THE INVESTIGATION OF SOME DRUGS BY EPR TECHNIQUE
Farhad O.HAMADAMEEN
M.Sc. THESIS DEPARTMENT OFphysics
DIYARBAKIR February 2016BAZI İLAÇLARIN EPR TEKNİĞİ İLE İNCELENMESİ
Farhad O.HAMADAMEEN
YÜKSEK LİSANS TEZİ FIZIK ANABİLİM DALI
DİYARBAKIR Şubat 2016
FEN BĠLĠMLERĠ ENSTĠTÜSÜ MÜDÜRLÜĞÜ DĠYARBAKIR
Farhad O. HAMADAMEEN tarafından yapılan “ Bazı ilaçların EPR tekniği ile
incelenmesi ” konulu bu çalıĢma, jürimiz tarafından Fizik Anabilim Dalında YÜKSEK LĠSANS tezi olarak kabul edilmiĢtir.
Jüri Üyeleri
BaĢkan : Prof. Dr. ġemsettin OSMANOĞLU
Üye : Prof. Dr. Mehmet DOĞRU
Üye : Doç. Dr.Murat AYDIN
Tez Savunma Sınavı Tarihi: .../.../...
Yukarıdaki bilgilerin doğruluğunu onaylarım.
.../.../...
Doç. Dr. Mehmet Yıldırım Enstitü Müdürü
UNIVERSITY OF DICLE
INSTITUTE OF NATURAL AND APPLIED SCIENCES DIYARBAKIR
The investigation of some drugs by EPR technique, Submitted by Farhad O.HAMADAMEEN in partial fulfillment of the requirements for the degree of Master of Science in physics.
Examination Committee:
Title Name & Surname Signature
Chairman (Supervisor) : Prof. Dr. ġemsettin OSMANOĞLU
Member : Prof. Dr. Mehmet DOĞRU
Member : Doç. Dr.Murat AYDIN
Date of Thesis Defense: / /
I approve accuracy of the above information.
Assoc. Prof. Dr. Mehmet YILDIRIM
MANAGER OF THE INSTITUTE (SALE)
I
I want to express our best thanks to almighty Allah who has given us enough strength to carry out and accomplish our research on time.I would like to express our deep gratitude to (Prof. Dr. ġemsettin OSMANOĞLU) for supervising our research.
I would also to like thank my Asst. Prof. Dr. Kerem SÜTÇÜ for all help during writing my thesis.
I would like to express our appreciation to all my instructors who tried to teach us any things useful during these for two years.
I would like to thank anyone who even taught us one word .Thanks to our parents and my brothers and sisters.
I would like to thank my best friend (Hemn). Thanks to all of our friends.
II CONTENTS ... II ÖZET ... III ABSTRACT ………... IV LIST OF TABLES ………... V LIST OF FIGURES ………. VI 1. INTODUCTION ………... 1
1.1. Paramagnetizm and Magnetization………. 2
1.1.1. Electron Pramagnetic Resonance Theory, Angular Momentum, Spine Momentum…. 3 1.1.2. Energy of a Dipole in Magnetic Field and EPR Resonsnce conditions ……… 8
1.2. Efect of Irradiation on substances ………. 12
1.3. Spin Hmiltonian……….. 14
1.3.1. Interaction between Electron Zeeman and Spin -Orbit ... 16
1.3.2. Hyperfine structure Interactions ... 17
1.3.3. Isotropic Hyperfine structure Intraction ... 20
2. PREVIOUS STUDIES ... 23
3. MATERIAL AND METHOD ... 29
3.1. EPR Spectrometry ... 29
3.2. Obatining spectrums, Measurments and Calculations ... 34
4. RESULT AND DISCUSSION ... 35
5. CONCLUSIONS AND OUTLOOKS ... 39
III
BAZI ĠLAÇLARIN EPR TEKNĠĞĠ ĠLE ĠNCELENMESĠ
YÜKSEK LĠSANS TEZĠ
Farhad O.HAMADAMEEN
DĠCLE ÜNĠVERSĠTESĠ FEN BĠLĠMLERĠ ENSTĠTÜSÜ
ANABĠLĠM DALI
2016
Bu çalıĢmanın amacı oda sıcaklığında (295 K) gama ile ıĢınlanmıĢ iki farklı ilaç örneğinde oluĢan serbest radikallerin EPR tekniği ile incelenmesidir. Ġlaç örnekleri gama ile 20 kGy doz değerinde ıĢınlandıktan sonra elde edilen EPR spektrumları simüle spektrumlarla karĢılaĢtırıldı. Deneysel ve simüle spektrumlar incelenerek oluĢan serbest radikallerin yapıları belirlendi. Her iki ilaç örneğine ait ıĢınlanmamıĢ ve ıĢınlanmıĢ EPR spektrumları incelendiğinde ıĢınlama sonucu ilaç yapılarının radyasyona aĢırı duyarlı oldukları belirlendi. Çünkü gama ıĢınları örneklerdeki karbon ve hidrojen bağlarını kırarak iyonlar yani serbest radikaller oluĢturdu.
IV
THE INVESTIGATION OF SOME DRUGS BY EPR TECHNIQUE
M.Sc. THESIS Farhad O.HAMADAMEEN DEPARTMENT OF PHYSICS
INSTITUTE OF NATURAL AND APPLIED SCIENCES UNIVERSITY OF DICLE
2016
The main purpose of this work was to find out the stability of free radicals induced by irradiating two types of drugs (Lamotrigine & Flurbiprofen) with gamma rays at room
temperature (295k).
The two drugs have been irradiated with gamma rays with the energy up to 20 kGy at room temperature and the produced EPR spectrum has been compared to the theoretical EPR spectrum before irradiation to find the type of free radicals produced after irradiation. Using the two EPR spectrum, irradiated and un-irradiated, we found out that the two
pharmaceutical active ingredients diseases are very sensitive to irradiation in solid state because gamma rays can breakdown the hydrogen and the carbon bonds and the drugs become ions, which means that free radicals are produced.
V
Table No. Description Page
Table 1.1. Periodic Table ………. 3
Table 3.1. Various frequency band values in terms of wavelength and frequency ….… 29
Table 4.1. Chemical structure, radical and EPR parameters of LA ……… 36
VI
Figure No. Description Page
Figure 1.1. L angular momentum of a particle, which is charged with q, of mass m moving with constant linear velocity v on an orbital with a θ radius and orbital
magnetic moment μ ………..……….. 5
Figure 1.2. Energy of a classical magnetic dipole as a function of θ between magnetic field
and dipole moment ………... 9
Figure 1.3. The direction of electron spin vector between Ms = -1/2 and Ms = +1/2 in the magnetic field. Spin vector makes a precession movement around the magnetic field and its projection in the direction of field is Sz = ± ½ h……….. 10
Figure 1.4. a) Splitting of energy levels of electrons in the external magnetic field b) Absorption signal when the resonance condition is provided
c) First derivative of the absorption signal ………... 12
Figure 1.5. Electromagnetic Spectrum ……… 14
Figure 1.6. Dipolar interactions between the spin of electron and spin of nucleus……. 19 Figure 3.1. Diagram of an EPR spectrometer working at X band ……… 32 Figure 4.1. a) Theoreticl EPR of (Lamotrigine)
b) Experimental EPR of the (Lamotrigine) ………... 37
Figure 4.2. a) Theoreticl EPR of (flurbiprofen) , b) Experimental EPR of the (flurbiprofen) ……….. 38
VII EPR : Electron Paramagnetic Resonance ESR : Electron Spine Resonance
AFC : Automatic Frequency Control FDD : Phase Sensitive Detector LA : Lamo trigine
1
1. INTRODUCTION
Substances with the presence of unpaired atomic electrons in their atomic or molecular orbitals are defined as paramagnetic substances. Paramagnetic substances with no transition elements are called radicals. According to modern atomic theory, electrons spin around the nucleus and its axis. This rotational movement around its axis is called spin movement. In the absence of magnetic field, spins of unpaired electrons are random, while they are oriented in parallel and anti-parallel to the field in a magnetic field. These two orientations correspond to two different energy levels; electron spins parallel to the field are at high energy levels and electron spins antiparallel to the field are at low energy levels, respectively. If the system is energized as the energy of these two energy levels, transitions may occur between spin states (Atherton, 1973; Caringto et al., 1969). The spectroscopy branch examines this type of transitions is called either Electron Paramagnetic Resonance (EPR) or Electron Spin Resonance (ESR).
Since EPR transitions are (10μeV-140μeV) at microwave energy levels, it only deals with transitions between states of spin. In addition to the magnetic field applied to the system from outside, local magnetic fields created around the nucleus by nucleus spins around unpaired electrons also affect these spin transitions. These transitions are observed in the EPR spectrum. The width of the lines in the EPR spectrum and the structure of these lines provide important information about the orbit of the unpaired electrons, their environments they interact with and their positions.
Electron spin resonance was discovered by Russian physicist Evgeni Zavoisky resonance in 1945. Paramagnetic substances in their gas, liquid or solid forms can be examined by EPR. In the early years, it was only used in the solution of some problems in fundamental physics. In the following years, it has started to be used in a detailed examination of the electronic structure of paramagnetic ions in crystals with different symmetries.
In all kinds of spectroscopies, there is a system based on the determination of the energy levels of atoms, molecules and nuclei. These energy levels are determined by
2
interactions occurring between the substance and nuclei. Interactions can occur within the limits of the electromagnetic spectrum. Thus, spectroscopies are located in electromagnetic spectrum with interactions formed as absorption or release at frequencies matched with rotation, vibration, electronic transition and spin movements of molecules, atoms and nuclei. The majority of the information about molecular structures is obtained from the analysis of absorption spectra. Analysis of such spectrum can be performed by measuring the attenuation of the electromagnetic radiation beam passing through a sample material by wavelength or frequency of the radiation.
Lines or bands in the spectrum represent transitions between energy levels of the molecules. Therefore, each line is a measure of the difference between two energy levels. Substances that can be examined through EPR may be compounds and complexes containing transition elements as well as some radicals created by some chemical and physical methods. Radicals can be created by chemical and physical methods with methods such as γ and X – rays irradiation (radiolysis), ultraviolet beam irradiation (photolysis), holding against the high-energy particles, applying temperature and pressure.
EPR spectrometers work according to the principle of fixed microwave frequency and variable magnetic fields due to some technical reasons. In this study, powder crystals of two drugs compounds with a great pharmaceuticals significance were irradiated with gamma rays. Paramagnetic centers formed by irradiation were examined by EPR method and spin Hamiltonian parameters were determined.
1.1. Paramagnatizm and Magnetization
Magnetization is a characteristic showing the magnetic properties of substances. As is known, substances are located in one of the following three classes; diamagnetic, paramagnetic and ferromagnetic classes. For example, substances with permanent magnetic dipole moment are called ferromagnetic materials. Some substances don’t constantly exhibit dipole moment in their normal states, but they become magnetic when placed in a magnetic field.
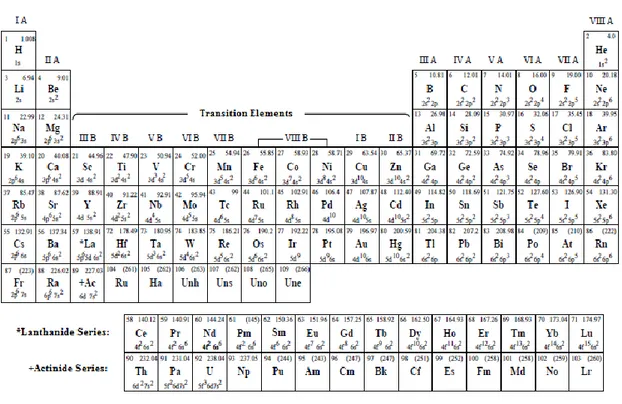
3
Some atoms have also magnetic moment since one of their inner shells is not filled. Such atoms are called transition elements in the periodic table given in Table 1.1.
Table 1.1 Periodic Table
1.1.1. Electron Paramagnetic Resonance Theory, Angular Momentum, Spin and Magnetic Moment
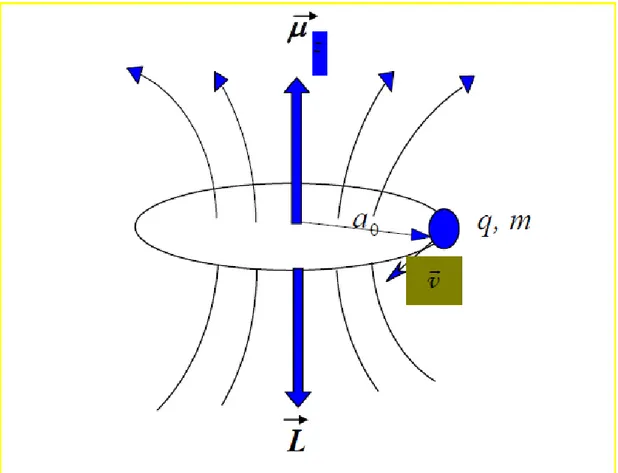
Classic physics states that angular momentum can take continuous values and angular momentum vector of a particle of a mass m moving with constant velocity v is always perpendicular to the plane of rotation. On the other hand, according to quantum mechanics, the angular momentum is a vectorial quantity that can take some certain discrete and quantized values.
The classical angular momentum of a particle of a mass m moving with constant velocity v on a non-relative xy-plane is as follows;
4
In this equation, m is the mass of the particle; v is the velocity and is the radius of the rotation. If these particle has an electric charge q, then the current occurring as a result of orbital movement of the particle is,
In this current, there is a magnetic field occurring perpendicular to the plane of rotation. This is equivalent to the magnetic dipole moment (Figure 1.1). If the area remaining in the orbit plane of the particle is represented by S, the magnetic dipole moment of the particle is as follows;
5
______________________________________________________________________
Figure 1.1 L angular momentum of a particle, which is charged with q, of mass m moving with constant linear velocity v on an orbital with ao radius and orbital magnetic moment μ.
If we combine this equation with Equation 1.1.1., it can be written as;
6 By using the definition
z component of orbital angular momentum is quantized and the magnitude of Lz component:
Where, Ml takes (2l + 1) values between +l and –l and known as orbital magnetic angular quantum number. If we plug Equation 1.1.8 in the Equation 1.1.7,
Spin dipole moment of an electron arises due to its charge distribution. If orbital dipole moment approach is taken as a basis and experimental result of spin quantum number is used as S=1/2, the magnitude of spin angular momentum:
7
If we take the relationship between spin magnetic moment and spin angular momentum similar to the state of orbits, then;
The z component of the spin magnetic moment;
Where, Ms is the spin quantum number and takes (2S+1) values from +S to –S. If there is no orbital movement in the electron spin angular momentum, g = 2 and it is non-dimensional (Bransden et al., 1989). The total magnetic moment is contributed by both orbital and spin angular momentum. g, which called Landé g factor or spectroscopic splitting factor, contains this contribution and provides information about the electronic orbit of rotation.
For the magnetic moment of electron and nucleus, Equation 1.1.12 can be written as follows;
8
Where, the charge of electron is –e and charge of the nucleus is +e. In the Equations 1.1.14a and 1.1.14b, S and I are the electron and nucleus spin angular momentum vectors, respectively; me and mp are the masses of electron and proton; β and βN are Bohr magnetons of electron and nucleus and they are equal to β =
9.27408x10-21 erg/G and βN = 5.05095x10-24 erg/G. Magnetic moments of nucleus and electron have opposite signs since their charges are also opposite to each other.
1.1.2. Energy of a Dipole in a Magnetic Field and EPR Resonance Conditions
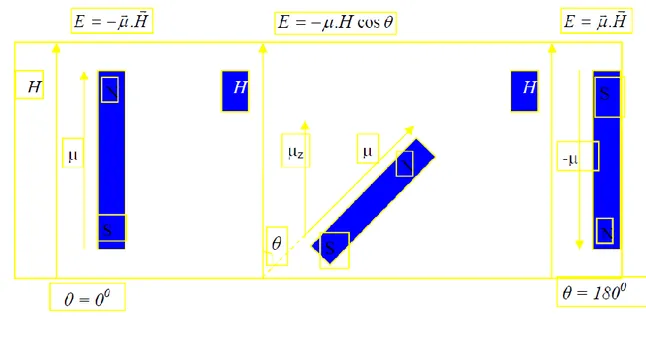
If a system with μ dipole moment is placed in a magnetic field with H intensity,
Affects the system. The work done by this; in other words, the interaction energy between magnetic dipole moment and the field;
Where, θ is the angle between magnetic field vector H and magnetic moment vector μ. In most of the first definitions of EPR, a free electron is compared with a small magnet stick with magnetic moment μ placed in a magnetic field of H (Figure 1.2) (Wertz et al., 1972).
9
______________________________________________________________________
Figure 1.2 Energy of a classical magnetic dipole as a function of θ between magnetic field and dipole moment.
If the moment in Equation 1.1.16 caused by the spin of electron, the energy can be written as
By using the Equation 1.1.14a. If the magnetic field is selected in the direction of +z, in case of H = H z k, electron spin is going to be quantized in the direction of z. For the electron with S=1/2 spin, Sz component is shown by ms and since Ms= -S, -S+1, , S-1, +S = 2S+1, energy level number is found as 2(1/2)+1= 2; Ms takes two values as -1/2, +1/2 (Figure 1.3). In this case, Equation 1.1.17 can be written as,
10
Figure 1.3 The direction of electron spin vector between Ms = -1/2 and Ms = +1/2 in the magnetic field.
Spin vector makes a precession movement around the magnetic field and its projection in the direction of field is Sz = ± ½ h.
Energy takes two values based on quantization status of electron spin;
These energy levels are in layers before applying the magnetic field. When magnetic field is applied, these layers are removed and the energy levels are separated from each other proportionally depending on the magnetic field applied. The difference between two energy levels given in Equations 1.1.19a and 1.1.19b is (Atherton, 1973);
11
If a microwave equal to the energy difference between these levels carrying an energy of ΔE = hν is sent to the electron;
Electron is stimulated to upper energy level. Microwave fields cause transitions of spins at two Zeeman energy levels to the Boltzmann distribution. Meanwhile, a signal is observed since electron absorbs energy. This is the necessary and sufficient condition of Electron Paramagnetic Resonance. The first derivative or second derivative absorption curve is usually drawn due to technical reasons (Figure 1.4) (BaĢkan, 2002). EPR transitions are observed at HBzB magnetic field that meets the resonance conditions and frequency ν. g = 2.0023 for a free electron, and EPR transition is observed when HBzB = 3390 Gauss for a microwave with a frequency value of ν = 9.5 GHz.
12
______________________________________________________________________ Figure 1.4. a) Splitting of energy levels of electrons in the external magnetic field
b) Absorption signal when the resonance condition is provided c) First derivative of the absorption signal.
1.2. Effect of Irradiation on Substances
When diamagnetic substances that don’t have paramagnetic properties are exposed to irradiation for a while, structural defects or in other words, radicals with paramagnetic properties can created. Free radicals are highly reactive (unstable) chemical products that contain an unpaired electron in one of atomic or molecular orbitals (Southarn et al., 1993). These highly reactive substances tend to make other atoms and molecules unstable by exchanging electron with atoms and molecules and changing their chemical structures. Therefore, radicals make other molecules unstable by reacting with these molecules (Thomas, 1995). It is known that carbon-centered radicals are reactive species interact with the DNA (Augusto, 1993).
13
Free radicals can be created by natural metabolic ways in the body. The main natural events creating free radicals are mitochondrial electron transfer, hexose monophosphate way, metabolism of xenobiotics, biosynthetic and biochemical degradation events (Fridowich, 1978; Ozturk et al., 2001). However, the carbon-centered radicals don’t occur in high concentrations occur in normal metabolisms (Augusto et al., 1992). They are formed in pathologic events such as oxidative stress and genetic disorders affecting the metabolisms of carbohydrates or amino acids (Çeken, 2005).
Free radicals are necessary for life. Electron transfer is the basis of energy production and many other metabolic functions (ġentürk, 2004). If free radicals are not neutralized, they may cause serious damage in the body. It is known that radicals shorten the life, lead to the development of chronic diseases and they have a very great importance in the aging period (Aydın, 2006).
In the studies conducted on radicals in EPR, irradiating the substances for formation of the radicals is one of the indispensable methods. Because radical formation is very rare other than irradiation. In the irradiation of the samples, rays in the electromagnetic spectrum such as γ-rays, X-rays, UV-rays and high energy β particles are used (Figure 1.5). Decomposition in the structure is directly associated with factors such as type of rays, irradiation time and irradiation dose.
Chemical bonds are broken in the solids as a result of irradiation. As a result of this process, some atoms or atom groups can be trapped in crystal lattice regions. In addition, molecules are stimulated and ionized due to irradiation. Trapped particles may not be paramagnetic all the time and can lose their paramagnetic properties in the regions they dragged. Besides, radiation creates cracks in the crystal lattice by breaking the bonds and atoms, electrons and atom groups can be trapped in these cracks. Paramagnetic centers trapped for solid lattices are turned into diamagnetic centers by recombining or creating some other bonds depending on environmental effects such as temperature and pressure (Tapramaz, 1991). Although the life time of radicals can be very short, it may take many years though. In order to observe short-lived radicals in EPR, in situ irradiation procedure is performed (record spectra during irradiatio n).
14
__________________________________________________________________ Figure 1.5 Electromagnetic Spectrum
In irradiation with particles, defects are formed on the surface of the material when particles are stopped at layers close to the surface of the material. Particles used in the irradiation process can form new structures and cause abnormalities by interacting with the material. In the irradiation with high-energy photons, they scattered in the substance (change of direction), lose energy (reduced frequency) or lose their intensity (decreased number) due to photoelectric effect, Compton Effect and production of electron-positron. In the irradiation with high-energy photons, defects occur not only on the surface, but also almost every part of the substance. Furthermore, since no additive particles are entered into substance, the nature of the substance is generally maintained. For these reasons, photons like γ-rays, X-rays, UV-rays are particularly preferred in the studies related to irradiation disorders.
1.3. Spin Hamiltonian
Spin Hamiltonian was first used in 1951 by Abraham and Pryce (Birey, 1989). The result found in Eq. 1.1.21 was found by considering only electron-Zeeman interaction. However, Hamiltonian, which means the paramagnetic center in the crystal or ions in a magnetic field, consists of some terms. Important Hamiltonian terms for EPR;
15 Let’s explain the terms in Equation 1.3.1;
Electron Zeeman Interaction Energy between external magnetic field and electron spin.
Electron Zeeman Interaction Energy between external magnetic field and nucleus spin.
Interaction energy of the hyper-fine structure between nucleus spin and electron spin.
Interaction energy of the hyper-fine structure between two or more electron spins.
Nucleus quadrupole interaction energy between nucleus spins.
Interaction energy of spin-orbit.
In some special cases, in addition to these terms, some terms such as temperature-dependent spin, magnetic field-orbit and crystal field can be added. The proper terms are considered depending on the topic of interest, while others are ignored. This choice completely depends on the nature of the problem.
The data obtained from EPR spectroscopy is obtained depending on some variables of the above mentioned spin Hamiltonian and properties of these variables. The most widely used and useful variables are:
16 a) Place of the line (g-factor)
b) Distance between lines (hyperfine structure interaction constant),
c) Number of lines (the number of nuclei with hyperfine structures)
d) Line intensity (area under the resonance line)
1.3.1. Interaction between Electron Zeeman and Spin-Orbit
In the majority of the atoms, there is a μ magnetic moment due to the orbital movement of the electrons. If an external H magnetic field is applied on this atom, the energy level of the atom changes as – μ.H. Splitting of the spectra of atoms with the help of an external magnetic field is called the Zeeman Effect.
The g factor in electron paramagnetic resonance is a measure of the difference between H magnetic fields applied on local field and the sample. Therefore, g factor of unpaired electrons on paramagnetic molecules is different. The resonance condition for a free electron is hν = geβH and ge=2. g-factor becomes spectroscopic splitting factor since electron is affected by local fields other than H magnetic field as in a radical or complex. The difference between the field applied and local field is reserved in the g-factor and ge is replaced with g under resonance conditions. Thus, if electron is not on a molecular orbit, g = ge and electron belongs to an atom, g = gj; in other words, Lange becomes g-factor.
Electron Zeeman or magnetic field electron spin spin interaction Hamiltonian;
Where, g factor takes the value g = 2 for pure spin movement. Although ge should be equal to 2 since a free radical doesn’t have an orbit, ge= 2.0023 due to the effect of relative movement. According to the solutions of Relative Dirac equations, these values were found as ge= 2.002319288 theoretically (Harriman, 1978).
17
Since an unpaired electron in a paramagnetic center has a particular orbit, g-factor takes different values due to both spin and orbit contributions. The contribution of spin-orbit interaction to g-factor is given as
By considering spin-orbit or Russell-Sounders couplings.
In this expression, S (S+1) = S2 refers to square of spin angular momentum, L (L+1) = L2 refers to square of orbital angular momentum and J (J+1) = J2 refers to square of total angular momentum vectors in h2 unit (Mezbacher, 1970; Atherton, 1973). δgr is the correction term comes from relative movement.
If we consider spin Hamiltonian in Equation 1.3.2 in terms of not only electron-Zeeman term, but also as receiving contribution from spin-orbit and magnetic field-orbit interaction, it can be written as;
Where, ge is the Lande-g factor of the free electron and λ is the spin-orbit interaction constant. The third term is term-orbit interaction term and it doesn’t have a notable contribution to Hamiltonian except transition elements (Aydın, 2006).
1.3.2. Hyperfine Structure Interactions
When an electron not paired with a paramagnetic center or radical is interacted with an externally applied magnetic field, a single line is observed in the EPR spectrum. In this case, information is obtained about only g value of the structure. If there are
18
multiple lines in the spectrum, there are different influences leading to formation of this spectrum. In order to explain the existence of these interactions, a molecule containing a single unpaired electron is considered. For the electron in such a molecule, the first interaction is caused by the close nuclei. Since these nuclei have angular momentum, their spin quantum numbers should be one of the values 0, 1/2, 1, 3/2...
If there is a nucleus of I ≠ 0 next to the unpaired electron, a magnetic field will be created due to the magnetic moment of the nucleus. Therefore, the electron in the molecule will be effected by not only external magnetic field applied but also local magnetic field created by nucleus. Thus, the total magnetic field acting on the electrons;
H
tot=H
ex+ H
local(1.3.5)
Where, H is the magnetic field applied from the outside, Hç is the local magnetic field generated by the nucleus. The interaction between unpaired electrons and nucleus is called hyperfine structure interaction.
If we consider the hyperfine structure interaction between unpaired electrons and nucleus as a dipole-dipole interaction; the component of local magnetic field in the direction of external magnetic field generated at the location of the electron by the nucleus;
Where, μNz is the component of magnetic moment in the direction of z, θ is the angle between z-axis and the direction of nucleus-electron and r is the distance between electron and nucleus (Figure 1.6). Curves in the figure are magnetic flux lines.
19
______________________________________________________________________ Figure 1.6 Dipolar interaction between the spin of electron and spin of nucleus.
Local magnetic field is largely depending on orientation according to Equation 1.3.6. If electron has equal orientations as in the s atomic orbit, the local magnetic field is zero since;
Therefore, since the unpaired electron in the hydrogen atom is located on the 1s orbit, the local magnetic created by the proton will be zero and splitting of hyperfine structure will not be observed. However, splitting of hyperfine structure has an independent component other than orientation. The source of this split cannot be dipolar interaction. The interaction between an unpaired electron and proton independent from orientation is called either spin-spin interaction or Fermi interaction.
The hyperfine structure interaction may be either isotopic or anisotropic (dipole-dipole interaction between unpaired electron and nucleus depends on orientation). Since the quantum number of nucleus MI takes (2I +1) values, the local magnetic field created by nucleus will take (2I +1) values. Therefore, PR resonance lines obtained will split into (2I +1) lines.
20
1.3.3. Isotropic Hyperfine Structure Interaction
Since the hyperfine structure interaction between electron and nucleus is dipole-dipole interaction between two spins, considering two dipole-dipoles with magnetic moments equal to 1 μ and 2 μ; according to classical electromagnetic theory, one these moments will generate a magnetic field at the location of the other. This interaction between two dipoles is called Hamiltonian;
When there are N dipoles in the system, the total local field generated by all these dipoles is considered. The energy corresponding to dipole-dipole interaction in such a system;
⃑⃑⃑⃑⃑⃑⃑⃑⃑⃑⃑ (1.3.9)
The local field on the electron can be either added to the external magnetic field or substituted from it depending on the angle θ. According to Equation 1.3.9, local magnetic field is largely dependent on orientation. Since electron is not fixed in a single point in space, the total local magnetic field affecting the electron will be close to the average value if it is calculated by considering all orientations in space.
If electron has equal orientations as in the s atomic orbit, the average cos2θ over a spherical surface for average value of the local field can be written as;
H
locallocal
21
If these values are plugged in Equation 1.3.29, Hlocal disappears. Since electron distribution in the s orbit is spherically symmetric, it can be said that the source of splitting of hyperfine structure is not dipolar interaction. For the occurrence of Fermi interaction, the possibility of electron to be next to the nucleus shouldn’t be zero. The presence of s atomic orbits of the electron meets this condition. However, electrons on p, d, f … atomic orbits don’t meet this condition. Because all p, d, f … orbits have nodes in the nucleus. According to Fermi, isotropic interaction energy of a system with electrons is;
|ψ(0)|2
is the probability of having electrons in the nucleus. The interaction energy of magnetic dipole moments of electron and nucleus is;
In terms of spin vectors.
If constants are shown with a;
a is the isotropic splitting of hyperfine structure and its probability to be in the nucleus is proportional to |ψ(0)|2
P. This value is a measure of the difference between consecutive transitions in the presence of hyperfine interaction.
22
Theoretically, in many paramagnetic ions and free radicals, isotopic fine structure interaction should be observed. However, if the baseline determining a magnetic system is interacted with the stimulated level of the system due to the mutual repulsion between electrons, this occurs as structural interaction in the system. As a result of this structural interaction, an electron distribution occurs at the stimulated levels. In case the stimulated level of magnetic field is similar to the s atomic orbit, isotropic fine structure splitting occurs (Gordy, 1981).
23
2. PREVIOUS STUDIES
In the first study conducted on radicals formed by exposure of amino acids to high energy irradiation, Ghosh et al. irradiated single crystals of glycine with gamma rays and recorded EPR spectra at room temperature (Ghosh et al., 1959). In the analyses of spectrums, the paramagnetic center that is considered to be generated as a result of irradiation in the structure was attributed to the NH3+-ĊH-COO− radical and determined that it was interacted with nitrogen nucleus of the unpaired electron, and three identical protons of the nitrogen isotropically and with α proton anisotropically. Isotropic hyperfine constants were calculated as aN = 3.5 G, aNH=18.9 G, aα = 26.8 G.
In another study, McConnell et al. investigated the decomposition seen in the malonic acid single crystals as a result of irradiation by EPR method (McConnell et al., 1960). The identity of radical generated in the structure was expressed as ĊH(COOH)2 with the help of EPR spectrum. They have determined that the interaction between unpaired electron and α proton is anisotropic and isotropic hyperfine structure constant was measured as a= 22.5 G. g value of the radical was found to be a little anisotropic and gor = 2.0031. The spin density of unpaired electron on the α carbon atom was found as Q = 22.5 G in the equation of aα = Qρα and spin intensity was found as Qρα ≈ 1.
Heller et al. irradiated the single crystals of β-succinic acid with X-rays and determined the radical formed in the structure as a result of irradiation as HOOCCH2ĊHCOOH (Heller et al., 1960). They have determined that methylene protons in the radical are not identical, hyperfine structure constant of these is nearly isotropic and ranged between 28.6-35.7 G. They have also found that unpaired electrons and α proton anisotropically interact with each other and isotropic hyperfine structure constant is aα = 25.7 G.
In another study conducted by Morton et al. (1961), single crystals of L-alanine irradiated by gamma rays were investigated by using EPR technique. In the analysis of spectrum, the parametric center considered to be formed in the structure was attributed to the NH2 CH3ĊHCCOOH radical. Unpaired electron was found to be anisotropically
24
interacted with α proton, almost isotropically interacted with methyl protons and isotropic hyperfine constants were calculated as aα = 19.6 G ve aCH3 = 25.1 G.
Horsfield et al. have recorded EPR spectrums of single crystals of L-alanine- field by irradiating them with gamma rays at 77K (Horsfield et al., 1961-a). They observed that spectrums consist of 12 lines with 1:1:2:2:1:1:1:1:2:2:1:1 intensity distribution and the radical considered to be formed after irradiation was CH3ĊHCOOH. They determined that methyl protons were not identical and measured isotropic hyperfine structure constants as aβ(1) = 42.8 G, aβ(2) = 27.1 G, aβ(3) = 5 G and isotropic hyperfine structure constant of α proton as aα = 22.1 G, respectively. In the study conducted at 100 K and 200 K, a radical similar to the one seen in the single crystals of L-α- field was observed (Horsfield et al., 1962).
In another study, defects formed in the single crystals of α-amino isobutyric acid as a result of irradiation was examined by using EPR technique (Horsfield et al., 1961-b). The defect formed in the structure due to the irradiation was attributed to the (CH3)2ĊCOOH radical. It was found that the unpaired electron had nearly isotropic interactions with six identical protons of the methyl group and hyperfine structure constant was aCH3 = 23.4 G. In addition, g value of the radical was a little an-isotropic and the average g was gort = 2.0029.
In another study, paramagnetic centers formed in the single crystals of DL- valine, D-valine and L-valine irradiated by X-rays were investigated by EPR method (Shields et al., 1967). The identity of the radical generated in the structure was defined as (CH3)2ĊCH(+NH3)COO-. They have also found that hyperfine structure constant was nearly isotropic and its value was ranged between 22.4-24.4 G. Hyperfine structure constant and g value of nitrogen nucleus had a little anisotropic characteristics and the values were measured as aN = 7.7 G, gor = 2.0032, respectively.
In the study of Sinclair et al., single crystals of L- alanine field was irradiated with X-rays and the paramagnetic center formed in the structure was examined at low and room temperatures by EPR method (Sinclair et al., 1967). The paramagnetic center formed in the structure due to the irradiation was attributed to the CH3ĊHCOOH radical. They have determined that the unpaired electron had an anisotropic interaction
25
with α proton and isotropic interaction with a methyl proton at low temperature, and measured hyperfine structure constants as aα= 15.1 G ve aβ (1) P= 18.8 G, respectively. In the room temperature, hyperfine structure constant of α proton was anisotropic and hyperfine structure constant of methyl protons was isotropic and their isotropic values were calculated as aα= 19.0 G, aβ= 25.8 G, respectively.
In the study of Ogawa et al., the single crystals of glutamic acid and glutamic acid hydrogen chloride were irradiated by gamma rays and recorded their EPR spectrums at a temperature ranged between 77 and 300 K (Ogawa et al., 980). In the spectrums measured at 77K, the radical formed in the structure was defined as HOOCCH2CH2CH(NH3+)Ċ O OH and isotropic hyperfine structure constant was measured as aβ = 8.5 G. In the analysis of spectrums at temperature ranged from 140 to 170 K, the paramagnetic center formed in the structure after irradiation was considered as either HOOCCH2CH2ĊHCOOH or HOOCĊHCH2CH(NH3+)COOH radicals. They have determined that the unpaired electron had anisotropic interaction with α proton and isotropic interaction with β(1) and β(2) protons and isotropic hyperfine structures were measured as aα = 23.3 G, aβ(1) = 45.8 G, aβ(2) = 23.9 G, respectively. In the spectrums measured at 200K, the radical formed in the structure was defined as HOOCCH2CH2Ċ(NH3 + )COOH. The unpaired electron had anisotropic interactions with β(1)
and β (1) protons and isotropic hyperfine structure constants were found as aβ(1) = 28.1 G, aβ (2) = 20.2 G. In the spectrums measured at 300K, the radical formed in the structure was defined as HOOCĊHCH2CHNH2COOH. They have determined that the unpaired electron had anisotropic interaction with α proton and isotropic interaction with β proton and measured the hyperfine structure constants as aα = 22.0 G, aβ (1) = 26.0 G, aβ(2) = 5.0 G, respectively.
In another study, the aqueous solutions of Al6O4(OH)10(Leucine)2.5H2O were subjected to X-Rays and their spectrums were recorded at room temperature and 77 K (Laslo et al., 1991). The radicals formed in the structure was defined as (CH3)2ĊCH2CHNH3 + COO -. The unpaired electron was observed to be interacted with seven identical β(1)
protons and one non-identical β(2) proton. The hyperfine structure constants were found to be aβ(1) = 23.0 G, aβ(2) = 7.0 G and g = 2.0034.
26
In another study, the single crystals of N-acetyl-L-glutamic acid, L-glutamic acid and DL-glutamic acid and hydrogen chloride were irradiated with gamma rays and their EPR spectrums were recorded at 100 and 300 K (Zengin et al., 1996). In the analysis of spectrums, the radicals formed in the single crystal of N-acetyl-L-glutamic acid were determined as CH3COΝH (I) and Ċ3H5 (II). In (I) radical, hyperfine structure constants of hydrogen proton and nitrogen nucleus were found to be varying and their isotropic values were measured as aN = 6.0 G, aH = 89.0 G and g value as g = 2.0043, respectively. The radicals formed in the single crystal of L-glutamic acid and DL-glutamic acid were determined as ĊH (I) and ΝH2 (II). The isotropic hyperfine structure constant of (I) radical was found as aα = 83.0 G and g = 2.0042, respectively. The isotropic hyperfine structure constants of (I) radical was found as aN =19.5 G, aH = 10.0 G and g = 2.0042, respectively.
In the study of Koksal et al., the single crystals of Nα-acetyl-L-glutamic acid (NALGA) and Nα-acetyl-L- glutamine acid (NALG) were irradiated with gamma rays and their paramagnetic centers were examined by EPR method at room temperature (Koksal et al., 1997). In the analyses of spectrums, the defect formed in the structure of NALGA was attributed to the HOOCCH2CH2Ċ(NHCOCH3)COOH radical. They have determined that the unpaired electron had anisotropic interactions with one of the β protons and nitrogen nucleus and isotropic hyperfine constants were found as aβ (1) = 36.5 G, aN =5.6 G, and g = 2.0016, respectively. The radicals formed in the structure of
NALG were attributed to the NH2COCH2Ċ(NHCOCH3)COOH (I) and
NH2COCH2CH2CH(NHCOCH3)ĊOOH(II) radicals. In the (I) radical, the unpaired electron had anisotropic interaction with N nucleus and isotropic interaction with one of the β protons and anisotropic hyperfine structure constants were found as aβ (1) = 40.0 G, aN = 11.0 G, and g = 1.9910. In the (II) radical, the unpaired electron was found to be interacted with a proton attached to the adjacent carbon and the hyperfine structure constant was found to be varying between aβ (1) = 3.0-6.0 G. The value of g was also calculated as g = 1.9880.
In the study of Osmanoglu et al., the single crystals of 2.2 dimethyl succinic acid were irradiated with gamma rays and their EPR spectrums were recorded at room temperature (Osmanoğlu et al., 2002). In the analysis of spectrums, the defect formed in
27
the structure was attributed to the ĊHCH2 radical. They have determined that the unpaired electron had an anisotropic interaction with α proton and isotropic interaction with β proton and measured hyperfine structure constants as aα = 24.4 G, aβ = 8.2 G, respectively. Furthermore, g value of the radical was found to be anisotropic and the isotropic value was found to be g = 1.9971.
In the study, the single crystals of Nα- acetyl -L-histidine monohydrate were irradiated with gamma rays and the defects formed in the structure were examined by EPR method (Kent et al., 2003). In the analysis of spectrums, the defect formed in the structure was attributed to the R1- ΝH (I), R2- NĊH (II) and C2H2ĊH2 (III) radicals. Hyperfine structure constants of all three radicals were found to be almost isotropic and they were calculated as aH = 130.0 G, aN =10.0 G and g = 2.0045 for radical (I); aH = 90.0 G, aN =10.0 G, ve g = 2.0042 for radical (II); and aH = 30.0 G, aβ =10.0 G and g = 2.0047 for radical (III), respectively.
Osmanoğlu et al. have irradiated the powder crystals of acetyl and carbamyl -β- methyl choline chloride with gamma rays and recorded their EPR spectrums at room temperature (Osmanoğlu et al, 2003-a). As a result of the irradiation, both radicals in both substances were found as [Me3NĊMeCH2OCOMe]CI. The hyperfine structure constants were found as aCH3 = 23.0 G, aCH = 21.0 G, aN =9.6 G and g = 2.0048.
In the study of Baskan et al., the paramagnetic center formed as a result of irradiation of single crystals of NBαB- monochloroacetyl -α- aminoisobutyric acid with gamma rays was examined by EPR method (Baskan et al., 2004). The identity of the radical considered to be formed in the structure after irradiation was determined as (CH3)2ĊCOOH. They have determined that the unpaired electron had anisotropic interactions with four identical protons of (CH3)2 group protons and isotropic interaction with a single proton. The hyperfine structure constants were determined to be aCH3CH = 16.6 G, aCH = 6.5 G, respectively. The g value of the radical had anisotropic characteristics and its average value was found to be g = 2.0085.
In another study, the single crystals of L-glutamine hydrogen chloride and N-carbamyl L-glutamic acid were irradiated with gamma rays and their paramagnetic centers were examined by EPR method at room temperature (Osmnanoğlu et al.,
2005-28
a). In the analyses of spectrums, the paramagnetic center formed in the single crystals of L-glutamine hydrogen chloride resulting from irradiation was attributed to CH (I) and H2 (II) radicals. In the (I) radical, the hyperfine structure constant of α proton and g value were found to be anisotropically varying and their isotropic values were found to be a= 86.0 G, g= 20037, respectively.
29
3. MATERIAL AND METHOD 3.1. EPR Spectrometry
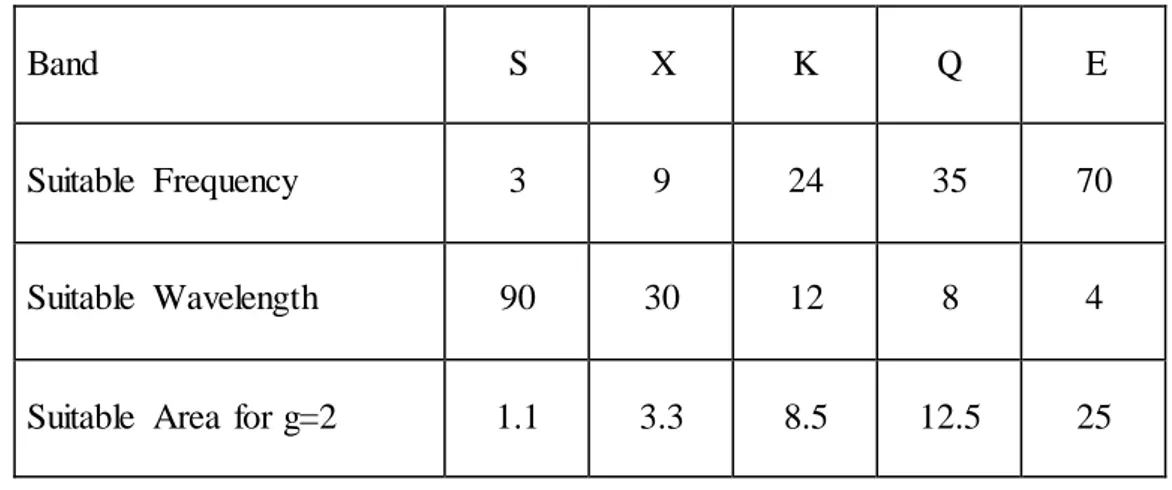
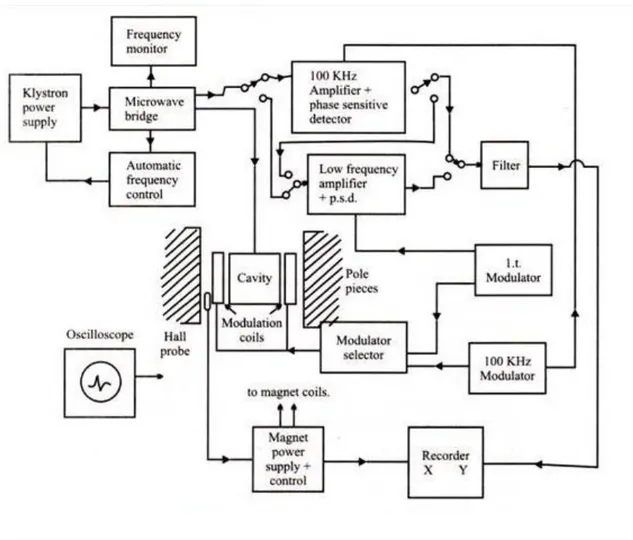
EPR spectrometer must be designed to provide hν = gβH condition. Since EPR transitions are done between 40 GHz microwave frequencies, magnetic field should be suitable to these values. In the resonance condition, variables are microwave frequency and magnetic field. Frequency is kept constant in continuous wave EPR spectroscopy for technical reasons and transitions are observed by changing the magnetic field. Because although it is easy to change the magnetic field with high precision, it is quite difficult to continuously change the microwave frequency. EPR spectrometers have a microwave source that can transmit microwave at a certain frequency range and varying magnetic field source. EPR spectrometers are made in various frequency bands (Table 3.1).
Table 3.1.Various frequency band values in terms of wavelength and frequency
Band S X K Q E
Suitable Frequency 3 9 24 35 70
Suitable Wavelength 90 30 12 8 4
Suitable Area for g=2 1.1 3.3 8.5 12.5 25
In Figure 3.1, diagram of a continuous-wave EPR spectrometer is given. Principles and functions of the elements shown in the diagram are as follows;
Electromagnets comprise a pair of coils wound on the ferromagnetic cores
30
The Klystron is an electron tube produces a low power stable microwave within
a narrow bandwidth. Its working principle is based on the modulation of speed of electrons accelerated under a potential difference at a frequency in the microwave region. Electrons with decreasing and increasing speeds emit electromagnetic waves at this frequency. The frequency is determined by changing the cavity size of klystron. Frequency can be changed within a small frequency range by changing the effective volume of this cavity mechanically. This process is necessary for the integration of resonant cavity with changing resonance frequency.
Waveguide is a component of microwave transmission. It is made with highly
conductive metals or alloys at various geometrical cross sections depending on the purpose of use. The most widely used and efficient ones have rectangular cross sections.
Ferrite isolator is a one-way microwave component connecting klystron to
waveguide and preventing noise going back into the klystron from guide-cavity system.
Variable attenuator consists of a microwave absorber added to the guide
system. It weakens the power of the microwaves going to the resonant cavity through the guide system. Power attenuation is measured in units of dB. If the microwave power produced by the klystron is PBKB, if the desired power to be sent to the sample is PB δB;
Magic T or wave rotator enables microwave coming from klystron directed to
the cavity and the returning wave turned back after being paired with the sample in the cavity directed to the sensors.
Screw tuner or iris is a component that enables the impedance agreement
31
system and cavity is obtained by inserting a conductor screw to the gap between guide and cavity.
Resonant cavity is a rectangular prism shaped component placed in the sample
(may be in different geometries). It creates static waves at TB102 B mode by reflecting from walls of polarized microwave cavity coming from the guide system. Magnetic field component of the static wave is perpendicular to the external magnetic field. Since the magnitude of static waves is similar to the magnitude of sound waves, sound waves also cause vibration and microphonic noise of cavity walls by coming to the cavity resonance as well as microwaves. Therefore, cavity walls should be thin and flexible. Cavity should be made of good conductor with small thermal expansion coefficient that is suitable for heating and cooling of the sample and its irradiation on the cavity. The quality factor showing the efficiency of the cavity is Q factor. This factor should be high as much as possible and it can be shown as follows (Weil at al., 1994);
Q = (2π (Maximum microwave power in the cavity)) / Lost energy in each rotation (3.1.2)
32
______________________________________________________________________
Figure 3.1. Diagram of an EPR spectrometer working at X band
The magnetic field modulation is the alternative magnetic field applied in a
direction parallel to the static magnetic field and provided by small bobbins placed in both sides of the cavity. This field with small amplitude (between 1 mg – 50 G) and low frequency (25 kHz - 100 kHz) is necessary to avoid spins in the static magnetic field not to be saturated. Field modulation cause microwave, which was reflected after interaction with the sample in the cavity, to be modulated at modulation frequency.
Crystal sensor is made of doped semiconductor crystals and converts
microwave into current. Since it is modulated in the frequencies of the microwave field modulation and AFC signal, the output current is alternative current in these frequencies.
Automatic frequency control (AFC) enables klystron to produce microwave at
33
plate of the klystron and it causes microwave to be modulated in this frequency. This signal separated from the crystal detector output current with a bandpass filter is applied to one of the inputs of FDD. FDD provides a DC voltage proportional to the to the phase shift between the main AFC signal applied to the other input and sensor output signal and shifts of microwave frequency are prevented by applying this voltage on the klystron acceleration phase plate.
Phase sensitive detector (FDD) is an electronic circuit with two inputs and one
output. It gives a DC output voltage proportional to the phase difference between the input signals with same frequencies. There are two phase sensitive detectors in the EPR spectrometer; one in the AFC system and the other is in the field modulation. It is connected to the output of FDD output spectrometer connected to the field modulation system.
Output units can be an oscilloscope used to observe spectrums, a
potentiometric chart plotter or a computer. One of the inputs of FDD giving the output signal is connected to the modulation field signal generator and the other one is connected to the signal at modulation frequency separated from crystal sensor with a band pass filter.
In the resonance cavity, microwave matches the Gaussian or Lorentzian distribution around sample resonance field value is absorbed. Therefore, the amplitude of the crystal sensor output signal varies according to the Gaussian or Lorentzian distribution around the resonance field. However, the amplitude of the other input signal of FDD is always constant. In addition, the phase difference between two signals on the rising edge of the absorption curve is 00, while the phase difference is 1800 in the falling edge, respectively. As a result, FDD output will be same as DC voltage absorption curve.
Spectrums in this study were obtained from Varian E-109 Line Century Series EPR spectrometer located in the EPR laboratory of Ondokuz Mayıs University, Faculty of Arts and Sciences and Bruker model EPR spectrometer located in Turkey Atomic Energy Agency (TAEK).
34
3.2. Obtaining Spectrums, Measurements and Calculations
Single crystal or powder states of the sample are considered while performing experiments with EPR spectrometer, the sample used is considered. Spectrums of powder samples are obtained by placing them into 4-5 mm diameter quartz tube.
For all samples used in the study, power tracking was performed within 2 and 200 mW range of the microwave. Spectrums in optimum conditions were recorded by selecting suitable modulation field amplitude, scanning field and speed.
Corrections of shifts in the microwave frequency of the spectrometer were made according to the g value (g = 2.0036) of DPPH (Diphenylpicrylhydrazyl) radical.
Since the spectrum is the center of all orientations for powder samples, the spectrum is formed by superposition of all these orientations. The resulting shapes of the EPR spectrum gives information about the symmetry of environment, where the radical generated by irradiation is placed. The analysis of spectrums of powder crystals is performed from intensities and shapes of the lines. The shapes of these lines in the powder spectrums can vary depending on the symmetry of the structure, where paramagnetic ion or radical is placed, as well as changing intensity distribution of the lines.
35
4.Result and Discussion
Lamotrigine is an Anti-epileptic Agent and pain-relieving properties. The physiologic effect of lamotrigine is by means of Decreased Central Nervous System Disorganized Electrical Activity. With increases the action of gamma-aminobutyric acid, an inhibitory neurotransmitter, which may end result in a decrease of pain-related transmission of signals along nerve fibers. This agent may also prevent voltage-gated sodium channels, suppress glutamate release, and prevent serotonin reuptake.
Flurbiprofen is an antiinflammatory with antipyretic, pain-relieving, and antifungal activity.It has been shown to reduce bone resorption in periodontal disease by preventing carbonic anhydrase.Fluibiprofen is a cyclooxygenase (COX) inhibitor,This results in a reduction of arachidonic acid conversion into prostaglandins that are involved in the inflammation, pain, swelling and fever .The EPR investigation of lamotrigine and Flurbiprofen have not been studied so far, and we have undertaken a study of this drugs.
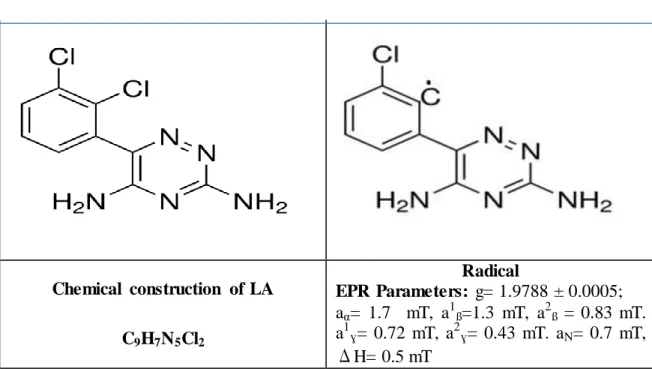
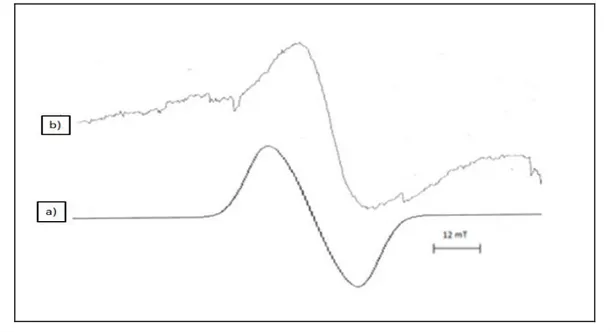
The EPR spectrum of LA (Lamotrigine) is a singlet with the g= 1.9788 ± 0.0005. The linewidth is on the order of 0.5 mT. The many- lined spectrum superimposed on the alkyl spectrum shown in Fig.4.1(a) This spectrum can be attributed to the radical presented in Table1, with the hyperfine parameters
a
α= 1.7 mT,a
1ß=1.3 mT,a
2ß = 0.83 mT.a
1ɣ= 0.72 mT,
a
2ɣ= 0.43 mT.a
N= 0.7 mT. The magnitude of β –proton splitting on the dihedral angle (θ),the theory of β hyperfine interactions,a
ßH= Bo + B1 cos2 θ
The constants Bo and B1 have been experimentally determined as (0-3.2) and (32.6-50),respectively .
a
ßH =3.2+32×1/2 =17 gauss
this value is in good agreement with the observed spectrum.The hyperfine splittings of Lamotrigine are not observable due to line broadening, and therefore difficult to interpret. The EPR spectrum of LA consists of an intense broad singlet due
36
to five proton of hyperfine splitting. Broad EPR spectrum with simulated was determined. The similar spectrum of linewidths 0.5 mT was obtained for NEHCl.
The g value, hyperfine constants and linewidths obtained as a result of simulation are given in Table 4.1.
Table 4.1.Chemical structure, radical and EPRparameters of LA
Chemical construction of LA C9H7N5Cl2 Radical EPR Parameters: g= 1.9788 ± 0.0005; aα= 1.7 mT, a1ß=1.3 mT, a2ß = 0.83 mT. a1ɣ= 0.72 mT, a2ɣ= 0.43 mT. aN= 0.7 mT, ΔH= 0.5 mT
The g-factor of free radical in the studied drugs is determined in the range of 2.0118- 2.0020. The computer simulation of EPR spectra of LA as shown Fig.4.1 (b). The results indicate that LA sample belong to ß-Blockers very sensitive towards ionizing radiation.
37
Figure 4.1. a) Theoreticl EPR of (Lamotrigine) ,b) Experimental EPR of the (Lamotrigine) The radicals attributed to these spectra are listed in Table 2. In the dose value of 20 kGy behavior of FB (flurbiprofen) is shown in Fig.4.2 (a) The spectrum consists of single lines, and the radical for this spectrum is attributed to the hydrogen abstraction from the C-H in the molecule of FB (Table 4.2).
Table 4.2.Chemical structure, radical and EPRparameters of FB
Chemical construction of FB C6H5C6H3(F)CH(CH3)CO2H Radical EPR Parameters: g= 1.9583 ± 0.0005; aα= 2.3 mT, aß= 2.2 mT, a1ß= 2.1 mT, a2ß= 1.6 mT, aɣ= 0.72 mT, ΔH= 0.5 mT
38
At 295K, the hyperfine interactions of the unpaired electron with the three methyle protons and other protons of nearly equal magnitude can be taken as a 1ß= 2.3 mT,
a
2ß= 2.2 mT,
a
3ß= 2.1 mT,a
1ɣ= 1.6 mT,a
2ɣ= 0.72 mT. This interaction can be under stood from hyperfine coupling constant reaction of the protonsa
ß = Bo +B1cos2θBo is the spin polarization contribution (Bo = 0-3.56), B1 is the hyperfine conjugative contibution (46 gauss)
a
ß =0+46.1/2 =23 gaussA simulation of the flurbiprofen spectrum is obtained in Fig.4.2(b), using above the hyperfine coupling constants. The experimental and simulated EPR spectra are found to agree well with each other Fig.{4(a), 5(b)}.
Figure 4.2 a) Theoreticl EPR of (flurbiprofen) , b) Experimental EPR of the (flurbiprofen) The measured g value is g= 1.9583 ± 0.0005. The hyperfine constants are similar to those carboxyl and alkyl radicals.
39
5. Conclusions and Outlooks
The radicals formed in irradiated drugs showed that main radicals came from breaking of a C-H bond. Identification of the radicals were confirmed by simulation values. The irradiated samples of LA and FB exhibited an EPR spectrum with single, two pharmaceutical active ingredients diseases determined very sensitive to irradiation in solid state.
The LA sample was irradiated in the dose rate of 20 kGy. The resonance signals of LA and FB with microwave power and modulation amplitude were investigated. The ESR spectrum of LA was observed. EPR spectroscopy has been developed to measure accumulated doses of ionising radiation absorbed. The measurement of the amount of radiation doses used in drugs is very important for human health. The relative amounts and spectroscopic parameter values of the suggested radical was also determined from the simulation calculations. EPR spectra of LA sample exhibit only one singlet line with g=1.9788± 0.0005
The EPR spectrum recorded after irradiation present singlet line. EPR spectra is very important for identification of the radiation treatment of drug sample. EPR spectroscopic measurements at mainly at 295K were performed to obtain the ESR spectra from the radicals produced on FB ɣ-ray irradiation. Considering all the results obtained from the room temperature ESR measurements of ɣ-irradiated FB drug proves to be very suitable system for understanding the mechanism of radiation action in the solid state. Free radical is formed at room temperature ɣ- irradiation on drug. This study gives for the first time a detailed analysis of the influence of dose and room temperature together on the distribution of free radical formed on FB drug.
41
6. REFERENCES
Augusto, O., Netto, L.E.S. and Gomes, L.F., 1992. DNA Alkylation Carbon-Centered Radials, UBrazilian J. Med. Biol. Res., 25U, 1171-1183.
Augusto, O., 1993. Alkylation and Cleavage of DNA by Carbon-Centered Radical Metabolites, UFree Radical Biol.& Med., 15U, 329-336.
Armstrong, W.A. and Humphreys, W.G., 1967. Amino Acid Radicals Produced Chemically in Aqueous Solutions. Electron Spin Resonance Spectra and Relation to Radiolysis Products, UCanadian J. Chem., 45U, 2589-2597.
Atherton, N.M., 1973, Electron Spin Resonance Theory and Application, John- Wiley and Sons Inc., New-York.
Aydın, M., 2006. Investigation Ġmino and Amino Acids in Compound-Induced Gamma Rays EPR with Free Radicals, D.Ü. College of Science. Inst., PhD Dissertation,Diyarbakir.
BaĢkan, M.H., 2004. Investigation Irradiated by γ-ray Some Isobutyric EPA Acid Derivatives, D.Ü.College of Science. Inst., PhD Dissertation, Diyarbakir.
BaĢkan, M.H and Osmanoğlu, ġ., 2004. EPR of Gamma Irradiated NBαB- Monochloroacetyl-α-Aminoisobutyric Acid, UZ. Naturforsch., 59a,U 665-668.
Birey, M., 1989. Investigation Organic and inorganic substances through γ-radiation and UV photolysis of free radicals generated by electron spin resonance methods College of Science G.Ü. College of Science. , PhD Dissertation, Ankara.
Bransden B. H., Joachaın C. J., 1989 Atomic and Molecular Physics, it Translated by: Fevzi Köksal, Hasan GümüĢ, Ondokuz Mayıs University. Publications, Broadcasting no 43, Samsun.
Carrıngton, A. and Mclachlan, A.D., 1969. Introduction to Magnetic Resonance With Aplications to Chemitry and Chemical Physics. New York.
CYR. N. and LIN, W.C., 1959. Electron Spin Resonance of Sigma-Electron Radical in X-Ray-Irradiated Malonamide, UJ. Chem. Phys., 50U, 3701-3706.
Çeken, B., 2005. Cutting of DNA with Synthetic Materials, D.Ü. College of Science. Enst., MSc Dissertation, Diyarbakir.
Dicle, I.Y., Investigation 2005. Some Amino Acid Derivatives with ESR, D.Ü. College of Science., MSc Dissertation, Diyarbakir.
Frıdowich, I., 1978. The Biology of Oxygen Free Radicals, UScience, 201U, 875-880.53