Contents lists available atScienceDirect
Results in Physics
journal homepage:www.elsevier.com/locate/rinp
Evaluation of the shielding parameters of alkaline earth based phosphate
glasses using MCNPX code
O. Agar
a, Z.Y. Khattari
b, M.I. Sayyed
c, H.O. Tekin
d,e, S. Al-Omari
b, M. Maghrabi
b, M.H.M. Zaid
f,⁎,
I.V. Kityk
gaKaramanoğlu Mehmetbey University, Department of Physics, 70100 Karaman, Turkey bDepartment of Physics, Faculty of Science, Hashemite University, Zarqa, Jordan cDepartment of Physics, Faculty of Science, University of Tabuk, Tabuk, Saudi Arabia
dUskudar University, Vocational School of Health Services, Radiotherapy Department, Istanbul 34672, Turkey
eUskudar University, Vocational School of Health Services, Department of Nuclear Technology and Radiation Protection, Istanbul, Turkey fDepartment of Physics, Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
gInstitute of Optoelectronics and Measuring Systems, Faculty of Electrical Engineering, Czestochowa University of Technology, 17 Armii Krajowej Str., 42-200
Czestochowa, Poland A R T I C L E I N F O Keywords: Phosphate glass Radiation Attenuation coefficients XCom
Effective atomic number MCNPX
A B S T R A C T
Glass systems of composition 16XO–3Al2O3–6CuO–20Na2O–55P2O5(where X = Sr, Ca, Mg and Ba mol%) have
been investigated to check its potential utilize as gamma ray shielding material. Different shielding quantities namely mass attenuation coefficient (µ/ρ), effective atomic number (Zeff), half value layer (HVL) and mean free path (MFP) have been evaluated using MCNPX code and XCOM program at different photon energies between 0.015 and 10 MeV. The obtained data revealed good agreement between the µ/ρ values derived from XCOM and MCNPX code (version 2.6.0). It is found that the mass attenuation coefficients of BaO-doped phosphate glass are higher than those of the other alkaline earth elements–doped glasses, whereas MgO possess the lowest values. This indicates that the BaO-doped phosphate glass is the superior gamma radiation attenuator among the studied glass samples. The gamma shielding performance of the glasses under study has been compared to some com-mercial glasses and different concrete samples in terms of MFP.
Introduction
High energy ionization radiation is frequently utilized for various purposes such as elemental analysis with non-destructive photoactiva-tion analysis (PAA) technique [1,2], medical diagnostics and therapy [3], irradiation response test of metal-oxide-semiconductors (MOS) capacitor[4,5], food processing[6,7]etc. In case of longer radiation expose to tissues of living beings, it causes major damages such as ra-diation sickness, cancer, mutation, and even death. Also, at genetic damages level, these affects the future of living organs. From this point, it has always been one of major issues taking the necessary precautions to reduce the amount of radiation emitted from radiation sources that imposed on employees and patients at hospital in case of nuclear medicine applications, medicinal research centers and investigation of shielding materials[8].
Unlike lead (Pb) and standard concrete materials, glass matrices have alternative and promising radiation shielding properties while
transparent to these radiations. These unique properties are emerged primely form their highflexibility of composition, ease of fabrication, high thermal stability, high dielectric constant and low crystallization ability[9–11].
There are many research works on gamma ray shielding properties for various glass systems in the literature[12–20]. For example, Tijani et al.[12]studied the radiation shielding performance of Er2O3
-ZnO-TeO2glass system at 20, 30, 40 and 60 keV photon energies. The
au-thors observed that the Er2O3-ZnO-TeO2 glass samples posses better
radiation shielding properties than concrete. Singh et al.[13]reported the exposure buildup factor (EBF) of the bismuth borosilicate glass systems by utilizing the Geometrical Progression (G-P) method. They compared the EBF of the bismuth borosilicate glasses with those of Pb and steel–magnetite concrete.
Kirdsiri et al.[14]measured the mass attenuation coefficient (μ/ρ) experimentally at 662 keV for silicate glass systems including PbO, BaO and Bi2O3. These authors reported that the values ofμ/ρ were enhanced
https://doi.org/10.1016/j.rinp.2018.11.054
Received 2 November 2018; Received in revised form 16 November 2018; Accepted 17 November 2018
⁎Corresponding author.
E-mail address:mhmzaid@upm.edu.my(M.H.M. Zaid).
Available online 22 November 2018
2211-3797/ © 2018 The Authors. Published by Elsevier B.V. This is an open access article under the CC BY license (http://creativecommons.org/licenses/BY/4.0/).
by the increment of PbO and Bi2O3content. In another work, Ruengsri
et al. [15]used the melt quenching method to fabricate the barium borosilicate glasses from rice husk ash and studied the gamma ray shielding features for the synthesized glass samples. As concluded by the authors, theμ/ρ and effective atomic numbers of the glasses were increased with the addition of BaO. Recently, El-Mallawany et al.[16] carried out the MCNP5 computer code to investigate the radiation shielding characteristic of many tellurite glasses in the form of TeO2
-V2O5-ZnO, TeO2-V2O5-CeO2and TeO2-V2O5-TiO2. These authors
com-pared the MCNP5 results with those obtained by XCOM software and a good agreement between MCNP5 and XCOM results was found. Fur-thermore, Sayyed et al.[17]prepared ZnO-B2O3-PbO glasses by solid
state reaction technique and determined some important physical properties such as density, molecular weight and molar volume of the prepared glasses. Moreover, they investigated the radiation shielding characterization for the prepared glasses applying MCNPX code in the energy range of 0.356–1.33 MeV and the gamma ray shielding char-acterization for these glassy compounds. Finally, they have compared their properties with those of different commercial window glasses [17]. In another recent work, Sayyed and Lakshminarayana[18] uti-lized the melt quenching method to prepare optical glasses with nom-inal composition of B2O3-SiO2-TeO2-ZnO-Li2O-BaO. These authors
re-ported on the structural and thermal properties for this glass system using different techniques such as XRD, FTIR, SEM/EDAX and TGA/ DSC. Also, they investigated the radiation shielding effectiveness for these glasses by calculating the essential parameters viz. µ/ρ, Zeff, EBF
and half value layer (HVL). The authors deduced that the substitution of BaO improves the shielding capability of the synthesized samples against gamma photon.
The basic superiority of phosphate among other oxide glasses such as borate, silicate, etc. is its capability to both maintain amorphous and host the high concentration transition metal ions. Moreover, phosphate glasses having unique physical and chemical structural and composi-tional capabilities that simplify tailoring chemical substance and phy-sical behavior[19]. However, since it suffers from poor chemical dur-ability, in general, makes such glasses inappropriate for several practical applications. Therefore, it should be developed by doped one or more of multivalent oxides namely Al2O3, ZnO, SnO, PbO, Fe2O3, etc.
These additives support the formation of Zn–O–P, Al–O–P, Fe–O–P, Sn–O–P, Pb–O–P chemical bonds and thus, considerably improve its chemical durability[20–22]. In this context, the optical and structural characteristics of XO–Al2O3–CuO–Na2O–P2O5 glass system have
pre-viously reviewed by Farouk et al.[20]and the chemical compositions of the glassy systems in their work have been discussed in details.
The basic objective of the present study is to determine the photon shielding features of 16XO–3Al2O3–6CuO–20Na2O–55P2O5 glass
sys-tems (where X = Sr, Ca, Mg and Ba mol%) by carrying out XCOM software for a wide range of photon energies between 0.015 and 10 MeV and compare the radiation shielding capability of the glasses under investigation in terms of mean free path with those of different concretes reported in the literature. Also, by comparing them with the radiation shielding glasses developed by SCHOTT company.
Materials and methods
The radiation shielding characteristics of various alkaline earth elements based phosphate glasses have been calculated using XCOM program. The formula of the investigated alkaline earth elements based phosphate glasses is 16XO–3Al2O3–6CuO–20Na2O–55P2O5,
where X represents the used alkaline earth elements (X = Ba, Sr, Mg, and Ca mol %). The densities of the glass samples were taken from Ref. [20]. The density and the weight fraction of elements in glass samples are listed inTable 1.
For the present alkaline earth elements based phosphate glasses, we calculated theμ/ρ using Monte Carlo method. To achieve this purpose, Monte Carlo N-Particle Transport Code System-extended (MCNPX) was
performed for the determination of µ/ρ for the selected glasses em-ploying the Lambert-Beer law.Fig. 1shows the 3-D view (obtained from MCNPX Visual Editor VE) of gamma-ray attenuation setup of MCNPX with several basic simulation instruments i.e. point isotropic radiation source, attenuator glass sample, Pb collimator for primary radiation beam, F4 tally mesh detectionfield and Pb blocks to prohibit scattered radiation. In this simulation study, F4 tally detectionfield was placed on the same line with a distance 70 cm from point isotropic radiation source. The F4 tally was used to count the gamma rays inter the de-tector per MeV cm2s−1. This type of tally (F4) gives the average photon
flux in the detection field. The studied glass material was located be-tween the point isotropic radiation source and the F4 tally mesh (de-tectionfield) at a distance 50 cm from the source. To acquire the var-iation in average photonflux caused from the glass thickness of in F4 detection field, each average photon flux has been recorded from MCNPX outputfile and has been ploted to obtain the linear attenuation coefficients considering the Beer-Lambert law. Finally, each obtained linear attenuation coefficients has been divided to glass density to ob-tain mass attenuation coefficients in different photon energies. The cell structure of modeled glasses have been considered as a different cell in the inputfile. The cell design of MCNPX code has a structure that changes according to material properties. Therefore, the cell structure has been defined as a different glass sample with the different elemental mass fractions as well as different densities, for each calculation (See Table 1). The MCNPX material card block can provide the definition of material properties considering the mass fractions of each element ac-cording to the shape required by the MCNPX code. To model a cell with a certain material properties, it should be defined as Mn in the material card. In the present investigation, each glass shielding material has been defined with the chemical properties in material card block. Results and discussion
The linear attenuation coefficient (μ) in cm−1 for a given glass
material follows Beer Lambert law[23,24]:
= − μ x lnI I0 (1) where I and I0 are the transmitted and initial photon intensities,
re-spectively, x is the thickness of the glass sample (cm). Theoretical va-lues of µ/ρ (in cm2/g) can be calculated for glass system with a
com-pound and mixture by utilizing XCOM computer software package[25] based on the mixture law. It provides both total and partial attenuation coefficients as well as cross sections of photon interaction mechanisms for about 100 elements[26,27]:
∑
= = μ μ ρ w μ ρ ( ) m i i (2) where wiis the weight fraction of each element in the glass system.The obtained values of µ/ρ using MCNPX and XCOM at defined energies (i.e., 0.015, 0.02, 0.05, 0.1, 0.5, 1, 5 and 10 MeV energies) are shown inFig. 2. All the calculated values are found to be in good agreement.
The results of total µ/ρ for the four glass samples under study over wide energy range of 0.015–10 MeV are demonstrated graphically in Fig. 3. The trend of the µ/ρ curve presented inFig. 3overlaps with the results reported by Bagheri et al.[28]who investigated the µ/ρ and some other related parameters of silicate glasses containing Bi2O3, PbO,
and BaO. It is quite clear fromFig. 3that the values of µ/ρ depend on the type of alkaline earth elements. Additionally, the µ/ρ values were calculated by MCNPX code and compared with those obtained by XCOM at some enenrgies.
Obviously, the µ/ρ has comparatively high values in the low energy zone (8.4, 9.76, 9.99 and 18.32 cm2/g for MgO, CaO, SrO and BaO
with further increase in the photon energy, and tend to be almost constant between 0.8 and 3 MeV. This tend can be interpreted ac-cording to the radiation physical concepts. Briefly, gamma radiation can interact with the glass sample depending on the energy of the Table 1
Density and weight fraction of elements in the selected glass samples.
Code O Na Al P Cu Mg Ca Sr Ba ρ(g/cm3)
MgO 0.4979 0.0878 0.0155 0.3253 0.0364 0.0371 0 0 0 2.6 CaO 0.4862 0.0857 0.0151 0.3176 0.0355 0 0.0598 0 0 2.62 SrO 0.4540 0.0801 0.0141 0.2966 0.0332 0 0 0.1220 0 2.8 BaO 0.4246 0.0749 0.0132 0.2774 0.0310 0 0 0 0.1789 2.99
Fig. 1. The simulation geometry of this study.
Fig. 2. Variation of mass attenuation coefficients (cm2/g) of alkaline earth based phosphate glasses with photon energy calculated by MCNPX and XCOM.
gamma photon and the atomic number (Z) of the sample in three me-chanisms such as photoelectric absorption (PE), Compton scattering (CS) and pair production (PP)[29]. In the low energy zone (also known as photoelectric zone), the PE is the domination process and the µ/ρ
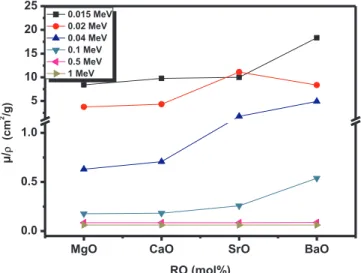
values vary with the photon energy and the atomic number as 1/E3.5 and Z4respectively, therefore we noticed the relatively high µ/ρ values in this zone. The incoherent CS is the dominant mechanism of inter-action for moderate energy photons. The cross section of CS is related to the energy and atomic number as 1/E and Z respectively[30,31]. For this reason, we can see that all the selected samples have almost the same µ/ρ values between 0.8 and 3 MeV. Additionally, it is clear from Fig. 3that the values of µ/ρ BaO-doped phosphate glass are higher than those of the other alkaline earth elements–doped glasses except for energy range between 0.02 and 0.03 MeV. The present sharp increase in the photoelectric effect is due to photoelectric absorption around the K-absorption edge of Sr which occurs at 0.0161 MeV. These observed discontinuities are illustrated inFig. 4. This Figure shows that there is a quite similar trend of CaO and MgO–doped phosphate glasses due to their close densities to each other, namely 3.35 and 3.58 g/cm3, re-spectively.
The variation of µ/ρ versus Ba, Sr, Mg, and Ca alkaline earth ele-ments at selected energies is shown inFig. 4. The Figure indicates that BaO-doped phosphate glass has higher µ/ρ values than the rest of glasses investigated in this work, except at 0.02 MeV where at this energy the SrO-doped phosphate glass has the highest µ/ρ. This ob-servation can be understood easily since the K-edge absorption of Sr is very close to 0.02 MeV, therefore there is higher chance of photoelectric absorption for SrO- doped phosphate at this energy. As it is known, BaO has the highest density (5.72 g/cm3) among the current studied alkaline
earth elements (i.e., MgO, CaO and SrO), therefore has a high effective atomic number in addition it’s a non-toxic compound. Thus, this oxide based glass systems have both strong absorption of X- and gamma ray photons and greater Compton interaction cross section as shown in Fig. 4 [27,32]. The previous study on BaO-doped glasses confirmed an increase in µ/ρ values with concentration increasing and showed that this glassy system has a better shielding efficiency than the standard concretes such as limonite-limonite, basalt-magnetite and hematite-serpentine in[33,34].Fig. 4is also indicted that the values of µ/ρ are nearly the same for 0.5 and 1 MeV and the difference in µ/ρ is notable for high energy values.
Using the above µ/ρ value, we can calculate the total photon in-teraction cross section (σt) as follow[10,35]:
= ∑ σ μ A n N t m i i i A (2)
where NAis the Avogadro number,Aiandniare the atomic weight of
the element i and number of formula units, respectively. The total atomic cross section (σa) in cm2/atom can be obtain by the following
equation: = ∑ σ σ n a t i i (3)
wherewiAirepresents the atomic weight of the element i in the glass
system.
The total electronic cross-section (σe) in cm2/electron is calculated
as follows:
∑
= σ N μ f A Z 1 ( ) e A i m i i i i (4)whereZiand fiare the atomic number and fractional abundance of the
element i.
The effective atomic number (Zeff) is derived from the
above-men-tionedσaandσe[36]: = Z σ σ eff a e (5)
Fig. 5displays the behavior of Zeffvariations for phosphate glass
systems containing Mg, Ca, Sr and Ba alkaline earth elements de-pending on the photon energy. The Zeff has minimum values at
0.4 < E < 4 MeV while maximum values are found between energy Fig. 4. Dependence of µ/ρ (cm2/g) values on alkaline earth elements.
Fig. 5. The Zefffor the glass samples.
range of 0.02–0.06 MeV. Similar to variations in µ/ρ, the sudden jumps is obtained at 0.03 MeV and 0.05 MeV for obvious reasons. These jumps are expressed in terms of K-absorption edges of Sr and Ba respectively. The trend in Zeffvariations becomes almost independent of energy for
all investigated glasses for the photon energy range 0.6–4 MeV, while Zeffstarts increasing in a small rate between 5 and 10 MeV. This
ob-served variations may connected to the dominance of CS mechanism (between 0.6 and 4 MeV) and Pair production (PP) between 5 and 10 MeV. It's well-known that higher Zeffvalues are essential for a better
ionization radiation shielding material with increasing possibilities of photons interaction with the target. It is obvious FromFig. 5that glassy matrix containing BaO exhibits a higher Zeffwhereas MgO has the lower
Zeff. This indicates that the BaO-doped phosphate glass is a superior
gamma radiation attenuator among the selected glass samples. Besides, the half value layer (HVL) represents the thickness at which the transmitted intensity is 50% the incident intensity. And the mean free path (MFP) is the average distance a photon can travel before in-teracting with the target. HVL and MFP values of the glass systems are estimated using result ofμ through the following equations[37,38]:
= HVL ln μ 2 (6) and = MFP μ 1 (7) The HVL thickness for the selected alkaline earth elements based phosphate glass systems have been estimated to directly test their photon attenuation effectiveness.Fig. 6indicates the variations of HVL against the photon energies mastering the same trend in the entire photon energy range. There is a notable decreasing in the HVL values in the order MgO, CaO, SrO and BaO. It is found that the HVL thickness is the highest for glasses containing MgO and CaO with ρ = 3.35 and 3.58 g/cm3 and the lowest for glass systems having BaO with
ρ = 5.72 g/cm3. It can be easily observed that the HVL values decrease
with density increasing; hereby materials with high density should be selected to achieve superior shielding.
Shielding performance needs to be compared with previously re-ported materials in the literature in order to explore both the possibility for the improvements as a shielding material as well as the improved characteristics of the selected glass systems. Therefore, the MFP thick-nesses of glass systems consisted of CaO/MgO/SrO/ BaO–Al2O3–CuO–Na2O–P2O5have been compared to several
commer-cial shielding glass samples developed by SCHOTT company[39]and concretes (ordinary, ilmenite–limonite, basalt–magnetite and hemati-te–serpentine)[40]as shown inFig. 7a and b. The MFPs for the four glass samples studied here are lower than RS253-G18 glass, ordinary and hematite–serpentine concretes while they are higher than those of RS323-G19 glass and basalt–magnetite concrete samples. Furthermore,
there is quite similarity between the present thicknesses and ilmeni-te–limonite concrete.
Conclusions
In the present work, the µ/ρ of
16XO–3Al2O3–6CuO–20Na2O–55P2O5(where X = Sr, Ca, Mg and Ba
mol%) glasses have been investigated using XCOM software and MCNPX code. The obtained µ/ρ results are in good agreement with each other. Thus, this study represents that MCNPX is favourable to be car-ried out as an reliable and alternative technique as experimental work for radiation shielding purposes. The data showed that the µ/ρ and Zeff
of BaO-doped phosphate glass are higher than those of the other alka-line earth elements–doped glasses. Besides, we calculated the HVL of the present glasses and discussed the variation of this parameter with photon energy. The values of MFP of the glasses studied here are compared with commercial glasses and concrete sample. The MFPs for the four glass samples studied are lower than RS253, RS253-G18 glasses, ordinary and hematite–serpentine concretes. Therefore, we conclude that these glasses are better shielding materials than other available commercial glasses and concrete samples. Consequently, since the investigated glasses have the advantage of being transparent to visible light, it can be particularly quite beneficial for a lot shielding applications in which being in point of the radiation sources are ap-propriate.
Acknowledgements
This study was supported by Karamanoglu Mehmetbey University (Project Numbers: 13-M-17) and thefinancial support from Universiti Putra Malaysia (UPM) under Inisiatif Putra Muda (IPM) are gratefully acknowledged. The Hashemite University is also acknowledged for the generous support.
References
[1] Agar O, Boztosun I, Segebade C. Multielemental analysis of some soils in Karaman by PAA using a cLINAC. Appl Radiat Isot 2017;122:57–62.https://doi.org/10. 1016/j.apradiso.2017.01.011.
[2] Kavun Y, Boztosun I, Dapo H, Maraş I, Segebade C. Determination of the Sr/Ca ratio of tooth samples by photoactivation analysis in Southern Turkey. Radiochim Acta 2018.https://doi.org/10.1515/ract-2017-2918.
[3] Matsunaga S, Shuto T, Kawahara N, Suenaga J, Inomori S, Fujino H. Gamma Knife surgery for brain metastases from colorectal cancer. J Neurosurg 2011;114:782–9.
https://doi.org/10.3171/2010.9.JNS10354.
[4] Kahraman A, Yilmaz E. Irradiation response of radio-frequency sputtered Al/ Gd2O3/p-Si MOS capacitors. Radiat Phys Chem 2017;139:114–9.https://doi.org/ 10.1016/j.radphyschem.2017.04.003.
[5] Kahraman A, Yilmaz E, Aktag A, Kaya S. Evaluation of radiation sensor aspects of Er2O3MOS capacitors under zero gate bias. IEEE Trans Nucl Sci 2016;63:1284–93.
https://doi.org/10.1109/TNS.2016.2524625.
[6] Wang XB, Wang CN, Zhang YC, Liu TT, Lv JP, Shen X, et al. Effects of gamma
radiation on microbial, physicochemical, and structural properties of whey protein model system. J Dairy Sci 2018.https://doi.org/10.3168/jds.2017-14085. [7] Gölge E, Ova G. The effects of food irradiation on quality of pine nut kernels. Radiat
Phys Chem 2008.https://doi.org/10.1016/j.radphyschem.2007.06.005. [8] Obaid SS, Gaikwad DK, Pawar PP. Determination of gamma ray shielding
para-meters of rocks and concrete. Radiat Phys Chem 2018;144:356–60.https://doi.org/ 10.1016/j.radphyschem.2017.09.022.
[9] Singh H, Singh K, Gerward L, Singh K, Sahota HS, Nathuram R. ZnO-PbO-B2O3glasses as gamma-ray shielding materials. Nucl Instruments Methods Phys Res Sect B Beam Interact Mater Atoms 2003;207:257–62.https://doi.org/10.1016/ S0168-583X(03)00462-2.
[10] Sayyed MI. Bismuth modified shielding properties of zinc boro-tellurite glasses. J Alloys Compd 2016;688:111–7.https://doi.org/10.1016/j.jallcom.2016.07.153. [11] Sayyed MI. Investigations of gamma ray and fast neutron shielding properties of tellurite glasses with different oxide compositions. Can J Phys 2016;94:1133–7.
https://doi.org/10.1139/cjp-2016-0330.
[12] Tijani SA, Kamal SM, Al-Hadeethi Y, Arib M, Hussein MA, Wageh S, et al. Radiation shielding properties of transparent erbium zinc tellurite glass system determined at medical diagnostic energies. J Alloys Compd 2018;741:293–9.https://doi.org/10. 1016/j.jallcom.2018.01.109.
[13] Singh VP, Badiger NM, Chanthima N, Kaewkhao J. Evaluation of gamma-ray ex-posure buildup factors and neutron shielding for bismuth borosilicate glasses. Radiat Phys Chem 2014;98:14–21.https://doi.org/10.1016/j.radphyschem.2013. 12.029.
[14] Kirdsiri K, Kaewkhao J, Chanthima N, Limsuwan P. Comparative study of silicate glasses containing Bi2O3, PbO and BaO: radiation shielding and optical properties. Ann Nucl Energy 2011;38:1438–41.https://doi.org/10.1016/j.anucene.2011.01. 031.
[15] Ruengsri S, Insiripong S, Sangwaranatee N, Kaewkhao J. Development of barium borosilicate glasses for radiation shielding materials using rice husk ash as a silica source. Prog Nucl Energy 2015;83:99–104.https://doi.org/10.1016/j.pnucene. 2015.03.006.
[16] El-Mallawany R, Sayyed MI, Dong MG. Comparative shielding properties of some tellurite glasses: part 2. J Non Cryst Solids 2017;474:16–23.https://doi.org/10. 1016/j.jnoncrysol.2017.08.011.
[17] Sayyed MI, Rammah YS, Abouhaswa AS, Tekin HO, Elbashir BO. ZnO-B2O3-PbO glasses: synthesis and radiation shielding characterization. Phys B Condens Matter 2018;548:20–6.https://doi.org/10.1016/j.physb.2018.08.024.
[18] Sayyed MI, Lakshminarayana G. Structural, thermal, optical features and shielding parameters investigations of optical glasses for gamma radiation shielding and defense applications. J Non Cryst Solids 2018;487:53–9.https://doi.org/10.1016/j. jnoncrysol.2018.02.014.
[19] Metwalli E, Karabulut M, Sidebottom DL, Morsi MM, Brow RK. Properties and structure of copper ultraphosphate glasses. J Non Cryst Solids 2004;344:128–34.
https://doi.org/10.1016/j.jnoncrysol.2004.07.058.
[20] Farouk M, Samir A, El Okr M. Effect of alkaline earth modifier on the optical and structural properties of Cu2+ doped phosphate glasses as a bandpassfilter. Phys B Condens Matter 2018;530:43–8.https://doi.org/10.1016/j.physb.2017.11.013. [21] Shih PY, Chin TS. Effect of redox state of copper on the properties of
P2O5-Na2O-CuO glasses. Mater Chem Phys 1999.https://doi.org/10.1016/S0254-0584(99) 00070-X.
[22] Chahine A, Et-Tabirou M, Elbenaissi M, Haddad M, Pascal JL. Effect of CuO on the structure and properties of (50–x/2)Na2O-xCuO-(50–x/2)P2O5glasses. Mater Chem Phys 2004;84:341–7.https://doi.org/10.1016/j.matchemphys.2003.11.009. [23] Eke C, Agar O, Segebade C, Boztosun I. Attenuation properties of radiation shielding
materials such as granite and marble againstγ-ray energies between 80 and 1350
keV. Radiochim Acta 2017.https://doi.org/10.1515/ract-2016-2690.
[24] Sayyed MI, Tekin HO, Kılıcoglu O, Agar O, Zaid MHM. Shielding features of con-crete types containing sepiolite mineral: comprehensive study on experimental, XCOM and MCNPX results. Results Phys 2018;11:40–5.https://doi.org/10.1016/j. rinp.2018.08.029.
[25] Gerward L, Guilbert N, Jensen KB, Levring H. WinXCom– A program for calculating X-ray attenuation coefficients. Radiat Phys Chem 2004.https://doi.org/10.1016/j. radphyschem.2004.04.040.
[26] Sayyed MI, Elmahroug Y, Elbashir BO, Issa SAM. Gamma-ray shielding properties of zinc oxide soda lime silica glasses. J Mater Sci Mater Electron 2017;28:4064–74.
https://doi.org/10.1007/s10854-016-6022-z.
[27] Kaur P, Singh D, Singh T. Heavy metal oxide glasses as gamma rays shielding material. Nucl Eng Des 2016;307:364–76.https://doi.org/10.1016/j.nucengdes. 2016.07.029.
[28] Bagheri R, Moghaddam AK, Shirmardi SP, Azadbakht B, Salehi M. Determination of gamma-ray shielding properties for silicate glasses containing Bi2O3, PbO, and BaO. J Non Cryst Solids 2018;479:62–71.https://doi.org/10.1016/j.jnoncrysol. 2017.10.006.
[29] Kurudirek M. Heavy metal borate glasses: potential use for radiation shielding. J Alloys Compd 2017;727:1227–36.https://doi.org/10.1016/j.jallcom.2017.08.237. [30] Gaikwad DK, Sayyed MI, Obaid SS, Issa SAM, Pawar PP. Gamma ray shielding
properties of TeO2-ZnF2-As2O3-Sm2O3glasses. J Alloys Compd 2018;765:451–8.
https://doi.org/10.1016/j.jallcom.2018.06.240.
[31] Sayyed MI, Tekin HO, Altunsoy EE, Obaid SS, Almatari M. Radiation shielding study of tellurite tungsten glasses with different antimony oxide as transparent shielding materials using MCNPX code. J Non Cryst Solids 2018.https://doi.org/10.1016/j. jnoncrysol.2018.06.022.
[32] Singh S, Kumar A, Singh D, Thind KS, Mudahar GS. Barium-borate-flyash glasses: as radiation shielding materials. Nucl Instruments Methods Phys Res Sect B Beam Interact Mater Atoms 2008.https://doi.org/10.1016/j.nimb.2007.10.018. [33] Tuscharoen S, Kaewkhao J, Limsuwan P. Development of BaO:B2O3:flyash glass
system for gamma-rays shielding materials. Prog Nucl Sci Tech 2011;1:110–3. [34] Chanthima N, Kaewkhao J, Limsuwan P. Study of photon interactions and shielding
properties of silicate glasses containing Bi2O3, BaO and PbO in the energy region of 1 keV to 100 GeV. Ann Nucl Energy 2012;41:119–24.https://doi.org/10.1016/j. anucene.2011.10.021.
[35] Obaid SS, Sayyed MI, Gaikwad DK, Pawar PP. Attenuation coefficients and exposure buildup factor of some rocks for gamma ray shielding applications. Radiat Phys Chem 2018.https://doi.org/10.1016/j.radphyschem.2018.02.026.
[36] Sayyed MI, Qashou SI, Khattari ZY. Radiation shielding competence of newly de-veloped TeO2-WO3 glasses. J Alloys Compd 2017.https://doi.org/10.1016/j. jallcom.2016.11.160.
[37] Shamshad L, Rooh G, Limkitjaroenporn P, Srisittipokakun N, Chaiphaksa W, Kim HJ, et al. A comparative study of gadolinium based oxide and oxyfluoride glasses as low energy radiation shielding materials. Prog Nucl Energy 2017.https://doi.org/ 10.1016/j.pnucene.2016.12.014.
[38] Un A, Sahin Y. Determination of mass attenuation coefficients, effective atomic and electron numbers, mean free paths and kermas for PbO, barite and some boron ores. Nucl Instruments Methods Phys Res Sect B Beam Interact Mater Atoms 2011.
https://doi.org/10.1016/j.nimb.2011.04.011.
[39] SCHOTT. <http://www.schott.com/advanced_optics/english/products/ opticalmaterials/special-materials/radiation-shielding-glasses/index.html> . (Accesed 03 September 2018) 2018.
[40] Bashter II. Calculation of radiation attenuation coefficients for shielding concretes. Ann Nucl Energy 1997;24:1389–401.https://doi.org/10.1016/S0306-4549(97) 00003-0.