monomer was recorded on a Varian Cary Eclipse Fluorescence Spec-trophotometer. Monomer was electropolymerized via cyclic voltam-metry on platinum disc working electrode (vs Ag/AgCl reference elec-trode). For spectroelectrochemical studies, polymerization was carried out on ITO (Delta Tech. 8–12, 0.7 cm × 5 cm) coated glass working electrode (vs Ag wire) and the spectroelectrochemical measurements were collected using Carry 60 model UV–Vis spectrometer combined with Gamry. All polymerizations were conducted in TBABF4-ACN/DCM
(2:1 v/v) electrolytic medium, and the polymer behaviour was in-vestigated in TBABF4-ACN. H NMR and C NMR spectra of the monomer
were recorded on a Bruker NMR Spectrometer (DPX-400) in CDCl3. For
electrochromic device fabrication, gel electrolyte was prepared ac-cording to literature [24].
2.1. Synthesis of monomer (EBE)
The monomer synthesis was achieved in two steps which were shown inScheme 1. Before all the synthesis steps, stannyl derivative of the donor group (EDOT) were prepared under conventional metallation
conditions [25]. 2.1.1. Step 1 synthesis
4,7-dibromobenzo[c] [1,2,5]thiadiazole was reduced to di-bromobenzene form using NaBH4 in ethanol, according to general
procedure [26]. The resulting intermediate product (3,6-di-bromobenzene-1,2-diamine) was used to react with the same equivalent cyclohexane-1,2-dione (1.5 mmol) in 20 mL diethyl ether along with the catalytic amount of acetic acid (0.5 mL). Reaction was kept under reflux and followed by thin layer chromotography (TLC). After the re-action completed, the solvent was evaporated under vacuo and purified over column chromatography using 20 Hexane/1Ethylacetate. The product was obtained as pale yellow solid.
2.1.1.1. (6,9-dibromo-1,2,3,4-tetrahydrophenazine). (Yield: 35%) 1H
NMR: (CDCl3) d: 7.74 ppm (d, 2H); 3.17 ppm (d, 4H); 1.98 ppm (d,
4H).13C NMR: 156.3; 139.6; 132.2; 122.9; 33.1; 22.6. (1H NMR and13C
NMR spectra were given in Supp. Info.,Figs. S1 and S2.) 2.1.2. Step 2 synthesis
The donor-acceptor-donor coupling was achieved via Stille coupling reaction. The general procedure was carried out as follows: 1 eq. of 6,9-dibromo-1,2,3,4-tetrahydrophenazine, 2.1 eq. of stannylated EDOT and 1% eq. of Pd(II) catalyst were added in freshly distilled toluene. Solution was stirred and refluxed at about 120 °C under inert atmo-sphere for two days by watching with TLC. The solvent was removed under vacuo and the crude product was extracted over DCM and water. After drying over magnesium sulphate, further purification was achieved by column chromatography (3 hexane/1 DCM) and the pro-duct was obtained as pale orange solid.
2.1.2.1. EBE. (Yield: 45%) 1H NMR: (CDCl
3) d: 8.51 ppm (s, 2H);
6.51 ppm (s, 2H); 4.38–4.29 ppm (m, 8H); 3.24 ppm (m 4H); 2.06 ppm (m, 4H); 13C NMR: 151.8; 141.3; 140.1; 137.6; 128.3;
127.1; 113.6; 102.7; 64.9; 64.4; 32.5; 22.9. (1H NMR and 13C NMR
spectra were given in Supp. Info.,Figs. S3 and S4.)
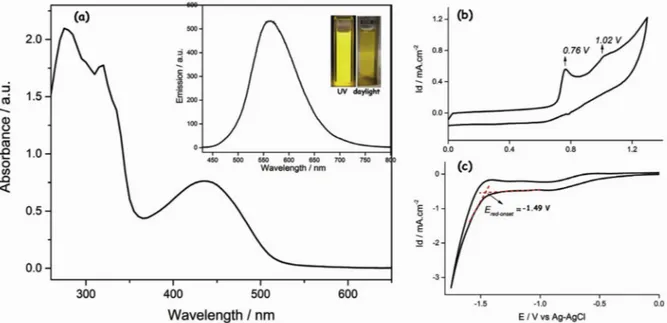
Fig. 1. a) UV–Vis spectrum of EBE recorded in DCM. Inset of (a): Emission spectrum of EBE in DCM (λexc= 430 nm) and photos of the monomer solution under daylight and UV-light. b) CV of the monomer during oxidation between 0.0 V and 1.3 V potentials. c) CVof the monomer during reduction between 0.0 V and −1.75 V potentials. (Both oxidation and reduction CVs were collected in 0.1 M TBABF4– ACN/DCM electrolytic solution at a scan rate of 100 mV/s)
Fig. 2. a) CVs recorded during
electro-polymerization of EBE on Pt-disc WE, con-taining 0.0323 M monomer solution. b) CV of P(EBE), recorded the potentials between −0.5 V and 1.3 V. c) Reduction behaviour of P(EBE), recorded the potentials between 0.0 V and −1.65 V. (Both oxidation and reduction CVs of the polymer were collected in 0.1 M TBABF4-ACN monomer free elec-trolytic solution, at a scan rate of 100 mV/s)
Fig. 3. a) CVs of P(EBE) recorded at the scan rates of 25, 50, 75, 100, 125, 150, 175, 200 mV s−1. b) Charge amounts and current intensities both in anodic and cathodic region as a function of scan rate. c) CVs of P(EBE) after 500 and1000 switchings at a scan rate of 100 mV s−1.
3. Results and discussion
3.1. Spectroscopic and electrochemical properties of the monomer (EBE) Fig. 1a shows the electronic absorption and fluorescence spectra of the monomer recorded in DCM. EBE gave 2 absorption peaks with having maximum at 320 nm and 436 nm. Donor-acceptor-donor based organic molecules typically results in dual band absorption spectrum, where low energy band attributes to intramolecular charge transfer between donor and acceptor units while high energy band indicates a π → π* transition [27,28]. Fluorescence spectrum of the monomer was also measured in DCM at the excitation wavelength of 430 nm, resulting in an emission maximum at 562 nm. The dilute monomer solution ap-pears as yellow under daylight and emits fluorescent yellow color under UV light (See insets ofFig. 1a). Exhibiting a fluorescent color of the material under UV light indicates the existence of a conjugation on the molecule.
Electrochemical behaviour of the monomer was monitored by re-cording its cyclic voltammograms (CV) both in anodic and cathodic regions and the corresponding redox behaviour was given inFig. 1b and c, respectively. The CV of EBE revealed two irreversible oxidation peaks at 0.76 V and 1.02 V. In the cathodic region, monomer exhibited an irreversible reduction having an onset at −1.49 V. The electrochemical band gap of the monomer was measured from the onsets of the oxi-dation (at 0.72 V) and reduction (−1.49 V) potentials and found to be 2.21 V.
3.2. Electrochemical polymerization of EBE on platinum disc electrode: electrochemical properties of P(EBE)
Electrochemical polymerization of EBE was carried out in a work-station of three-electrode system. Pt disc electrode (0.02 cm2area) was
used as a working electrode (WE) vs. Ag/AgCl reference. 0.0323 M monomer solution is used for polymerization, containing TBABF4 –
ACN/DCM electrolytic solution. Electropolymerization was achieved using cyclic voltammetry technique by applying 10-repetitive scans between −0.2 V and 1.3 V potentials and the resulting cyclic voltam-mograms were depicted inFig. 2a. Increasing the peak intensities in each completed scan indicates the polymer formation on WE surface. After the polymerization complete, polymer containing WE was washed in ACN and taken into monomer free electrolytic solution (0.1 M TBABF4in ACN).Fig. 2b shows the cyclic voltammogram of P(EBE). As
seen from the figure, the polymer film has two reversible oxidation peaks, the first one is quite clear at 0.46 V. After that, the second
Fig. 4. Capacitive behaviour of P(EBE), scanning between +0.4 V and 1.1 V
potentials.
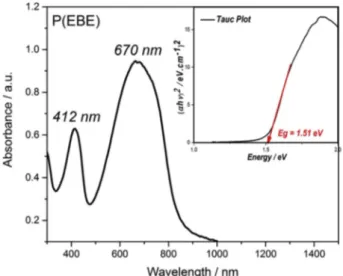
Fig. 5. UV–Vis spectrum of P(EBE) film on ITO-glass WE. Inset: The optical
band gap calculation using Tauc Plot.
Fig. 6. a) UV–Vis spectra of P(EBE) monitored during the anodic scan from −0.8 V to 1.0 V, at a scan rate of 20 mV s−1, b) Colors of the polymer film at different potentials. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
oxidation peak can be observed at 0.86 V. Moreover, the polymer film scanned in negative potentials to −1.65 V and gave a reduction with having an onset at −1.4 V (Fig. 2c). The electrochemical band gap value was calculated from the onsets of the oxidation (0.13 V) and re-duction (−1.4 V) potentials and found to be 1.53 V.
P(EBE) film was scanned at different scan rates increasing from 25 to 200 mV s−1, in order to see the effectiveness of charge-discharge
behaviour with increasing scan rates (Fig. 3a). According to the cyclic voltammograms, charge/discharge amounts and anodic/cathodic cur-rent responses were monitored as a function of scan rate. Amount of charge during oxidation and reduction measured from the integrating program of cyclic voltammetry on GAMRY potentiostat. As seen in Fig. 3b, charge amounts showed almost no change even at high scan
rates, indicating a very effective charging/discharging ability of P (EBE). This phenomenon can be explained with the inverse relationship between the current and time. Faster scan rates shorten the cycling times and result in higher current intensities. As a result, charge amount for each scan remains constant (Q = I × t).
Moreover, current responses were found to be directly related to the scan rates, indicating a well adhering electroactive polymer film on WE surface. These results also indicates a nondiffusional redox process of P (EBE) (See inset ofFig. 3b).
In order to see the electrochemical stability upon many switchings, the polymer film was switched between its redox states (at potentials of −0.2 V and 0.7 V) via square wave potential method with 5 s intervals. The cyclic voltammograms recorded before switching and after 500 and 1000 switchings were shown in Fig. 3c. P(EBE) was saved its 95% charge/discharge intensity and 94% of its current intensity after 1000 switching, indicating a high electrochemical stability.
The capacitive response of the polymer film, to see if the polymer keeps its rectangular shaped CV with increasing scan rates, was also investigated by recording the voltammograms at various scan rates in the potential range of +0.4–1.1 V. The related voltammograms are shown inFig. 4. P(EBE) was saved the direct relation of anodic and cathodic current intensity as a function of scan rate and the CVs mostly saved their rectangular shapes, indicating the capacitive ability of P (EBE) [29]. Only 14% charge lost was observed in both half reactions. 3.3. Electrochemical polymerization of EBE on ITO-glass electrode: spectroelectrochemical properties of P(EBE)
After revealing the electrochemical properties of P(EBE), the polymer film was re-obtained on ITO-glass WE (vs Ag wire as a pseudo-reference electrode) in order to investigate the optical properties. 0.0216 M monomer solution is used for polymerization, containing TBABF4– ACN/DCM electrolytic solution. 10 repetitive potential
cy-clings between the potentials of −0.2 V and 1.3 V were applied for electropolymerization. After the polymer film was obtained, the WE was washed in ACN and taken into monomer free solution of 0.1 M TBABF4– ACN. The area of the polymer film on ITO WE calculated as
1.4 cm2 (CVs during electropolymerization and CV of the resulting polymer film on ITO WE is given in Supp. Info.,Fig. S5.)
P(EBE) film revealed two well separated absorption bands, with having maximum at 412 nm and 670 nm in its neutral state (Fig. 5). Dual absorption before 500 nm and after 600 nm in the visible region is a fingerprint of the green color.
The optical band gap (Eg-optical) was calculated using Tauc Plot method and found to be 1.5 eV (Inset ofFig. 5) [30,31]. (Eg-optical was also calculated from the onset of the lower energy band (1240/823) and found to be the same.) Noted that the band gap value determined from electrochemistry (in Section3.2) is in agreement with the value de-termined from spectroelectrochemistry.
When the polymer film being oxidized, change in the optical
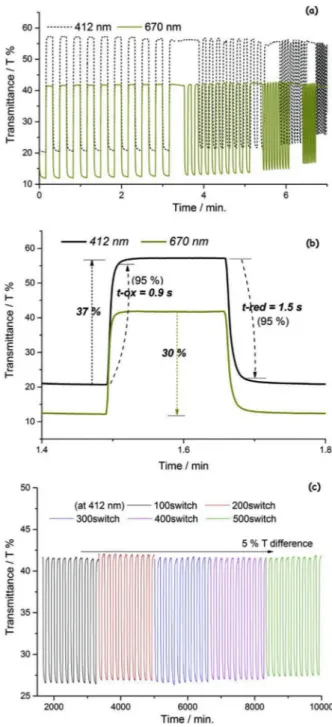
Fig. 7. a) Optical contrast as a function of time at 412 nm and 670 nm, applying
the potentials of −0.8 V and 1.0 V with 10, 5, 2 and 1 s intervals, b) 5th curve of 10 s switching, used for revealing the percent transmittance, response times and coloration efficiency, c) Optical stability test of P(EBE) under the constant potentials of −0.2 V and 0.7 V for 500 switchings.
Table 1
Properties of P(EBE) and the similar polymers in the literature. Polymers ʎmax
(nm) Eg-optical(eV) T % t-switching (s) ColorsNeutral to Oxidized P(EBE) 412
670 1.5 3730 0.90.6 Green toTransmissive (green) [19]
PDEQ 410660 1.4 3630 10.72 Blue-green toTransmissive [20]
PBEAQ 435670 1.5 22.510 1.51.5 Green toTransmissive(blue) [21]
PHED 470790 1.1 2716 0.91.2 Green to Gray [22]
absorption was monitored by UV–Vis spectrometry and the resulting spectra were depicted inFig. 6a. Upon electrochemical oxidation of the film, two neutral bands lost their intensity, accompanying by a for-mation of a new charge carrier band beyond 800 nm, indicating ap-pearance of the charge carriers or polarons [32]. Upon further oxida-tion, another band beyond 950 nm started to appear, most probably attributing the bipolaron formation [33]. Polymer film is dark cyanish-green below −0.5 V (L: 27.2; a*: 12.4; b: 8.7), in its fully neutral state. When potential becomes −0.5 V, the color becomes more green (L: 35.4; a*: 14.3; b: 6.5). At 0.1 V, semi-transmissive (L: 42.7; a*: 6.1; b: 0.80) and after 0.6 V, fully transmissive green is observed (L: 52.8; a*: 2.5; b: 3.4).
It is important to determine the change in optical contrast of the polymer film between its oxidized and neutral states (T %). Besides, other optical properties such as response times (t-ox and t-red) and coloration efficiency (CE) can also be extracted from the optical transmittance data. In order to reveal these properties, square wave input potentials of −0.8 V and 1.0 V were applied on the polymer film at selected wavelengths in 10 s, 5 s, 2 s and 1 s intervals and the re-sulting visible transmittance was shown in Fig. 7a. At 412 nm and 670 nm, T % of P(EBE) was measured as 37% and 30%, respectively. As seen fromFig. 7a, when switching times are fastened, the oxidation transmittance levels keep their intensity while some loss can be seen in the reduction transmittance levels. (especially switchings shorter than 2 s). It can be concluded that the polymer film can be oxidized in a very short time but reduction requires relatively more time than oxidation. Fig. 7b shows the response time measurement on the fifth transmittance curve. 95% of the optical density was used for measuring t-ox and t-red, where human eye is more sensitive. P(EBE) oxidation and reduction times were measured as 0.9 s and 1.5 s, respectively at 412 nm. As ob-served inFig. 7a, reduction time was found to be longer than oxidation time. On the other hand, at 670 nm, t-ox and t-red were calculated as 0.6 s and 1.2 s, respectively.
Optical stability of P(EBE) was also tested under the constant po-tentials of −0.2 V and 0.7 V with 5 s intervals. After 500 switchings, 95% of the optical transmittance was saved, indicating a good optical stability of the polymer film (Fig. 7c).
The amount of electrochromic color formed or bleached by the charge consumed is the characteristic of an electrochromic material at a specific wavelength, called “coloration efficiency (CE)”. For P(EBE), CE values were determined according to literature [34,35]. At 95% of the optical density, CEs of P(EBE) were calculated as 128 cm2/C and
200 cm2/C, at 412 nm and 670 nm, respectively, indicating a
remark-able coloration ability.
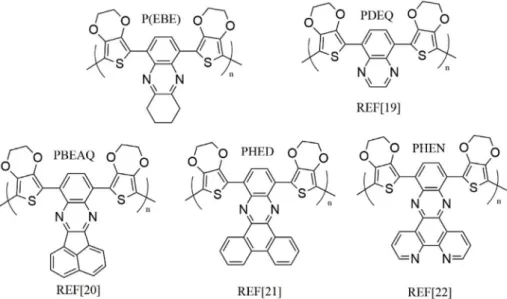
Fig. 8. Chemical structures of P(EBE) and of the similar polymers in the literature.
Fig. 9. UV–Vis spectra of ECD during an anodic potential scan (from −1.1 V to
1.1 V) at a scan rate of 20 mV s−1. Insets: CV of ECD and the photos of the ECD in two distinct redox states.
Fig. 10. Optical stability test of ECD, measured at 410 nm, under square
In order to compare the properties of P(EBE) in terms of the elec-tron withdrawing quinoxaline unit, EDOT containing molecules having similar acceptor skeleton existing in the literature were searched. Table 1demonstrates those polymer films and summaries their basic optical properties. Rather than electrochromics with bis-substituted quinoxalines [14–18] or other aimed quinoxaline and EDOT based polymers [36–40], electron withdrawing quinoxaline based heterocyles (characterized as electrochromics) were especially compared [19–22]. Structures of these polymers were shown inFig. 8. PDEQ contains bare quinoxaline as an acceptor and reveals similar optical properties to P (EBE). As mentioned in the related reference [19], the polymer ex-hibited blue-green color in the neutral state, since PDEQ absorption maxima (both higher and lower energy transitions) shifted toward 600 nm which is the absorption maximum of EDOT itself. In other words, the valley between two absorption bands is at about 440 nm, makes the polymer bluish. For P(EBE), the valley between the visible absorption bands is at 480 nm, making P(EBE) greener than PDEQ. Among all the polymers in Fig. 8, P(EBE) demonstrated the fastest switching time and the highest optical contrast at both wavelengths. On the other hand, PHED and PHEN contains fused benzene rings on qui-noxaline unit, which brings a stronger acceptor property and probably a better donor-acceptor match, resulting in a lower optical band gap. 3.4. Electrochromic device (ECD) application of P(EBE)
For ECD fabrication, P(EBE) and poly(3,4-ethylenedioxythiophene), PEDOT, films on ITO WE were used as the anodically and the cath-odically coloring materials, respectively. Neutral state P(EBE) and oxidized state PEDOT films were sandwiched, sealed via gel electrolyte and dried overnight.Fig. 9shows the spectroelectrochemical study of ECD. During a potential scan from −1.1 V to 1.1 V, absorption band related to PEDOT film at about 600 nm decreased its intensity and the characteristic peaks coming from P(EBE) became intense, attributing to cross oxidation-reduction processes of two polymers. Resulting cyclic voltammogram and the colors of ECD can be seen in the inset ofFig. 9. Upon switching, ECD changed its color from blue to green, as expected simply from the color-mixing phenomenon.
Optical contrast was also measured at three wavelengths coming from both polymer films and found as 5%, 2% and 4% at 410 nm, 600 nm and 700 nm, respectively. As seen fromFig. 9, the relatively lower optical contrasts were obtained at 600 nm and 700 nm with re-spect to that of at 412 nm, due to overlapping of the re-spectra at these optical bands (For change in the optical contrast of ECD, see Sup. Info., Fig. S6).
Optical stability was also tested for ECD at 412 nm, by reducing and oxidizing the device at constant potentials of −1.0 V and 1.0 V (Fig. 10). After 400 switchings, ECD saved its whole optical contrast with just showing an upward shift. This phenomenon may be attributed to easier oxidation but relatively slower reduction times of ECD. Be-sides, upward shift may also show the achievement of the equilibrium of the device at its first 200 switchings.
4. Conclusion
A novel EDOT and cyclohexylquinoxaline based conjugated mole-cule was synthesized via Stille coupling reaction. The electrochromic polymer film was obtained electrochemically and characterized in terms of its electrochemical and spectroelectrochemical properties. The resulting polymer possesed dark cyanish-green in its fully neutral state (below −0.5 V), more green in the neutral state (−0.5 V) and very transmissive at the oxidized state (0.6 V), with having fast switching times at its both higher and lower optical energy bands. Moreover, the polymer film showed excellent electrochemical stability upon 1000 switchings, by saving its 94% of electroactivity. Moreover, 95% of optical transmittance of the polymer remained upon 500 switching on ITO-glass working electrode, indicating a good optical stability. Finally,
dual type ECD of the polymer with EDOT worked well, possesing green and blue colors in different redox states. In the light of these results, P (EBE) is reported as a promising candidate for electrochromic appli-cations.
Acknowledgement
We thank to Prof. Dr. Ahmet M. Onal (Middle East Technical University) for his academic support.
Appendix A. Supplementary data
Supplementary data to this article can be found online athttps:// doi.org/10.1016/j.orgel.2019.105398.
References
[1] J. Jensen, F.C. Krebs, From the bottom up – flexible solid state electrochromic devices, Adv. Mater. 26 (2014) 7231–7234https://doi.org/10.1002/adma. 201402771.
[2] F.M. Kelly, L.L. Meunier, C. Cochrane, V. Koncar, Polyaniline: application as solid state electrochromic in a flexible textile display, Displays 34 (2013) 1–7https://doi. org/10.1016/j.displa.2012.10.001.
[3] R.J. Mortimer, A.L. Dyer, J.R. Reynolds, Electrochromic organic and polymeric materials for display applications, Displays 27 (2006) 2–18https://doi.org/10. 1016/j.displa.2005.03.003.
[4] R. Rauh, Electrochromic windows: an overview, Electrochim. Acta 44 (1999) 3165–3176 1999https://doi.org/10.1016/S0013-4686(99)00034-1. [5] R.G. Mortimer, Electrochromic materials, Chem. Soc. Rev. 26 (1997) 147–156
https://doi.org/10.1039/CS9972600147.
[6] H. Zhou, L. Yang, A.C. Stuart, S.C. Price, S. Liu, W. You, Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7 % efficiency, Angew. Chem. 123 (2011) 3051–3054https://doi.org/10.1002/anie.201005451. [7] S. Song, Y. Jin, S.H. Kim, J.Y. Shim, S. Son, I. Kim, K. Lee, H. Suh, Synthesis and characterization of polyfluorenevinylene with cyano group and carbazole unit, J. Polym. Sci., Polym. Chem. Ed. 47 (2009) 6540–6551https://doi.org/10.1002/pola. 23697.
[8] C. Yang, J.Y. Kim, S. Cho, J.K. Lee, A.J. Heeger, F. Wudl, Functionalized metha-nofullerenes used as n-type materials in bulk-heterojunction polymer solar cells and in field-effect transistors, J. Am. Chem. Soc. 130 (2008) 6444–6450https://doi. org/10.1021/ja710621j.
[9] L. Shen, L. Du, S. Tan, Z. Zang, C. Zhao, W. Mai, Flexible electrochromic super-capacitor hybrid electrodes based on tungsten oxide films and silver nanowires, Chem. Commun. 52 (2016) 6296–6299https://doi.org/10.1039/C6CC01139J. [10] L.B. Groenendaal, G.P. Zotti, H. Aubert, S.M. Waybright, J.R. Reynolds,
Electrochemistry of poly(3,4‐alkylenedioxythiophene) derivatives, Adv. Mater. 15 (2003) 855–879https://doi.org/10.1002/adma.200300376.
[11] G. Sonmez, C.K.F. Shen, F. Rubin, F. Wudl, A red, green and blue (RGB) polymeric electrochromic device (PECD): the dawning of the PECD era, Angew. Chem. Int. Ed. 43 (2004) 1498–1502https://doi.org/10.1002/anie.200352910.
[12] I. Perepichka, E.L. Levillain, J. Roncali, Effect of substitution of 3,4-ethylenediox-ythiophene (EDOT) on the electronic properties of the derived electrogenerated low band gap conjugated polymers, J. Mater. Chem. 14 (2004) 1679–1681https://doi. org/10.1039/B403818E.
[13] P. Wang, Z. Xie, Z. Hong, J. Tang, O. Wong, C. Lee, N. Wong, S. Lee, Synthesis, photoluminescence and electroluminescence of new 1H-pyrazolo[3,4-b]quinoxa-line derivatives, J. Mater. Chem. 13 (2003) 1894–1899https://doi.org/10.1039/ B302972G.
[14] G.E. Gunbas, A. Durmus, L. Toppare, Could green be greener? Novel donor–-acceptor‐type electrochromic polymers: towards excellent neutral green materials with exceptional transmissive oxidized states for completion of RGB color space, Adv. Mater. 20 (2008) 691–695https://doi.org/10.1002/adma.200890012. [15] E. Kose Unver, S. Tarkuc, Y. Arslan Udum, C. Tanyeli, L. Toppare, The effect of the
donor unit on the optical properties of polymers, Org. Electron. 12 (2011) 1625–1631https://doi.org/10.1016/j.orgel.2011.06.004.
[16] S. Özdemir, A. Balan, D. Baran, Ö. Dogan, L. Toppare, Green to highly transmissive switching multicolored electrochromes: ferrocene pendant group effect on elec-trochromic properties, React. Funct. Polym. 71 (2011) 168–174https://doi.org/10. 1016/j.reactfunctpolym.2010.11.024.
[17] S. Tarkuc, Y. Arslan Udum, L. Toppare, Tuning of the neutral state color of the p-conjugated donor–acceptor–donor type polymer from blue to green via changing the donor strength on the polymer, Polymer 50 (2009) 3458–3464https://doi.org/ 10.1016/j.polymer.2009.05.042.
[18] Z. Xu, M. Wang, W. Fan, J. Zhao, H. Wang, The synthesis of new donor–acceptor polymers containing the 2,3-di(2-furyl) quinoxaline moiety: fast-switching, low-band-gap, p- and n-dopable, neutral green-colored materials, Electrochim. Acta 160 (2015) 271–280https://doi.org/10.1016/j.electacta.2015.02.033.
[19] A. Durmus, G.E. Gunbas, L. Toppare, New, highly stable electrochromic polymers from 3,4-Ethylenedioxythiophene-bis-substituted quinoxalines toward green poly-meric materials, Chem. Mater. 19 (2007) 6247–6251https://doi.org/10.1021/
cm702143c.
[20] R. Matsidik, X. Mamtimin, H.Y. Mi, I. Nurulla, Synthesis and properties of polymer from bis-3,4-ethylenedioxythiophene substituted acenaphthenequinoxaline, J. Appl. Polym. Sci. 118 (2010) 74–80https://doi.org/10.1002/app.32376. [21] S. Tarkuc, Y. Arslan Udum, L. Toppare, Molecular architecture: another plausible
pathway toward a low band gap polymer, J. Electroanal. Chem. 643 (2010) 89–93
https://doi.org/10.1016/j.jelechem.2010.03.003.
[22] S. Tarkuc, E. Kose Unver, Y. Arslan Udum, L. Toppare, Multi-colored electrochromic polymer with enhanced optical contrast, Eur. Polym. J. 46 (2010) 2199–2205
https://doi.org/10.1016/j.eurpolymj.2010.08.002.
[23] X. Mamtimin, R. Matsidik, A. Obulda, S. Sidik, I. Nurulla, Solid-state polymerization of EDOT derivatives containing acenaphthenequinoxaline ring, Fibers Polym. 14 (2013) 1066–1072https://doi.org/10.1007/s12221-013-1066-7.
[24] A. Cirpan, A.A. Argun, C.R.G. Grenier, B.D. Reevesa, J.R. Reynolds, Electrochromic devices based on soluble and processable dioxythiophene polymers, J. Mater. Chem. 13 (2003) 2422–2428https://doi.org/10.1039/B306365H. [25] Q. Hou, Q. Zhou, Y. Zhang, W. Yang, R. Yang, Y. Cao, Synthesis and
electro-luminescence properties of high-efficiency saturated red emitter based on copoly-mers from fluorene and 4,7-di(4-hexylthien-2-yl)-2,1,3-benzothiadiazole, Macromolecules 37 (2004) 6299–6305https://doi.org/10.1021/ma049204g. [26] S. Soylemez, S.O. Hacioglu, S. Demirci Uzun, L. Toppare, A low band gap
benzi-midazole derivative and its copolymer with 3,4-Ethylenedioxythiophene for elec-trochemical studies, J. Electrochem. Soc. 162 (2015) H6-H14https://doi.org/10. 1149/2.0311501jes.
[27] S. Mondal, M. Konda, B. Kauffmann, M.K. Manna, A.K. Das, Effects of donor and acceptor units attached with benzoselena-diazole: optoelectronic and self-assem-bling patterns, Cryst. Growth Des. 15 (2015) 5548–5554https://doi.org/10.1021/ acs.cgd.5b01179.
[28] R. Sen, S.O. Singh, P. Johari, Strategical designing of donor-acceptor-donor based Organic molecules for tuning their linear optical properties, J. Phys. Chem. A 122 (2018) 492–504https://doi.org/10.1021/acs.jpca.7b07381.
[29] L. Chen, C.Z. Yuan, H. Dou, B. Gao, S.Y. Chen, X.G. Zhang, Synthesis and electro-chemical capacitance of core–shell poly (3,4-ethylenedioxythiophene)/poly (so-dium 4-styrenesulfonate)-modified multiwalled carbon nanotube nanocomposites, Electrochim. Acta 54 (2009) 2335–2341https://doi.org/10.1016/j.electacta.2008.
10.071.
[30] A.B. Murphy, Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting, Sol. Energy Mater. Sol. Cells 91 (2007) 1326–1337https://doi.org/10.1016/j.solmat. 2007.05.005.
[31] B.D. Viezbicke, S. Patel, B.E. Davis, D.P. Birnie, Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system, Basic Solid State Phys. 252 (2015) 700–1710https://doi.org/10.1002/pssb.201552007. [32] J. Bakalis, A.R. Cook, S. Asaoka, M. Forster, U. Scherf, J.R. Miller, Polarons, com-pressed polarons, and bipolarons in conjugated polymers, J. Phys. Chem. C 118 (2014) 114–125https://doi.org/10.1021/jp408910a.
[33] R.R. Chance, J.L. Brédas, R. Silbey, Bipolaron transport in doped conjugated polymers, Phys. Rev. B 29 (1984) 4491–4495https://doi.org/10.1103/PhysRevB. 29.4491.
[34] C. Bechinger, M.S. Burdis, J.G. Zhang, Comparison between electrochromic and photochromic coloration efficiency of tungsten oxide thin films, Solid State Commun. 101 (1997) 753–756https://doi.org/10.1016/S0038-1098(96)00703-X. [35] C.L. Gaupp, D.M. Welsh, R.D. Rauh, J.R. Reynolds, Composite coloration efficiency measurements of electrochromic polymers based on 3,4- alkylenedioxythiophenes, Chem. Mater. 14 (2002) 3964–3970https://doi.org/10.1021/cm020433w. [36] D. Aldakov, P. Anzenbacher, Sensing of aqueous phosphates by polymers with dual
modes of signal transduction, J. Am. Chem. Soc. 126 (2004) 4752–4753https://doi. org/10.1021/ja039934o.
[37] U.t. Bulut, M. Kolay, S. Tarkuc, L. Toppare, Dibenzophenazine derivatives as visible photosensitizers for diaryliodonium salts, J. Polym. Sci. Part A: Pol. Chem. 49 (2011) 3299–3303https://doi.org/10.1002/pola.24766.
[38] D. Aldakov, P. Anzenbacher Jr., Dipyrrolyl quinoxalines with extended chromo-phores are efficient fluorimetric sensors for pyrophosphate, Chem. Commun. 12 (2003) 1394–1395https://doi.org/10.1039/B301362F.
[39] P.l. Anzenbacher Jr., K. Jursikova, D. Aldakov, M. Marquez, R. Pohl, Materials chemistry approach to anion-sensor design, Tetrahedron 60 (2004) 11163–11168
https://doi.org/10.1016/j.tet.2004.08.060.
[40] D. Gedefaw, M. Prosa, M. Bolognesi, M. Seri, M.R. Andersson, Recent development of quinoxaline based polymers/small molecules for organic photovoltaics, Adv. Energy Mater. 7 (2017) 1700575https://doi.org/10.1002/aenm.201700575.