Full Terms & Conditions of access and use can be found at
http://www.tandfonline.com/action/journalInformation?journalCode=tlip20
ISSN: 1758-4299 (Print) 1758-4302 (Online) Journal homepage: http://www.tandfonline.com/loi/tlip20
Metabolic and cardiovascular effects of berberine:
from preclinical evidences to clinical trial results
Arrigo Cicero & Sibel Ertek
To cite this article: Arrigo Cicero & Sibel Ertek (2009) Metabolic and cardiovascular effects of berberine: from preclinical evidences to clinical trial results, Clinical Lipidology, 4:5, 553-563 To link to this article: https://doi.org/10.2217/clp.09.41
Copyright 2009 Taylor and Francis Group LLC
Published online: 18 Jan 2017.
Submit your article to this journal
Article views: 579
Metabolic and cardiovascular effects of
berberine: from preclinical evidences to clinical
trial results
Atherosclerosis is by far the single most impor‑ tant pathological process in the development of cardiovascular diseases (CVDs), which are the most common causes of morbidity and mortality in developed nations [1]. The main risk factors of CVD are already known and reversing the exposition to these risk factors is associated with a significant reduction of the CVD risk. This is particularly evident when hypercholesterol‑ emia is treated: a 1 mmol/l decrease in LDL‑C is associated with a 20% risk reduction of CVD events [2]. On the other hand, a large amount of residual risk remains, even in patients adequately treated with the available antihypercholester‑ olemic drugs, mainly because of their limited action on lipid fractions other than LDL‑C and on other sub‑clinical risk factors, such as insu‑ lin resistance and obesity [3]. Moreover, with the best‑available treatments, a large number of patients at high risk for CVD events still do not reach the lipid targets suggested by the most widely accepted international guidelines for CVD prevention [4]. Therefore, a propor‑ tion of patients refuse to continue the standard treatment owing to intolerance [5], or search for alternative treatments due to statin‑related side effects [6].
In this context, this report aims to evaluate the metabolic properties of the natural alka‑ loid berberine, and its potential application to CVD prevention.
Berberine sources & chemical characteristics
Berberine is a plant quaternary ammonium salt from the group of isoquinoline alkaloid (2,3‑methylenedioxy‑9,10‑dimethoxypro‑ toberberine chloride; C20H18NO4+) with a
molar mass of 336.36122 g/mol. It is highly concentrated in the roots, rhizomes and stem bark of various plants including Coptis chien-sis (Huanglian), Rhizoma coptidis, Hydrastis canadensis (golden seal), Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry), Berberis aristata (tree turmeric), Tinospora cor-difolia, Copthidis rhizome, Arcangelisia flava and Cortex rhellodendri (Figure 1)[7].
It is traditionally used for its supposed anti‑ microbial effects and as a treatment for diabetes in traditional Chinese, Indian and middle‑east folk medicine. Its chemical structure has qua‑ ternary base and it is commercially prepared for clinical application as salts, such as berberine chloride or berberine sulphate [7].
Berberine antihyperlipidemic effects
The metabolic effects of berberine have been widely investigated during the last few years. With regard to the lipid metabolism, the lipid‑lowering effect of berberine appears to be mainly due to stabilization of the hepatic LDL‑C receptor (LDLR) by an ERK‑ dependent mechanism and also by increasing
Berberine is a plant alkaloid with various biological activities. A large body of literature support different pharmacological actions of berberine that could be interesting in the management of metabolic diseases associated with high cardiovascular disease risk, such as mixed hyperlipidemia, insulin resistance, metabolic syndrome and Type 2 diabetes. Numerous preclinical in vitro and in vivo studies support all these effects. Moreover, it seems that berberine also exerts anti-inflammatory and antiproliferative effects that could play a role in the development of atherosclerosis and its clinical consequences. Recently, the metabolic effects of berberine have also been demonstrated in humans, opening new perspectives for the use of this molecule in patient therapy. Larger and longer studies need to be carried out to implement the definition of the therapeutic role of berberine in humans.
KEYWORDS: berberine n cardiovascular disease n diabetes n hypercholesterolemia Arrigo F Cicero† & Sibel Ertek1
†Author for correspondence:
‘GC Descovich’ Atherosclerosis & Metabolic Diseases Research Center, Internal Medicine, Aging & Kidney Diseases Department, Sant’Orsola, Malpighi Hospital, University of Bologna, Via Massarenti, 9, 40138 Bologna, Italy Tel.: +39 349 855 8017 Fax: +39 051 390 646 afgcicero@cardionet.it
1Ufuk University, Ankara,
transcriptional activity of the LDLR promoter by the c‑Jun N‑terminal kinase pathway [8,9]. Moreover, berberine induces decreased tran‑ scription of the proprotein convertase subtili‑ sin/kexin type 9 (PCSK9) gene, where PCSK9 post‑transcriptionally downregulates the LDLR by binding to the receptor’s EGF repeat A on the cell surface and shuttling the LDLR to the lysosomes for degradation [10].
Recently, it was demonstrated that berberine exerts LDLR mRNA stabilization effects, medi‑ ated by reducing the LDLR mRNA 3´‑untrans‑ lated region binding of heterogenous nuclear ribonucleoprotein I and KH‑type splicing regulatory protein, which are key modulators of LDLR mRNA stability in liver cells [11].
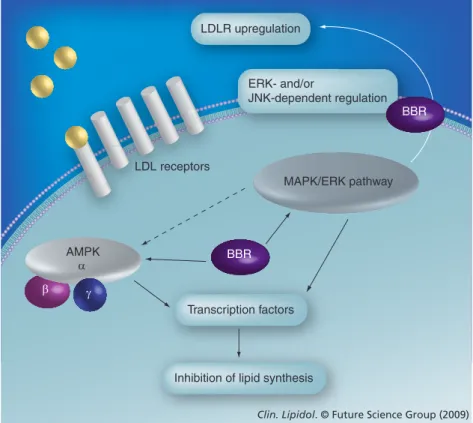
In addition to LDLR upregulation, other mechanisms of action were proposed (Box 1). In 3T3L1 cells, leptin, transcription factors, such as sterol regulatory element‑binding protein‑1c and CCAAT enhancer‑binding protein‑a, PPAR‑g, fatty acid synthase, ace‑ tyl‑CoA carboxylase, acyl‑CoA synthase and lipoprotein lipase were reduced by berberine treatment [12]. Berberine also activates AMPK,
while blocking the MAPK/ERK pathway, resulting in inhibition of lipid synthesis; its action on AMPK is eliminated by the MEK inhibitor, suggesting a link between these two pathways (Figure 2)[13].
The antihyperlipidemic effects of berberine have also been confirmed by some clinical tri‑ als (Table 1), which mainly involved patients affected by mixed hyperlipidemia treated with berberine 500–1500 mg/day. A mean 25% LDL‑C and 25% TG reduction were observed in mild mixed hyperlipidemic patients [14], in Type 2 diabetics [15,16] and in patients affected by liver diseases [17].
The antihyperlipidemic effect of berberine has been observed to be synergistic with other nutraceuticals inhibiting cholesterol synthesis, such as monakolins and policosanols. In the study carried out on Caucasian hyperlipid‑ emic subjects, a small randomized clinical trial compared 40 patients randomized to berber‑ ine alone 500 mg/day versus berberine 500 mg combined with policosanol 10 mg and red yeast extract 3 mg/day for 4 weeks. Reduction in triglycerides was 26% in the combination and 22% in the berberine group, and for LDL‑C the reduction was 20% in berberine and 25% in combination [18]. A similar effect has been observed by adding berberine treatment to simvastatin in the therapy of hyperlipidemic patients [19]. Overall the berberine antihyper‑ lipidemic effect does not appear to be directly dose‑related.
Effects of berberine on insulin resistance & fatty acid metabolism
Beyond the impressive effect of berberine on lipid metabolism, recent evidences suggest that it also has a relevant impact on glucose homeostasis. In fact, in cultured human liver cells and rat skeletal muscle berberine increases
N O O CH3O OCH3 + Cl -Figure 1. Berberine.
Box 1. Berberine actions involved in plasma lipid level modulation.
Inhibition of proprotein convertase subtilisin/kexin type 9 mRNA transcription n Stabilization of LDLR on the liver cell membranes
Stabilization of liver LDLR mRNA n AMPK activation
– Suppression of adipogenesis – Induction of LDLR expression n MAPK inhibition
– LDLR upregulation
– Inhibition of lipid synthesis probably in conjunction with AMPK pathway n Modulatory effects on peroxisome proliferator-activated receptor (PPAR) -a and -g
insulin receptor mRNA expression through protein kinase C‑dependent activation of its promoter [20]. Because it is supposed that ber‑ berine acts as an insulin‑sensitizing agent [21], its activity has been compared with that of metformin in different animal models. In rat models of Type 2 diabetes, berberine showed similar or better plasma fasting glucose and LDL‑C lowering and better homeostasis model assessment of insulin resistance (HOMA‑IR) than metformin by a mechanism involving retinol binding protein‑4 and glucose trans‑ porter (GLUT)‑4 [22,23]. Moreover, in the 3T3‑L1 adipocytes model of insulin resistance, increased GLUT‑4 levels in both normal and insulin‑resistant cells and AMPK activity is related to GLUT‑1‑mediated glucose uptake [24]. Inhibition of mitochondrial glucose oxi‑ dation by berberine and increased AMP:ATP ratio causes AMPK activation and stimulation of glycolysis [25]. In the same cellular model, berberine also reversed IKKb Ser181 and insu‑ lin receptor substrate‑1 Ser307 phosphorylation, and improved insulin‑stimulated glucose trans‑ port and reversed free fatty‑acid (palmic acid in this study) induced insulin resistance [26]. Unlike insulin, berberine‑induced glucose uptake in 3T3L1 adipocytes was not inhibited by phosphatidylinositol 3‑kinase inhibitor or p38 MAPK inhibitor, and ERK‑kinase inhibitor decreased berberine‑stimulated glucose uptake only by 32%. Berberine did not induce Akt phosphorylation opposite to insulin, but ber‑ berine action was totally inhibited by the tyro‑ sine kinase inhibitor genistein, suggesting that berberine increases GLUT‑1 activity. Finally, berberine increased AMPK and acetyl coen‑ zyme A carboxylase phosphorylation [27]. Since berberine‑induced glucose uptake is inhibited both by AMPK inhibitor (compound C) and p38 MAPK inhibitor (SB202190), berberine acts via the AMP–AMPK–p38 MAPK pathway and this may also account for its antilipidemic effects [28]. Berberine also inhibits the transcrip‑ tion of PPARg genes, thus inhibiting adipocyte differentiation [29,30].
Mechanisms of action other than insulin‑ sensitization are probably involved in berber‑ ine’s antidiabetic action. In rats with streptozo‑ cin‑induced diabetes, berberine administered at doses of 120 mg/kg/day for 5 weeks was asso‑ ciated with an increase in glucagon‑like pep‑ tide‑1 levels in plasma and intestine, plasma insulin levels and pancreas b‑cell number [31]. Moreover, berberine may have protective
effects for diabetes by directly protecting b cells through amelioration of cellular antioxi‑ dant status [32]. Furthermore, berberine may inhibit sucrase and maltase activity with an acarbose‑like effect [33].
Berberine also has complex effects on glucose homeostasis. Some preclinical evidence shows that berberine inhibits mitochondrial functions (by inhibition of mitochondrial respiratory com‑ plex I [34]), stimulates glycolysis, activates the AMPK pathway, suppresses adipogenesis, has antiobesity effects and induces LDLR expres‑ sion, which are important mechanisms for insulin resistance and lipid metabolism [26,35].
Berberine also acts as a secretagogue agent, as demonstrated in diabetic rats, where this effect has been compared with that of the sul‑ phonylurea, glibenclamide [36]; in this test, berberine enhanced glucose‑stimulated insu‑ lin secretion in a dose‑dependent manner and increased both mRNA and protein expression
β AMPK α γ BBR LDL receptors MAPK/ERK pathway
Clin. Lipidol. © Future Science Group (2009)
ERK- and/or
JNK-dependent regulation LDLR upregulation
Transcription factors
Inhibition of lipid synthesis
BBR
Figure 2. Cellular actions of berberine in lipid metabolism. AMPK (previously
termed HMG-CoA reductase kinase) plays a key role in cellular energy homeostasis, as a cellular sensor for cellular ATP levels. Binding of AMP to its catalytic a subunit activates the complex that regulates cellular lipid and glucose metabolisms via several transcription factors. As it regards lipid metabolism, AMPK inhibits Acetyl CoA carboxylase by activation of a and g subunits, resulting in decreased lipid synthesis. Among three MAPK groups (ERK1/2, p38MAPK, JNK), which are primarily involved in cellular differentiation and the cell cycle, JNK is important in LDLR upregulation and also MAPK/ERK may have a link with AMPK pathway in cellular lipid metabolism.
of hepatic nuclear factor 4 a and glycokinase activity, providing an insulinotropic effect dif‑ ferent from sulfonylureas. Other studies do not support this effect as relevant for berber‑ ine‘s action on glucose metabolism [23,37]. The effects of berberine on insulin resistance and intra cellular glucose metabolism are summa‑ rized in Figure 3. Berberine could also exert some protective effects on diabetes‑induced nephro pathy, through controlling blood glu‑ cose, reduction of oxidative stress and inhibi‑ tion of the activation of the polyol pathway, as observed in diabetic rats [38], and also by inhibiting fibronectin and collagen synthesis, partly via the p38 MAPK signal pathway in glomerular mesangial cells [39].
Beyond this large preclinical literature, data on human glucose metabolism are preliminary. However, at least two randomized clinical tri‑ als are available investigating the antidiabetic effect of berberine as monotherapy [15,16] or when added to standard antidiabetic treatments [16]. When added to metformin, berberine made it possible to obtain a significant decrease in HbA1% (‑2%), fasting plasma glucose (‑44%), postprandial glucose (‑45%), fasting plasma insulin (‑28%) and HOMA index (‑44.7%).
Vascular & antihypertensive effects of berberine
Vasorelaxant effects of berberine have been observed in different rat models [40–42]. Vasodilatator effects of berberine are the result of its action on both endothelium and vascular smooth muscle. At low concentra‑ tions (<1×10–6 M) the berberine‑mediated
aortic relaxation appears to be dependent on endothelium but at higher concentrations to be independent of intact endothelium [43,44]. Other mechanisms suggested to be involved in the vasorelaxant effect of berberine are an angiotensin‑converting enzyme (ACE) inhibi‑ tory effect and direct release of NO/cGMP from rat aortic rings [45], a1‑adrenoreceptor antagonistic action in rat and rabbit aorta [46], potentiation of acetylcholine [42], activation of K+ channels and the inhibition of intracellu‑
lar calcium release, blocking l‑type calcium channels [47]. Berberine increases expression of endothelial nitric oxide synthase mRNA and inhibits expression of inducible nitric oxide synthase mRNA in gastric tissue in studies on ethanol‑induced gastric ulcers in mice [48]. Activation of tetrapentylammonium‑, 4‑ami‑ nopyridine‑ and Ba2+‑sensitive K+ channels,
Table 1. Clinical efficacy of berberine as an antihyperlipidemic agent.
Author No. of patients Diagnosis Study design Dose tested Study duration LDL-C reduction (%) TG reduction (%) Ref. Kong et al. (2004) 91 Mild mixed hyperlipidemia Double-blind, RCT 500 mg b.i.d. 12 weeks -20% (-25% in untreated patients) -28% (-35% in untreated patients) [14] Cicero et al. (2007) 40 Moderate mixed hyperlipidaemia Single-blind, RCT 500 mg u.i.d. 4 weeks -20% -22% [18] Zhang et al. (2008)
116 Type 2 diabetes and mixed hyperlipidemia Double-blind, RCT 500 mg u.i.d. 12 weeks -25% -30% [15] Yin J et al. (2008)
84 Newly diagnosed Type 2 diabetes and poorly controlled Type 2 diabetes Double-blind, RCT 500 mg t.i.d. 13 weeks -20% NR [16] Kong WJ et al. (2008) 63 Hypercholesterolemia Double-blind, RCT 500 mg b.i.d. 8 weeks -24% (-32% + simvastatin 20 mg) -22% (-39% + simvastatin 20 mg) [19] Zhao W et al. (2008) 70 Hyperlipidemia in: a) Hepatitis B (35) b) Hepatitis C (18) c) Cirrhosis (17) Open study 500 mg b.i.d. 12 weeks -21% -19% -22% -21% -24% -17% [17] Cicero et al. (2008) 40 Hypercholesterolemia in intolerance to more than one statin Open study 500 mg u.i.d. + Phytostanols 2 gr u.i.d. 24 weeks -32% -13% [6]
inhibition of intracellular Ca2+ release from
caffeine‑sensitive pools or a direct relaxant effect, are also likely to be responsible for the berberine‑induced endothelium‑independent relaxation [44].
The vasodilatator effects of berberine were not observed at low concentrations (below 1×10–6 M) of berberine on methylene blue‑pre‑
treated rat aorta, but at higher concentrations aortic relaxation was observed irrespective of such a nitric oxide inhibitor [43].
Blood pressure increase could also be pre‑ vented by the above‑cited nephroprotective effect demonstrated in rat models [38,39].
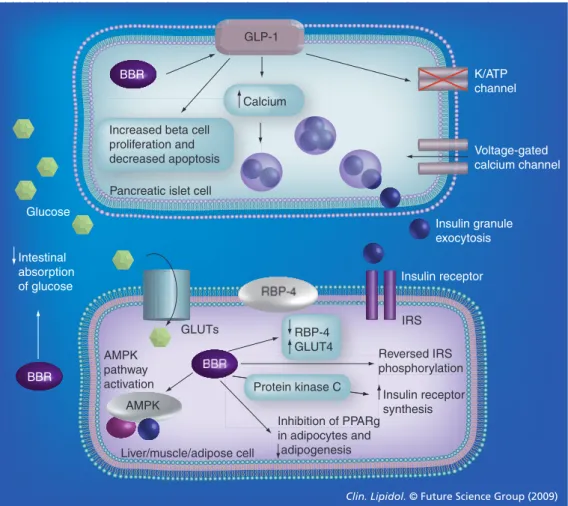
Berberine also has some vascular‑protecting actions that could preserve the endothelial func‑ tions maintaining the vessels more reactive and elastic properties. In fact, berberine inhibits PDGF‑induced vascular smooth muscle cell growth via activation of AMPK/p53/p21(Cip1) signaling, while activating Ras/Rac1/Cyclin D/ Cdks and suppressing PDGF‑stimulated migra‑ tion by Rac1 and Cdc42 inhibition, finally causing antiproliferative and antimigratory effects [49]. In rat glomerular mesangial cells, berberine inhibits fibronectin and collagen syn‑ thesis partly via the p38MAPK pathway [39]. It also prevents the migration and regrowth of GLP-1 K/ATP channel Voltage-gated calcium channel BBR BBR BBR
Increased beta cell proliferation and decreased apoptosis
Calcium
Pancreatic islet cell
Intestinal absorption of glucose Liver/muscle/adipose cell GLUTs Glucose IRS Insulin receptor AMPK Reversed IRS phosphorylation Insulin receptor synthesis RBP-4 GLUT4 Protein kinase C Inhibition of PPARg in adipocytes and adipogenesis AMPK pathway activation
Clin. Lipidol. © Future Science Group (2009) Insulin granule exocytosis
Figure 3. Effects of berberine on glucose metabolism. Berberine affects glucose metabolism
increasing insulin secretion, stimulating glycolysis, suppressing adipogenesis, inhibiting
mitochondrial function, activating the AMPK pathway and increasing glycokinase activity. Berberine also increases GLUT-4 and GLP-1 levels. GLP-1 receptors are important in islet cell survival: upon their activation, adenylyl cyclase is activated and cAMP generated, leading to activation of second messenger pathways and closure of ATP-dependent K+ channels. Increased intracellular potassium
causes depolarization, and calcium influx through the voltage dependent calcium channels occurs. This intracellular Ca2+ increase stimulates the migration and exocytosis of the insulin granules. In
glucose-consuming tissues, such as adipose, liver or muscle cells, berberine affects both GLUT-4 and RBP-4 in favor of glucose uptake into the cell, stimulates glycolysis by AMPK activation and also have effects on PPARg molecular targets and phosphorylation of IRS-1, finally resulting in decreased insulin resistance.
BBR: Berberine; GLP-1: Glucagon-like peptide-1; GLUT: Glucose transporter; IRS: Insulin receptor substrate; RBP: Retinol binding protein.
smooth muscle cells to mechanically trauma‑ tized sites by inactivating the MEK1,2/ERK/ Egr1 signaling pathway, decreasing Erg1, cFos, cyclin D and PDGF‑A levels [50]. Activation of ERK1/2 pathway (one of three MAPK groups) was also demonstrated by its inhibition on lyso‑ phosphatidylcholine‑stimulated vascular smooth muscle cell proliferation and migration [51].
As it regards human data, in a study on 20 volunteers, berberine 400 mg three times a day for a month induced upregulation of number and function of erythrocyte progenitor cells due to NO production [52]. It was found that 1‑month treatment with berberine (400mg/day, three times a day) in 15 healthy volunteers induces mobilization of circulating endothelial progenitor cells with CD34/KDR double positivity in small arteries [53]. Furthermore, in a recent study carried out on 14 healthy subjects, berberine markedly decreased circulating endothelial microparticles, which are associated with endothelial dysfunc‑ tion, and this may also contribute to the beneficial effects of berberine on endothelium [54].
Other berberine activities with potential relevance in cardiovascular disease prevention
It has been shown that berberine does not only have indirect myocardium protective effects by modulating lipid metabolism, glucose homeosta‑ sis and blood pressure, but it also directly acts on the heart at different levels.
In fact, berberine has sympathetic activity modulator effects on myocardium. In rats with experimentally induced cardiac hypertrophy by suprarenal aortic constriction, berberine decreases plasma noradrenaline and adrenaline levels and adrenaline in ventricular tissue, improved car‑ diac contractility with shortened time to reach the maximum rate from beginning of contraction and reduced size of left ventricular myocardium [55,56]. In a dog ischemic heart failure model, intra‑ venous berberine administration increased cardiac output, decreased left ventricular end diastolic pressure and systemic vascular resistance [57].
Berberine blocks ATP‑sensitive and voltage sensitive K+/ATP channels and causes shorten‑
ing of the action potential duration and effective refractory period, thus it mainly has class III antiarrythmic effect [58].
Examination of hemodynamic parameters in humans revealed similar results with increased cardiac index, increased left ventricular ejec‑ tion fraction, decreased systemic and pulmo‑ nary vascular resistance and left ventricular
end‑diastolic pressures [59]. In a clinical trial, berberine decreased the frequency and com‑ plexity of ventricular premature complexes and increased the left ventricular ejection fraction in chronic heart failure patients [60]. In 24–48 h ambulatory monitoring of 100 patients with ventricular tachyarrythmias, berberine caused 50% or greater reduction in ventricular prema‑ ture contractions in 62% of patients and 90% or more reduction in 38% [61].
Some studies showed that berberine has a significant antiplatelet effect [62], explained by inhibition of arachidonic acid metabolism and calcium influx [63]. However, recent evidences suggest that berberine can also have a prothrom‑ botic effect, enhancing tissue factor activity [64]. The anti‑inflammatory effect occurs by inhi‑ bition of COX‑2 via the ERK1/2 signaling path‑ way; JNK pathway in high doses and NF‑kB activation may also have a role [65,66].
The antioxidant [67] and antiproliferative effects of berberine on vascular smooth muscle cells and animal studies also suggest it has a pos‑ sible preventive action on atherosclerosis and post‑treatment restenosis development [49–51].
Berberine pharmacokinetics: implications for safety
Berberine has low bioavailability in animal models (<5%) and bowel p‑glycoprotein appears to contribute to its poor absorption, actively expelling the alkaloid from the lumen muco‑ sal cells [7]; in human volunteers after a single administration the Cmax is 394.7 µgl‑1, the t‑peak
2.37 h and the AUC 0‑∞ 3028 3 µgl‑1·h‑1[68].
This could at least partly explain why berber‑ ine efficacy is not strongly dose‑related and why for the most part its known side effects for com‑ mon doses are gastrointestinal [69]. High berber‑ ine doses have been also associated with arterial hypotension, dyspnoea, flu‑like symptoms and cardiac damage (Box 2)[70].
Site marker competitive experiments indicated that berberine binds to human albumin and that this binding primarily took place in subdo‑ main IIA [71]. Since berberine has a strong affin‑ ity for albumin and other circulating proteins, it also displaces from its binding sites drugs such as warfarin, thiopental and tolbutamide, whose high plasma levels increase their toxicity [72]. In a similar way, berberine displaces bilirubin from albumin; for this reason and because it poten‑ tially cause uterine contraction and miscarriage, its use is not suggested during pregnancy and breastfeeding of jaundice infants [73].
Studying the pharmacokinetics of unbound berberine in a rat noncompartmental model, Tsai et al. found that berberine is transported to bile through active transportation and it is metabolized by the p450 enzyme system in the liver [74]. In rats its main metabolic site is the liver, and intestinal bacterial flora plays a role in enterohepatic circulation of berberine and its conjugated metabolites. Berberine is metabo‑ lized in the liver with Phase I demethylation and Phase II glucuronidation and a very small amount of unchanged berberine is eliminated in urine [75]. Berberine has four main metabo‑ lites identified in rats: berberrubine, thalifendine, demethyleneberberine and jatrorrhizine, all of which have glucuronide conjugates [76] whose activity has not been adequately investigated. In humans the main urinary metabolites are jatror‑ rhizine‑3‑sulfate, demethyleneberberine‑2‑sulfate and thalifendine‑10‑sulfate [77].
Similar to other alkaloids contained in Hydrastis canadensis extracts (i.e., hydrastine and canadine), berberine may inhibit p450 2E1‑like [78] and 1A2 [79]. This inhibition is not related to a significant increase in pharmacological interac‑ tion since the most part of available drugs are not metabolized by these enzymatic systems.
On the other hand, berberine can markedly increase blood levels of cyclosporine A because of cytochrome p450 3A4 inhibition in liver and gut wall and an increase in gastric emptying time causing increased cyclosporine A (CSA) bioavailability and reduced metabolism [80]. In a clinical study, the coadministration of berberine (2 g/day, three times a day) to renal transplant recipients who take cyclosporine 3 mg/kg twice daily resulted in mean AUC of CsA increasing by 34.5% and the mean half‑life of cyclosporine increasing by 2.7 h [81].
In rats it was observed that intestinal p‑glyco‑ protein and organic cation transport inhibitors are involved in active berberine efflux from the
liver, while the coadministration of berberine and cyclosporine or quinidine significantly decreased berberine levels in the bile, while its glucuronidation was not affected by proben‑ ecid [74]. It also inhibits cytochrome 1A1, thus potentially interacting with drugs metabolized by this cytochrome isoform [82].
Berberine may also antagonize paclitaxel [83], thereby reducing its uptake and resistance, by a multidrug‑resistance gene mechanism. The LD50 of berberine sulfate is 25 mg/kg in mice [84], but that of Berberis vulgare is mod‑ erately high (LD50 = 2.6 ± 0.22 g/kg twice weekly in mice) [70]; these data support the use of only highly purified and concentrated berberine formulation.
However, since berberine has strong DNA‑ binding properties owing to its chemical struc‑ ture of a quarternary ammonium compound, the suspicion regarding its genotoxic, mutagenic or recombinogenic activities is important. In the study of Pasqual et al., this alkaloid induced cytotoxic and cytostatic effects in dividing repair‑deficient Saccharomyces cerevisae strains and induced some frameshift and mitochon‑ drial mutations, but these were not considered as potent mutagenic effects [85]. In a relatively more recent study, quarternary protoberberine alkaloids were tested by new techniques and it found that they may interact with nucleic acids as intercalators or minor groove binders [86]. Although so far there is no study to show such action in human cells, berberine needs to be tested for its potential risk on genetic damage in humans.
Finally, a recent preclinical study also directs attention to the possible interaction of berber‑ ine with the coagulation system [87], but fur‑ ther evidences have to confirm and clarify this potential adverse effect, because previous reports highlighted a berberine potential antithrombotic effect [88].
Box 2. Summary of potential adverse effects and cautions about berberine.
n Gastrointestinal discomfort n Constipation n Gastritis n Hypotension n Dyspnea n Flu-like symptoms n Cardiotoxicity n Genotoxicity potential
n Drug interactions (explained in the text) n Jaundice in infants
Executive summary
Introduction
n Best-available treatments still largely miss the accepted guideline targets for patients at high cardiovascular disease (CVD) risk, and statin-related side effects also necessitate the search for alternative treatments for hyperlipidemia.
Berberine sources & chemical characteristics
n Berberine is a plant quaternary ammonium salt from the group of isoquinoline alkaloids, highly concentrated in the roots, rhizomes and stem bark of various plants.
Berberine antihyperlipidemic effects
n The lipid-lowering effect of berberine has been confirmed in animals and humans and appears to be mainly due to LDL-C receptor upregulation, AMPK activation, MAPK/ERK pathway blockage and proprotein convertase subtilisin/kexin type 9 inhibition.
Effects of berberine on insulin resistance & fatty acid metabolism
n Berberine improves glucose homeostasis in animals and humans: the possible mechanism of actions involved in this effect are the increase in insulin receptor mRNA expression, the reduction of retinol binding protein-1, the increase in glucose transporter-4 and glucagon-like peptide-1, the decrease in PPARg in adipocytes and a secretagogue activity.
Other berberine activities with potential relevance in cardiovascular disease prevention
n Berberine directly protect endothelium and has angiotensin-converting enzyme inhibitory effects, improving hemodynamic parameters in both animal and human studies.
Berberine pharmacokinetics: implications for safety
n Berberine has a low bioavailability and liver metabolism. Its tolerability and safety appear to be good at standard dosages for human use and the pharmacokinetic interaction relatively rare. However, further longer and larger clinical studies have to be carried out to confirm berberine’s potential usefulness in human therapy.
Bibliography
Papers of special note have been highlighted as: • of interest
•• of considerable interest
1 Müller‑Nordhorn J, Binting S, Roll S, Willich SN: An update on regional variation in cardiovascular mortality within Europe.
Eur. Heart J. 29(10), 1316–1326 (2008).
2 Baigent C, Keech A, Kearney PM et al.; Cholesterol Treatment Trialists’ (CTT) Collaborators: efficacy and safety of cholesterol‑lowering treatment: prospective meta‑ana lysis of data from 90,056
participants in 14 randomised trials of statins.
Lancet 366(9493), 1267–1278 (2005).
3 Fruchart JC, Sacks F, Hermans MP et al.: The Residual Risk Reduction Initiative: a call
to action to reduce residual vascular risk in patients with dyslipidemia. Am. J. Cardiol. 102(Suppl. 10), K1–K34 (2008). 4 Sénécal M, Fodor G, Gagné C et al.:
Limitations of statin monotherapy for the treatment of dyslipidemia: a projection based on the Canadian Lipid Study –
Observational. Curr. Med. Res. Opin. 25(1), 47–55 (2009).
Conclusion
Numerous preclinical studies and some well‑car‑ ried out clinical trials strongly support the poten‑ tial use of berberine as a powerful insulin‑sen‑ sitizing agent with relevant antihyperlipidemic effects and vascular‑protective action. Further long‑term randomized clinical trials have to be carried out in order to better delineate the clini‑ cal indication and the safety profile of the drug.
Future perspective
The modern investigation on berberine phar‑ macological activity is exploding and numerous scientific evidences are in press and reported at international congresses.
The near future perspective is the isolation or neosynthesis of berberine analogs with a higher bioavailability. Then it will be probable that the hypothesis on the berberine mechanism of action on glucose and lipid metabolism will be clarified and partially unified: it is in fact possible that berberine acts as a modulator of a gene involved in more actions or of a gene‑regulator (e.g., the upstream stimulatory factors). However,
the effect on PCSK9 modulation is already a potential therapeutic target, because it makes the combined use of berberine and the available antihyperlipidemic drugs possible.
Then, the antihyperlipidemic and antidiabetic effects of berberine have to be related to mark‑ ers of improvement in organ damage in humans (i.e., by evaluating their effect on microalbumin‑ uria or arterial stiffness). Finally, longer trials are needed to better evaluate the safety profile of the molecule, when administered alone or in association with other antihyperlipidemic or antidiabetic drugs.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a finan-cial interest in or finanfinan-cial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pend-ing, or royalties.
No writing assistance was utilized in the production of this manuscript.
5 Jacobson TA: Toward ‘pain‑free’ statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin. Proc. 83(6), 687–700 (2008).
6 Cicero AFG, Ertek S: Natural sources of antidyslipidaemic agents: is there an evidence‑based approach for their prescription? Med. J. Nutr. Metab. 1(2), 85–93 (2008).
7 Birdsall TC, Kelly GS: Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Altern. Med. Rev. 2, 94–103 (1997).
8 Abidi P, Zhou Y, Jiang JD, Liu J: Extracellular signal regulated kinase‑dependent
stabilization of hepatic low density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler. Thromb. Vasc. Biol. 25(10), 2170–2176 (2005).
9 Lee S, Lim HJ, Park JH et al.: Berberine induced LDLR up‑regulation involves JNK pathway. Biochem. Biophy. Res. Commun. 362(4), 853–857 (2007).
10 Cameron J, Ranheim T, Kulseth MA, Leren TP, Berge KE: Berberine decreases PCSK9 expression in HepG2 cells.
Atherosclerosis 201(2), 266–273 (2008).
11 Li H, Chen W, Zhou Y et al.: Identification of mRNA binding proteins that regulate the stability of LDL receptor mRNA through AU‑rich elements. J. Lipid Res. 50, 820–831 (2009).
12 Choi BH, Ahn IS, Kim YH et al.: Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3‑L1 adipocyte. Exp. Mol. Med. 38(6), 599–605 (2006).
13 Brusq JM, Ancellin N, Grondin P et al.: Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J. Lipid Res. 47(6), 1281–1288 (2006)
14 Kong W, Wei J, Abidi P et al.: Berberine is a novel cholesterol lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10(12), 1344–1351 (2004).
nn Very complex and complete study, the first that proposed a new lipid-lowering mechanism of action to explain the biological effects of berberine confirmed by a randomized clinical trial.
15 Zhang Y, Li X, Zou D et al.: Treatment of Type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin.
Endocrinol. Metab. 93(7), 2559–2565 (2008).
16 Yin J, Xing H, Ye J: Efficacy of berberine in patients with Type 2 diabetes mellitus.
Metabolism 57(5), 712–717 (2008).
17 Zhao W, Xue R, Zhou ZX, Kong WJ, Jiang JD: Reduction of blood lipid by berberine in hyperlipidemic patients with chronic hepatitis or liver cirrhosis. Biomed.
Pharmacother. 62(10), 730–731 (2008).
n Confirms berberine’s tolerability in patients affected by liver disease, which often limits the prescription of conventional
hypocholesterolemic drugs.
18 Cicero AF, Rovati LC, Setnikar I:
Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol‑lowering agents. A single‑blind clinical investigation. Arzneimittelforschung 57(1), 26–30 (2007).
19 Kong WJ, Wei J, Zuo ZY et al.: Combination of simvastatin with berberine improves the lipid‑lowering efficacy. Metabolism 57(8), 1029–1037 (2008).
n Demonstrates the summatory effect of berberine with statin as lipid-lowering agent.
20 Kong WJ, Zhang H, Song DQ et al.: Berberine reduces insulin resistance through protein kinase C‑dependent up‑regulation of insulin receptor expression. Metabolism 58(1), 109–119 (2009).
21 Ko BS, Choi SB, Park SK et al.: Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol. Pharm.
Bull. 28(8), 1431–1437 (2005).
22 Zhang W, Xu YC, Guo FJ, Meng Y, Li ML: Antidiabetic effects of cinnamaldehyde and berberine and their impacts on retinol binding protein 4 expression in rats with Type 2 diabetes mellitus. Chin. Med. J. (Eng.) 121(21), 2124–2128 (2008).
23 Yin J, Hu R, Chen M et al.: Effects of berberine on glucose metabolism in vitro.
Metabolism 51(11), 1439–1443 (2002).
24 Kim SH, Shin EJ, Kim ED et al.: Berberine activated GLUT‑1‑mediated glucose uptake in 3T3‑L1 adipocytes. Biol. Pharm. Bull. 30(11), 2120–2125 (2007).
25 Yin J, Gao Z, Liu D, Liu Z, Ye J: Berberine improves glucose metabolism through induction of glycolysis. Am. J. Physiol.
Endocrinol. Metab. 294(1), E148–E156
(2008).
26 Yi P, Lu FE, Xu LJ, Chen G, Dong H, Wang KF: Berberine reverses free‑fatty‑ acid‑induced insulin resistance in 3T3‑L1 adipocytes through targeting IKKb.
World J. Gastroenterol. 14(6), 876–883
(2008).
27 Zhou L, Yang Y, Wang X et al.: Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism 56(3), 405–412 (2007).
28 Cheng Z, Pang T, Gu M et al.: Berberine stimulated glucose uptake in L6 myotubes includes both AMPK and p 38 MAPK.
Biochim. Biophys. Acta 1760(11), 1682–1689
(2006) .
29 Huang C, Zhang Y, Gong Z et al.: Berberine inhibits 3T3L1 adipocyte differentiation through the PPARg pathway. Biochem.
Biophys. Res. Comun. 348(2), 571–578
(2006).
30 Wang SH, Wang WJ, Wang XF, Chen W: Effects of Astragalus polysaccharides and berberine on carbohydrate metabolism and cell differentiation in 3T3‑L1 adipocytes.
Zhongguo Zhong Xi Yi Jie He Za Zhi 24(10),
926–928 (2004).
31 Lu SS, Yu YL, Zhu HJ et al.: Berberine promotes glucagons‑like peptide‑1 (7–36 amide) secretion in streptozocin‑induced diabetic rats. J. Endocrinol. 200(2), 159–165 (2009)
32 Zhou J, Zhou S, Tang J et al.: Protective effect of berberine on b cells in streptozotocin‑ and high carbohydrate/ high‑fat‑diet‑induced diabetic rats. Eur.
J. Pharmacol. 606(1–3), 262–268
(2009).
33 Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY: The involvement of p‑glycoprotein in berberine absorption. Pharmacol. Toxicol. 91(4), 193–197 (2002).
34 Turner N, Li JY, Gosby A et al.: Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP‑activated protein kinase and improve insulin action. Diabetes 57(5), 1414–1418 (2008).
35 Yin J, Zhang H, Ye J: Traditional Chinese medicine in treatment of metabolic syndrome.
Endocr. Metab. Immune. Disord. Drug Targets
8(2), 99–111(2008).
36 Wang ZQ, Lu FE, Leng SH et al.: Facilitating effects of berberine on rat pancreatic islets through modulating hepatic nuclear factor 4 a expression and glucokinase activity.
World J. Gastroenterol. 14 (39), 6004–6011
(2008).
37 Leng S, Lu F, Xu L: Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion.
Acta Pharmacol. Sin. 25(4), 496–502
(2004).
38 Liu WH, Hei ZQ, Nie H et al.: Berberine ameliorates renal injury in streptozocin‑ induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin. Med. J. 121(8), 706–712 (2008).
39 Liu W, Tang F, Deng Y et al.: Berberine reduces fibronectin and collagen accumulation in rat glomerular mesangial cells cultured under high glucose condition.
Mol. Cell. Biochem. 325(1–2), 99–105
(2009).
40 Fatehi‑Hassanabad Z, Jafarzadeh M, Tarhini A, Fatehi M: The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother. Res. 19(3), 222–225 (2005).
41 Peychev L: Pharmacological investigation on the cardiovascular effects of Berberine vulgaris on tested animals. Pharmacia 52(1), 118–121 (2005).
42 Chun YT, Yip TT, Lau KL, Kong YC, Sankawa U: A biochemical study on hypotensive effects of berberine in rats. Gen.
Pharmacol. 10(3), 177–182 (1979).
43 Wong KK: Mechanism of the aortic relaxation induced by low concentrations of berberine. Planta Med. 64(8), 756–757 (1998).
44 Ko WH, Yao XQ, Lau CW et al.: Vasorelaxant and antiproliferative effects of berberine. Eur. J. Pharmacol. 399(2–3), 187–196 (2000).
45 Kang DG, Sohn EJ, Kwon EK et al.: Effects of berberine on angiotensin‑ converting enzyme and NO/cGMP system in vessels. Vascul. Pharmacol. 39(6), 281–286 (2002).
46 Olmez E, Ilhan M: Evaluation of the a‑adrenoceptor antagonistic action of berberine in isolated organs.
Arzneimittelforschung 42(9), 1095–1097
(1992).
47 Chiou WE, Yen MH, Chen CF: Mechanism of vasodilatory effect of berberine in rat mesenteric artery. Eur. J. Pharmacol. 204(1), 35–40 (1991).
48 Pan LR, Tang Q, Fu Q, Hu BR, Xiang JZ, Qian JQ: Roles of nitric oxide in protective effect of berberine in ethanol‑induced gastric ulcer mice. Acta Pharmacol. Sin. 26(11), 1334–1338 (2005).
49 Liang KW, Yin SC, Ting CT et al.: Berberine inhibits platelet‑derived growth factor‑ induced growth and migration partly through an AMPK–dependent pathway in vascular smooth muscle cells. Eur. J. Pharmacol. 590(1–3), 343–354 (2008).
50 Liang KW, Ting CT, Yin SC et al.: Berberine suppresses MEK/ERK‑dependent Egr‑1 signalling pathway and inhibits vascular smooth muscle regrowth after in vitro mechanical injury. Biochem. Pharmacol. 71(6), 806–817 (2006).
51 Cho BJ, Im EK, Kwon JH et al.: Berberine inhibits the production of
lysophosphatidylcholine‑induced reactive oxygen species and the ERK1,2 pathway in smooth muscle cells. Mol. Cell 20(3), 429–434 (2005).
52 Xu MG, Wang JM, Chen L et al.: Berberine‑ induced upregulation of endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology 112(4), 279–286 (2008).
n Demostrates a direct vascular anti-aging effect of berberine in humans.
53 Xu MG, Wang JM, Chen L, Wang Y, Yang Z, Tao J: Berberine‑induced mobilization of circulating endothelial progenitor cells improves human small artery elasticity. J. Hum. Hypertens. 22(6), 389–393 (2008).
54 Wang JM, Yang Z, Xu MG et al.: Berberine induced decline in circulating CD31+/
CD42‑ microparticles is associated with
improvement of endothelial function in humans. Eur. J. Pharmacol. 614(1–3), 77–83 (2009).
55 Hong Y, Hui SS, Chan BT, Hou J: Effect of berberine on catecholamine levels in rats with experimental cardiac hypertrophy. Life Sci. 72(22), 2499–2507 (2003).
56 Hong Y, Hui SC, Chan TY, Hou JY: Effect of berberine on regression of pressure overload induced cardiac hypertrophy in rats. Am. J. Chin. Med. 30(4), 589–599 (2002).
57 Huang WM, Yan H, Jin JM, Yu C, Zhang H: Beneficial effects of berberine on
hemodynamics during acute ischemic left ventricular failure in dogs. Chin. Med. J. 105(12), 1014–1019 (1992).
58 Wang YX, Zheng YM, Zhou XB: Inhibitory effects of berberine on ATP‑sensitive K+
channels in cardiac myocytes. Eur.
J. Pharmacol. 316(2–3), 307–315 (1996).
59 Marin‑Neto JA, Maciel BC, Secches AL, Gallo L Jr: Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin. Cardiol. 11(4), 253–260 (1988).
n First clinical test of berberine in heart failure.
60 Zeng XH, Zeng XJ, Li YY: Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 92(2), 173–176 (2003).
61 Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y: Cardiovascular actions of berberine. Cardiovasc. Drug Rev. 19(3), 234–244 (2001).
62 Huang CG, Chu ZL, Wei SJ, Jiang H, Jiao BH: Effects of berberine on arachidonic acid metabolism in rabbit platelets and endothelial cells. Thromb. Res. 106(4–5), 223–227 (2002).
63 Huang CG, Chu ZL, Yang ZM: The progress in pharmacological researches on berberine. Comun. Inform. Pharm. 9(4), 10–12 (1991).
64 Holy EW, Akhmedov A, Lüscher TF, Tanner FC: Berberine a natural lipid‑lowering drug, exerts prothrombic effects on vascular cells. J. Mol. Cell. Cardiol. 46(2), 234–240 (2009).
65 Guo Y, Wang QZ, Li FM, Jiang X, Zuo YF, Wang L: Biochemical pathways in the antiatherosclerotic effect of berberine.
Chin. Med. J. (Eng.) 121(13), 1197–1203
(2008).
66 Pandey MK, Sung B, Kunnumakkara AB
et al.: Berberine modifies cysteine 179 of
IkBa kinase, suppresses nuclear factor‑k‑B‑ regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 68(13), 5370–5379 (2008).
67 Hwang JM, Wang CJ, Chou FP et al.: Inhibitory effect of berberine on tert‑butyl‑ hydroperoxide‑induced oxidative damage in rat liver. Arch. Toxicol. 76(11), 664–670 (2002).
68 Li BX, Zhang MS, Bao LH: Study of pharmacokinetics of berberine after oral administration in human being. J. Haerbin
Med. Univ. 29, 382–385 (1995)
69 Yuan J, Shen XZ, Zhu XS: Effect of berberine on transit time of human small intestine.
Zhongguo Zhong Xi Yi Jie He Za Zhi 14(12),
718–720 (1994).
70 Imanshahidi M, Hosseinzadeh H: Pharmacological and therapeutic effects of
Berberis vulgaris and its active constituent,
berberine. Phytother. Res. 22(8), 999–1012 (2008).
71 Hu YJ, Liu Y, Xiao XH: Investigation of the interaction between berberine and human serum albumin. Biomacromolecules DOI: 10.1021/bm801120k (2009) (Epub ahead of print).
72 Tan YZ, Wu AC, Tan BY et al.: Study on the interactions of berberine displace other drug from their plasma proteins binding sites. Chin. Pharmacol. Bull. 18, 576–578 (2002).
73 Chan E: Displacement of bilirubin from albumin by berberine. Biol. Neonat. 63(4), 201–208 (1993).
74 Tsai PL, Tsai TH: Hepatobiliary excretion of berberine. Drug. Metab. Dispos. 32(4), 405–412 (2003).
75 Zuo F, Nakamura N, Akao T, Hattori M: Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ‑free rats determined by liquid chromatography/ion trap mass spectrometry.
Drug Metab. Dispos. 34(12), 2064–2072
(2006).
76 Chen CM, Chang HC: Determination of berberine in plasma, urine and bile by high performance liquid chromatography.
J. Chromatogr. 665(1), 117–123 (1995).
77 Pan JF, Yu C, Zhu DY et al.: Identification of three sulfate‑conjugated metabolites of berberine chloride in healthy volunteers’ urine after oral administration. Acta Pharmacol.
Sin. 23(1), 77–82 (2002).
78 Raner GM, Cornelius S, Moulick K et al.: Effects of herbal products on human cytochrome P450(2E1) activity. Food Chem.
Tox. 45(12), 2359–2365 (2007).
79 Zhao X, Zhang JJ, Wang X, Bu XY, Lou YQ, Zhang GL: Effect of berberine on hepatocyte proliferation inducible nitric oxide synthase expression, cyrochrome 450 2E1 and 1A2
activities in diethylnitrosamine‑ and phenobarbital‑treated rats. Biomed.
Pharmacother. 62(9), 567–572 (2008).
80 Xin HW, Wu XC, Li Q et al.: The effects of berberine on the pharmacokinetics of cyclosporine A in healthy volunteers. Methods
Find. Exp. Clin. Pharmacol. 28(1), 25–29
(2006).
81 Wu X, Li Q, Xin H, Yu A, Zhong M: Effects of berberine on the blood concentration of cyclosporine A in renal transplanted recipients: clinical and pharmacokinetic study. Eur. J. Clin. Pharmacol. 61(8), 567–572 (2005).
82 Vrzal R, Zdarilova A, Ulrichova J et al.: Activation of the aryl hydrocarbon receptor by berberine in HepG2 and H4IIEcells: biphasic effect on CYP1A1. Biochem.
Pharmacol. 70(6), 925–936 (2005).
83 Lin HL, Liu TY, Wu CW, Chi CW: Berberine modulates expression of mdr1 gene product and the responses of the digestive tract cancers to Paclitaxel. Br. J. Cancer. 81(3), 416–422 (1999).
84 Sabir M, Bhide NK: Study of some pharmacological actions of berberine. Indian
J. Physiol. Pharmacol. 15(3), 111–132 (1971).
85 Pasqual MS, Lauer CP, Moyna P, Henriques JA: Genotoxicity of the
isoquinoline alkaloid berberine in prokaryotic and eukaryotic organisms. Mutat. Res. 286(2), 243–252 (1993).
86 Grycova L, Dostal J, Marek R: Quarternary protoberberine alkaloids. Phytochemistry 68(2), 150–175 (2007).
87 Holy EW, Akhmedov A, Lüscher TF, Tanner FC: Berberine, a natural lipid‑ lowering drug, exerts prothrombotic effects on vascular cells. J. Mol. Cell. Cardiol. 46(2), 234–240 (2009).
88 Chu ZL, Huang CG, Xu ZP. The anti‑platelet effect of berberine and its mechanism.
Zhongguo Zhong Xi Yi Jie He Za Zhi 14(8),