Contents lists available atScienceDirect
Computational Biology and Chemistry
journal homepage:www.elsevier.com/locate/cbacResearch Article
Multifunctional approaches to provide potential pharmacophores for the
pharmacy shelf: Heracleum sphondylium L. subsp. ternatum (Velen.)
Brummitt.
Ahmet Uysal
a,⁎, Omer Yilmaz Ozer
a, Gokhan Zengin
b, Azzurra Stefanucci
c, Adriano Mollica
c,
Carene Marie Nancy Picot-Allain
d, Mohamad Fawzi Mahomoodally
daDepartment of Medicinal Laboratory, Vocational School of Health Services, Selcuk University, Konya-Turkey bSelcuk University, Science Faculty, Department of Biology, Campus, 42250, Konya, Turkey
cDepartment of Pharmacy University“G. d’Annunzio” of Chieti-Pescara, 66100, Chieti-Italy dUniversity of Mauritius, Faculty of Science, Department of Health Sciences Réduit, Mauritius
A R T I C L E I N F O Keywords: Hogweed In silico Acetylcholinesterase Antimicrobial Phenolic Industrial uses A B S T R A C T
Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. commonly known as“hogweed” is traditionally used to manage several human ailments. This investigation assessed, for thefirst time, the enzyme inhibitory properties, antioxidant activity, phytochemical profile, antimutagenic, and antimicrobial potential of the ethyl acetate, methanol, and water extracts of H. sphondylium. We also established the possible interactions of iden-tified phenolic compounds with cholinesterases, amylase, glucosidase, and tyrosinase using in silico docking studies. Chlorogenic acid was found in high amounts in the methanol extract of H. sphondylium. The methanol extract was an effective inhibitor of acetylcholinesterase (1.70 mg galantamine equivalent (GALAE)/g extract) while the ethyl acetate extract showed pronounced inhibitory action against butyrylcholinesterase (1.77 mg GALAE/g extract). The extracts exhibited low inhibition against amylase (0.12-0.84 mmol acarbose equivalent (ACAE)/g extract) and a more pronounced inhibition against glucosidase (2.29–3.65 mmol ACAE/g extract). In silico results showed that rutin and quercetin (-70.4 and -72.2 Kcal/mol, for rutin and quercetin respectively) docked to the enzymatic cavity of acetylcholinesterase but these phenolic compounds showed less affinity with butyrylcholinesterase (-15.0 and -5.2 Kcal/mol, for rutin and quercetin respectively). The extracts did not induce any mutations on the bacterial strains, while they have excellent antimutagenic capacity against well-known mutagens (inhibition values 98%, 97% and 96%). The methanol extract (0.78 mg/ml) showed moderate anti-fungal activity while the ethyl acetate extract (0.78–3.12 mg/ml) showed weak to moderate antimicrobial ac-tivity. This study provides valuable baseline data which might serve for the development of future pharmaco-phores for the management of human ailments.
1. Introduction
The genus Heracleum belonging to Apiaceae family, contains more than 120 species (Babak et al., 2016). Several Heracleum species have been used for medicinal and culinary purposes. For instance, H. per-sicum is used as a spice,flavoring, carminative, antiseptic, digestive, analgesic, anticonvulsant, flatulence, stomachs, epilepsy, and pain killer; H. sprengelianum is used against sunburn, skin diseases, and tumor; H. rigens is used to manage urinary disorders, cough, hyper-acidity, wound, abdominal disorders, cardiac diseases, vomiting, con-stipation, stomachic, diarrhea, headache, phlegm, gastric disorders, and indigestion (Babak et al., 2016).
Heracleum sphondylium L. subsp. ternatum, also known as“hogweed” is commonly found in Europe, part of Asia and North America (Senejoux et al., 2013). This herbaceous species has a hollow stem, pinnate leaves, and whiteflowers (Benedec et al., 2017). The roots and aerial parts of H. sphondylium have been used in traditional medicine as aphrodisiac, vasodilator, tonic, antihypertensive, sedative, and to heal wounds. H. sphondylium has also been used to treat diarrhea, dysentery, dyspepsia, digestive, and menstrual problems (Cieśla et al., 2008). The dichloromethane extract of H. sphondylium, which has been tradition-ally used to manage hypertension, exhibited vasorelaxant properties by inhibiting Ca2+ mobilization and modulating K+ channels con-ductivity, responsible for regulating membrane potential of vascular
https://doi.org/10.1016/j.compbiolchem.2018.11.018
Received 17 September 2018; Received in revised form 29 October 2018; Accepted 19 November 2018
⁎Corresponding author.
E-mail address:ahuysal@selcuk.edu.tr(A. Uysal).
Available online 20 November 2018
1476-9271/ © 2018 Elsevier Ltd. All rights reserved.
smooth muscle cells (Senejoux et al., 2013). The ethanol and aqueous extracts of H. sphondylium showed antimicrobial activity against Sta-phylococcus aureus, Enterococcus feacalis, Escherichia coli, Pseudomonas aeruginosa, Listeria monocytogenes, Shigella, Streptococcus pyogenes, Cor-ynobacterium diphtheria, Candida albicans, and Candida krusei (Ergene et al., 2006). The essential oil of H. sphondylium seeds, rich in 1-octanol and octyl butyrate, showed significant antimicrobial activity (İşcan et al., 2003). Octyl acetate and octyl butyrate were the most abundant aliphatic esters identified in the essential oil of H. sphondylium fruits (Maggi et al., 2014). n-Octyl acetate, n-octyl caproate, a mixture of hydrocarbons (n-pentacosane, n-heptacosane, n-octacosane n-non-acosane, n-triacontane, n-hentriacontane), ceryl alcohol, β-sitosterol, furanocoumarin, and bergapten were identified from the petroleum extract of H. sphondylium seeds (Lawrie et al., 1968). The ethanol ex-tracts of H. sphondyliumflower and leaves (168.94 and 116.22 μg/ml, respectively for DPPH assay) showed potent antioxidant activities (Benedec et al., 2017). In this respect, this plant shows immense po-tential to be domesticated and used industrially.
However, there is still a dearth of scientific data regarding the biological properties of H. sphondylium. In this sense, the aim of the current study was to investigate the antioxidant, enzyme inhibitory, antimicrobial, and antigenotoxic properties of H. sphondylium extracts as well as their phytochemical profiles. In silico experiments were per-formed to provide new insights on interactions between phenolics and target enzymes.
2. Materials and methods
2.1. Plant material and preparation of extracts
The aerial parts of H. sphondylium subsp. ternatum were collected at theflowering stage from Başarakavak Town (Konya, Turkey) in July 2017. Taxonomic identification of the plant material was confirmed by the senior taxonomist Dr. Evren Yildiztugay, of the Department of Biotechnology, Selcuk University, Konya, Turkey. The plant samples were air dried for 10 days at room temperature.
2.2. Extraction procedures
Ten grams of dried plant samples were extracted in 200 ml of or-ganic solvents, namely ethyl acetate and methanol, at 25 °C for 24 h. Obtained extracts were thenfiltered and concentrated using a rotary evaporator in vacuo. Water extracts were prepared with 5 g of the samples which were boiled with 100 ml of distilled water for 20 min and lyophilized at −80 °C for 48 h. The residues were kept at + 4 °C until analysis.
2.3. Total metabolite contents determination and HPLC analysis
The total phenolics andflavonoids contents were evaluated using Folin-Ciocalteu and AlCl3assays, respectively, according to previously published methods (Slinkard and Singleton, 1977;Zengin et al., 2016). Results were expressed as gallic acid equivalent (GAE) and rutin equivalent (RE), respectively.
RP-HPLC-DAD (Shimadzu Scientific Instruments, Kyoto, Japan) was used to detect phenolic components in the studied extracts. Eclipse XDB C-18 reversed-phase column (250 mm × 4.6 mm length, 5μm particle size, Agilent, Santa Clara, CA, USA) was used as stationary phase at 30 °C. The detailed information’s were reported in a previous study (Movahhedin et al., 2016). Identification and quantification of phenolic components were done by comparison with standards. The amount of each phenolic compound was expressed asμg per gram of extract using external calibration curves, which were obtained for each phenolic standard at correspondent absorption maximas for specific phenolic class (i.e. phenolic acids,flavonoids).
2.4. Evaluation of antioxidant capacity
The antioxidant potential of the samples was determined using the DPPH, ABTS, FRAP, CUPRAC, metal chelating, and phosphomo-lybdenum assays (Grochowski et al., 2017). The antioxidant activity was expressed as Trolox equivalent (TE), whereas EDTA was used as reference for metal chelating assay.
2.5. Key enzymes inhibitory effects
The inhibition activities of the samples against acetylcholinesterase (AChE, from Electric ell acetylcholinesterase, Type-VI-S, EC 3.1.1.7), butyrylcholinesterase (BChE, from horse serum butyrylcholinesterase, EC 3.1.1.8), tyrosinase (from mushroom, EC 1.14.18.1),α-glucosidase (from Saccharomyces cerevisiae, EC 3.2.1.20), andα-amylase (from ex-porcine pancreas, EC 3.2.1.1) were measured using the protocols pub-lished byGrochowski et al. (2017).
2.6. In silico assays 2.6.1. Enzymes preparation
The enzymes crystal structures employed for the in silico experi-ments were selected from the RCSB PDB database (Berman et al., 2000): acetylcholinesterase pdb: 4 × 3C (Pesaresi, 2016) in complex with its standard inhibitor, butyrylcholinesterase pdb: 4BDS (Nachon et al., 2013) complexed with the inhibitor tacrine, tyrosinase pdb: 2Y9X (Ismaya et al., 2011) complexed with tropolone, α-glucosidase pdb:3AXI (Yamamoto et al., 2011) complexed with maltose a-amylase, complezed. The rawfiles have been initially polished with UCSF Chi-mera (Pettersen et al., 2004) then the enzymes were prepared by using the automatic functionalities (Sastry et al., 2013) implemented in Schrodinger Maestro suite 2017-1 (Release, 2018), the missing side chains and loops were added and the heteroatoms protonation states at pH 7.4 have been generated by using Prime module embedded in Maestro 2017-1 (Jacobson et al., 2002). The non-catalytic waters, ions, and other molecules have been eliminated. The states of copper atoms present in the tyrosinase enzymatic pocket have been prepared for coordinative bonds.
2.6.2. Ligand preparation
The most abundant phenolic compounds identified in the extracts of H. sphondylium were employed for the in silico experiments, namely gallic acid, protocatecuic acid, (+)-catechin, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid, vanillin, p-coumaric acid, ferulic acid, o-coumaric acid, rutin, quercetin, luteolin, kaempferol, and apigenin. These compounds were obtained from the Zinc Database, were loaded in Schrodinger Maestro 2017-1 (Release, 2018), and prepared with LigPrep tool (Schrödinger, 2017), protonated at pH 7.4 by ionizer fol-lowed by a minimization using the OPLS3 parameters (Harder et al., 2015). The crystallographic ligands were also prepared in the same manner and re-docked for self-docking validation to the respective enzyme, in order to have a direct comparison of the value and to va-lidate the docking method. The RMSD of the self-docking from the crystallographic poses was < 1.3 Å. Also acarbose, has been selected for the docking as standard inhibitor forα-glucosidase and α-amylase. 2.6.3. Molecular docking
Glide docking tool of Maestro suite 2017 was employed for the docking study. The grids for the docking experiments were calculated by Glide (Friesner et al., 2006) using the crystallographic ligand as center the box, and manually selecting the size of 24 × 24 × 24 ang-stroms. Metal coordination was allowed for the two copper ions present in the tyrosinase enzymatic pocket. Dockings were conducted with the eXtra Precision scoring function present in Glide and the best scoring pose was kept and post-docking minimized; also the freeΔG energy was obtained from the best posed by the MM/GBSA method (Genheden and
Ryde, 2015) using the Prime module, following the consolidated pro-cedure reported our research group (Mollica et al., 2018).
2.7. Antimutagenic evaluation
The toxic dose levels of the extracts were determined according to Dean et al. (1985). Non toxic doses of the plant extracts were de-termined as 10,000, 5000, and 2500μg/plate for water and methanol extracts; 1000, 500 and 250μg/plate doses for ethyl acetate extract and these concentrations were tested in the Ames test.
In this experiment, mutagenic activity was evaluated by the Salmonella/microsome assay described by Maron and Ames (1983). Two His−mutant strains of S. typhimurium TA98 and S. typhimurium TA100 were obtained from The Research Laboratory of Microbiology, Science Faculty, Selcuk University, Konya, Turkey. At the beginning of the assays, standard mutations of the S. typhimurium strains were tested and revertant colony numbers were calculated (Mortelmans and Zeiger, 2000). The modified plate incorporation method was performed with and without S9 mix (Zengin et al., 2014).
The extracts determined as non toxic and non mutagenic were subject to antimutagenicity experiment using the Ames test (Zengin et al., 2014). The formula presented below was used to evaluate the inhibition rates of mutagenicity:
[(A-B)/(A-C)]× 100, where A = No. of his. revertants in the ab-sence of sample, B = No. of his. revertants in the preab-sence of sample, C = spontaneous revertants (Zengin et al., 2014)
The antimutagenicity was evaluated as‘strong’ > 40%, ‘moderate’ when the rates were ranging between 25–40% and ‘weak’ when the ratios of inhibition less than 25% (Negi et al., 2003).
2.8. Antimicrobial potential
The antimicrobial activity of the extracts of H. sphondylium against Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (MSSA-ATCC 25923, MRSA-ATCC 43300), Klebsiella pneumoniae (ATCC 70603), Salmonella enteritidis (ATTC 13076), Sarcina lutea (ATCC 9341), Streptococcus mutants (NCTC 10449), Enterococcus faecalis (ATCC 29212), Salmonella typhimurium (NRRLE 4463), Yersina enterocolitica (ATCC 1501), Proteus mirabilis (ATCC 25933), Listeria monocytogenes (NRRL B33314), Staphylococcus epidermidis (NRRL B-4268), Enterobacter aerogenes (ATCC 13048), Candida albicans (NRRL Y-417), Candida parasilopsis, was investigated. The broth microdilution method was used to determine the lowest concentration of the extract that inhibits the macroscopic growth of microorganisms (MIC) (Zengin et al., 2014). The turbidity of bacterial cultures were adjusted to 108CFU/ml (0.5 McFarland). The inoculums used in the test were adjusted to 105CFU/ml. An aliquot of 100μl of Mueller-Hinton Broth was distributed to each well of micro plates. A volume of 100μl of plant extract was added into the first wells. Then, 100μl from first wells was transferred to 7 consecutive wells for dilu-tion and then, the extract-broth medium in microplate was inoculated with equal amount of each bacterium (100μl) and the mixture was incubated at 37 °C for 24 h. Solutions of the tested extracts were pre-pared at concentrations ranging from 6.25 to 0.002 mg/ml. Gentamicin was used as control antibiotic. For the assignation of microbial growth, 20μl of 2,3,5-Triphenyl-tetrazolium chloride (0.5%) was added to each well and incubated for 30 min again at same temperature.
2.9. Statistical analysis
All experiments were performed in triplicates and results were ex-pressed as the mean value ± SD. Statistical analysis were performed using one-way ANOVA following by Tukey’s. To detect differences be-tween the extracts SPSS v. 17.0 program was used. The values of p < 0.05 were considered to be statistically significant.
3. Results and discussion
The total phenolic and flavonoid contents of the ethyl acetate, methanol, and water extracts of H. sphondylium are presented in Table 1. As observed, the water extract showed highest phenolic and flavonoid content (30.06 mg GAE/g extract and 23.94 mg RE/g extract, respectively). Besides, the phenolic profile of the extracts was char-acterized by RP-HPLC-DAD. Epicatechin, syringic acid, sinapic acid, benzoic acid, hesperidin, rosmarinic acid, eriodictyol, and cinnamic acid were not identified in H. sphondylium extracts (results not shown). FromFig. 1, we observed that the methanol extract had the highest concentration of chlorogenic acid (7488μg/g extract), followed by the water (2546μg/g extract) and ethyl acetate extract (406 μg/g extract). Chlorogenic acid has also been identified in the roots and stems of Romanian H. sphondylium (Benedec et al., 2017). Indeed, the biological properties of chlorogenic acid have attracted much scientific attention. This ester of caffeic and quinic acids is known to scavenge free radicals responsible for DNA damage and also upregulated the expression of genes responsible for the activation of the immune system (NCI, 2018). It has also been postulated that chlorogenic acid regulated glucose and lipid metabolism and thus might provide alternative therapeutic stra-tegies for the treatment of diabetes, cardiovascular disease, and obesity (Tajik et al., 2017). FromFig. 1(in also supplementary material), it was noted that the water extract contained higher concentration of p-cou-maric acid. Thisfinding was in line with a previous study conducted by Galanakis et al. (2013)who reported that p-coumaric acid was more soluble in water compared to ethyl acetate and methanol.
We then investigated the possible inhibitory action of the different extracts of H. sphondylium against enzymes considered as pharma-tar-gets for the management of Alzheimer’s disease (AD), epidermal hy-perpigmentation, and diabetes type 2. As presented in Table 2, the methanol extract, having higher galantamine equivalent value, was a potent inhibitor of AChE. Maintaining normal level of acetylcholine, a neurotransmitter responsible for memory and cognition in the brain, is the main therapeutic approach to manage AD (Jiang et al., 2017). In-hibiting the activity of AChE, having principal physiological function the hydrolysis of acetylcholine, is thus the most promising strategy for the treatment of AD. The methanol extract of H. sphondylium contained high amounts of chlorogenic acid, which has been previously reported to exert neuroprotective action via the inhibition of AChE activity in the hippocampus and frontal cortex of mice (Kwon et al., 2010). The ac-tivity of BChE, the secondary acethycholine-hydrolysing enzyme, was found to increase in the brain (hippocampus and temporal cortex) of elderly individuals, highlighting the loss of episodic memory and cog-nitive decline in AD patients (Bono et al., 2015). In the present study, the ethyl acetate extract of H. sphondylium showed pronounced in-hibitory action against BChE. Apigenin (762 μg/g extract), found in considerable amounts in the ethyl acetate extract, was reported to re-versibly inhibit human BChE (Katalinić et al., 2010). Mounting epide-miological evidences indicate that diabetes type 2 increases the risk of developing neurodegenerative diseases, suggesting the causative role of hyperglycemia in the pathogenesis of AD (Baglietto-Vargas et al., 2016). Diabetes type 2 induces modifications in vascular function and structure, insulin signaling, glucose metabolism, and β-amyloid/tau metabolisms, thereby enhancing neurodegeneration (Shinohara and
Table 1
Total phenolic andflavonoid contents of H. sphondylium extracts.
Extracts Total phenolic content (mg GAE/g extract)
Totalflavonoid content (mg RE/g extract)
Ethyl acetate 25.12 ± 1.52 5.01 ± 0.30
Methanol 21.69 ± 0.70 17.28 ± 0.50
Water 30.06 ± 0.45 23.94 ± 0.15
Values expressed are means ± S.D. of three parallel measurements. GAE: Gallic acid equivalent; RE: Rutin equivalent.
Sato, 2017). In the present study, we assessed the ability of H. sphon-dylium extracts to inhibit two key enzymes targeted for the management of diabetes type 2, namely, amylase, and glucosidase. Amylase is re-sponsible for the hydrolysis of ingested polysaccharides to oligo-saccharides, which will be hydrolyzed to monosaccharides by glucosi-dase (Picot et al., 2017). The inhibition of these enzymes is considered as an alternative therapeutic strategy for controlling blood glucose level in diabetes patients. However, several gastrointestinal disturbances are associated with the excessive amylase inhibition (Zengin et al., 2018). Thus, hypoglycemic agents having mild amylase inhibitory activity and potent glucosidase inhibitory action are preferred. In this work, we found that H. sphondylium extracts exhibited low inhibition against amylase (0.12-0.84 mmol ACAE/g extract) and a more pronounced in-hibition against glucosidase (2.29–3.65 mmol ACAE/g extract). Among the studied extracts, the ethyl acetate extract was the most active against glucosidase (Table 2). Interestingly, the ethyl acetate extract was rich in apigenin, which is reported to reversibly inhibit glucosidase in a non-competitive manner (Zeng et al., 2016). Apart from apigenin, the ethyl acetate extract also contained significant amounts of luteolin (382 μg/g extract) compared to the methanol and water extracts (Table 3). Indeed, the ability of luteolin to inhibit both amylase and glucosidase, has been reported previously (Kim et al., 2000).
We also investigated into the ability of the H. sphondylium extracts to inhibit tyrosinase, which is a melanogenic enzyme within melano-cytes responsible for the production of melanin by converting o-dopa-quinone to dopachrome (Zengin et al., 2018). Epidermal hy-perpigmentation conditions such as chloasma, freckles, age spots, melasma, and pigmented acne scars are of particular concern and are usually treated by the administration of medicines or medicinal cos-metics containing depigmenting agents (Rezaei et al., 2018). Tyrosinase
inhibitors are clinically used to treat a number of dermatological con-ditions linked to the accumulation of melanin and are the major in-gredients of depigmentation products (Chai et al., 2017). In this work, only the water extract significantly inhibited tyrosinase. Phenolic pro-file assessments revealed that protocatechuic acid, (+)-catechin, and quercetin, were only identified in the water extract. Protocatechuic acid, isolated from pear extract significantly suppressed cellular tyr-osinase activity in melanoma cells (Truong et al., 2017). Besides, ca-thechin was reported to inhibit tyrosinase expression by B16 melanoma cells (Sato and Toriyama, 2009). Recently, quercetin was found to in-hibit tyrosinase in a reversible and competitive manner (Fan et al., 2017).
Cellular homeostasis is orchestrated by the constant production and removal of reactive oxygen species, and thus provides protection against the onset of oxidative stress-induced complications (Prasad et al., 2018). Oxidative stress is associated to more than 100 patholo-gies, including diabetes type 2, AD, and epidermal hyperpigmentation problems (Hassan et al., 2017). Phenolic compounds from plants dis-play potent antioxidant properties. In this study, we assessed the anti-oxidant capacity of the ethyl acetate, methanol, and water extracts of H. sphondylium using multiple in vitro bio-assays. As shown inTable 3, the water extract of H. sphondylium possessed strong radical scavenging, reducing power, and metal chelating properties. The water extract was a good scavenger of DPPH and ABTS radicals (88.28 and 192.26 mg TE/ g extract, respectively). CUPRAC and FRAP assays were employed to evaluated the reducing potential of the extracts. Likewise, the water extract showed potent reducing properties (165.50 and 148.95 mg TE/g extract, for CUPRAC and FRAP assays, respectively). Protocatecheuic acid, present in the water extract only, was previously reported as a chelating agent (Doble and Kumar, 2005). It should be noted that the
Fig. 1. Phenolic profile of H. sphondylium extracts established by RP-HPLC-DAD.
Table 2
Enzyme inhibitory properties of H. sphondylium extracts.
Extracts AChE inhibition (mg GALAE/ g extract)
BChE inhibition (mg GALAE/ g extract)
Amylase inhibition (mmol ACAE/g extract)
Glucosidase (mmolACAE/g extract)
Tyrosinase inhibition (mg KAE/ g extract)
Ethyl acetate 1.12 ± 0.14 1.77 ± 0.09 0.84 ± 0.02 3.65 ± 0.15 na
Methanol 1.70 ± 0.06 1.30 ± 0.13 0.45 ± 0.03 2.29 ± 0.15 na
Water 0.21 ± 0.04 na 0.12 ± 0.01 3.31 ± 0.09 17.05 ± 1.30
Values expressed are means ± S.D. of three parallel measurements. GALAE: Galatamine equivalent; KAE: Kojic acid equivalent; ACAE: Acarbose equivalent; na: not active.
quantitative phenolic content estimation showed that the water extract had the highest amount of phenolics (Table 1). However, we observed some contradictory results regarding metal chelating properties in the literature due to the presence of non-phenolic chelators such as poly-saccharides, proteins or peptides (Wang et al., 2009)
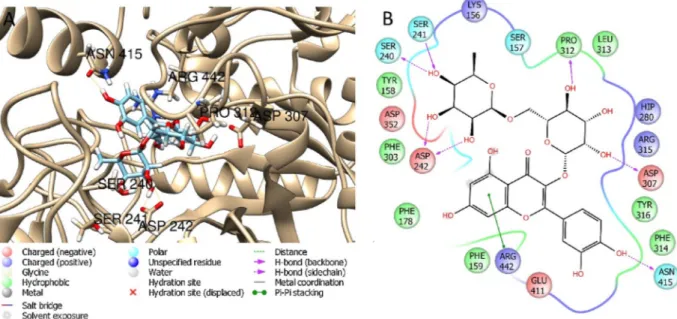
Based on the presence of bioactive substances on each extract and taking the different distribution, the most relevant phenolic compounds were selected for the docking study on the enzyme pools also used in this paper for the biological assays (Fig. 2). The best ranked pose and ΔG free binding energy of the considered phenolic compounds have been calculated by molecular mechanic (MM/GBSA) method and the results are reported inTable 4. Results evidenced that rutin and quer-cetin (-70.4 and -72.2 Kcal/mol, for rutin and querquer-cetin respectively) docked to the enzymatic cavity of AChE but these compounds showed less affinity with BChE (-15.0 and -5.2 Kcal/mol, for rutin and quercetin respectively). Likewise, recently published data demonstrated that rutin and quercetin have a good inhibitory potential toward AChE and were less potent against BChE (Ademosun et al., 2016).Fig. 3showed the best docked poses obtained when rutin was docked to AChE and BChE. Additionally, docking studies revealed that rutin showed favorable in-teractions with glucosidase, with aΔG of -68.2 Kcal/mol (Fig. 4).Li et al. (2009)have also reported that rutin showed inhibitory activity against glucosidase in low microMolar range. Docking studies revealed that luteolin showed high affinity to amylase and tyrosinase (Fig. 5). Evidence from the literature support the higher affinity of luteolin for amylase compared to glucosidase (Tadera et al., 2006). Ourfindings are in agreement with Loizzo et al. (2012)which reported that luteolin interfered with the tyrosinase activity by acting as competitive sub-strate (Fig. 5). However, it is noteworthy mentioning that the observed enzymatic inhibition may arise from the concerted synergistic action of
several phenolic compounds.
Table 5shows the possible mutagenic action of three extracts of H. sphondylium, observed in S. typhimurium TA98 and TA100 with and without S9 mix. In the Ames assay, positive control mutagens increased the number of His+revertant colonies in two mutant strains, in the presence and absence of metabolic activation system. On the other hand, no significant increase in revertant colonies was observed. In the presence of H. sphondylium extracts, no base pair substitution or frame-shifts mutations of the bacterial strains was observed. The anti-mutagenic potential of each extract was determined as inhibition ratios against 4- NPDA and 2-AF for TA98 strain; SA and 2-AA for TA100 strain (Table 6). The methanol extract revealed strong antimutagenicity against 4-nitro-O-phenylenediamine (4-NPDA) for TA98 strain without S9 mix. Inhibition ratios were determined as 49%, 47% and 46% for three concentration of the methanol extract. After addition of S9 mix, only 10,000μg/plate concentration of the extract showed strong anti-mutagenicity against 2-amino flourene with a rate of 47%, while 5000μg/plate dose was moderate antimutagenic (33%) and 2500 μg/ plate dose was weak antimutagenic (23%). For TA 100 strain, the methanol extract had no antimutagenicity at all test doses against so-dium azide in the absence of S9 mix except for 10,000μg/plate dose which was assessed as moderate antimutagenic (25%). When combined with 2-amino anthracene, the methanol extract ameliorated the muta-genic effects of this chemical and excellent inhibition rates (97%, 94% and 92%, respectively) against this mutagen were observed at all test doses (Table 6).
The water extract of H. sphondylium can be considered as strong antimutagenic against 4-NPDA for TA98 with 45%, 47% and 47% in-hibition rates at all test doses (without S9), respectively (Table 6). It can be stated from this point that the water extract was effective against
Table 3
Antioxidant properties of H. sphondylium extracts.
Extracts Phosphomolybdenum (mmol TE/ g extract) DPPH (mg TE/g extract) ABTS (mg TE/g extract) CUPRAC (mg TE/g extract) FRAP (mg TE/g extract)
Metal chelating activity (mg EDTAE/g extract) Ethyl acetate 1.51 ± 0.05 18.21 ± 0.68 63.67 ± 1.85 96.64 ± 3.35 43.94 ± 1.32 17.03 ± 0.28 Methanol 1.07 ± 0.11 52.63 ± 1.39 119.14 ± 1.43 116.64 ± 0.48 91.47 ± 1.22 14.00 ± 0.37
Water 1.06 ± 0.02 88.28 ± 0.66 192.26 ± 2.08 165.50 ± 4.27 148.95 ± 5.92 19.43 ± 0.03
Values expressed are means ± S.D. of three parallel measurements. TE: Trolox equivalent; EDTAE: EDTA equivalent.
frame shift mutations caused by 4-NPDA. Except for the lowest dose (2500μg/plate) others exhibited strong antimutagenicity against 2-AF without S9 mix with rates of 47% and 43%. The water extract of H. sphondylium was not effective against mutagenic effects of sodium azide and 2-amino anthracene for TA100 both in the presence and absence of S9 enzymes. Inhibition rates, determined as 8%, 3% and 2% without S9; 9%, 8%, and 10% with S9, were not enough to assess them as anti-mutagenic agent (Table 6).
When the ethyl acetate extract was used, it was defined that 1000 and 500μg doses of extract manifested strong antimutagenicity against 4-NPDA with a rate of 86% and 86% for TA98 strain without S9 mix. The lowest dose had no antimutagenic potential without metabolic activation. Associated with 2-AF, inhibition rates decreased at the doses of 1000 and 500μg from 86% to 63% and 59%, respectively. But they were still strong antimutagenic against 2-AF. Surprisingly, inhibition rates of the lowest dose (250μg/plate) increased by the addition of metabolic activation from 18% to 54% and was determined as strong antimutagenic. Ethyl acetate extract displayed excellent antimutagenic activities against SA with inhibition rates of 96%, 94%, and 98%, spectively for TA100 strain without S9 mix. Similarly, this extract re-vealed excellent inhibition against base pair exchange mutations oc-curred by 2-AA after addition of metabolic activation (96%, 90%, and 79%). This implies that the ethyl acetate extract of H. sphondylium al-leviated the mutagenic actions of potential chemicals and it had sig-nificant antimutagenic capacity in Ames test (Table 6).
In the present study, H. sphondylium extracts were investigated for their potential mutagenic and antimutagenic activities against well-known mutagens. Methanol, water, and ethyl acetate extracts showed strong antimutagenicity against 4-nitro-phenylene diamine with S9 mix on TA98 strain. Upon activation with metabolic enzyme, the extracts revealed moderate to strong antimutagenic activity against 2-amino flourene. The ethyl acetate extract was the most effective, showing the following % inhibition values 96%, 94%, 98%, against sodium azide without metabolic activation system on TA100 strain. H. sphondylium extracts, except the water extract, exhibited strong antimutagenicity against 2-amino anthracene (79%–97% inhibition values) after addition of S9 mix for TA100. HPLC-DAD analyses revealed the presence of chlorogenic acid. In a study conducted byYamada and Tomita (1996), it was reported that chlorogenic acid strongly improved the mutagenic activity of some mutagens such as Trp-P-I, Glu-P-2, 4-NQO and 2-AF
with rates of > 40%, reaching 96%, on S. typhimurium TA98 strain. Belkaid et al. (2006)reported the inhibitory action of chlorogenic acid on microsomal glucose-6-phosphate translocase, thereby acting as an anti-cancer agent. Chlorogenic acid was also reported to inhibit en-doplasmic reticulum glucose-6-phosphate transport on brain tumor cells. In another study, chlorogenic acid prevented micronucleus for-mation in mice bone narrow subjected to gamma ray (Abraham et al., 1993). Thesefindings suggest that chlorogenic acid, identified in H. sphondylium extracts, might be responsible for the observed anti-mutagenic activity.
Ethyl acetate extracts showed weak to moderate antimicrobial ac-tivity at concentrations ranging from 0.78 to 3.12 mg/ml (Table 7). The ethyl acetate extract (0.78 mg/ml) of H. sphondylium showed activity against Streptococcus mutans. The methanol extract (0.78 mg/ml) showed moderate antifungal activity against two Candida species. H. sphondylium ethyl acetate and methanol extracts showed weak to moderate antimicrobial activities. The methanol extract exhibited moderate antifungal capacity against Candida albicans and Candida parasilopsis. The phenolic profiling by RP-HPLC-DAD has shown that kaempferol was identified in the ethyl acetate and methanol extracts of H. sphondylium (Table 2). Indeed, the antimicrobial activity of kaemp-ferol has been reported (Del Valle et al., 2016;Tatsimo et al., 2012).
Several studies have reported that the extracts, essential oils, and purified compounds from various Heracleum species have significant antimicrobial activities (Babak et al., 2016). In a study carried out by Maggi et al. (2014)the essential oil of H. sphondylium subsp. ternatum was assayed against gram-negative and gram-positive bacteria. Water and ethanol extracts of Heracleum sphondylium subsp. artvinense were tested byErgene et al. (2006)for their antimicrobial activities against 8 bacteria and 2 yeasts, showed moderate antimicrobial activity. 4. Conclusion
This study elucidated the inhibitory action of different extracts of Heracleum sphondylium on key enzymes involved in Alzheimer’s disease, diabetes type 2, and epidermal hyperpigmentation conditions. Additionally, in silico molecular docking studies were performed to provide an additional insight of the interaction of phenolic compounds identified in the different extracts with the studied enzymes. The me-thanol extract, rich in chlorogenic acid, revealed to be a good inhibitor
Table 4
Docking score of the best pose obtained by Glide XP scoring function,ΔG binding energy (Kcal/mol) calculated by Prime.
Phenolic compound AChE BChE Amylase Glucosidase Tyrosinase
XP ΔG XP ΔG XP ΔG XP ΔG XP ΔG
Positive control (self-docking validation)
Tacrine-nicotinamide −8.1 −41.0 – – – – – – – – Tacrine – – −6.9 −32.2 – – – – – – r-nitrophenyl-a-D-maltoside – – – – −8.5 −41.9 – – – – tropolone – – – – – – – – −4.5 −26.3 maltose – – – – – – −10.0 −17.1 – – Acarbose – – – – −13.6 −37.7 −13.2 −28.2 – – Gallic acid −5.8 −25.2 −2.8 −9.9 −4.5 −4.7 −4.3 −18.8 −2.8 −9.9 Prorocatecheuic acid −5.8 −17.9 −4.4 −10.5 −3.4 −4.5 −5.9 −17.9 −4.4 −10.9 (+)-Catechin −9.3 −42.1 −4.8 −27.6 −6.9 −33.6 −9.3 −42.3 −4.9 −27.6 p- hydoxybenzoic acid −3.8 −21.9 −3.7 −5.0 −5.7 −13.4 −3.8 −21.3 −3.7 −5.1 Chlorogenic acid −9.5 −37.5 −7.9 −18.2 −8.6 −29.3 −9.4 −37.7 −7.9 −18.2 Caffeic acid −7.2 −8.8 −4.5 −27.7 −6.1 −10.6 −7.2 −8.7 −4.9 −0.5 Vanilin −5.9 −27.9 −4.7 −37.5 −6.1 −23.1 −5.9 −27.2 −4.7 −37.5 p- coumaric acid −5.9 −20.4 −3.2 −9.3 −5.3 −1.7 −5.9 −20.2 −3.2 −9.5 Ferulic acid −6.2 −21.6 −3.9 −3.1 −5.8 −7.4 −6.2 −28.2 −3.9 −3.1 o- coumaric acid −6.3 −21.2 −3.7 −0.5 −4.9 −13.5 −6.3 −21.9 −3.7 −2.8 Rutin −13.2 −70.4 −15.0 −34.6 −11.5 −55.7 −13.2 −68.2 −9.6 −30.6 Quercetin −9.9 −72.2 −5.2 −32.6 −8.6 −29.3 −9.9 −67.0 −5.2 −32.7 Luteolin −9.9 −49.4 −5.5 −32.6 −9.1 −44.0 −9.9 −49.4 −5.5 −32.7 Kaempferol −9.2 −54.0 −4.4 −30.2 −6.3 −22.9 −9.2 −54.0 −4.5 −30.2 Apigenin −9.5 −49-7 −4.0 −30.4 −7.8 −38.6 −9.5 −49.6 −4.0 −30.4
Fig. 3. Best pose and interaction diagrams of rutin docked to AChE (A–B) and to BChE (C–D).
Fig. 5. Best pose and interaction diagrams of luteolin docked to amylase (A–B) and to tyrosinase (C–D).
Table 5
Mutagenicity of H. sphondylium extracts towards S. typhimurium TA98 and TA100 strains with and without S9.
Concentration (μg/plate) Number of His+Revertants/plate
TA 98 TA 100
S9 (-) S9 (+) S9 (-) S9 (+)
aNegative Control 100μl/plate 26 ± 2 39 ± 2 113 ± 11 174 ± 3
bPositive Control 510 ± 25 3203 ± 141 1548 ± 46 4306 ± 151 Methanol extract 0 32 ± 5 37 ± 4 150 ± 5 174 ± 6 10,000 50 ± 8 35 ± 4 171 ± 19 203 ± 10 5000 42 ± 7 29 ± 3 138 ± 6 180 ± 6 2500 29 ± 3 31 ± 2 135 ± 5 204 ± 5 Water extract 0 32 ± 5 37 ± 4 150 ± 5 174 ± 6 10,000 22 ± 2 29 ± 7 130 ± 24 165 ± 3 5000 28 ± 3 31 ± 2 124 ± 5 177 ± 18 2500 28 ± 5 26 ± 1 141 ± 11 189 ± 13
Ethyl acetate extract 0 32 ± 5 37 ± 4 150 ± 5 174 ± 6
1000 53 ± 8 45 ± 4 192 ± 15 189 ± 15
500 49 ± 6 39 ± 3 196 ± 12 188 ± 18
250 32 ± 2 34 ± 5 169 ± 9 182 ± 16
a Negative control: DMSO (100μl/plate) was used as negative control for S. typhimurium TA98 and TA100 both in the presence and absence of S9.
b Positive controls: 2-Aminofluorene (7.5 μg/plate) was used as positive indirect mutagen in the presence of S9 mix; 4-nitro-O-fenilendiamine (5 μg/plate) was
used as positive direct mutagen in the absence of S9 mix for S. typhimurium TA98 strain; 2-Aminoanthracene (5μg/plate) was used as positive indirect mutagen in the presence of S9 mix; Sodium azide (5μg/plate) was used as positive direct mutagen in the absence of S9 mix for S. typhimurium TA100.
of AChE. However, docking studies showed that rutin and quercetin possessed high binding energy when docked to AChE. Thus, we might conclude that the observed inhibitory action of the methanol extract on AChE might be due to the synergistic action of phenolic compounds present. The methanol extract also showed potent antioxidant proper-ties on a battery of in vitro assays. AChE activity and oxidative stress play major role in the onset and progression of Alzheimer’s disease. Besides, the methanol extract showed strong antimutagenicity against 4-nitro-O-phenylenediamine, a powerful direct-acting mutagen, and 2-amino anthracene, a pro-mutagen. Heracleum sphondylium also showed moderate antifungal activity against Candida albicans and Candida parasilopsis. Data collected from this study support the need for further investigation geared towards the isolation of phytochemicals from the methanol extract of H. sphondylium for the development of novel pharmacophores.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the
online version, at doi:https://doi.org/10.1016/j.compbiolchem.2018. 11.018.
References
Abraham, S.K., Sarma, L., Kesavan, P.C., 1993. Mutat. Res. 303, 109–112.
Ademosun, A.O., Oboh, G., Bello, F., Ayeni, P.O., 2016. J. Evid. Complementary Altern. Med. 21, 11–17.
Babak, B.M., Leila, D., Gokhan, Z., 2016. Compr. Rev. Food Sci. Food Saf. 15, 1018–1039.
Baglietto-Vargas, D., Shi, J., Yaeger, D.M., Ager, R., LaFerla, F.M., 2016. Neurosci. Biobehav. Rev. 64, 272–287.
Belkaid, A., Currie, J.C., Desgagnes, J., Annabi, B., 2006. Cancer Cell. Int. 6.
Benedec, D., Hanganu, D., Filip, L., Oniga, I., TIPERCIUC, B., Olah, N.-K., Gheldiu, A.-M., Raita, O., Vlase, L., 2017. Farmacia 65, 252–256.
Berman, H., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T., Weissig, H., Shindyalov, I., Bourne, P., 2000. Nucleic Acids Res. 28, 235–242.
Bono, G.F., Simão-Silva, D.P., Batistela, M.S., Josviak, N.D., Dias, P.F.R., Nascimento, G.A., Souza, R.L.R., Piovezan, M.R., Souza, R.K.M., Furtado-Alle, L., 2015. Neurochem. Int. 81, 57–62.
Chai, W.-M., Lin, M.-Z., Song, F.-J., Wang, Y.-X., Xu, K.-L., Huang, J.-X., Fu, J.-P., Peng, Y.-Y., 2017. Int. J. Biol. Macromol. 102, 425–430.
Cieśla, Ł., Bogucka-Kocka, A., Hajnos, M., Petruczynik, A., Waksmundzka-Hajnos, M., 2008. J. Chrom. A. 1207, 160–168.
Table 6
Antimutagenicity and percentage inhibition of H. sphondylium extracts towards S. typhimurium TA98 and TA100 strains with and without metabolic activation (S9) against direct and indirect mutagens.
Concentration (μg/plate) Number of His+Revertants/plate
TA 98 TA 100
S9 (-) % inhibition S9 (+) % inhibition S9 (-) % inhibition S9 (+) % inhibition
aNegative Control 100μl/plate 23 ± 2 38 ± 3 113 ± 11 190 ± 9
bPositive Control 532 ± 25 0 3203 ± 136 0 1602 ± 42 0 4306 ± 250 0 Methanol extract 0 32 ± 5 37 ± 4 150 ± 6 192 ± 13 10,000 289 ± 12 49 1710 ± 215 47 1668 ± 27 25 326 ± 11 97 5000 295 ± 16 47 2153 ± 239 33 1493 ± 29 8 425 ± 19 94 2500 303 ± 8 46 2470 ± 91 23 1449 ± 26 11 536 ± 10 92 Water extract 0 32 ± 5 37 ± 4 150 ± 6 192 ± 13 10,000 306 ± 6 45 2068 ± 233 44 1488 ± 96 8 3917 ± 186 9 5000 298 ± 14 47 1850 ± 122 43 1555 ± 37 3 3980 ± 260 8 2500 297 ± 15 47 2335 ± 182 27 1573 ± 69 2 3902 ± 347 10
Ethyl acetate extract 0 32 ± 5 37 ± 4 150 ± 6 192 ± 13
1000 103 ± 16 86 1223 ± 111 63 214 ± 14 96 348 ± 46 96
500 103 ± 10 86 1345 ± 147 59 240 ± 19 94 596 ± 40 90
250 444 ± 12 18 1495 ± 38 54 175 ± 3 98 1063 ± 56 79
a
Negative control: DMSO (100μl/plate) was used as negative control for S. typhimurium TA98 and TA100 both in the presence and absence of S9.
b Positive controls: 2-Aminofluorene (7.5 μg/plate) was used as positive indirect mutagen in the presence of S9 mix; 4-nitro-O-fenilendiamine (5 μg/plate) was
used as positive direct mutagen in the absence of S9 mix for S. typhimurium TA98 strain; 2-Aminoanthracene (5μg/plate) was used as positive indirect mutagen in the presence of S9 mix; Sodium azide (5μg/plate) was used as positive direct mutagen in the absence of S9 mix for S. typhimurium TA100.
Table 7
Minimum Inhibitory Concentrations (MIC) of Heracleum extracts determined against pathogen microorganisms by broth microdilution test.
Strains MIC values (mg/ml)
Water Ethyl acetate Methanol Gentamicin (μg/ml)
Escherichia coli ATCC 25922 – 3.12 3.12 2.44
Pseudomonas aeruginosa ATCC 27853 – 3.12 1.56 9.76
Staphylococcus aureus (MSSA) ATCC 25923 – 3.12 3.12 2.44
Klebsiella pneumoniae ATCC 70603 – 3.12 3.12 2.44
Staphylococcus aureus (MRSA) ATCC 43300 – 3.12 3.12 78.12
Salmonella enteritidis ATTC 13076 – 3.12 3.12 4.88
Sarcina lutea ATCC 9341 – 3.12 1.56 4.88
Streptococcus mutants NCTC 10449 – 0.78 1.56 < 2.44
Enterococcus faecalis ATCC 29212 – 1.56 3.12 2.88
Candida parasilopsis – 3.12 0.78 312.5
Salmonella typhimurium NRRLE 4463 – 3.12 3.12 < 2.44
Yersina enterocolitica ATCC 1501 – 3.12 3.12 < 2.44
Proteus mirabilis ATCC 25933 – 3.12 3.12 < 2.44
Listeria monocytogenes NRRL B33314 – 3.12 3.12 4.88
Staphylococcus epidermidis NRRL B-4268 – 3.12 3.12 < 2.44
Candida albicans NRRL Y-417 – 3.12 0.78 312.5
Dean, B.J., Brooks, T.M., Hodsonwalker, G., Hutson, D.H., 1985. Mutat. Res. 153, 57–77.
Del Valle, P., García-Armesto, M.R., de Arriaga, D., González-Donquiles, C., Rodríguez-Fernández, P., Rúa, J., 2016. Food Control 61, 213–220.
Doble, M., Kumar, A., 2005. Biotreatment of Industrial Effluents. Butterworth-Heinemann, Burlington, pp. 169–175.
Ergene, A., Guler, P., Tan, S., Hamzaoglu, E., Duran, A., 2006. Afr. J. Biotechnol. 5, 1087.
Fan, M., Zhang, G., Hu, X., Xu, X., Gong, D., 2017. Food Res. Int. 100, 226–233.
Friesner, R.A., Murphy, R.B., Repasky, M.P., Frye, L.L., Greenwood, J.R., Halgren, T.A., Sanschagrin, P.C., Mainz, D.T., 2006. J. Med. Chem. 49, 6177–6196.
Galanakis, C.M., Goulas, V., Tsakona, S., Manganaris, G.A., Gekas, V., 2013. Int. J. Food Prop. 16, 382–396.
Genheden, S., Ryde, U., 2015. Expert Opin. Drug Discov. 10, 449–461.
Grochowski, D.M., Uysal, S., Aktumsek, A., Granica, S., Zengin, G., Ceylan, R., Locatelli, M., Tomczyk, M., 2017. Phytochem. Lett. 20, 365–372.
Harder, E., Damm, W., Maple, J., Wu, C., Reboul, M., Xiang, J.Y., Wang, L., Lupyan, D., Dahlgren, M.K., Knight, J.L., 2015. J. Chem. Theory Comput. 12, 281–296.
Hassan, W., Noreen, H., Rehman, S., Gul, S., Kamal, M.A., Kamdem, J.P., Zaman, B., da Rocha, J.B.T., 2017. Curr. Top. Med. Chem. 17, 1336–1370.
Ismaya, W.T., Rozeboom, H.J., Weijn, A., Mes, J.J., Fusetti, F., Wichers, H.J., Dijkstra, B.W., 2011. Biochemistry 50, 5477–5486.
İşcan, G., Demirci, F., Kürkçüoǧlu, M., Kıvanç, M., Başer, K.H.C., 2003. Zeitschrift für Naturforschung C 58, 195–200.
Jacobson, M.P., Kaminski, G.A., Friesner, R.A., Rapp, C.S., 2002. J. Phys. Chem. B 106, 11673–11680.
Jiang, Y., Gao, H., Turdu, G., 2017. Bioorg. Chem. 75, 50–61.
Katalinić, M., Rusak, G., Domaćinović Barović, J., Šinko, G., Jelić, D., Antolović, R., Kovarik, Z., 2010. Eur. J. Med. Chem. 45, 186–192.
Kim, J.-S., Kwon, C.-S., SoN, K.H., 2000. Biosci. Biotechnol. Biochem. 64, 2458–2461.
Kwon, S.-H., Lee, H.-K., Kim, J.-A., Hong, S.-I., Kim, H.-C., Jo, T.-H., Park, Y.-I., Lee, C.-K., Kim, Y.-B., Lee, S.-Y., Jang, C.-G., 2010. Eur. J. Pharmacol. 649, 210–217.
Lawrie, W., McLean, J., Garby, El, Younes, M., 1968. Phytochemistry 7, 2065–2066.
Li, Y.Q., Zhou, F.C., Gao, F., Bian, J.S., Shan, F., 2009. J. Agric. Food Chem. 57, 11463–11468.
Loizzo, M., Tundis, R., Menichini, F., 2012. Compr. Rev. Food Sci. Food Saf. 11, 378–398.
Maggi, F., Quassinti, L., Bramucci, M., Lupidi, G., Petrelli, D., Vitali, L.A., Papa, F., Vittori, S., 2014. Nat. Prod. Res. 28, 1354–1363.
Maron, D.M., Ames, B.N., 1983. Mutat. Res. 113, 173–215.
Mollica, A., Zengin, G., Durdagi, S., Ekhteiari Salmas, R., Macedonio, G., Stefanucci, A., Dimmito, M.P., Novellino, E., 2018. J. Biomol. Struct. Dyn. 1–15.
Mortelmans, K., Zeiger, E., 2000. Mutat. Res-Fund. Mol. M. 455, 29–60.
Movahhedin, N., Zengin, G., Bahadori, M.B., Sarikurkcu, C., Bahadori, S., Dinparast, L., 2016. Ind. Crop. Prod. 94, 89–96.
Nachon, F., Carletti, E., Ronco, C., Trovaslet, M., Nicolet, Y., Jean, L., Renard, P.-Y., 2013. Biochem. J. 453, 393–399.
NCI, 2018.https://www.cancer.gov/publications/dictionaries/cancer-drug/def/ chlorogenic-acid.
Negi, P.S., Jayaprakasha, G.K., Jena, B.S., 2003. Food Chem. 80, 393–397.
Pesaresi, A.L., D, 2016.http://wwwrcsborg/pdb/explore/exploredo?structureId=4X3C.
Pettersen, E.F., Goddard, T.D., Huang, C.C., Couch, G.S., Greenblatt, D.M., Meng, E.C., Ferrin, T.E., 2004. J. Comp. Chem. 25, 1605–1612.
Picot, M.C.N., Bender, O., Atalay, A., Zengin, G., Loffredo, L., Hadji-Minaglou, F., Mahomoodally, M.F., 2017. Biomed. Pharmacother. 89, 342–350.
Prasad, N., Ramteke, P., Dholia, N., Yadav, U.C.S., 2018. In: Jungraithmayr, W., Bagchi, D. (Eds.), Immunity and Inflammation in Health and Disease. Academic Press, pp. 341–362.
Release, S., 2018. Maestro, Schrödinger, LLC, New York, NY, 2018.
Rezaei, M., Mohammadi, H.T., Mahdavi, A., Shourian, M., Ghafouri, H., 2018. Int. J. Biol. Macromol. 108, 205–213.
Sastry, G.M., Adzhigirey, M., Day, T., Annabhimoju, R., Sherman, W., 2013. J. Comput. Aided Mol. Des. 27, 221–234.
Sato, K., Toriyama, M., 2009. Molecules 14, 4425–4432. Schrödinger, 2017. New York.
Senejoux, F., Demougeot, C., Cuciureanu, M., Miron, A., Cuciureanu, R., Berthelot, A., Girard-Thernier, C., 2013. J. Ethnopharmacol. 147, 536–539.
Shinohara, M., Sato, N., 2017. Neurochem. Int. 108, 296–302.
Slinkard, K., Singleton, V.L., 1977. Am. J. Enol. Viticult. 28, 49–55.
Tadera, K., Minami, Y., Takamatsu, K., Matsuoka, T., 2006. J. Nutr. Sci. Vitaminol. 52, 149–153.
Tajik, N., Tajik, M., Mack, I., Enck, P., 2017. Eur. J. Nutr. 56, 2215–2244.
Tatsimo, S.J., de Tamokou, J.D., Havyarimana, L., Csupor, D., Forgo, P., Hohmann, J., Kuiate, J.R., Tane, P., 2012. BMC Res. Notes 5, 158.
Truong, X.T., Park, S.-H., Lee, Y.-G., Jeong, H.Y., Moon, J.-H., Jeon, T.-I., 2017. Int. J. Mol. Sci. 18, 1809.
Wang, T., Jonsdottir, R., Ólafsdóttir, G., 2009. Food Chem. 116, 240–248.
Yamada, J., Tomita, Y., 1996. Biosci. Biotech. Bioch. 60, 328–329.
Yamamoto, K., Miyake, H., Kusunoki, M., Osaki, S., 2011. J. Biosci. Bioeng. 112, 545–550.
Zeng, L., Zhang, G., Lin, S., Gong, D., 2016. J. Agric. Food Chem. 64, 6939–6949.
Zengin, G., Nithiyanantham, S., Locatelli, M., Ceylan, R., Uysal, S., Aktumsek, A., Selvi, P.K., Maskovic, P., 2016. Eur. J. Integr. Med. 8, 286–292.
Zengin, G., Senkardes, I., Mollica, A., Picot-Allain, C.M.N., Bulut, G., Dogan, A., Mahomoodally, M.F., 2018. Comput. Biol. Chem. 75, 111–119.